Abstract
Velo-cardio-facial syndrome/DiGeorge syndrome (VCFS/DGS), the most common micro-deletion disorder in humans, is characterized by craniofacial, parathyroid and thymic defects as well as cardiac outflow tract malformations. Most patients have a similar hemizygous 3 million base pair deletion on 22q11.2. Studies in mouse have shown that Tbx1, a T- box containing transcription factor present on the deleted region, is likely responsible for the etiology of the syndrome. Furthermore, mutations in TBX1 have been found in rare non-deleted patients. Despite having the same sized deletion, most VCFS/DGS patients exhibit significant clinical variability. Stochastic, environmental and genetic factors likely modify the phenotype of patients with the disorder. Here, we review mouse genetics studies which may help identify genetic modifiers for VCFS/DGS.
Keywords: DiGeorge syndrome, Velo- cardio- facial syndrome, genetic modifiers, Tbx1
Introduction
Velo- cardio- facial syndrome (VCFS; MIM: 192430) (Shprintzen et al. 1978)/DiGeorge syndrome (DGS; MIM: 188400) (DiGeorge 1965), with an incidence of 1 in 2–4,000 (Burn and Goodship 1996; Robin and Shprintzen 2005), is the most common microdeletion syndrome seen in humans. It is characterized by multiple developmental anomalies, including craniofacial (cleft palate, velo-pharyngeal insufficiency), thymic and parathyroid defects as well as cardiovascular [outflow tract (OFT) and aortic arch] malformations (Robin and Shprintzen 2005). All these defects derive from the pharyngeal apparatus, a temporary embryological structure lateral to the developing head. The pharyngeal apparatus consists of bilaterally symmetric structures called pharyngeal arches, which are composed of cells from all three germ layers and the neural crest. Neural crest cells (NCCs) migrate from the adjacent closing neural tube into the pharyngeal arches to surround the non-neural crest mesoderm (Graham 2003).
Mouse models of VCFS/DGS
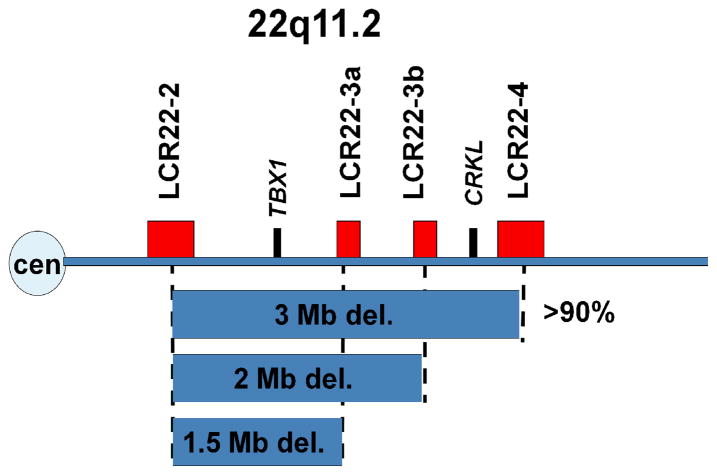
Approximately 85–90% of the patients have a similar 3 Mb (million base pair) deletion within the 22q11.2 region (Edelmann et al. 1999; Shaikh et al. 2000) which contains about 40 genes (Figure 1). Some patients have a nested 1.5 Mb deletion, containing about 30 genes (Figure 1). It has been hypothesized that one or more genes in the deleted interval are responsible for the etiology of the syndrome, perhaps by regulating the development of the pharyngeal apparatus into their derivative structures. Since the physical malformations in VCFS/DGS patients occur often, as individual birth defects in the general population, determining the molecular basis of the disorder would shed light into the causes of more common, non-syndromic anomalies.
Figure 1.
Human 22q11.2 genomic region. The horizontal line represents chromosome 22, with the centromere on the left. The recurrent chromosomal deletions (represented by blue boxes) share common breakpoints on 22q11.2. The breakpoints are in segmental duplications termed LCR22s (red rectangles). The location of TBX1 and CRKL is indicated by the black rectangles.
In order to elucidate the molecular basis of the syndrome, it was necessary to turn to model organisms. Mice carrying varied sized deletions in the region of synteny to the 22q11.2 region were generated using the Cre/loxP system, to identify candidate genes (Lindsay et al. 2001; Merscher et al. 2001). Mice carrying a 1.2 Mb (Df1/+) or 1.5 Mb (Lgdel/+) deletion in the region of synteny to the human 22q11.2 region, had mild defects similar to the patients. Defects in these mice were rescued by crossing with mice carrying 1–2 copies of a bacterial artificial chromosome (BAC) containing four human genes from the region, including TBX1 (GP1BB, PNUTL1, TBX1 and WDR14) (Merscher et al. 2001). Thus, the critical interval for these malformations, was reduced to four genes (Merscher et al. 2001). Of the four, Tbx1, a member of the T-box family of transcription factors, is the only one expressed specifically in the affected structures making it the most likely candidate (Lindsay et al. 2001; Merscher et al. 2001). Tbx1 was inactivated in the mouse and while heterozygotes survived in normal Mendelian ratios and were mildly affected, homozygous mice died at birth with major defects in all the pharyngeal structures relevant to the syndrome (Jerome and Papaioannou 2001; Lindsay et al. 2001; Merscher et al. 2001). Tbx1 +/− mice were phenotypically similar to 1.5 Mb deleted mice, ruling out other genes in the 1.5 Mb deleted interval as having a major effect. Tbx1 −/− mice had a cleft palate, absent parathyroid and thymus glands as well as a common outflow tract (Jerome and Papaioannou 2001). Thus, Tbx1 is a major candidate for the etiology of the physical malformations occurring in the syndrome. Inactivating mutations in TBX1 were found in rare non-deleted patients with VCFS/DGS (Paylor et al. 2006; Yagi et al. 2003) and one of them is demonstrated to act as a loss of function mutation in cell culture (Stoller and Epstein 2005).
Even though most VCFS/DGS patients have the same 3 Mb deletion, the phenotype is highly variable (Ryan et al. 1997). Phenotypes seen in the disorder vary from life threatening cardiovascular anomalies to mild craniofacial defects and learning disabilities. Stochastic, environmental and genetic factors likely modify the phenotype. This review focuses on genetic factors that modify the phenotypic spectrum of the physical malformations in the syndrome. Evidence for genetic factors or modifiers derives from genetic studies in animal models in the form of genetic background effects of Tbx1 mutations, phenocopies and gene interactions.
Background effects of mouse models of DGS/VCFS
Using a mouse model of the disorder carrying a 1.2 Mb deletion, referred to as Df1/+, it was found that the penetrance of cardiovascular and thymic defects was strongly affected by the genetic background (Taddei et al. 2001). Interestingly, it was shown that allelic variation within the haploid Df1/+ genes did not alter the frequency of the malformations, suggesting that genes in this region of synteny to 22q11.2 are not major modifiers of these anomalies. These findings suggest the presence of modifier genes elsewhere in the genome, perhaps on other chromosomes (Taddei et al. 2001). Nonetheless, it is still possible that genes on the remaining allele, such as in the larger 3 Mb region could influence the expressivity of the disorder, since not many genetic backgrounds were assessed, thereby limiting the analysis for genetic variation.
The pharyngeal arch arteries, although starting out as bilaterally symmetrical, become remodeled to the adult asymmetric organization. The remodeling of the pharyngeal arch arteries are particularly vulnerable to genetic insults. The left fourth pharyngeal arch artery forms a key part of the aortic arch, and when disrupted; an interrupted aortic arch type B, a common feature of VCFS/DGS arises. Differences in penetrance of fourth pharyngeal arch artery hypoplasia, seen in Tbx1+/− embryos at E10.5 has been shown to be background dependant, again suggesting the presence of modifier genes (Zhang et al. 2005).
Phenocopies and gene interactions
One important goal is to identify genes that can modify the phenotype in VCFS/DGS patients. One way to identify genes is to examine mouse mutants that carry null alleles with similar defects as in Tbx1 −/− mutant mice. These would represent phenocopies of the syndrome. Some of these genes might in fact be in the genetic pathway, upstream or downstream from Tbx1. Tbx1 is expressed in the pharyngeal arch endoderm and mesoderm, as well as temporarily in the distal pharyngeal arch ectoderm (Chapman et al. 1996; Zhang et al. 2005), but is not expressed in the NCCs. Since NCC ablation also results in phenocopies of the syndrome (Bockman et al. 1990; Kirby and Waldo 1995), it is likely that Tbx1 may regulate genes that signal to NCCs to promote their growth and differentiation. It is possible that single nucleotide polymorphisms (SNPs) in these genes might alter the expressivity of the syndrome, perhaps influencing NCC function, thus serving as genetic modifiers. We discuss below publications describing potential VCFS/DGS modifiers.
Fibroblast growth factor 8 (Fgf8)
Fgf8 belongs to the Fibroblast growth factor (Fgf) family of signaling molecules which play important roles in development by regulating cell proliferation, migration and differentiation (Bottcher and Niehrs 2005). Mouse embryos hypomorphic for Fgf8 display the complete array of craniofacial, thymic, parathyroid, as well as cardiovascular phenotypes seen in VCFS/DGS (Abu-Issa et al. 2002; Frank et al. 2002). Like Tbx1, Fgf8 is expressed in the epithelia and mesoderm, but not in NCCs (Ilagan et al. 2006; Macatee et al. 2003; Park et al. 2006). Fgf8 is downregulated in the pharyngeal endoderm of Tbx1−/− embryos and Fgf8+/−; Tbx1+/− double heterozygous embryos have an increased penetrance of aortic arch remodeling anomalies when compared with Tbx1+/− embryos, indicating that Tbx1 and Fgf8 genetically interact (Vitelli et al. 2002). Fgf8 modifies the fourth PAA recovery from arterial growth delay, seen in Tbx1+/− embryos (Vitelli et al. 2006).
Conflicting evidence exists for the genetic interaction between Tbx1 and Fgf8 in outflow tract (OFT) development. As in a Tbx1 deficient background, OFT- specific suppression of a transgene harboring a Tbx1- dependant Fgf8 enhancer is seen; it has been proposed that Tbx1-Fgf8 interaction occurs in the OFT (Hu et al. 2004). Cre-mediated excision of an Fgf8 conditional allele in the Tbx1 expression domain gives rise to cardiovascular defects involving the OFT and proximal great vessels (Brown et al. 2004). However, deletion of an Fgf8 conditional allele from Tbx1- positive cells using a Tamoxifen- inducible Tbx1- driven Cre allele does not result in OFT anomalies (Vitelli et al. 2006). Deletion of the Fgf8 conditional allele with a constitutively active Cre driver under the control of endogenous Tbx1 regulatory elements will help provide clarification of the phenotypic differences observed between the two studies. Overall, studies on genetic interactions are complex to dissect with the caveats and limitations of the Cre/loxP system, including lack of complete inactivation or ectopic Cre expression, and thus require further confirmation in additional model organisms or humans.
Fibroblast growth factor 10 (Fgf10)
Tissue interactions between the NCCs and the non-neural crest mesoderm are required to form a normally septated cardiac OFT. The first heart field mesoderm forms the linear heart tube, while the secondary heart field (SHF) mesoderm forms the OFT, right ventricle and parts of the atria (Kelly et al. 2001; Mjaatvedt et al. 2001; Waldo et al. 2001). The core mesoderm of the pharyngeal apparatus and surrounding lateral, splanchnic mesoderm comprises the SHF (Kelly et al. 2001; Mjaatvedt et al. 2001; Waldo et al. 2001). The NCCs form the septum between the aorta and pulmonary trunk, while the SHF forms the OFT itself. Fgf10, another member of the Fgf family of signaling molecules, is co-expressed with Tbx1 in the core mesoderm and anterior heart field (AHF), a subset of the SHF. Even though in situ expression analysis and in vitro cell culture data (Xu et al. 2004) suggested that Fgf10 may be downstream of Tbx1 in the AHF; surprisingly, the Fgf10+/−;Tbx1+/− mice did not have any significant cardiovascular anomalies (Aggarwal et al. 2006; Kelly and Papaioannou 2007). In fact, Fgf10 −/− mice do not have OFT defects either (Marguerie et al. 2006). It is likely that the Fgfs, many co-expressed, serve redundant functions. To test whether Tbx1, Fgf8 and Fgf10 genetically interact, we performed intercrosses with heterozygous mice (Aggarwal et al. 2006). Mice that were Tbx1 +/−; Fgf8 +/−; Fgf10 +/− did not show a significant increase in defects observed in Tbx1 +/−; Fgf8 +/− embryos and they did not have significant OFT defects (Aggarwal et al. 2006). It is possible that further reduction in dosage would be important to observe a genetic interaction, or hemizygosity of additional Fgf ligand genes are required. Conditional deletion of the AHF expressed Fgfs in the Tbx1 null background may reveal whether Fgf signaling is downstream of Tbx1 in the development of the OFT.
Gbx2
Gbx2, a homeobox- containing transcription factor is necessary for the specification of the mid- hindbrain organizer in mice (Wassarman et al. 1997). Gbx2 null mutant embryos have cardiovascular defects associated with abnormal development of the fourth pharyngeal arch arteries including interrupted aortic arch type B, right aortic arch and retroesophageal right subclavian artery (Byrd and Meyers 2005). Other VCFS/DGS related defects like overriding aorta, ventricular septal defects and craniofacial anomalies are also seen in these mutants. Furthermore, Fgf8 and Gbx2 interact genetically during cardiovascular and pharyngeal arch development (Byrd and Meyers 2005). These findings suggest that GBX2 may be a modifying locus for VCFS/DGS.
Pitx2
Pitx2, a bicoid- like homeobox gene, is co- expressed with Tbx1 in the SHF during early pharyngeal development. Pitx2 is particularly interesting in that it is required to establish right-left asymmetry (Piedra et al. 1998). The isoform that is asymmetrically expressed is termed Pitx2c. Since OFT defects observed in Pitx2 mouse mutants are similar to those in VCFS/DGS patients, it is possible that they might act in the same pathway. Supporting this hypothesis, Pitx2+/−; Tbx1+/− double heterozygous embryos showed an increased penetrance of VCFS/DGS related cardiovascular defects, albeit with reduced penetrance (Nowotschin et al. 2006). This suggests that the two genes may genetically interact in the SHF. It was also shown that Tbx1, with the synergistic action of Nkx2.5, directly activates the Pitx2c asymmetric enhancer (Nowotschin et al. 2006) PITX2c may thus be a genetic modifier of the cardiovascular defects seen in VCFS/DGS.
Crkl
Crkl is an adaptor- protein- encoding gene located within the 3 Mb region commonly deleted in VCFS/DGS. Crkl null mutant mice display some VCFS/DGS related thymic, parathyroid and cardiovascular, defects (Guris et al. 2001). Compound heterozygosity of Crkl and Tbx1, in mice, results in an increase in the penetrance and expressivity of VCFS/DGS related defects compared to Tbx1+/− or Crkl+/− mice (Guris et al. 2006). Of interest, Crkl is an adaptor protein of the Fgf-FgfR activated complex, and thus promotes the intracellular response of Fgf signaling. Relevant to this, Crkl +/−; Fgf8 +/− mice showed VCFS/DGS related defects as well (Moon et al. 2006). Furthermore, Tbx1 +/−; Fgf8 +/−; Crkl +/− triple heterozygous mice have more severe defects than double heterozygous mutants, supporting a significant genetic interaction (Guris et al., 2006). This indicates that CRKL may be a genetic modifier of the syndrome. It is of particular interest since both TBX1 and CRKL are deleted in most patients (Figure 1). It is likely that a recessive mutation in CRKL could greatly increase the severity of the syndrome.
Retinoic acid
In addition to genetic influences, environmental exposure can produce phenocopies of the syndrome. Retinoic acid (RA), the active vitamin A derivative, is important for various developmental processes in vertebrates. Balancing activities of RA- synthesizing enzymes RALDH (retinaldehyde dehydrogenases) and Cyp26 (RA- catabolizing cytochrome P450 hydroxylases) determine the tissue distribution of RA (Duester 2000; Reijntjes et al. 2004). Human fetuses which are exposed to retinoids during gestation phenocopy VCFS/DGS (Rosa et al. 1986).
Perturbation of RA levels have been shown to cause a downregulation of Tbx1 expression in the avian embryo (Roberts et al. 2005). It has also been demonstrated that administration of too much RA to zebrafish embryos causes downregulation of Tbx1, as well (Zhang et al. 2006). It is thus speculated that there is a genetic link between Tbx1 and RA signaling during pharyngeal apparatus development.
Retinaldehyde dehydrogenase 2 (RALDH2), a member of the aldehyde dehydrogenase family, is involved in RA synthesis by converting retinaldehyde to RA. Mice carrying one hypomorphic allele and one null allele (Raldh2neo/−) display VCFS/DGS related thymic, parathyroid and cardiovascular defects including a persistent truncus arteriosus (PTA), ventricular septal defect (VSD) and aortic arch artery remodeling defects (Vermot et al. 2003). The expression pattern of Tbx1, as determined by RNA in situ hybridization, was normal in these mutants but the expression of Fgf8 was altered in both the ectoderm and endoderm in the posterior pharyngeal region of the mutants (Vermot et al. 2003). Thus, it is possible that both Tbx1 and Raldh2 regulate Fgf8 expression in the pharyngeal endoderm.
Further interactions occur between RA, Tbx1 and Crkl. Defects seen in the Crkl+/−; Tbx1+/− embryos may be traced to ectopic RA signaling seen in the double heterozygous mutants (Guris et al. 2006). Interestingly, by genetically reducing the amount of RA in these mutants; the penetrance of thymic hypoplasia was significantly reduced (Guris et al. 2006). Thus, genes involved in RA metabolism and signaling may act as modifiers of the syndrome.
RA is converted to more polar metabolites by enzymes of the Cyp26 family (Fujii et al. 1997). Blocking Cyp26 function in the chick embryo using R115866, a specific inhibitor of Cyp26 enzyme function, results in a dose- dependant phenocopy of VCFS/DGS (Roberts et al. 2006). The caudal pharyngeal arches were missing in early embryos and the late stage embryos show heart defects including common arterial trunk and perimembranous ventricular septal defects (Roberts et al. 2006). These are similar to the defects seen in the Tbx1 null mutant mouse. This supports the hypothesis that genes involved in RA metabolism modify the phenotypic spectrum of VCFS/DGS. What is particularly compelling is the possibility that molecules that modulate RA levels could in fact be considered as therapeutics, in the future, to perhaps influence early embryonic development.
Vascular endothelial growth factor (Vegf)
Vascular endothelial growth factor (Vegf) has an important role in vasculogenesis and angiogenesis (Ferrara and Davis-Smyth 1997). Three different isoforms of Vegf exist which contain 120, 164 and 188 amino acids respectively. Mice expressing only the Vegf120 variant (Vegf120/120) or only the Vegf 188 variant (Vegf188/188) exhibit VCFS/DGS related cardiovascular malformations (Stalmans et al. 2003). Vegf120/120 mice also exhibit craniofacial, thymic and parathyroid defects commonly seen in VCFS/DGS. Relevant to this review, Tbx1 mRNA was reduced in the pharyngeal arches of the Vegf120/120 mutants. It was also shown that reduced levels of vegf increased the risk for aortic arch artery and pharyngeal cartilage defects in a tbx1-knockdown zebrafish model. Analysis of the allelic frequencies of three single nucleotide polymorphisms (SNPs) in the 5′ untraslated region of VEGF revealed that the −2578A/−1154A/−634G haplotype was more prevalent in VCFS/DGS cases with cardiovascular defects than in controls. Comparison of the distribution of the −1154A allele and the −2578A/−1154A/−634G haplotype in patients with and without cardiovascular defects, showed increased prevalence of both the variant −1154A allele and the −2578A/−1154A/−634G haplotype in patients with than without cardiovascular defects. Thus, genetic data in mouse, zebrafish and humans indicate that VEGF is a modifier of the cardiovascular defects in VCFS/DGS (Stalmans et al. 2003). Nonetheless, this work should be replicated in an independent larger cohort of VCFS/DGS patients.
Transforming growth factor beta (Tgfβ)
Specific inactivation of transforming growth factor beta (Tgfβ) signaling by conditional inactivation of the TGFβ receptor type II (TβRII) gene in neural crest stem cells results in VCFS/DGS related defects. It was shown that neural crest differentiation rather than migration or cell survival was defective in these mutants. Also, it was demonstrated that Tgfβ signaling is required for phosphorylation of Crkl in neural crest cells (Wurdak et al. 2005). These data indicate that Tgfβ signaling may thus modify the VCFS/DGS phenotype. Nevertheless, Tbx1 +/−; Tgfb2 +/− mice did not show signs of a genetic interaction in our lab (Liao, et al., unpublished). Further work needs to be performed to understand potential genetic relationships with this pathway.
Chordin
Mouse mutants which are homozygous null for Chordin, an antagonist of bone morphogenetic protein (Bmp) signaling, show a phenotype characteristic of VCFS/DGS. This includes craniofacial defects, cleft palate and reduction of the external ear, aplasia of the thymus and parathyroid glands as well as cardiovascular defects including persistent truncus arteriosus and aortic arch artery remodeling malformations. Both Tbx1 and Fgf8 were reduced in the endoderm of these mutants, indicating that both genes may act downstream of Chrd in the same regulatory pathway (Bachiller et al. 2003). These data indicate that loss of Chrd function has phenotypic effects very similar to those of VCFS/DGS, despite the fact that Chrd is located outside the 22q11 deletion interval. This indicates that polymorphisms in the CHRD gene may modify the phenotype of VCFS/DGS as well.
Sonic hedgehog (Shh)
Sonic hedgehog (Shh) is one of three mammalian homologues of the extracellular signaling molecule, Hedgehog. Shh−/− embryos exhibit abnormal cardiovascular development which includes a single outflow tract and aortic arch anomalies like right sided aortic arch and abnormal subclavian arteries (Washington Smoak et al. 2005). These defects are commonly seen in VCFS/DGS and in Tbx1−/− mutant embryos. Tbx1 expression is reduced in Shh mutant mice (Garg et al. 2001). It was found that Shh may regulate Tbx1 expression through the Fox family of transcription factors (Foxc2), binding to an upstream regulatory region in the Tbx1 locus (Garg et al. 2001; Yamagishi et al. 2003). These findings suggest a regulatory relationship between Shh and Tbx1 and also that SHH is a possible modifying locus for VCFS/DGS.
Conclusions and future prospects
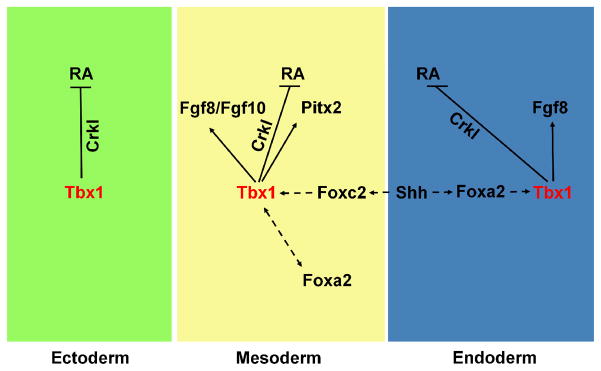
Studies in model organisms are gradually elucidating genes, which may act as modifying loci for VCFS/DGS. Given the variable expressivity seen in the syndrome, it is possible to hypothesize that the genetic loci described above can modify the phenotype in the syndrome. Some of the genes can fit into a genetic pathway of Tbx1 (Figure 2). In this pathway, Tbx1 serves to restrict RA signaling via Crkl (Guris et al., 2006) in all three germ layers where it is expressed (Figure 2). In addition, it acts to regulate Fgfs including Fgf8 and Fgf10, required in the AHF/SHF for the development of the OFT. Tbx1 also regulates Fgf8 in the endoderm which could signal to adjacent NCCs thereby activating pathways leading to cell proliferation. Finally, the Shh pathway is shown in the endoderm and mesoderm acting upstream of Tbx1 (Garg et al., 2001). Future studies should be directed at: (i) testing the in vivo significance of genetic interactions between these genes and Tbx1 in model organisms (ii) using association studies to test candidate genes as modifiers of VCFS/DGS and (iii) identify regions containing genetic modifiers by performing whole genome association studies in a large cohort of VCFS/DGS patients.
Figure 2.
Hypothetical genetic pathways of Tbx1 in the development of the pharyngeal apparatus. Tbx1 is expressed in the pharyngeal ectoderm (green), mesoderm (yellow), and endoderm (blue). In the mesoderm, the gene activates fibroblast growth factor (FGF) family members Fgf8 and Fgf10, as well as Pitx2 (arrows). Tbx1 is also implicated in an auto-regulatory loop with Foxa2 in the pharyngeal mesoderm (double-headed dashed arrows). In the endoderm, the gene activates Fgf8. Sonic hedgehog (Shh), from the endoderm, regulates expression of Tbx1 in the mesoderm and in the endoderm, possibly through regulation of Foxc2 and Foxa2, respectively (dashed arrows). Tbx1 together with Crkl negatively regulates activation of the retinoic acid (RA) signaling pathways in all three germ layers (arrows).
Acknowledgments
We would like to acknowledge Ms. Marilana Petti for critical review of this manuscript. This work was supported by grants from NHLBI (R01HL084410-01A1), NIDCD (R01DC05186-03) and the March of Dimes (FY2005-443) (B.E.M.).
References
- Abu-Issa R, Smyth G, Smoak I, et al. Fgf8 is required for pharyngeal arch and cardiovascular development in the mouse. Development. 2002;129(19):4613–4625. doi: 10.1242/dev.129.19.4613. [DOI] [PubMed] [Google Scholar]
- Aggarwal VS, Liao J, Bondarev A, et al. Dissection of Tbx1 and Fgf interactions in mouse models of 22q11DS suggests functional redundancy. Hum Mol Genet. 2006;15(21):3219–3228. doi: 10.1093/hmg/ddl399. [DOI] [PubMed] [Google Scholar]
- Bachiller D, Klingensmith J, Shneyder N, et al. The role of chordin/Bmp signals in mammalian pharyngeal development and DiGeorge syndrome. Development. 2003;130(15):3567–3578. doi: 10.1242/dev.00581. [DOI] [PubMed] [Google Scholar]
- Bockman DE, Redmond ME, Kirby ML. Altered development of pharyngeal arch vessels after neural crest ablation. Ann N Y Acad Sci. 1990;588:296–304. doi: 10.1111/j.1749-6632.1990.tb13219.x. [DOI] [PubMed] [Google Scholar]
- Bottcher RT, Niehrs C. Fibroblast growth factor signaling during early vertebrate development. Endocr Rev. 2005;26(1):63–77. doi: 10.1210/er.2003-0040. [DOI] [PubMed] [Google Scholar]
- Brown CB, Wenning JM, Lu MM, et al. Cre-mediated excision of Fgf8 in the Tbx1 expression domain reveals a critical role for Fgf8 in cardiovascular development in the mouse. Dev Biol. 2004;267(1):190–202. doi: 10.1016/j.ydbio.2003.10.024. [DOI] [PubMed] [Google Scholar]
- Burn J, Goodship JA. In: Congenital heart disease. Rimoin DLCJM, Pyeritz RE, editors. Churchill Livingstone; 1996. pp. 767–828. [Google Scholar]
- Byrd NA, Meyers EN. Loss of Gbx2 results in neural crest cell patterning and pharyngeal arch artery defects in the mouse embryo. Dev Biol. 2005;284(1):233–245. doi: 10.1016/j.ydbio.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Chapman DL, Garvey N, Hancock S, et al. Expression of the T-box family genes, Tbx1-Tbx5, during early mouse development. Dev Dyn. 1996;206(4):379–390. doi: 10.1002/(SICI)1097-0177(199608)206:4<379::AID-AJA4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- DiGeorge A. A new concept of the cellular basis of immunity. J Pediatr. 1965;67:907–908. [Google Scholar]
- Duester G. Families of retinoid dehydrogenases regulating vitamin A function: production of visual pigment and retinoic acid. Eur J Biochem. 2000;267(14):4315–4324. doi: 10.1046/j.1432-1327.2000.01497.x. [DOI] [PubMed] [Google Scholar]
- Edelmann L, Pandita RK, Morrow BE. Low-copy repeats mediate the common 3-Mb deletion in patients with velo-cardio-facial syndrome. Am J Hum Genet. 1999;64(4):1076–1086. doi: 10.1086/302343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18(1):4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- Frank DU, Fotheringham LK, Brewer JA, et al. An Fgf8 mouse mutant phenocopies human 22q11 deletion syndrome. Development. 2002;129(19):4591–4603. doi: 10.1242/dev.129.19.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Sato T, Kaneko S, et al. Metabolic inactivation of retinoic acid by a novel P450 differentially expressed in developing mouse embryos. Embo J. 1997;16(14):4163–4173. doi: 10.1093/emboj/16.14.4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg V, Yamagishi C, Hu T, et al. Tbx1, a DiGeorge syndrome candidate gene, is regulated by sonic hedgehog during pharyngeal arch development. Dev Biol. 2001;235(1):62–73. doi: 10.1006/dbio.2001.0283. [DOI] [PubMed] [Google Scholar]
- Graham A. Development of the pharyngeal arches. Am J Med Genet A. 2003;119(3):251–256. doi: 10.1002/ajmg.a.10980. [DOI] [PubMed] [Google Scholar]
- Guris DL, Duester G, Papaioannou VE, et al. Dose-dependent interaction of Tbx1 and Crkl and locally aberrant RA signaling in a model of del22q11 syndrome. Dev Cell. 2006;10(1):81–92. doi: 10.1016/j.devcel.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Guris DL, Fantes J, Tara D, et al. Mice lacking the homologue of the human 22q11.2 gene CRKL phenocopy neurocristopathies of DiGeorge syndrome. Nat Genet. 2001;27(3):293–298. doi: 10.1038/85855. [DOI] [PubMed] [Google Scholar]
- Hu T, Yamagishi H, Maeda J, et al. Tbx1 regulates fibroblast growth factors in the anterior heart field through a reinforcing autoregulatory loop involving forkhead transcription factors. Development. 2004;131(21):5491–5502. doi: 10.1242/dev.01399. [DOI] [PubMed] [Google Scholar]
- Ilagan R, Abu-Issa R, Brown D, et al. Fgf8 is required for anterior heart field development. Development. 2006;133(12):2435–2445. doi: 10.1242/dev.02408. [DOI] [PubMed] [Google Scholar]
- Jerome LA, Papaioannou VE. DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nat Genet. 2001;27(3):286–291. doi: 10.1038/85845. [DOI] [PubMed] [Google Scholar]
- Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev Cell. 2001;1(3):435–440. doi: 10.1016/s1534-5807(01)00040-5. [DOI] [PubMed] [Google Scholar]
- Kelly RG, Papaioannou VE. Visualization of outflow tract development in the absence of Tbx1 using an FgF10 enhancer trap transgene. Dev Dyn. 2007;236(3):821–828. doi: 10.1002/dvdy.21063. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Waldo KL. Neural crest and cardiovascular patterning. Circ Res. 1995;77(2):211–215. doi: 10.1161/01.res.77.2.211. [DOI] [PubMed] [Google Scholar]
- Lindsay EA, Vitelli F, Su H, et al. Tbx1 haploinsufficieny in the DiGeorge syndrome region causes aortic arch defects in mice. Nature. 2001;410(6824):97–101. doi: 10.1038/35065105. [DOI] [PubMed] [Google Scholar]
- Macatee TL, Hammond BP, Arenkiel BR, et al. Ablation of specific expression domains reveals discrete functions of ectoderm- and endoderm-derived FGF8 during cardiovascular and pharyngeal development. Development. 2003;130(25):6361–6374. doi: 10.1242/dev.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marguerie A, Bajolle F, Zaffran S, et al. Congenital heart defects in Fgfr2-IIIb and Fgf10 mutant mice. Cardiovasc Res. 2006;71(1):50–60. doi: 10.1016/j.cardiores.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Merscher S, Funke B, Epstein JA, et al. TBX1 is responsible for cardiovascular defects in velo-cardio-facial/DiGeorge syndrome. Cell. 2001;104(4):619–629. doi: 10.1016/s0092-8674(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Mjaatvedt CH, Nakaoka T, Moreno-Rodriguez R, et al. The outflow tract of the heart is recruited from a novel heart-forming field. Dev Biol. 2001;238(1):97–109. doi: 10.1006/dbio.2001.0409. [DOI] [PubMed] [Google Scholar]
- Moon AM, Guris DL, Seo JH, et al. Crkl deficiency disrupts Fgf8 signaling in a mouse model of 22q11 deletion syndromes. Dev Cell. 2006;10(1):71–80. doi: 10.1016/j.devcel.2005.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowotschin S, Liao J, Gage PJ, et al. Tbx1 affects asymmetric cardiac morphogenesis by regulating Pitx2 in the secondary heart field. Development. 2006;133(8):1565–1573. doi: 10.1242/dev.02309. [DOI] [PubMed] [Google Scholar]
- Park EJ, Ogden LA, Talbot A, et al. Required, tissue-specific roles for Fgf8 in outflow tract formation and remodeling. Development. 2006;133(12):2419–2433. doi: 10.1242/dev.02367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paylor R, Glaser B, Mupo A, et al. Tbx1 haploinsufficiency is linked to behavioral disorders in mice and humans: implications for 22q11 deletion syndrome. Proc Natl Acad Sci U S A. 2006;103(20):7729–7734. doi: 10.1073/pnas.0600206103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedra ME, Icardo JM, Albajar M, et al. Pitx2 participates in the late phase of the pathway controlling left-right asymmetry. Cell. 1998;94(3):319–324. doi: 10.1016/s0092-8674(00)81475-0. [DOI] [PubMed] [Google Scholar]
- Reijntjes S, Gale E, Maden M. Generating gradients of retinoic acid in the chick embryo: Cyp26C1 expression and a comparative analysis of the Cyp26 enzymes. Dev Dyn. 2004;230(3):509–517. doi: 10.1002/dvdy.20025. [DOI] [PubMed] [Google Scholar]
- Roberts C, Ivins S, Cook AC, et al. Cyp26 genes a1, b1 and c1 are down-regulated in Tbx1 null mice and inhibition of Cyp26 enzyme function produces a phenocopy of DiGeorge Syndrome in the chick. Hum Mol Genet. 2006;15(23):3394–3410. doi: 10.1093/hmg/ddl416. [DOI] [PubMed] [Google Scholar]
- Roberts C, Ivins SM, James CT, et al. Retinoic acid down-regulates Tbx1 expression in vivo and in vitro. Dev Dyn. 2005;232(4):928–938. doi: 10.1002/dvdy.20268. [DOI] [PubMed] [Google Scholar]
- Robin NH, Shprintzen RJ. Defining the clinical spectrum of deletion 22q11.2. J Pediatr. 2005;147(1):90–96. doi: 10.1016/j.jpeds.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Rosa FW, Wilk AL, Kelsey FO. Teratogen update: vitamin A congeners. Teratology. 1986;33(3):355–364. doi: 10.1002/tera.1420330315. [DOI] [PubMed] [Google Scholar]
- Ryan AK, Goodship JA, Wilson DI, et al. Spectrum of clinical features associated with interstitial chromosome 22q11 deletions: a European collaborative study. J Med Genet. 1997;34(10):798–804. doi: 10.1136/jmg.34.10.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh TH, Kurahashi H, Saitta SC, et al. Chromosome 22-specific low copy repeats and the 22q11.2 deletion syndrome: genomic organization and deletion endpoint analysis. Hum Mol Genet. 2000;9(4):489–501. doi: 10.1093/hmg/9.4.489. [DOI] [PubMed] [Google Scholar]
- Shprintzen RJ, Goldberg RB, Lewin ML, et al. A new syndrome involving cleft palate, cardiac anomalies, typical facies, and learning disabilities: velo-cardio-facial syndrome. Cleft Palate J. 1978;15(1):56–62. [PubMed] [Google Scholar]
- Stalmans I, Lambrechts D, De Smet F, et al. VEGF: a modifier of the del22q11 (DiGeorge) syndrome? Nat Med. 2003;9(2):173–182. doi: 10.1038/nm819. [DOI] [PubMed] [Google Scholar]
- Stoller JZ, Epstein JA. Identification of a novel nuclear localization signal in Tbx1 that is deleted in DiGeorge syndrome patients harboring the 1223delC mutation. Hum Mol Genet. 2005;14(7):885–892. doi: 10.1093/hmg/ddi081. [DOI] [PubMed] [Google Scholar]
- Taddei I, Morishima M, Huynh T, et al. Genetic factors are major determinants of phenotypic variability in a mouse model of the DiGeorge/del22q11 syndromes. Proc Natl Acad Sci U S A. 2001;98(20):11428–11431. doi: 10.1073/pnas.201127298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermot J, Niederreither K, Garnier JM, et al. Decreased embryonic retinoic acid synthesis results in a DiGeorge syndrome phenotype in newborn mice. Proc Natl Acad Sci U S A. 2003;100(4):1763–1768. doi: 10.1073/pnas.0437920100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitelli F, Taddei I, Morishima M, et al. A genetic link between Tbx1 and fibroblast growth factor signaling. Development. 2002;129(19):4605–4611. doi: 10.1242/dev.129.19.4605. [DOI] [PubMed] [Google Scholar]
- Vitelli F, Zhang Z, Huynh T, et al. Fgf8 expression in the Tbx1 domain causes skeletal abnormalities and modifies the aortic arch but not the outflow tract phenotype of Tbx1 mutants. Dev Biol. 2006;295(2):559–570. doi: 10.1016/j.ydbio.2006.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldo KL, Kumiski DH, Wallis KT, et al. Conotruncal myocardium arises from a secondary heart field. Development. 2001;128(16):3179–3188. doi: 10.1242/dev.128.16.3179. [DOI] [PubMed] [Google Scholar]
- Washington Smoak I, Byrd NA, Abu-Issa R, et al. Sonic hedgehog is required for cardiac outflow tract and neural crest cell development. Dev Biol. 2005;283(2):357–372. doi: 10.1016/j.ydbio.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Wassarman KM, Lewandoski M, Campbell K, et al. Specification of the anterior hindbrain and establishment of a normal mid/hindbrain organizer is dependent on Gbx2 gene function. Development. 1997;124(15):2923–2934. doi: 10.1242/dev.124.15.2923. [DOI] [PubMed] [Google Scholar]
- Wurdak H, Ittner LM, Lang KS, et al. Inactivation of TGFbeta signaling in neural crest stem cells leads to multiple defects reminiscent of DiGeorge syndrome. Genes Dev. 2005;19(5):530–535. doi: 10.1101/gad.317405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Morishima M, Wylie JN, et al. Tbx1 has a dual role in the morphogenesis of the cardiac outflow tract. Development. 2004;131(13):3217–3227. doi: 10.1242/dev.01174. [DOI] [PubMed] [Google Scholar]
- Yagi H, Furutani Y, Hamada H, et al. Role of TBX1 in human del22q11.2 syndrome. Lancet. 2003;362(9393):1366–1373. doi: 10.1016/s0140-6736(03)14632-6. [DOI] [PubMed] [Google Scholar]
- Yamagishi H, Maeda J, Hu T, et al. Tbx1 is regulated by tissue-specific forkhead proteins through a common Sonic hedgehog-responsive enhancer. Genes Dev. 2003;17(2):269–281. doi: 10.1101/gad.1048903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhong T, Wang Y, et al. TBX1, a DiGeorge syndrome candidate gene, is inhibited by retinoic acid. Int J Dev Biol. 2006;50(1):55–61. doi: 10.1387/ijdb.052036lz. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Cerrato F, Xu H, et al. Tbx1 expression in pharyngeal epithelia is necessary for pharyngeal arch artery development. Development. 2005;132(23):5307–5315. doi: 10.1242/dev.02086. [DOI] [PubMed] [Google Scholar]