Synopsis
The interactions between hormones, epilepsy, and the medications used to treat epilepsy are complex, with tridirectional interactions which affect both men and women in various ways. Abnormalities of baseline endocrine status occur more commonly in people with epilepsy, and are most often described for the sex steroid hormone axis. Common symptoms include sexual dysfunction, decreased fertility, premature menopause, and polycystic ovarian syndrome. Antiepileptic drugs and hormones have a bidirectional interaction, with a decrease in the efficacy of hormonal contraceptive agents with some AEDs and a decrease in the concentration and efficacy of other AEDs with hormonal contraceptives. Endogenous hormones can influence seizure severity and frequency, resulting in catamenial patterns of epilepsy. However, this knowledge can be used to develop hormonal strategies to improve seizure control in people with epilepsy.
Keywords: Seizure, epilepsy, catamenial, progesterone, estrogen, hormone
I. HORMONAL ABNORMALITIES IN PEOPLE WITH EPILEPSY AND ON AEDS
Several alterations in different hormonal profile have been described for patients with epilepsy. The brain directly regulates hormonal status through hypothalamus – pituitary – endocrine gland feedback loops. Epilepsy itself, with both interictal and ictal effects, and the medications used to treat epilepsy can have direct effects on regulation of these hormone systems. Epilepsy and AEDs can target a number of substrates to affect hormone levels, including the limbic system, hypothalamus, pituitary, peripheral endocrine glands, liver, and adipose tissue [33]. Abnormalities of the sex steroid hormones have been described most frequently, but have also been reported for thyroid hormone levels, prolactin, and vitamin D.
A) Sex Steroid Hormone Axis
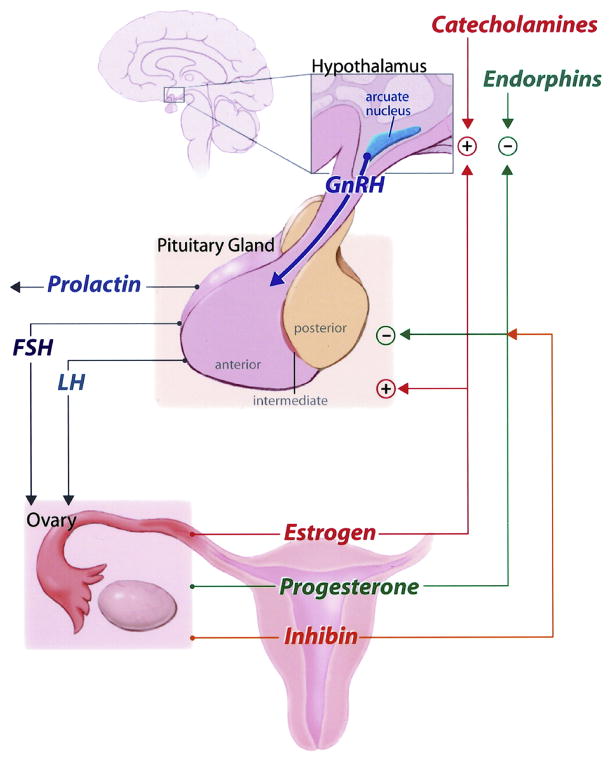
The sex steroid hormones directly affect brain function and excitability. Release of these hormones is controlled by the hypothalamic-pituitary-gonadal axis. The major sex steroid hormones are testosterone from the testis and estrogen and progesterone from the ovaries. The adrenal gland is another primary steroid endocrine gland with significant contribution to androgen synthesis. Peripheral conversion to other biologically active forms also occurs in skin or adipose tissue. The main estrogen in women during reproductive years is estradiol. A complex bidirectional feedback loop occurs with the hypothalamic-pituitary-ovarian axis (Figure 1). Gonadotropin-releasing hormone (GnRH) is secreted by the hypothalamus and stimulates the release of follicle-stimulating hormone (FSH) by the pituitary. FSH stimulates formation of the ovarian follicles, which secrete estradiol as they develop. FSH is inhibited whereas GnRH is stimulated by estrogen. This combination then leads to a surge of luteinizing hormone (LH), which induces oocyte maturation, ovulation, and conversion of the follicle into the corpus luteum. This marks the end of the follicular phase and the beginning of the luteal phase (Figure 2). Following ovulation, the corpus luteum secretes progesterone. The progesterone inhibits secretion of GnRH, FSH, and LH. If there is no pregnancy, the corpus luteum regresses and production of progesterone and estradiol declines. When progesterone secretion tapers off and GnRH inhibition decreases, the cycle repeats. Because of the cyclic nature of hormone release and the direct neuronal effects of estrogen and progesterone or metabolites, women are especially susceptible to the effects of these shifting hormones on seizure frequency and severity.
Fig 1.
Picture of hypothalamic - pituitary – ovarian axis
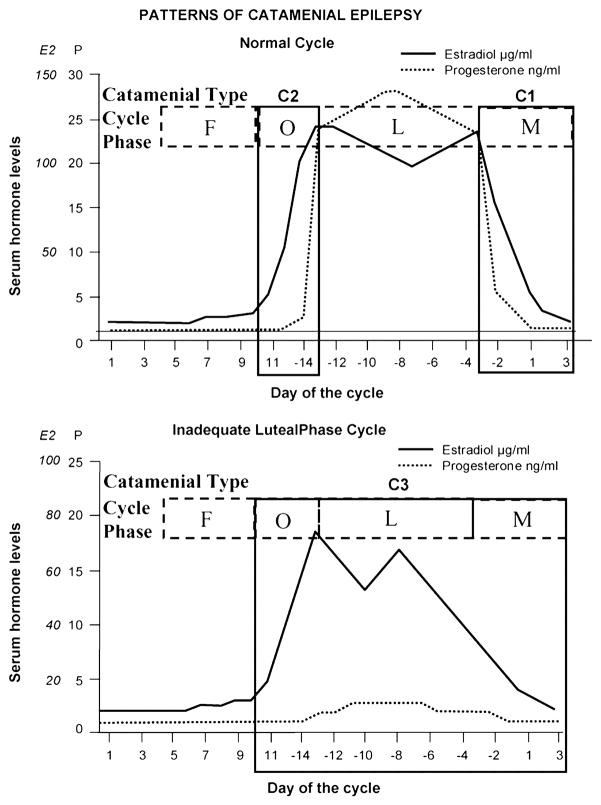
Fig. 2. Three patterns of catamenial epilepsy. During normal, ovulatory cycles, both perimenstrual (C1) and periovulatory (C2) patterns can occur in isolation or together. During inadequate luteral phase cycles, the (C3) pattern can occur with increased seizures during the entire second half of the cycle.
Day 1 is the first day of menstrual flow and ovulation usually occurs at Day 14 in normal cycles. Adapted from Herzog, A.G. [30]
The interaction between the sex steroid hormonal axis, epilepsy, and the medications used to treat epilepsy is complex, with tridirectional interactions which affect both men and women in various ways. Both interictal and ictal discharges have been proposed as altering the sex steroid hormonal axis at the level of the hypothalamus and the pituitary [31]. Alterations in the reproductive hormone axis has been reported to occur commonly in both women and men with epilepsy. The most common reported manifestations in men are decreased sexual function with decreased libido and/or impotence. Women often have menstrual cycle irregularities and may have increased risk of infertility or signs of hyperandrogenism [29]. Both epilepsy and antiepileptic drugs (AEDs) have been causally implicated[33].
B) Hypogonadism
Among the abnormal patterns of reproductive function described in patients with epilepsy, hypogonadotropic hypogonadism is one of the more common ones. Hypothalamic amenorrhea is the most extreme form of this in women with epilepsy, characterized by amenorrhea (no menses) associated with low gonadotropin and estrogen levels and diminished LH response to gonadotropin releasing hormone (GnRH), often tested with a GnRH challenge.
Herzog et al. [29] studied 50 consecutive women with temporal lobe epilepsy. Fifty-six percent had amenorrhea, oligomenorrhea, or abnormally long or short menstrual cycle intervals, and 68% of these women had clearly identifiable reproductive endocrine disorders with polycystic ovarian syndrome (PCOS) in 20%, hypoandrogenism in 12%, severe premature menopause in 4%, and functional hyperprolactinemia in 2%. The estimated frequency for hypoandrogenism in the general population of women is 1.5%. since there was no significant relationship between these menstrual disorder and the use of AEDs, the authors concluded that epilepsy itself may be a significant factor, which stimulated future investigations.
Hypogonadotopic hypogonadism has been found to be more common in women with right temporal lobe epilepsy compared to left temporal lobe epilepsy [36,46]. Herzog et al. [36] studied 30 women with unilateral temporal lobe interictal epileptiform dischages and reproductive disorders. EEG laterality was significantly different for women with PCOS (left sided discharges) and hypogonadotropic hypogonadism (right-sided discharges). Kalinin et al. [46] studied 80 women with temporal lobe epilepsy. They also reported that PCOS was more commonly associated with left TLE, and hypogonadotropic hypogonadims with right TLE. They also reported that a catamenial patter was more common in the women with left TLE.
Other studies supporting the effect of epilepsy itself on reproductive dysfunction include the finding that women with idiopathic generalized epilepsy have higher pulse frequency GnRH secretion than normal controls [8]. Quigg et al. [69] studied LH secretion patterns in 10 men with TLE and in control men. They reported that the pulsatile secretion of LH was abnormal in the TLE group; interictal effects consisted mainly in loss of circadian fluctuations in LH burst amplitude, whereas postictal effects consist of altered burst timing.
In addition to the effects of the underlying disorder of epilepsy and seizures, several studies describe effects on the medications used to treat epilepsy on reproductive endocrine function. Lossius et al. [52] performed a prospective, randomized, and double-blinded study of the effects on reproductive endocrine function of AED withdrawal in 160 men and women with epilepsy, followed for 1 year. Patients were randomized to withdrawal or no withdrawal of AEDs. Blood samples were obtained before and 4 months after withdrawal or no withdrawal in 130 subjects. They reported that reversible endocrine changes in sex steroid hormone levels were observed in both sexes after withdrawal of AEDs. Carbamazepine (CBZ) was the most commonly used drug and withdrawal led to significant increases in serum testosterone concentrations and free androgen index (FAI) in both genders. Abnormalities in these parameters can lead to sexual dysfunction in both men and women with epilepsy.
C) Sexual dysfunction (related to epilepsy and AED effects)
Antiepileptic drugs can have a major impact on circulating sex steroid hormone levels, with affects on sexual function. Herzog et al [38] studied men with localization-related epilepsy (LRE) on various AED regimens, on no AEDs, and control men without epilepsy. Sexual function scores (S-Scores) were obtained by a four-question self-administered questionnaire. Serum measurements included bioactive testosterone (BAT), bioactive estradiol ( BAE), BAT/BAE, sex hormone binding globulin (SHBG), and luteneizing hormone (LH). Gonadal efficiency was calculated as BAT/LH. Almost one-quarter of the men with epilepsy (MWE) had sexual dysfunction. S-scores were even lower in the men on enzyme-inducing AEDs (EIAEDs) compared to the MWE on lamotrigine (LTG) and compared to the control men. BAT levels correlated with S scores for men on EIAEDs, and were lower in this group as well as BAT/BAE, BAT/LH ratios, compared to control groups or the MWE on LTG.
A study of 40 men with refractory localization-related epilepsy by Kuba et al. [50] in the Czech Republic investigated sexual dysfunction and hormonal profiles. At least one of the types of sexual dysfunction was found in 55% of patients, with erectile dysfunction in 15%, orgasmic dysfunction in 15%, and loss of sexual desire in 40%. In the group of men with sexual dysfunction, there was an increase of FSH and SHBG, and a decrease of DHEAS and FAI in comparison with patients with normal sexual function. An increase in estrogen was also seen in men with erectile dysfunction. The observation was made that all patients with orgasmic dysfunction were being treated with CBZ, but when all patients with at least one type of sexual dysfunction were analyzed, a higher proportion were on VPA than CBZ.
Testosterone supplementation can be an effective treatment for hypogonadal men with epilepsy and sexual dysfunction. It may improve not only sexual function, but mood and even seizure frequency [33].
Morrell and Flynn [56] studied sexual function and hormones in women with epilepsy 18 to 40 years with localization-related epilepsy, primary generalized epilepsy (PGE), and nonepilepsy controls. Questionnaires were administered examining sexual experience, arousability, anxiety, and depression (Beck Depression Inventory). Endocrine assessment was performed during the early follicular phase. Compared to the controls, women with LRE had significant higher sexual dysfunction scores, lower mean arousal, and higher depression scores. Mean arousal scores were also lower in the PGE group. Women on EIAEDs, combined into one group, had statistically higher sexual dysfunction and lower sexual arousal compared to controls Overall, estradiol negatively correlated with sexual anxiety, and dehydroepiandrostone (DHEAS) negatively correlated with sexual dysfunction and positively correlated with sexual arousal.
Another study reported similar adverse findings of altered hormone blood levels in association with lower sexual quality of life in men with epilepsy, but interestingly, not women with epilepsy [55]. Seventy-nine patients with different types of epilepsy or no epilepsy completed the Derogatis Interview for Sexual Function-Self-Report Inventory and had blood drawn for measurement of hormone studies. In men, increasing sex hormone-binding globulin (SHBG) levels and duration of epilepsy decreased sexual quality of life. SHBG levels were related to enzyme-inducing antiepileptic drugs (EIAEDs) and age. Women did not demonstrate an association between any hormone levels and sexual quality of life.
In summary, men and women with epilepsy are susceptible to sexual dysfunction. Questions about sexual function should be part of the routine evaluation in the outpatient clinic. The etiologies of sexual dysfunction in men and women with epilepsy are multifactorial, but AED type is one contributing factor that can be modified when indicated and available evidence suggests non-EIAEDs show more favorable profiles.
D) Fertility and Childbirth rates
One study in North America compared fertility rates in 863 married adults with idiopathic/cryptogenic epilepsy and same-sex siblings without epilepsy. Reduced fertility rates occurred for both MWE and WWE after but not before the onset of epilepsy. Factors reducing fertility rates further were localization-related epilepsy and early age at onset [80].
Another study reported that the reduced rate of childbirths held in married women with epilepsy (69% of expected number of live-born children) but did not hold in married men with epilepsy [13]. A population- based cohort study in Finland (1985–2001) of patients with newly-diagnosed epilepsy (n=14,077) reported that childbirth rates were lower than in a reference cohort among men (hazard ratio = 0.58 (95% CI 0.54-0.62)) and women (HR=0.88 (0.83–0.93)) [2]. However, a comparable study from Iceland suggested no difference in childbearing rates for women with epilepsy [60]. Geographic and cultural differences are likely substantial factors and these will influence self-directed decisions. The “Ideal World” survey of Epilepsy Action UK women found that 33% of the women were not considering having children because of their epilepsy [12].
For men with epilepsy, abnormalities on semen analysis have been reported, including decreased sperm count, abnormal morphology, or impaired motility, with some reports as high as 90% of men with epilepsy [33].
E) Premature Menopause
Premature menopause is characterized by amenorrha, cessation of ovarian function and elevated gonadotropin levels. This premature ovarian failure occurs more commonly in women with epilepsy. Klein et al. [47] evaluated the incidence of premature ovarian failure in women with epilepsy in 50 women with epilepsy, aged 38–64yo, and compared to control women. Premature menopause was defined as amenorrhea for greater than 1 year with elevated Day 3 FSH levels in women younger than 42 years. Premature perimenopause was defined by the presence of perimenopausal symptoms. Of the women with epilepsy, 14% had premature perimenopause or menopause, compared to only 3.7% of the control women (p=0.042). They did not find an association with epilepsy duration, seizure severity, or AEDs, although women with premature menopause were more likely to have had catamenial exacerbation of their seizures than were women without POF (p=0.02).
Harden et al. [26] also found premature menopause in their multicenter cohort of women with epilepsy. The median age at menopause in the group of women with epilepsy was 47 years, compared to the median age of 51.4 years in the general US population US [22]. They also divided the patients into low, intermediate, and high seizure frequency groups; they found an increasingly lower age at menopause with a negative correlation between the age at menopause and seizure group based on estimated lifetime seizures (p = 0.014). They also found no influence of enzyme-inducing AEDs. The authors concluded that the association of lifetime number of seizures with the timing of cessation of reproductive cycling may occur due to disruption of hypothalamic and pituitary function directly by the seizures.
F) Polycystic Ovarian Syndrome
The polycystic ovarian syndrome (PCOS) is characterized by enlarged ovaries with multiple small cysts and a hypervascularized, androgen-secreting stroma leading to the associated signs of androgen excess (hirsutism, alopecia, acne), obesity, and menstrual-cycle disturbance (oligo- or amenorrhea) [4]. This common endocrine disturbance occurs in approximately 4–7% of women of reproductive age in the general population, but in 10–25% of women with epilepsy [6,33,48].
The definition of PCOS has been a source of debate and varies between Europe and North America, with a more strict criteria adopted by North America. Minimal diagnostic criteria were defined by a 1990 NIH Consensus Panel: 1) menstrual irregularity, 2) biochemical or clinical evidence of hyperandrogenism, 3) and exclusion of other diseases that could cause female hyperandrogenism (such as congenital adrenal hyperplasia) [54]. These North American minimal criteria do not include the need to identify the polycystic component of the ovaries by ultrasound. The signs and symptoms expressed by women with PCOS vary substantially (Table 1) [4]. There is a familial tendency with some recent discoveries of potential underlying genetic contributions with variants of luteneizing hormone (LH), the pathway for androgen biosynthesis and of regulation of expression of the insulin gene [4]. Women with PCOS are at long term risk of developing diabetes and cardiovascular disease.
Table 1.
Antiepileptic drug effects on hormonal contraceptive agents
| Lowers Hormone Levels | No Significant Effects |
|---|---|
| Carbamazepine | Ethosuximide |
| Oxcarbazepine | Gabapentin |
| Phenobarbital | Lacosamide |
| Phenytoin | Lamotrigine |
| Primidone | Levetiracetam |
| Rufinamide | Tiagabine |
| Topiramate | Valproate |
| Zonisamide |
One of the most influential factors that affect the expression of PCOS is weight; weight gain causes a worsening of symptoms, and weight loss improves the endocrine and metabolic profile. Yet women with PCOS are prone to eating disorder, possibly due to a link with leptin. This is one of the reasons why the interplay between epilepsy, the use of VPA, weight gain, and PCOS has been so difficult to tease out. The underlying epilepsy itself likely contributes to the expression of PCOS via effects on hypothalamic control of the menstrual cycle with the tendency toward more anovulatory cycles and oligomenorrhea. It is likely that both the underlying epilepsy disorder and AED treatment play roles in the development of PCOS [54].
Herzog, et. al. [41] was one of the first groups to publish on the finding of PCOS in women with epilepsy. He reported that PCOS occurs significantly more often in women with TLE than in the general female population and is associated with predominantly left-sided lateralization of interictal epileptic discharges. These findings suggest that the underlying epilepsy contributes to the development of PCOS via the hypothalamic-pituitary-gonadal axis. Bilo, et al. also reported a higher incidence of reproductive endocrine disorders including PCOS, but in women with idiopathic generalized epilepsy syndromes [7]. Neither group found an association with the type of AED being taken by the women.
Several studies by Isojarvi et al. reported that the higher incidence of PCOS in WWE was specifically associated with VPA use. One of the probable reasons for discrepancies in different studies is due to the differing definitions for PCOS. A study of 238 WWE in Finland on a variety of AEDs included vaginal ultrasonography and serum sex-hormone concentrations [42]. Unlike the North American criteria for PCOS, they reported on findings of polycystic ovaries as an isolated finding as well as elevated testosterone concentrations. The VPA monotherapy group included findings of polycystic ovaries (43%) and elevated testosterone concentrations without polycystic ovaries (17%). Notably, 80% of the group with that began VPA treatment before 20 years old had polycystic ovaries or hyperandrogenism
A later study by Isojarvi, et al. [44] enrolled 16 women taking VPA for epilepsy that had polycystic ovaries or hyperandrogenism, They were converted to lamotrigine (LTG) monotherapy and followed for one year (n=12 completers). The additional findings of weight gain, hyperinsulinemia, and lipid profiles were monitored. During the first year after drug conversion, the number of polycystic ovaries, BMI, fasting serum insulin and testosterone concentrations decreased, and the HDL/total cholesterol ratios increased.
A recent examination of 148 women with epilepsy (WWE) by epilepsy type and AED use by Lofgren, et.al. [51] reported a higher prevalence of reproductive endocrine disorders in women with idiopathic generalized epilepsy than control subjects, but the specific findings of hyperandrogenism, polycystic ovaries, and polycystic ovary syndrome were more prevalent in WWE on VPA than in WWE taking other drugs or control women. The use of VPA and younger age predicted the development of hyperandrogenism.
Morrell, et al. [57] studied 447 women prospectively with randomization to initiating 12 months of treatment with either VPA or LTG. More women in the VPA group than the LTG group developed ovulatory dysfunction (54% vs. 38%; p = 0.010) and more women in the VPA group than the LTG group developed PCOS (9 vs 2%; p = 0.007). Development of hyperandrogenism was more frequent with VPA than LTG among those initiating treatment at age younger than 26 years (44% vs. 23%; p = 0.002) but was similar if treatment was started at age 26 years or older (24% vs. 22%).
In conclusion, WWE are a group at risk for reproductive health disorders regardless of AED use. Questions about reproductive health should be part of the evaluation of women with epilepsy, both during the initial evaluation and periodically during follow-up visits. History should include menarche, menstrual patterns and regularity, fertility problems, as well as assessment of hirsutism, acne, and weight and height measurements [54]. When findings of reproductive disorder are obtained, one should consider referral to a reproductive endocrinologist, gynecologist or endocrinologist familiar with these issues. If women have signs of obesity, hyperandrogenism, of menstrual irregularities, then consideration should be given to avoidance of VPA use in women of reproductive age for these risks, in addition to the known increased risk for anatomical and neurodevelopmental teratogenecity [27]. Some experts suggest that women who are treated with VPA should not only be frequently monitored for weight gain, but also undergo laboratory investigation on a yearly basis for lipid and glucose metabolism, hyperandrogenism, and ultrasound for polycystic ovaries with more frequent monitoring if they develop menstrual pattern changes [54]. If PCOS develops, then the AED regimen should be reconsidered as to whether alternatives could still provide good seizure control given the long term health consequences and the potential to reverse the VPA-related risks by substituting VPA with another AED [44].
G) Thyroid
Several studies evaluating thyroid function status in patients with epilepsy suggest that there are no alterations related directly to epilepsy, but there can be some alterations in thyroid function tests associated with some of the AEDs. However, these laboratory abnormalites may not be clinically significant (Herzog 2008). A study by Isojarvi, et al. [45] of 90 men with epilepsy on either CBZ, VPA, or OXC and 25 control men demonstrated that serum thyroxine (T4) and free thyroxine (FT4) concentrations were low in men taking CBZ or OXC with 45% and 24% falling below the reference range, respectively, but serum triiodothyronine and thyrotropin (TSH) levels were normal. In men taking VPA, the concentrations of thyroid hormones and TSH were normal.
Another study in children with new-onset epilepsy [82] was performed prospectively with baseline thyroid testing prior to drug administration, followed by testing at 3, 6, and 12 months after initiation of CBZ or VPA; age-matched subjects served as controls. The epilepsy group included subjects with partial epilepsy and subjects with generalized epilepsy. Thyroid function tests were normal at baseline in all subjects. After 3 months and persisting until 12 months, CBZ-treated patients demonstrated significantly lower serum T4 and fT4 levels compared to baseline evaluation and control subjects, but normal serum T3 and TSH response to a TRH test. Some of the CBZ-treated children were withdrawn from CBZ for other reasons, and reevaluation 6 months later revealed renormalization of their values. All TFT values remained normal in the VPA group. Even with the alterations in the CBZ group, the authors concluded that the patients are euthyroid and therefore, thyroid hormone alterations are not associated with clinical or subclinical hypothyroidism.
Gomez, et al. [23] studied 96 patients on a variety of first-generation AEDs. They observed decreased T4 serum levels and free T4 index below the normal ranges (in 25 and 14 patients, respectively), decreased rT3, but normal levels of free T4, T3, and TSH. They concluded that the TSH was the best measurement to reflect the euthyroidism of these patients.
Studies of other AEDs, especially the enzyme-inducing AEDs, have demonstrated somewhat similar findings of a tendency toward lower serum concentrations of thyroxine (T4), free thyroxine, and thyroid-binding globulin while maintaining TSH at normal or slightly elevated levels [33]. Thyrotropin response to thyrotropin-releasing hormone is either unchanged or slightly increased as well [43,81].
Another study of 35 patients on long-term PHT or CBZ [81] reported that the mean concentrations of T4, FT4, FT3, and rT3, but not T3, of these patients were significantly lower than those of 19 controls of similar age and sex distribution. The mean serum TSH concentration was slightly but significantly higher in patients than in controls, but the serum TSH response to TRH was not significantly increased. Several other metabolic studies in these patients did not show other clinical signs of functional hypothyroidism and they assessed response to thyroxine treatment in a smaller subset of subjects. These authors concluded that, on the basis of all data from the cross-sectional and thyroxine treatment studies, patients receiving AEDs chronically are eumetabolic and do not need thyroxine supplementation.
Since clinically significant thyroid disorders occur very rarely with AED use, routine testing of thyroid function is generally not encouraged for patients on chronic AED therapy; exceptions would be when the patient exhibits potential signs of clinical hypo/hyperthyroidism or when the patient has a preexisting thyroid disorder. When thyroid function tests are obtained, any findings of lower concentrations of total or free thyroid hormones need to be considered in the context of the TSH, and other secondary metabolic effects [81]. Any benefit of thyroid supplementation needs to be weighed against the possible exacerbation of seizures in people with epilepsy.
H) Prolactin
Following seizures, a rise in serum prolactin levels has been reported [5] [10,68]. In one study of measurements of over 500 seizures, increased prolactin levels were measured in 88% of generalized tonic-clonic seizures, 78% of complex partial seizures, and 22% of simple partial seizures [5]. Absence seizures do not lead to a prolactin increase [7]. The mechanism is thought to be the propagation of the electrical activity (usually from the temporal lobe) to the hypothalamic-pituitary axis, causing the release of the hormone. A rise is seen in approximately 60% of complex partial seizures and may be related to the intensity and duration of the seizure; generalized convulsive seizures are even more likely to be associated with a postictal rise in prolactin [10]. Since prolactin does not usually rise after psychogenic seizures, some authors propose that postictal prolactin levels can be used to differentiate between epileptic and psychogenic seizures. However, caution should be used since a rise in prolactin is not detected after all epilepsy seizures, and timing of the blood draw is critical and should be within 30 minutes after the seizure. Only in the case of a prolactin rise may one conclude that an epileptic seizure had occurred. Additionally, prolactin may show a decrease in its postictal release, most commonly with flurries of seizures or status epilepticus.
I) Bone Health
Bone disease occurs in both men and women with epilepsy and even in some children with epilepsy. Aging women can be at greater risk with estrogen loss that occurs in menopause. AED exposure is a cause of secondary osteoporosis with decreased bone mineral density (BMD) secondary to poor bone accrual in children or accelerated bone loss in adults. Fracture rates are higher in persons with epilepsy, due to multifactorial causes including increased bone turnover with some AEDs, higher rates of osteopenia and osteoporosis, and falls due to seizures or medication side-effects.
One differential risk factor is AED selection. Studies have cited decreased bone mineral density, increased markers for bone turnover, and/or fractures in patients on the enzyme-inducing AEDS and valproic acid, but the most consistent finding has been changes in bone metabolism and increased turnover and lower bone mineral density associated with phenytoin use [61,63]. Pack, et. al [63] studied premenopausal WWE on different monotherapy regimens. They reported that calcium concentrations were significantly lower in subjects on either CBZ, PHT, or VPA monotherapy compared to women on LTG monotherapy (p = 0.008). Additionally, the PHT group demonstrated increases in markers of bone turnover.
All patients with epilepsy should be advised to receive at least the recommended daily allowance of calcium and vitamin D for their age, gender, and menopausal status. Recommendations vary for routine screening, and no consensus has been reached thus far. Some experts advise screening of 25-OH-vitamin D levels and bone mineral density screening for prolonged AED exposure, especially if other risk factors are present [62]. These screening measures should occur earlier if the woman is on phenytoin. If osteopenia is found, it is helpful to reinforce calcium and vitamin D daily requirements; if osteoporosis is found, then one should consider referral to an internist or endocrinologist for possible treatment interventions, such as with a biphosphonate. If the patient is on phenytoin, then conversion to another AED should be considered.
II. ANTIEPILEPTIC DRUGS AND HORMONAL CONTRACEPTIVE INTERACTIONS
A) Reduced efficacy of contraceptive hormones due to AEDs
Many of the AEDs induce the hepatic cytochrome P450 system, the primary metabolic pathway of the sex steroid hormones, and induce the production of sex hormone binding globulin (SHBG). This leads to a lower concentration of free hormone to exert its targeted action and a more rapid clearance of the steroid hormones. This can allow ovulation in women taking oral contraceptive pills (OCPs) or other hormonal forms of birth control and contribute to the relatively high number of unplanned pregnancies in this patient population [24]. One recommended strategy is to prescribe an OCP containing ≥ 50 micrograms of ethinyl estradiol daily. However, more popular trends in the general population of women of childbearing age have been to prescribe especially low concentration hormonal contraceptives (through OCPs or other routes such as vaginal rings). Patients should be warned that midcycle bleeding indicates possible OCP failure but that the absence of such bleeding is not an indication of adequate contraceptive effectiveness [24]. The transdermal patch and vaginal ring formulations also have higher failure rates with these AEDs. Intramuscular medroxyprogesterone provides higher dosages of progestin but still may require dosing at 8–10 week intervals rather than 12-week intervals. Two types of IUD are available (hormone releasing and copper). The Mirena IUD is impregnated with a progesterone, but since its effect is at the local level of the endometrium AEDs are unlikely to have an effect on its efficacy.
The 1998 guidelines by the American Academy of Neurology recommend use of an estradiol dose of 50 micrograms or its equivalent for 21 days of each cycle when using oral contraceptive agents with the enzyme-inducing AEDs [75], but no studies have addressed whether this improves contraceptive efficacy. A higher progestin component may be as important as the estrogen component to prevent ovulation, but the combined oral contraceptive pills with a higher estradiol dose do usually also have a higher progestin content. Since this may still not be adequate protection against pregnancy, a backup barrier method is recommended. Table 1 lists effects of the individual AEDs on hormonal contraceptive agents [24,49].
The two newest AEDs approved in the US have undergone interaction studies. Lacosamide studies of drug-drug interactions with a combination oral contraceptive pill containing ethinylestradiol and levonorgestrel demonstrated no pharmacokinetic interactions. Its primary route of elimination is renal excretion and biotransformation. Rufinamide has been studied for hormonal contraceptive interaction. Co-administration with a common combination pill resulted in a mean decrease in the AUC and the Cmax for both ethinyl estradiol and norethindrone. Therefore, additional non-hormonal forms of contraception are recommended.
Davis et al. [14] administered a cross-sectional questionnaire to women with epilepsy (18–44 years) in an urban, academic medical center. They classified methods of contraception as highly effective (≤10% experience pregnancy in the first year of typical use) or less effective (>10% will experience pregnancy in the first year of typical use). Half of the pregnancies in these women were unplanned, and among those who were sexually active in the past month, 74% used contraception but only 53% used highly effective methods of contraception (sterilization, intrauterine device, hormonal pill (any formulation), patch and injection). Among the participants using hormonal contraception, 29% were on EIAEDs, with probable increased risk of unplanned pregnancy. One of the concerns these authors raised was that overall, highly effective methods of contraception were underprescribed, such as intrauterine devices, intramuscular medroxyprogesterone, and even any form of OCPs. The effectiveness of OCPs in the setting of EIAEDs is still likely higher than that with condoms or withdrawal. This could be due to practitioner confusion about the interactions or worry about the possible effect of estrogen on seizure control. Despite the well-known effects of estrogen on lowering seizure threshold, observational studies have not shown that estrogen-containing OCs worsen seizures in women with epilepsy [24,83].
B) Influene of Exogeneous Hormones on AED concentrations
Studies in women with epilepsy have highlighted that during pregnancy clearances of AEDs increase [65]. Although serum concentrations of all AEDs are reduced during pregnancy, the magnitude of alterations in lamotrigine concentrations exceeds that described for the older AEDs, which are primarily eliminated via the cytochrome P450 system [64]. Approximately 90% of LTG undergoes hepatic glucuronidation, catalyzed by UGT1A4, an isozyme of the UGT family of enzymes. This elimination pathway appears particularly susceptible to activation during pregnancy, possibly as a result of direct effects of rising sex steroid hormone levels [66] The effect of sex steroid hormones on LTG metabolism is not unique to pregnancy. Several recent studies have demonstrated increased clearance in LTG in women on hormonal contraceptives. In a study of 22 women on LTG in combination with oral contraceptives (OC) and 30 women on LTG without OC, the LTG plasma levels relative to dose and body weight were significantly reduced by >50% with co-administration of oral contraceptives [78]. A more recent prospective study separated the contraceptive group according to whether the women were on ethinyl estradiol (EE)-containing preparations or progesterone-only containing compounds [74]. LTG serum concentration-to-dose ratio (CDR) was significantly lower in women using EE than in the control group; the CDR of the progesterone group was not different from controls. Of the women who switched from the control to the EE group, considerable reductions in their CDRs occurred, and an increase in CDR occurred in the two women who changed from EE to progesterone-containing compounds. It appears that it is the estrogen component of contraceptive preparations that induces the increase in LTG metabolism.
A doube-blind placebo-controlled trial demonstrated that cessation of OCPs leads to an 84% increase in the concentration of LTG, and the change in LTG concentration occurs within 1 week of the shift in OCP status [9]. A study of HRT in postmenopausal women with epilepsy also noted that subjects randomized to receive HRT had a decrease in lamotrigine levels of 25–30% while taking CEE/MPA [25]. These finding should be considered when treating women during their reproductive and postmenopausal years. Initiation or discontinuation of estradiol-containing compounds may necessitate LTG serum concentration monitoring and dosage adjustment. Although none of these studies directly evaluated whether the alterations in LTG pharmacokinetics were clinically relevant with regard to seizure control or toxic side-effects, it is unlikely that a controlled, randomized study would be performed to demonstrate seizure worsening with lowering of LTG serum concentrations by coadministration of exogenous ethinyl estradiol. An observational study in women on LTG during pregnancy did demonstrate that lower LTG concentrations that occurred with the rising endogenous sex steroid hormone concentrations were predictive of worsening seizure frequency [66].
The discovery that glucuronidation can be activated by hormonal shifts may apply to other AEDs. Metabolism of VPA is 30–50% by glucuronidation, and 50–60% of the clearance of oxcarbazepine (OXC) is via glucuronidation. Similar observations about increased clearance with possible worsening seizure control, have been made about OXC during pregnancy, but reports about effects of concomitant OCP use are lacking [1,65]. Findings of a study of VPA with OCP use in women were that total and unbound VPA concentrations were higher during the OCP-free interval than during the OCP interval [21]. VPA clearance increased by 21.5% for total VPA and 45% for free VPA. The authors also commented the magnitude of the change varies across individuals, being potentially clinically relevant in some cases and recommended monitoring of serum VPA concentrations when adding or discontinuing OCPs and possibly during the on-off intervals of an OCP cycle.
A recent study of VPA and LTG use with OCPs also demonstrated that total VPA and LTG levels were lower (23% and 33%, respectively) on active OCP than on inactive pill [37]. The authors also noted that with endogenous hormone fluctuations during normal menstrual cycles not on OCPs, serum LTG concentrations were lower during the mid-luteal phase, but the finding was not significant.
III. HORMONAL INFLUENCE ON SEIZURE SUSCEPTIBILITY
Both female patients with epilepsy and their treating physicians have observed a tendency for seizures to cluster in relationship to the menstrual cycle during the reproductive years. This phenomen has been the source of much investigation in humans with epilepsy and in animal studies. It has been attributed to the neuroactive properties of sex steroid hormones and the cyclic variation of their serum levels. Results of animal studies of the effects of 17beta-estradiol (estradiol) and progesterone are compelling. Estradiol has been shown to increase seizure activity, while progesterone has been shown to have anticonvulosant properties [20]. Many cellular and molecular mechanisms have been identified contributing to the changes in brain excitability mediated by these hormones.
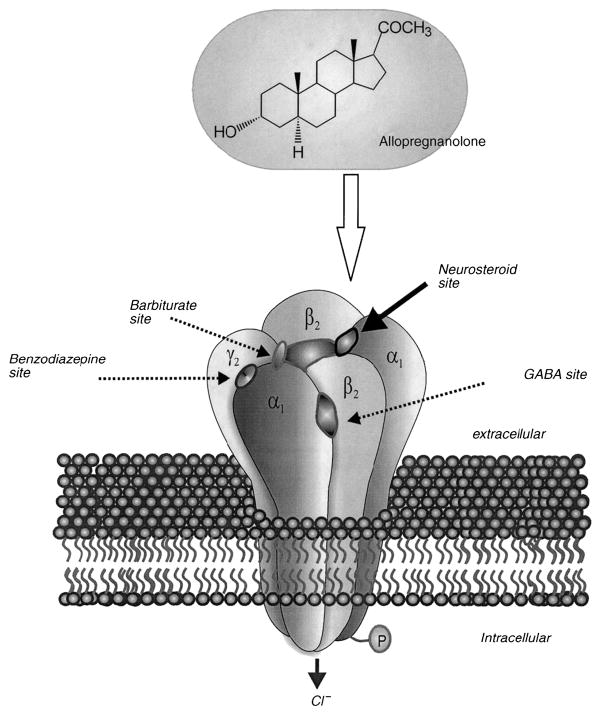
In animal studies, estrogen is generally proconvulsant [70]. Estradiol can create new cortical seizure foci when applied topically, activate preexisting cortical foci, increase the severity of pentylenetetrazol-induced seizures, lower the electroshock seizure threshold, and increase the number of dendritic spines and excitatory synapses on hippocampal CA1 pyramidal cells. Mechanisms are complex and likely include estrogen receptor-mediated effects, and action at glutamate and NMDA receptors. Progesterone, conversely, generally has an anticonvulsant effect [70,73,79]. Animal studies demonstrate that progesterone (PROG) elevates the seizure threshold, suppresses kindling, and decreases interictal spikes caused by cortically-applied penicillin. Potential mechanisms include action at the progesterone receptor, but also through metabolism to GABAA receptor modulating neuroactive steroids. Several studies suggest that allopregnanolone (ALLO) is the primary compound responsible for decreased seizure susceptibility when PROG is elevated and the anticonvulsant actions of PROG administration [79]. Putative steroid binding sites have been described on the GABAA receptor (Figure 2).26
Although many NAS can be synthesized in the brain from precursors such as PROG, experimental evidence demonstrates that blood levels correlate with seizure frequency and that administration of neuroactive steroids systemically can provoke or protect against seizures or the development of epilepsy }[72,73].29,30 Even natural fluctuations in sex steroid hormone levels during the menstrual cycle can affect not only epilepsy, but other neuropsychiatric symptoms including premenstrual syndrome, perimenstrual migraines, and dysphoria. Withdrawal from progesterone after chronic administration to rats via subcutaneous route results in increased seizure susceptibility [71].
Androgens can also worsen seizures and some are felt to be proconvulsant in experimental models [76], but given that major fluctuations do not occur except during puberty and gradually with aging, a regulatory role on seizure control is harder to demonstrate in men with epilepsy.
A) Catamenial Epilepsy during the Reproductive Years
In women with epilepsy during the reproductive years, a correlation has been observed between the cyclic monthly levels of estrogen and progesterone and seizure frequency [3,40]. Catamenial epilepsy is the term applied when the pattern of seizure occurrence in a woman fluctuates with the menstrual cycle. The reported prevalence of a catamenial epilepsy among women varies due to differing definitions and few formal studies, with reports that it affects between 20–70% of women with epilepsy [15,16,19] [30]. Work by Herzog et al. has led to a more uniform acceptance of the definition of catamenial epilepsy as a two-fold increase in daily seizure frequency during specific phases of the menstrual cycle [30].
Herzog et al. [40] studied a cohort of 184 women with medically-refractory localization-related epilepsy and were able to define three distinct patterns of catamenial epilepsy based on statistical evaluation of seizure frequency in women during their reproductive years. The definitions are based on Day 1 as the first day of menstrual flow and ovulation is presumed to occur 14 days before the subsequent onset of menses (Day −14). Seizures and menses were charted and midluteal serum progesterone levels were obtained on day 22. Progesterone concentrations >5 ng/ml were considered ovulatory. Cycles were divided into four phases: menstrual (M) = −3 to +3, follicular (F) = 4 to 9, ovulatory (O) = 10 to −13, and luteal (L) = −12 to −4 (Figure 3). Average daily seizure frequency for each phase was calculated and compared among phases, separately for ovulatory and anovulatory cycles. The seizures recorded during ovulatory cycles occurred with significantly greater average daily frequency during the menstrual and ovulatory phases than during the follicular and luteal phases, in patterns labeled perimenstrual (catamenial 1) and periovulatory (catamenial 2). For the anovulatory cycles, seizures occurred with lower frequency during the follicular phase than during all other phases, in a pattern labeled catamenial 3 by the authors (Figure 3). The authors went on to plot of the percentage of women with greater seizure frequency according the three patterns of catamenial epilepsy and evaluated points of inflection of the S-shaped distribution curves for optimal distinction between women with high and low susceptibility to hormonal influence. Based on these findings, these authors proposed a twofold increase in average daily seizure frequency during these phases in one of the three patters for the designation of catamenial epilepsy. In their patient cohort, one-third of women showed at least one of the three patterns of catamenial epilepsy.
Figure 3.
Schematic diagram of allopregnanolone and its neuroactive steroid binding site on the GABAA-BDZ receptor
A follow-up study by Herzog et al. [39] in 87 women found that 39% of women enrolling in a progesterone treatment trial demonstrated a catamenial pattern. This study assessed 87 women over a few menstrual cycles rather than just one cycle. Catamenial epilepsy designation was made if two of three cycles showed at least one of the three catamenial patterns. Another study by El-Khayat et al [16] of 42 Egyptian women with epilepsy (and 21 controls) evaluated the pattern of seizure occurrence and hormone levels. Additionally, pelvic ultrasound was performed near the time of ovulation. A catamenial pattern of seizures occurred in 31% of patients (53.8% C1 pattern; 46.15% C3 pattern). Patients with C3 pattern showed lower progesterone levels in the midluteal phase compared to patients with noncatamenial pattern, to those with C1 pattern or to controls. Patients with the C1 pattern had lower progesterone levels than controls in the menstrual phase. The patients with a catamenial pattern also had higher estradiol/progesterone ratios.
Although approximately one-third of women with localization-related epilepsy have a catamenial pattern, this cyclic pattern is often under-recognized or under-acknowledged by physicians treating women with epilepsy. Often the woman is the one to bring it up to her physician, but still too often her concerns are met by indifference or even doubt [58]. Perhaps one of the reasons is the perceived lack of treatment options. No similarly rigorous studies have been performed in women with idiopathic generalized epilepsy, but would be more difficulty given that seizures are often more readily controlled. Patients will often report, however, that their rare seizures only occur during the perimenstrual phase.
B) Epilepsy during other Reproductive Life Stages
Alterations in seizure patterns can occur with other major hormonal shifts, such as during puberty, pregnancy, and perimenopause. Some epilepsy syndromes are first expressed or worsen during puberty [11]. Perimenopause is marked by erratic and frequently high estrogen levels, while postmenopause is characterized by stable, low estrogen levels. A retrospective questionnaire study suggested that seizure frequency can increase with perimenopause and can improve once the menopausal transition is complete [28]. This alteration in seizure pattern was more likely to occur in women who experienced a catamenial pattern during their reproductive years. In the postmenopausal group, hormone replacement therapy (HRT) was significantly associated with an increase in seizures.
The latter finding led to design and implementation of a multicenter, double-blind, randomized, placebo-controlled trial of the effects of two doses of Prempro (CEE/MPA) (0.625mg of conjugated equine estrogens, plus medroxyprogesterone acetate, 2.5 mg) on seizure control [25]. Subjects received either placebo, single dose CEE/MPA, or double dose CEE/MPA. Twenty-one subjects were randomized after completing baseline. Five out of seven subjects (71%) on double-dose CEE/MPA had a worsening seizure frequency of at least one seizure type, compared to 4/8 (50%) on single dose CEE/MPA and 1/6 (17%) on placebo (p=0.05). Increasing CEE/MPA dose was also associated with an increase in the frequency of the subject s most severe seizure type (p=0.008) and of complex partial seizures (p=0.05). Despite the limited enrollment of this study, the conclusion could still be made that CEE/MPA was associated with a dose-related increase in seizure frequency in postmenopausal women with epilepsy.
This study in postmenopausal women with epilepsy was halted early due to findings from the Women’s Health Initiative (WHI) study: overall health risks after five years of follow-up clearly exceeded benefits from use of single dose CEE/MPA in healthy postmenopausal US women [77]. Although the released findings from the WHI study has resulted in decreased long-term HRT use for preventive health measures, HRT is still frequently used as treatment for perimenopausal symptom relief. The findings from study by Harden, et al. [25] raise caution even for the short-term use of CEE/MPA in women with epilepsy. However, synthetic progestins such as medroxyprogesterone acetate are not metabolized to the active neurosteroid allopregnanolone. Future studies of whether an estrogen with natural progesterone could circumvent the risk for increased seizures would be beneficial for women with epilepsy.
IV. Hormones as Therapies in People with Epilepsy
The benefit of hormonal therapy in the form of ACTH has long been recognized for infantile spasms, but it is only recently that investigation has extended to the use of sex steroid hormones or their analogs for the treatment of other epilepsies. An understanding of the effects of hormones on seizure susceptibility may provide an opportunity to use hormones as therapy in women with intractable epilepsy.
A) Cyclic Progesterone
Open-label studies of progesterone supplementation have shown promising results (Table 3). Herzog et al. studied 8 women with localization-related epilepsy and inadequate luteal phase cycles with catamenial exacerbation [32]. Progesterone was administered as vaginal suppositories during the premenstrual phase or entire second half of the cycle in doses of 50 to 400 mg BID as adjunctive therapy to standard AEDs. The average monthly seizur e frequency declined by 68% in the 3-month treatment period compared with the 3 months prior to therapy, with 6 of the 8 women experiencing decreased seizure frequency. Adverse effects included mild sleepiness and symptoms of depression, but resolved with lowering of the dose.
A later open-label trial by Herzog et al. [34] of 25 women with medically-refractory temporal lobe epilepsy included women with either anovulatory or ovulatory cycles and with the associated catamenial patterns. Progesterone was administered as oral lozenges, 200 mg tid, in relation to the pattern of seizure exacerbation. For perimenstrual exacerbation (C1 pattern) they were given for days 23 to 25 of each menstrual cycle and then tapered to discontinuation by day 28. For luteal phase exacerbation (C3 pattern) progesterone lozenges were given for days 15 to 25 of each menstrual cycle followed by the same taper schedule. The dosage of progesterone dosage was adjusted if needed to produce physiologic luteal range progesterone serum levels between 5 and 25 ng/ml 4 hours after taking a lozenge. Over 3 months of treatment, overall average monthly decline was 54% for complex partial and 58% for secondarily generalized tonic clonic seizures. Overall, 72% of the patients experienced a reduction of seizures. The investigators noted that progesterone was more efficacious when administered during the entire second half of the cycle for both groups with the different catamenial patterns, followed by gradual taper over 3 to 4 days. This observation formed the basis for future treatment trials.
Fifteen women from the treatment group in this open-label study were followed for over 3 years and continued to show improved seizure control [35]. Three of the women maintained seizure freedom and four had seizure reduction of >75%, while another eight had 50–75% seizure reduction. It is encouraging that despite a likely primary action at the GABAA-BDZ receptor complex (via conversion to allopregnanolone), tolerance to adjunctive progesterone does not appear to occur and the efficacy is maintained.
The findings from these open label trials, including details about which regimen seems to most effective for all patterns of catamenial epilepsy, have led to a prospective, double-blind, randomized, placebo-controlled, multicenter trial of use of cyclic adjunctive progesterone lozenges. Enrollment just ended and analysis of the data is pending. The population enrolled included women with medically-refractory focal-onset seizures, and included a group with a non-catamenial pattern and another group with any of the three catamenial patterns [39].
Progesterone lozenges can be obtained from almost all compounding pharmacies. It can be initiated as it is used in the treatment trials as 200mg TID on days 15 to 25, with a taper to 100 mg TID for 2 days, then 50 mg TID for one day, then off. However, it is often helpful to later individualize the dose based on response and progesterone levels with a target concentration of 20–40 ng/mL drawn 4 hours after dosing. Some women may require an even slower taper or addition of topical progesterone cream during other phases of the menstrual cycle to prevent withdrawal effects. Progesterone is also available in a micronized form in an oral capsule preparation that may be effective, but it has not been studied formally. One concern by experts is the possible large first-pass effect of hepatic metabolism which occurs following swallowing of the capsule. In addition to possible sedation, and changes in mood, progesterone can also be associated with breast tenderness, weight gain, irregular vaginal bleeding and sometimes constipation. Higher progesterone dosages may be required to achieve luteal-range levels in women who take EIAEDs.
If the currently ongoing multicenter, double-blind, randomized, placebo-controlled trial of cyclic progesterone lozenges in the treatment of women with localization-related epilepsy is positive, physicians will not only be able to acknowledge the effect of cycling hormones on seizures, but be able to use this relationship to their advantage in selecting treatment options.
B) Other hormone strategies for catamenial epilepsy
In addition to standard AED regimens, treatment strategies can include increasing the AED dose or adding another AED around the time of increased seizure susceptibility, or avoiding the cyclic variation in endogenous hormones by a continuous oral contraceptive pill or medroxyprogesterone acetate injections.
The use of adjusting conventional AED therapy during periods of seizure exacerbation has not been studied in a systematic manner or reported on, but is occasionally used by practitioners for women with catamenial seizure patterns. Intermittent benzodiazepines have been used for treatment of catamenial seizures, despite very few studies. One group reported on use of clobazam in a small group of patients a few decades ago, with moderately successful results [17,18]. Clobazam, 20 or 30 mg/day was given for 10 days around menstruation in successive menstrual cycles to 13 women who had responded favourably to this drug in the earlier short-term placebo controlled cross-over study. A little over 1/3 did very well seizure freedom around menstruation with retained benefit for over 3 years, another 1/3 had a moderate response, and another 1/3 had seizures or side effects leading to discontinuation of the clobazam. The most common adverse effects were sedation and depression.
Clobazam is is not available in the United States but can be obtained from pharmacies outside the US. Despite the lack of rigorous trials, it is a fairly popular treatment regimen for women with catamenial epilepsy in Europe, and is purported to be better tolerated than other benzodiazepines.
Another option is use of contraceptive hormones to eliminate the effect of cycling endogenous hormone concentrations. Again, despite the fact that his may be used in clinical practice as a treatment strategy, clinical trials are lacking. There have been isolated case reports, but a paucity of systematic trials or even observational studies reported in the literature [19]. Taking this theoretical benefit one step further, there may be an additional advantage of use of continuous dosing OCPs, with rare to no weeks of placebo administration.
Medroxyprogesterone acetate (MPA) is a synthetic progestin and is available as an oral form and as an intramuscular injection every 3 months. This suppresses normal ovulatory hormone cycling, and thus could theoretically improve catamenial epilepsy. Mattson et al. [53] studied 14 women with medically-refractory epilepsy for the effects of MPA. They were given the oral or depot-intramuscular form to achieve amenorrhea. Half the women were responders with seizure reduction and the group overall had a 39% reduction in seizure frequency. As is common for all women receiving depot-MPA, they had a 3- to 12-month delay in resumption of regular menses after treatment. In addition to this problem, in the general population, reported side effects are common with irregular menstrual bleeding, breast tenderness, weight gain, and depression. Another concern for women with epilepsy, especially if they are on an EIAED, is that depot-MPA is associated with increased risk for osteoporosis.
Other treatment strategies reported by experts include antiestrogens such as clomiphene citrate and gonadotropin-releasing homone analogues, which suppress ovarian and testicular steroidogenesis [19]. Although there are small pilot studies demonstrating some benefit for seizure control, they have not been studied in any series of > 15 patients and they carry significant gynecologic, metabolic, and bone health risks. When considered for use for seizure control, they should be administered with collaboration with a gynaecologist or endocrinologist.
C) Other neuroactive steroids
Ganaxolone is a neuroactive steroid analog, and is the synthetic 3β-methyl derivative of allopregnanolone [73]. It has positive allosteroic modulatory effect at the neurosteroid binding site on the GABAA receptor complex, which is distinct from the benzodiazepine binding site (Figure 2). It has beneficial activity in a broad range of animal models of epilepsy. Ganaxolone also has the advantage that it lacks hormonal activity, and thus can more readily be applied as a therapeutic agent across both genders and all ages than progesterone. Another advantage is that tolerance does not develop to GNX unlike the benzodiazepines, despite working at the same receptor complex.
The findings from a nonrandomized, open-label, pilot study in pediatric and adolescent subjects with refractory epilepsy have been reported [67]. Of the 15 subjects enrolled, 8 completed the trial and 3 continued in the compassionate-use extension period. The responder rate (> 50% reduction in seizure frequency) was 25%, and another 13% had a moderate response (25–50% reduction in seizure frequency). Of the 3 that entered the extension phase, one remained seizure-free for over 3.5 years of GNX administration. The medication was tolerated well, with somnolence being the most frequent side effect.
To date, 961 human subjects (135 children) have been exposed to GNX in safety and pharmacokinetic studies and in clinical trials (most of the latter In early Phase II studies)[73]. GNX has been well tolerated in adults and children, and has appeared to have promising therapeutic benefits in adult patients with localization-related epilepsy and children with infantile spasms. Sedation and somnolence was the most common reported side effect, but is dose-related and reversible. Additionally, preliminary reports include that women with catamenial epilepsy are particularly responsive to GNX, perhaps because it provides neurosteroid replacement in these women [73]. It is currently undergoing further development in infants with newly diagnosed infantile spasms, in women with catamenial epilepsy, and in adults with refractory partial-onset seizures [59].
V. SUMMARY
The relationships between hormones, epilepsy, and the medications used to treat epilepsy are complex, with tridirectional interactions which affect both men and women in various ways. Abnormalities of baseline endocrine status occur more commonly in people with epilepsy. Abnormalities are most often described for the sex steroid hormone axis, commonly presenting as sexual dysfunction in men and women with epilepsy and lower fertility. Other signs and symptoms in women with epilepsy include menstrual irregularities, premature menopause, and polycystic ovarian syndrome.
Ideal evaluation and care of adult patients with epilepsy should include considerations of the common hormonal aberrations that occur in this patient population. Questions about reproductive health disorders, sexual function, symptoms of thyroid disorders, and bone health should be part of the evaluation of all adult patients with epilepsy. Further laboratory or radiologic testing and referral to other specialists to participate in collaborative care may be warranted if underlying disorders are found, especially given that many of these hormone abnormalities can result in long term health risks as well as negatively impact quality of life.
Antiepileptic drugs and hormones have a bidirectional interaction that can impair the efficacy of contraceptive hormone treatments and of the AEDs. Endogenous hormones can influence seizure severity and frequency, resulting in catamenial patterns of epilepsy. However, this susceptibility to hormonal influences can be used to develop hormonal strategies to improve seizure control in women with epilepsy with use of cyclic progesterone supplementation or alteration of the endogenous hormone release. Additionally, development of the neurosteroid analog ganaxolone provides a novel approach that can be used across both genders and all age groups.
Table 2.
Summary of open-label trials of progesterone in women with catamenial epilepsy
| Medroxy-progesteronea | Progesterone Suppositoriesb | Progesterone Lozengesc | Progesterone Lozengesd | |
|---|---|---|---|---|
| Regimen | 5 mg to 10 mg qd days 15 to 28 of cycle | 100 mg to 200 mg tid days 15 to 28 of | 100 mg to 200 mg tid days 15 to 28 of cycle | 100 mg to 200 mg tid days 15 to 28 of cycle |
| Assessment | At 3 months | At 3 months | At 3 months | At 3 years |
| Subjects | 24 | 8 | 25 | 15 of original 25 |
| Number improved | 10 (42%) | 6 (75%) | 18 (72%) | 15 (100%/60% overall) |
| Seizure frequency | 10% | 68%e | −54%f CPS −58%e SGMS |
−62%f CPS −74%f SGMS |
Abbreviations: CPS, complex partial seizure; qd, daily; SGMS, secondary generalized motor seizure; tid, 3 times a day.
Results of 1983 study published in Herzog AG. Progesterone therapy in women with epilepsy: a 3-year follow-up. Neurology 1999;52(9);1917–8.
Data from Herzog AG. Intermittent progesterone therapy and frequency of complex partial seizures in women with menstrual disorders. Neurology 1986;36(12);1607–10.
Data from Herzog AG. Progesterone therapy in women with complex partial and secondary generalized seizures. Neurology 1995;45(9):1660–2.
Data from Herzog AG. Progesterone therapy in women with epilepsy: a 3-year follow-up. Neurology 1999; 52(9):1917–8.
P < .05.
P < .01.
Data from Herzog AG. AAN CONTINUUM: lifelong learning in neurology. Neuroendocrinology 2009;15(2):37–66.
Box 1.
Clinical manifestations of polycystic ovarian syndrome
| Signs and symptoms (proportion of patients affected) |
| Obesity (38%) |
| Menstrual disturbance (66%) |
| Oligomenorrhea (47%) |
| Amenorrhea (19%) |
| [Regular cycle 30%] |
| Hyperandrogenism (48%) |
| Infertility (73% of anovulatory infertility) |
| Symptomless–20% of those with polycystic ovaries |
| Hormone systems that might be disturbed |
| Insulin ↑ |
| Sex-hormone-binding globulin ↓ |
| Androgens (testosterone and androstenedione) ↑ |
| Luteinizing hormone ↑ |
| Prolactin ↑ |
| Possible late sequelae |
| Dyslipidemia |
| Low-density lipoprotein (LDL) ↑, High-density lipoprotein (HDL) ↓ |
| Triglycerides ↑ |
| Diabetes mellitus |
| Cardiovascular disease; hypertension |
| Endometrial carcinoma |
Data from Balen A. Pathogenesis of polycystic ovary syndrome—the enigma unravels? Lancet 999;354:966.
Acknowledgments
Supported in part by NIH NINDS RO3NS063233
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Seizure control and treatment in pregnancy: observations from the EURAP epilepsy pregnancy registry. Neurology. 2006;66:354. doi: 10.1212/01.wnl.0000195888.51845.80. [DOI] [PubMed] [Google Scholar]
- 2.Artama M, Isojarvi JI, Raitanen J, et al. Birth rate among patients with epilepsy: a nationwide population-based cohort study in Finland. Am J Epidemiol. 2004;159:1057. doi: 10.1093/aje/kwh140. [DOI] [PubMed] [Google Scholar]
- 3.B„ckstr”m T. Epileptic seizures in women related to plasma estrogen and progesterone during the menstrual dycle. Acta Neurologica Scandinavia. 1976;54:321. doi: 10.1111/j.1600-0404.1976.tb04363.x. [DOI] [PubMed] [Google Scholar]
- 4.Balen A. Pathogenesis of polycystic ovary syndrome--the enigma unravels? Lancet. 1999;354:966. doi: 10.1016/S0140-6736(99)00218-4. [DOI] [PubMed] [Google Scholar]
- 5.Bauer J. Epilepsy and prolactin in adults: a clinical review. Epilepsy Res. 1996;24:1. doi: 10.1016/0920-1211(96)00009-5. [DOI] [PubMed] [Google Scholar]
- 6.Bauer J, Cooper-Mahkorn D. Reproductive dysfunction in women with epilepsy: menstrual cycle abnormalities, fertility, and polycystic ovary syndrome. Int Rev Neurobiol. 2008;83:135. doi: 10.1016/S0074-7742(08)00007-X. [DOI] [PubMed] [Google Scholar]
- 7.Bilo L, Meo R, Nappi C, et al. Reproductive endocrine disorders in women with primary generalized epilepsy. Epilepsia. 1988;29:612. doi: 10.1111/j.1528-1157.1988.tb03770.x. [DOI] [PubMed] [Google Scholar]
- 8.Bilo L, Meo R, Valentino R, et al. Abnormal pattern of luteinizing hormone pulsatility in women with epilepsy. Fertil Steril. 1991;55:705. doi: 10.1016/s0015-0282(16)54234-4. [DOI] [PubMed] [Google Scholar]
- 9.Christensen J, Petrenaite V, Atterman J, et al. Oral contraceptives induce lamotrigine metabolism: evidence from a double-blind, placebo-controlled trial. Epilepsia. 2007;48:484. doi: 10.1111/j.1528-1167.2007.00997.x. [DOI] [PubMed] [Google Scholar]
- 10.Collins WC, Lanigan O, Callaghan N. Plasma prolactin concentrations following epileptic and pseudoseizures. J Neurol Neurosurg Psychiatry. 1983;46:505. doi: 10.1136/jnnp.46.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cramer JA, Gordon J, Schachter S, et al. Women with epilepsy: hormonal issues from menarche through menopause. Epilepsy Behav. 2007;11:160. doi: 10.1016/j.yebeh.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Crawford P, Hudson S. Understanding the information needs of women with epilepsy at different lifestages: results of the ‘Ideal World’ survey. Seizure. 2003;12:502. doi: 10.1016/s1059-1311(03)00085-2. [DOI] [PubMed] [Google Scholar]
- 13.Dansky LV, Andermann E, Andermann F. Marriage and fertility in epileptic patients. Epilepsia. 1980;21:261. doi: 10.1111/j.1528-1157.1980.tb04072.x. [DOI] [PubMed] [Google Scholar]
- 14.Davis AR, Pack AM, Kritzer J, et al. Reproductive history, sexual behavior and use of contraception in women with epilepsy. Contraception. 2008;77:405. doi: 10.1016/j.contraception.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Duncan S, Read CL, Brodie MJ. How common is catamenial epilepsy? Epilepsia. 1993;34:827. doi: 10.1111/j.1528-1157.1993.tb02097.x. [DOI] [PubMed] [Google Scholar]
- 16.El-Khayat HA, Soliman NA, Tomoum HY, et al. Reproductive hormonal changes and catamenial pattern in adolescent females with epilepsy. Epilepsia. 2008;49:1619. doi: 10.1111/j.1528-1167.2008.01622.x. [DOI] [PubMed] [Google Scholar]
- 17.Feely M, Calvert R, Gibson J. Clobazam in catamenial epilepsy. A model for evaluating anticonvulsants. Lancet. 1982;2:71. doi: 10.1016/s0140-6736(82)91691-9. [DOI] [PubMed] [Google Scholar]
- 18.Feely M, Gibson J. Intermittent clobazam for catamenial epilepsy: tolerance avoided. J Neurol Neurosurg Psychiatry. 1984;47:1279. doi: 10.1136/jnnp.47.12.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foldvary-Schaefer N, Falcone T. Catamenial epilepsy: pathophysiology, diagnosis, and management. Neurology. 2003;61:S2. doi: 10.1212/wnl.61.6_suppl_2.s2. [DOI] [PubMed] [Google Scholar]
- 20.Frye CA. Hormonal influences on seizures: basic neurobiology. Int Rev Neurobiol. 2008;83:27. doi: 10.1016/S0074-7742(08)00003-2. [DOI] [PubMed] [Google Scholar]
- 21.Galimberti CA, Mazzucchelli I, Arbasino C, et al. Increased apparent oral clearance of valproic acid during intake of combined contraceptive steroids in women with epilepsy. Epilepsia. 2006;47:1569. doi: 10.1111/j.1528-1167.2006.00629.x. [DOI] [PubMed] [Google Scholar]
- 22.Gold EB, Bromberger J, Crawford S, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153:865. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- 23.Gomez JM, Cardesin R, Virgili N, et al. Thyroid function parameters and TSH in patients treated with anticonvulsant drugs. An Med Interna. 1989;6:235. [PubMed] [Google Scholar]
- 24.Guberman A. Hormonal contraception and epilepsy. Neurology. 1999;53:S38. [PubMed] [Google Scholar]
- 25.Harden CL, Herzog AG, Nikolov BG, et al. Hormone replacement therapy in women with epilepsy: a randomized, double-blind, placebo-controlled study. Epilepsia. 2006;47:1447. doi: 10.1111/j.1528-1167.2006.00507.x. [DOI] [PubMed] [Google Scholar]
- 26.Harden CL, Koppel BS, Herzog AG, et al. Seizure frequency is associated with age at menopause in women with epilepsy. Neurology. 2003;61:451. doi: 10.1212/01.wnl.0000081228.48016.44. [DOI] [PubMed] [Google Scholar]
- 27.Harden CL, Meador KJ, Pennell PB, et al. Practice parameter update: management issues for women with epilepsy--focus on pregnancy (an evidence-based review): teratogenesis and perinatal outcomes: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology. 2009;73:133. doi: 10.1212/WNL.0b013e3181a6b312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harden CL, Pulver MC, Ravdin L, et al. The effect of menopause and perimenopause on the course of epilepsy. Epilepsia. 1999;40:1402. doi: 10.1111/j.1528-1157.1999.tb02012.x. [DOI] [PubMed] [Google Scholar]
- 29.Herzog A, Seibel M, Schomer D, et al. Reproductive endocrine disorders in women with partial seizures of temporal lobe orgin. Archives of Neurology. 1986;43:341. doi: 10.1001/archneur.1986.00520040029014. [DOI] [PubMed] [Google Scholar]
- 30.Herzog AG. Catamenial epilepsy: definition, prevalence pathophysiology and treatment. Seizure. 2008;17:151. doi: 10.1016/j.seizure.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 31.Herzog AG. Disorders of reproduction in patients with epilepsy: primary neurological mechanisms. Seizure. 2008;17:101. doi: 10.1016/j.seizure.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 32.Herzog AG. Intermittent progesterone therapy and frequency of complex partial seizures in women with menstrual disorders. Neurology. 1986;36:1607. doi: 10.1212/wnl.36.12.1607. [DOI] [PubMed] [Google Scholar]
- 33.Herzog AG. Neuroendocrinology: Epilepsy. Continuum Lifelong Learning Neurology. 2009;15:37. [Google Scholar]
- 34.Herzog AG. Progesterone therapy in women with complex partial and secondary generalized seizures. Neurology. 1995;45:1660. doi: 10.1212/wnl.45.9.1660. [DOI] [PubMed] [Google Scholar]
- 35.Herzog AG. Progesterone therapy in women with epilepsy: A 3-year follow-up. Neurology. 1999;52:1917. doi: 10.1212/wnl.52.9.1917-a. [DOI] [PubMed] [Google Scholar]
- 36.Herzog AG. A relationship between particular reproductive endocrine disorders and the laterality of epileptiform discharges in women with epilepsy. Neurology. 1993;43:1907. doi: 10.1212/wnl.43.10.1907. [DOI] [PubMed] [Google Scholar]
- 37.Herzog AG, Blum AS, Farina EL, et al. Valproate and lamotrigine level variation with menstrual cycle phase and oral contraceptive use. Neurology. 2009;72:911. doi: 10.1212/01.wnl.0000344167.78102.f0. [DOI] [PubMed] [Google Scholar]
- 38.Herzog AG, Drislane FW, Schomer DL, et al. Differential effects of antiepileptic drugs on sexual function and hormones in men with epilepsy. Neurology. 2005;65:1016. doi: 10.1212/01.wnl.0000178988.78039.40. [DOI] [PubMed] [Google Scholar]
- 39.Herzog AG, Harden CL, Liporace J, et al. Frequency of catamenial seizure exacerbation in women with localization-related epilepsy. Annals of Neurology. 2004;56:431. doi: 10.1002/ana.20214. [DOI] [PubMed] [Google Scholar]
- 40.Herzog AG, Klein P, Ransil BJ. Three patterns of catamenial epilepsy. Epilepsia. 1997;38:1082. doi: 10.1111/j.1528-1157.1997.tb01197.x. [DOI] [PubMed] [Google Scholar]
- 41.Herzog AG, Seibel MM, Schomer DL, et al. Reproductive endocrine disorders in women with partial seizures of temporal lobe origin. Arch Neurol. 1986;43:341. doi: 10.1001/archneur.1986.00520040029014. [DOI] [PubMed] [Google Scholar]
- 42.Isojarvi JI, Laatikainen TJ, Pakarinen AJ, et al. Polycystic ovaries and hyperandrogenism in women taking valproate for epilepsy. N Engl J Med. 1993;329:1383. doi: 10.1056/NEJM199311043291904. [DOI] [PubMed] [Google Scholar]
- 43.Isojarvi JI, Pakarinen AJ, Myllyla VV. Thyroid function in epileptic patients treated with carbamazepine. Arch Neurol. 1989;46:1175. doi: 10.1001/archneur.1989.00520470029021. [DOI] [PubMed] [Google Scholar]
- 44.Isojarvi JI, Rattya J, Vv M, et al. Valproate, lamotrigine, and insulin-mediated risks in women with epilepsy. Annals of Neurology. 1998;43:446. doi: 10.1002/ana.410430406. [DOI] [PubMed] [Google Scholar]
- 45.Isojarvi JI, Turkka J, Pakarinen AJ, et al. Thyroid function in men taking carbamazepine, oxcarbazepine, or valproate for epilepsy. Epilepsia. 2001;42:930. doi: 10.1046/j.1528-1157.2001.042007930.x. [DOI] [PubMed] [Google Scholar]
- 46.Kalinin VV, Zheleznova EV. Chronology and evolution of temporal lobe epilepsy and endocrine reproductive dysfunction in women: relationships to side of focus and catameniality. Epilepsy Behav. 2007;11:185. doi: 10.1016/j.yebeh.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 47.Klein P, Serje A, Pezzullo JC. Premature ovarian failure in women with epilepsy. Epilepsia. 2001;42:1584. doi: 10.1046/j.1528-1157.2001.13701r.x. [DOI] [PubMed] [Google Scholar]
- 48.Knochenhauer ES, Key TJ, Kahsar-Miller M, et al. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83:3078. doi: 10.1210/jcem.83.9.5090. [DOI] [PubMed] [Google Scholar]
- 49.Krauss GL, Brandt J, Campbell M, et al. Antiepileptic medication and oral contraceptive interactions: a national survey of neurologists and obstetricians. Neurology. 1996;46:1534. doi: 10.1212/wnl.46.6.1534. [DOI] [PubMed] [Google Scholar]
- 50.Kuba R, Pohanka M, Zakopcan J, et al. Sexual dysfunctions and blood hormonal profile in men with focal epilepsy. Epilepsia. 2006;47:2135. doi: 10.1111/j.1528-1167.2006.00851.x. [DOI] [PubMed] [Google Scholar]
- 51.Lofgren E, Mikkonen K, Tolonen U, et al. Reproductive endocrine function in women with epilepsy: the role of epilepsy type and medication. Epilepsy Behav. 2007;10:77. doi: 10.1016/j.yebeh.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 52.Lossius MI, Tauboll E, Mowinckel P, et al. Reversible effects of antiepileptic drugs on reproductive endocrine function in men and women with epilepsy--a prospective randomized double-blind withdrawal study. Epilepsia. 2007;48:1875. doi: 10.1111/j.1528-1167.2007.01147.x. [DOI] [PubMed] [Google Scholar]
- 53.Mattson RH, Cramer JA, Caldwell BV, et al. Treatment of seizures with medroxyprogesterone acetate: preliminary report. Neurology. 1984;34:1255. doi: 10.1212/wnl.34.9.1255. [DOI] [PubMed] [Google Scholar]
- 54.Meo R, Bilo L. Polycystic ovary syndrome and epilepsy: a review of the evidence. Drugs. 2003;63:1185. doi: 10.2165/00003495-200363120-00002. [DOI] [PubMed] [Google Scholar]
- 55.Molleken D, Richter-Appelt H, Stodieck S, et al. Sexual quality of life in epilepsy: correlations with sex hormone blood levels. Epilepsy Behav. 2009;14:226. doi: 10.1016/j.yebeh.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 56.Morrell MJ, Flynn KL, Done S, et al. Sexual dysfunction, sex steroid hormone abnormalities, and depression in women with epilepsy treated with antiepileptic drugs. Epilepsy Behav. 2005;6:360. doi: 10.1016/j.yebeh.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 57.Morrell MJ, Hayes FJ, Sluss PM, et al. Hyperandrogenism, ovulatory dysfunction, and polycystic ovary syndrome with valproate versus lamotrigine. Ann Neurol. 2008;64:200. doi: 10.1002/ana.21411. [DOI] [PubMed] [Google Scholar]
- 58.Morrell MJ, Sarto GE, Shafer PO, et al. Health issues for women with epilepsy: a descriptive survey to assess knowledge and awareness among healthcare providers. Journal of Womens Health & Gender-Based Medicine. 2000;9:959. doi: 10.1089/15246090050199982. [DOI] [PubMed] [Google Scholar]
- 59.Nohria V, Giller E. Ganaxolone. Neurotherapeutics. 2007;4:102. doi: 10.1016/j.nurt.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olafsson E, Hallgrimsson JT, Hauser WA, et al. Pregnancies of women with epilepsy: a population-based study in Iceland. Epilepsia. 1998;39:887. doi: 10.1111/j.1528-1157.1998.tb01186.x. [DOI] [PubMed] [Google Scholar]
- 61.Pack A. Bone health in people with epilepsy: is it impaired and what are the risk factors? Seizure. 2008;17:181. doi: 10.1016/j.seizure.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 62.Pack AM. Should patients with epilepsy be routinely screened for low bone mineral density? Nat Clin Pract Neurol. 2008;4:354. doi: 10.1038/ncpneuro0822. [DOI] [PubMed] [Google Scholar]
- 63.Pack AM, Morrell MJ, Marcus R, et al. Bone mass and turnover in women with epilepsy on antiepileptic drug monotherapy. Ann Neurol. 2005;57:252. doi: 10.1002/ana.20378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pennell PB. Antiepileptic drug pharmacokinetics during pregnancy and lactation. Neurology. 2003;61:S35. doi: 10.1212/wnl.61.6_suppl_2.s35. [DOI] [PubMed] [Google Scholar]
- 65.Pennell PB, Hovinga CA. Antiepileptic drug therapy in pregnancy I: gestation-induced effects on AED pharmacokinetics. Int Rev Neurobiol. 2008;83:227. doi: 10.1016/S0074-7742(08)00013-5. [DOI] [PubMed] [Google Scholar]
- 66.Pennell PB, Peng L, Newport DJ, et al. Lamotrigine in pregnancy: clearance, therapeutic drug monitoring, and seizure frequency. Neurology. 2008;70:2130. doi: 10.1212/01.wnl.0000289511.20864.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pieribone VA, Tsai J, Soufflet C, et al. Clinical evaluation of ganaxolone in pediatric and adolescent patients with refractory epilepsy. Epilepsia. 2007;48:1870. doi: 10.1111/j.1528-1167.2007.01182.x. [DOI] [PubMed] [Google Scholar]
- 68.Pritchard PB, 3rd, Wannamaker BB, Sagel J, et al. Serum prolactin and cortisol levels in evaluation of pseudoepileptic seizures. Ann Neurol. 1985;18:87. doi: 10.1002/ana.410180115. [DOI] [PubMed] [Google Scholar]
- 69.Quigg M, Kiely JM, Johnson ML, et al. Interictal and postictal circadian and ultradian luteinizing hormone secretion in men with temporal lobe epilepsy. Epilepsia. 2006;47:1452. doi: 10.1111/j.1528-1167.2006.00617.x. [DOI] [PubMed] [Google Scholar]
- 70.Reddy DS. Role of neurosteroids in catamenial epilepsy. Epilepsy Res. 2004;62:99. doi: 10.1016/j.eplepsyres.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 71.Reddy DS, Kim HY, Rogawski MA. Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia. 2001;42:328. doi: 10.1046/j.1528-1157.2001.10100.x. [DOI] [PubMed] [Google Scholar]
- 72.Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of neuroactive steroids in a rat model of catamenial epilepsy. Epilepsia. 2001;42:337. doi: 10.1046/j.1528-1157.2001.10200.x. [DOI] [PubMed] [Google Scholar]
- 73.Reddy DS, Rogawski MA. Neurosteroid replacement therapy for catamenial epilepsy. Neurotherapeutics. 2009;6:392. doi: 10.1016/j.nurt.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reimers A, Helde G, Brodtkorb E. Ethinyl estradiol, not progestogens, reduces lamotrigine serum concentrations. Epilepsia. 2005;46:1414. doi: 10.1111/j.1528-1167.2005.10105.x. [DOI] [PubMed] [Google Scholar]
- 75.Report of the Quality Standards Subcommittee of the American Academy of N: Practice parameter: management issues for women with epilepsy (summary statement) Neurology. 1998;51:944. doi: 10.1212/wnl.51.4.944. [DOI] [PubMed] [Google Scholar]
- 76.Rhodes ME, Frye CA. Androgens in the hippocampus can alter, and be altered by, ictal activity. Pharmacol Biochem Behav. 2004;78:483. doi: 10.1016/j.pbb.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 77.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 78.Sabers A, Ohman I, Christensen J, et al. Oral contraceptives reduce lamotrigine plasma levels. Neurology. 2003;61:570. doi: 10.1212/01.wnl.0000076485.09353.7a. [DOI] [PubMed] [Google Scholar]
- 79.Scharfman HE, MacLusky NJ. The influence of gonadal hormones on neuronal excitability, seizures, and epilepsy in the female. Epilepsia. 2006;47:1423. doi: 10.1111/j.1528-1167.2006.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schupf N, Ottman R. Reproduction among individuals with idiopathic/cryptogenic epilepsy: risk factors for reduced fertility in marriage. Epilepsia. 1996;37:833. doi: 10.1111/j.1528-1157.1996.tb00035.x. [DOI] [PubMed] [Google Scholar]
- 81.Tiihonen M, Liewendahl K, Waltimo O, et al. Thyroid status of patients receiving long-term anticonvulsant therapy assessed by peripheral parameters: a placebo-controlled thyroxine therapy trial. Epilepsia. 1995;36:1118. doi: 10.1111/j.1528-1157.1995.tb00470.x. [DOI] [PubMed] [Google Scholar]
- 82.Verrotti A, Laus M, Scardapane A, et al. Thyroid hormones in children with epilepsy during long-term administration of carbamazepine and valproate. Eur J Endocrinol. 2009;160:81. doi: 10.1530/EJE-08-0325. [DOI] [PubMed] [Google Scholar]
- 83.Vessey M, Painter R, Yeates D. Oral contraception and epilepsy: findings in a large cohort study. Contraception. 2002;66:77. doi: 10.1016/s0010-7824(02)00323-2. [DOI] [PubMed] [Google Scholar]