Abstract
Recently, a series of potent and selective neuronal nitric oxide synthase inhibitors containing two basic nitrogen atoms was reported (Ji, H.; Stanton, B. Z.; Igarashi, J.;, Li, H.; Martásek, P.; Roman, L. J.; Poulos, T. L.; Silverman, R. B. J. Am. Chem. Soc. 2008, 130(12), 3900–3914). In an effort to improve their bioavailability, three compounds (2a–c) were designed with electron-withdrawing groups near one of the basic nitrogen atoms to lower its pKa. Inhibition studies with these compounds showed that two of them not only retained most of the potency and selectivity of the best analogue of the earlier series, but also showed improved membrane permeability based on data from a cell-based assay.
Neuronal nitric oxide synthase (nNOS) catalyzes the oxidation of l-arginine to l-citrulline via the intermediate NG-hydroxy-l-arginine in the central nervous system (CNS), generating the highly reactive free radical neurotransmitter nitric oxide (NO).1–3 Significant research has shown that overproduction of NO from nNOS is implicated in various neurodegenerative diseases,4–5 including Parkinson’s,6–8 Alzheimer’s,9 Huntington’s10 diseases, and stroke.11 Since nNOS plays a critical role in the production of neuronal NO, it is considered to be a promising neurodegenerative therapeutic target.12–14
Although intense research efforts have been devoted to the design and development of small molecules to inhibit the activity of nNOS,15 none has been reported to enter clinical trials for neurodegenerative disease. There are two major challenges, in addition to high potency, involved in developing novel nNOS inhibitors. The first is the selectivity of inhibitors for nNOS over its closely related isozymes, endothelial NOS (eNOS) and inducible NOS (iNOS). eNOS is a key regulator of blood pressure and vascular tissues. It has been shown that inhibition of eNOS activity leads to high blood pressure and vascular effects.16 On the other hand, iNOS is the enzyme responsible for the generation of cytotoxic NO, playing an important role in the immune system.16 Therefore, when blocking the activity of nNOS, designed inhibitors should not interfere with the activity of either eNOS or iNOS.15 The second challenge for drugs that target nNOS, which primarily resides in the CNS, is the ability of the designed compounds to penetrate the blood brain barrier (BBB). The BBB is a unique barrier formed by brain capillary endothelial cells that are linked via tight junctions. Accordingly, drug 5 molecules need to cross the endothelial cell monolayer via the transcellular pathway to exhibit a pharmaceutical effect in the CNS.
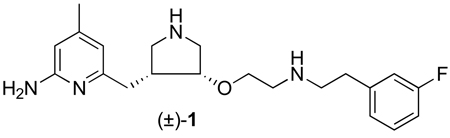
Our laboratory has previously reported the structure-based design, synthesis, and biological evaluation of a series of pyrrolidine-based nNOS selective inhibitors.17–19 Among them, 1 demonstrates great potency (Ki = 15 nM) and very high selectivity for nNOS over eNOS (2100 fold) and iNOS (630 fold). These data indicated that 1 is a promising candidate to be considered as a lead compound for neurodegenerative drugs.19
Although 1 exhibits high in vitro potency and selectivity, its therapeutic utility is limited by poor BBB permeability.19 We speculated that the two positive charges of 1 at physiological pH, derived from the two secondary amino groups, dramatically impaired the ability of 1 to penetrate the highly lipophilic BBB by passive diffusion.
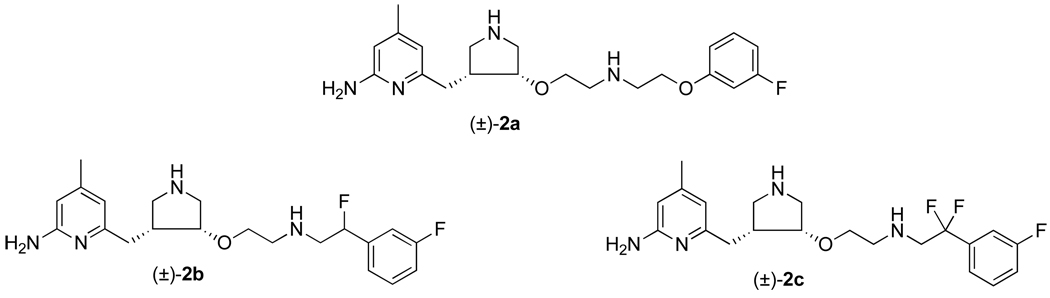
The low membrane permeability of 1 represents a major challenge for further investigation of this compound. To circumvent this problem, different strategies have been applied in an attempt to improve the bioavailability of 1.19–21 In this report, we describe the design, synthesis, and biological evaluation of a new series of analogues of 1 with electron-withdrawing groups (Figure 1) to lower the pKa of one of the basic nitrogens (2a–c), and demonstrate the possibility of using them as inhibitors with improved bioavailability.
Figure 1.
Chemical structures of 2a–c.
In the current design, different electron withdrawing groups, including ether (2a), monofluoro methylene (2b), and difluoro methylene (2c), were introduced at a vicinal position to the amino group in the lipophilic tail of 1. Chemical structures of these new inhibitors are closely related to that of 1, while the predicted pKa values of the protonated vicinal nitrogen atoms are significantly lower than the corresponding protonated nitrogen atom in 1 (Table 1). We anticipate that the additional electron withdrawing groups, which partially remove the positive charge from the vicinal nitrogen atom by induction, would decrease the cationic character of inhibitors 2a–c compared to 1, and therefore, improve membrane permeability of the inhibitors.
Table 1.
Physiochemical properties of inhibitors 1 and 2a–c.
| Compound | Mw | pKaa |
|---|---|---|
| 1 | 372 | 8.94 |
| 2a | 388 | 8.12 |
| 2b | 390 | 7.32 |
| 2c | 408 | 5.56 |
Lipophilicity data were calculated with ACD/LogD version 7.0, Advanced Chemistry Development, Inc., Toronto, Canada.
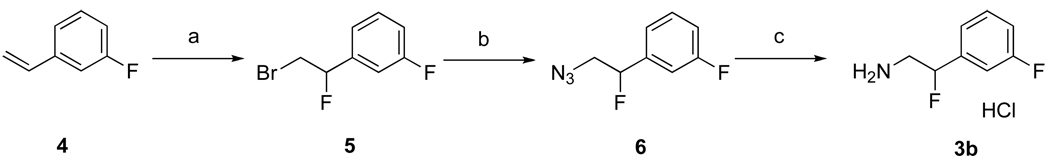
As shown in Scheme 1, the synthesis of 3b began with 3-fluorostyrene (4). Bromofluorination of 4 using NBS and Et3N·3HF generated 1-(2-bromo-1-fluoroethyl)-3-fluorobenzene (5) in high yields.22 Next, 5 was converted to azide (6) using NaN3 in DMSO at 65 °C in good yields.23 Finally, catalytic hydrogenation of 6 in a mixture of EtOH and 1 N HCl (2:1) provided 3b as a HCl salt in excellent yields.
Scheme 1.
Reagents and conditions: (a) NBS, Et3N·3HF, CH2Cl2, 0 °C to r.t., 12 h, 85%; (b) NaN3, DMSO, 65 °C, 4 h, 85%; (c) Pd(OH)2/C, H2, EtOH/1 N HCl (2:1), r.t., 24 h, 98%.
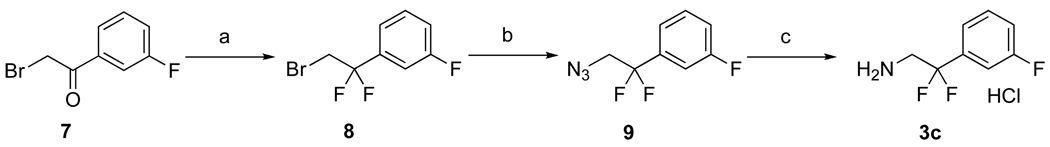
2,2-Difluoro-2-(3-fluorophenyl)ethanamine (3c) was synthesized as shown in Scheme 2. 2-Bromo-3’-fluoroacetophenone (7) was treated with diethylaminosulfur trifluoride (DAST) to give 1-(2-bromo-1,1-difluoroethyl)-3-fluorobenzene (8) in good yields.24 Next, 8 was allowed to react with NaN3 in DMSO at 110 °C to give azide (9) in good yields. Finally, 9 was subjected to catalytic hydrogenation under acidic conditions to give 3c as an HCl salt in excellent yields.
Scheme 2.
Reagents and conditions: (a) DAST (neat), rt, 7 days, 70%; (b) NaN3, DMSO, 110 °C, 1 h, 88%; (c) Pd(OH)2/C, H2, EtOH/1 N HCl (2:1), rt, 24 h, 91%..
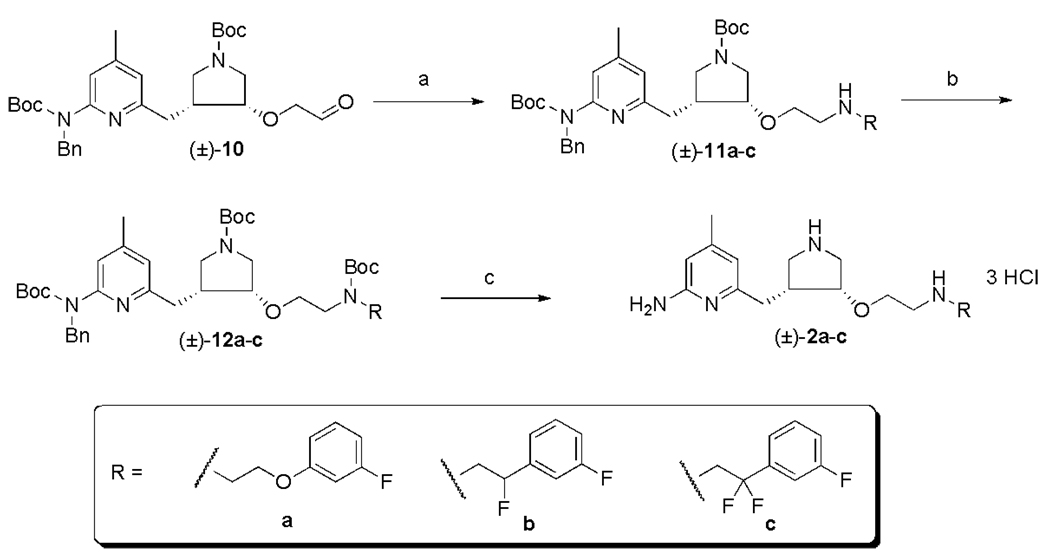
With 3a–c in hand (3a is commercially available), we finished the syntheses of inhibitors 2a–c using a three-step procedure (Scheme 3). First, reductive amination between 1019 and 3a–c using NaHB(OAc)3 gave 11a–c in modest yields. To simplify the purification process, the resulting secondary amines (11a–c) were protected with (Boc)2O to give 12a–c. Finally, the Bn-protecting group and the three Boc-protecting groups were removed at the same time in a mixture of EtOH and 12 N HCl (2:1) under high pressure catalytic hydrogenation conditions to provide 2a–c as HCl salts in good yields.
Scheme 3.
Reagents and conditions: (a) (i) 3a–c, THF, r.t., 5 min, (ii) NaHB(OAc)3, r.t., 3 h; (b) (Boc)2O, Et3N, MeOH, r.t., 12 h, 48–60% for two steps; (c) Pd(OH)2/C, H2, 2:1 EtOH/HCl (12 N), r.t., 500 psi, 40 h, 85–91%.
Inhibitors 2a–c were evaluated for in vitro inhibition against three isozymes of NOS: rat nNOS, bovine eNOS, and murine iNOS using known methods.25 Inhibitor 2a, with an electronegative oxygen atom inserted into the lipophilic tail of 1, is almost six-fold less potent for nNOS compared to the lead compound (1), while the selectivity of this inhibitor for nNOS over eNOS and iNOS decreases by ten-fold and three-fold, respectively. There are two likely reasons for these results. First, compound 2a is one atom longer than 1, which probably makes it too long to fit snugly into the active site of nNOS. Moreover, it has been shown previously that the positively charged amine functionality in the lipophilic tail plays an important role for tight binding of 1 to nNOS;19 therefore, partial removal of the positive charge from this group may impair the activity of inhibitors.19 Inhibitor 2b, with monofluoromethylene vicinal to one of the basic amino groups, shows very good potency for nNOS and excellent selectivity for nNOS over eNOS and iNOS. Given that the pKa of the vicinal amino group in 2b is lower than that in 2a, partial removal of the positive charge on that amino group cannot explain the lower potency of 2a. Finally, inhibitor 2c, with a difluoromethylene in the lipophilic tail of 1, exhibited a 2.2-fold drop in potency relative to 2b. The introduction of the strongly electron-withdrawing difluoromethylene group essentially completely removes the positive charge on the amino group in the lipophilic tail at neutral pH, which decreases its ability for an electrostatic interaction with the heme carboxylate. However, inhibitor 2c still showed excellent selectivity for nNOS over eNOS and iNOS.
The two best inhibitors (2b and 2c), together with the lead compound (1), were tested for their potency in a cell-based assay.26 This assay can provide information about their membrane permeability. The results are summarized in Table 3. The IC50 values for inhibitors 2b and 2c are compared to the lead compound (1) in the first ratio column. Compound 2b is 0.42 times as potent as 1; compound 2c is 0.19 times as potent as 1. The second ratio column compares IC50(cell) values for 2b and 2c relative to 1 in the cell-based assay, which should relate to the relative ability of the compounds to penetrate the cell membrane. Compound 2b is only 0.9 as potent as 1, which indicates that 2b crosses the cell membrane 2.1 times (0.9/0.42) better than 1. Compound 2c is 0.47 times as potent as 1 in the cell-based assay, indicating that 2c crosses the cell membrane 2.5 times (0.47/0.19) better than 1. This suggests that the difluoromethylene electron-withdrawing group of 2c, which decreases the pKa of the vicinal amino group compared to the monofluoromethylene group of 2b and no electron-withdrawing group of 1, improves cell permeability. On the other hand, the absolute potency of inhibitors may be sacrificed because of the importance of the positively charged amino group in binding to nNOS.
Table 3.
IC50 values of inhibitors in purified enzyme assay and cell-based assay.
| Compound | IC50 (µM)a | IC50(cell) (µM)a |
IC50(1)/ IC50(x) | IC50(1)(cell)/ IC50(x)(cell) |
|---|---|---|---|---|
| 1 | 0.13 | 9 | - | - |
| 2b | 0.31 | 10 | 0.42 | 0.9 |
| 2c | 0.70 | 19 | 0.19 | 0.47 |
The IC50 values represent at least duplicate measurements with standard deviations of ±10%.
In conclusion, a new series of potent and selective nNOS inhibitors (2a–2c) were designed and synthesized. Purified enzyme and cell-based evaluation of these new inhibitors led to the discovery of inhibitors 2b and 2c, which not only retain most of the activity of the lead compound (1), but also have improved membrane permeability.
Supplementary Material
Table 2.
Kia values of inhibitors for rat nNOS, bovine eNOS, and murine iNOS.
| Compound | nNOS (µM) | eNOS (µM) | iNOS (µM) | selectivityb | |
|---|---|---|---|---|---|
| n/e | n/i | ||||
| 119 | 0.015 | 31 | 9.5 | 2100 | 630 |
| 2a | 0.086 | 21 | 18 | 240 | 210 |
| 2b | 0.036 | 36 | 13 | 1000 | 360 |
| 2c | 0.080 | 62 | 52 | 780 | 650 |
The Ki values were calculated based on the directly measured IC50 values, which represent at least duplicate measurements with standard deviations of ±10%.
The ratio of Ki (eNOS or iNOS) to Ki (nNOS).
Acknowledgments
The authors are grateful to the National Institutes of Health (GM49725 to R.B.S. and GM52419 to Professor Bettie Sue Siler Masters, with whose laboratory P.M. and L.J.R. are associated) for financial support of this research. B.S.S.M. also is grateful to the Welch Foundation for a Robert A. Welch Foundation Distinguished Professorship in Chemistry (AQ0012). P.M. is supported by grants 0021620806 and 1M0520 from MSMT of the Czech Republic.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supporting Information: Detailed synthetic procedures and full characterization (1H NMR, 13C NMR and mass spectrum) of compounds 5–6, 8–9, 3b–c, 12a–c, and 2a–c are available.
References and Notes
- 1.Hall AV, Antoniou H, Wang Y, Cheung AH, Arbus AM, Olson SL, Lu WC, Kau CL, Marsden PA. J. Biol. Chem. 1994;269:33082. [PubMed] [Google Scholar]
- 2.Wang Y, Newton DC, Marsden PA. Crit. Rev. Neurobiol. 1999;13:21. doi: 10.1615/critrevneurobiol.v13.i1.20. [DOI] [PubMed] [Google Scholar]
- 3.Rosen GM, Tsai P, Pou S. Chem. Rev. 2002;102:1191. doi: 10.1021/cr010187s. [DOI] [PubMed] [Google Scholar]
- 4.Huang Z, Huang PL, Panahian N, Dalkara T, Fishman MC, Moskowitz MA. Science. 1994;265:1883. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- 5.Duncan AJ, Heales SJ. Mol. Aspects. Med. 2005;26:67. doi: 10.1016/j.mam.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Przedborski S, Jackson-Lewis V, Yokoyama R, Shibata T, Dawson VL, Dawson TM. Proc. Natl. Acad. Sci. U.S.A. 1996;93:4565. doi: 10.1073/pnas.93.10.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hantraye P, Brouillet E, Ferrante R, Palfi S, Dolan R, Matthews RT, Beal MF. Nat. Med. 1996;2:1017. doi: 10.1038/nm0996-1017. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Dawson VL, Dawson TM. Pharmacol. Ther. 2006;109:33. doi: 10.1016/j.pharmthera.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Dorheim M-A, Tracey WR, Pollock JS, Grammas P. Biochem. Biophys. Res. Commun. 1994;205:659. doi: 10.1006/bbrc.1994.2716. [DOI] [PubMed] [Google Scholar]
- 10.Norris PJ, Waldvogel HJ, Faull RLM, Love DR, Emson PC. Neuronsicence. 1996;72:1037. doi: 10.1016/0306-4522(95)00596-x. [DOI] [PubMed] [Google Scholar]
- 11.Sims NR, Anderson MF. Neurochem. Int. 2002;40:511. doi: 10.1016/s0197-0186(01)00122-x. [DOI] [PubMed] [Google Scholar]
- 12.Southan GJ, Szabo C. Biochem. Pharmacol. 1996;51:383. doi: 10.1016/0006-2952(95)02099-3. [DOI] [PubMed] [Google Scholar]
- 13.Babu BR, Griffith OW. Curr. Opin. Chem. Biol. 1998;2:491. doi: 10.1016/s1367-5931(98)80125-7. [DOI] [PubMed] [Google Scholar]
- 14.Hobbs AJ, Higgs A, Moncada S. Annu. Rev. Pharmacol. Toxicol. 1999;39:191. doi: 10.1146/annurev.pharmtox.39.1.191. [DOI] [PubMed] [Google Scholar]
- 15.Ji H, Erdal EP, Litzinger EA, Seo J, Zhu Y, Xue F, Fang J, Huang J, Silverman RB. In: Frontiers in Medicinal Chemistry. Reitz AB, Choudhary MI, editors. Volume 4. Bentham Science Publishers; 2009. p. 842. [Google Scholar]
- 16.Alderton WK, Cooper CE, Knowles RG. Biochem. J. 2001;357:593. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji H, Tan S, Igarashi J, Li H, Derrick M, Martásek P, Roman LJ, Vásquez-Vivar J, Poulos TL, Silverman RB. Ann. Neurol. 2009;65:209. doi: 10.1002/ana.21555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ji H, Li H, Martásek P, Roman L, Poulos TL, Silverman RB. J. Med. Chem. 2009;53:779. doi: 10.1021/jm801220a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawton GR, Ranaivo HR, Wing LK, Ji H, Xue F, Martesek P, Roman LJ, Watterson DM, Silverman RB. Bioorg. Med. Chem. 2009;17:2371. doi: 10.1016/j.bmc.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue F, Silverman RB. unpublished results. [Google Scholar]
- 21.Xue F, Ji H, Huang J, Fang J, Silverman RB. unpublished results. [Google Scholar]
- 22.Rosen TC, Yoshida S, Fröehlich R, Kirk KL, Haufe G. J. Med. Chem. 2004;47:5860. doi: 10.1021/jm049957t. [DOI] [PubMed] [Google Scholar]
- 23.Other solvents such as THF and DMF did not give satisfactory yields.
- 24.Lakshmipathi P, Crévisy C, Grée R. J. Comb. Chem. 2002;4:612. doi: 10.1021/cc0200179. [DOI] [PubMed] [Google Scholar]
- 25.Hevel JM, Marletta MA. Methods Enzymol. 1994;233:250. doi: 10.1016/s0076-6879(94)33028-x. [DOI] [PubMed] [Google Scholar]
- 26.Fang J, Silverman RB. Anal. Biochem. 2009;390:74. doi: 10.1016/j.ab.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.