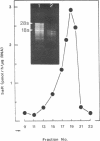
Abstract
The enzyme 4-ene-3-ketosteroid-5 alpha-oxidoreductase [5 alpha-reductase; 3-oxo-5 alpha-steroid delta 4-dehydrogenase, 3-oxo-5 alpha-steroid: (acceptor) delta 4-oxidoreductase, EC 1.3.99.5] plays a key role in androgen-dependent target tissues, where it catalyzes the conversion of testosterone to the biologically active dihydrotestosterone. The regulation of 5 alpha-reductase expression has not been studied at the molecular level as the enzyme is a membrane protein that is labile in cell-free homogenates. We developed a sensitive bioassay of the enzyme activity expressed in Xenopus oocytes microinjected with rat liver and prostate mRNA. After microinjection, incubation of intact oocytes in the presence of [3H]testosterone revealed the in ovo appearance of active 5 alpha-reductase. Polyadenylated RNA was fractionated by sucrose gradient centrifugation, and the enzymatic activity was shown to be encoded by a 1600- to 2000-base-pair fraction of hepatic poly(A)+ RNA. 5 alpha-Reductase mRNA was most efficiently translated when up to 80 ng of RNA was injected per oocyte. In the injected oocytes, 5 alpha-reductase mRNA was found to be a short-lived molecule (t1/2 = 2 hr), whereas its in ovo translatable 5 alpha-reductase protein exhibited stable enzymatic activity for over 40 hr. Moreover, the levels of translatable tissue-specific 5 alpha-reductase mRNAs as monitored in the Xenopus oocytes correlated with the variable 5 alpha-reductase activities in female rat liver, male rat liver, and prostate homogenates; the ratio of their specific activities was of 2500:630:1, respectively. Altogether, these results provide supporting evidence in favor of the transcriptional control of 5 alpha-reductase expression in rat tissues.
Full text
PDF
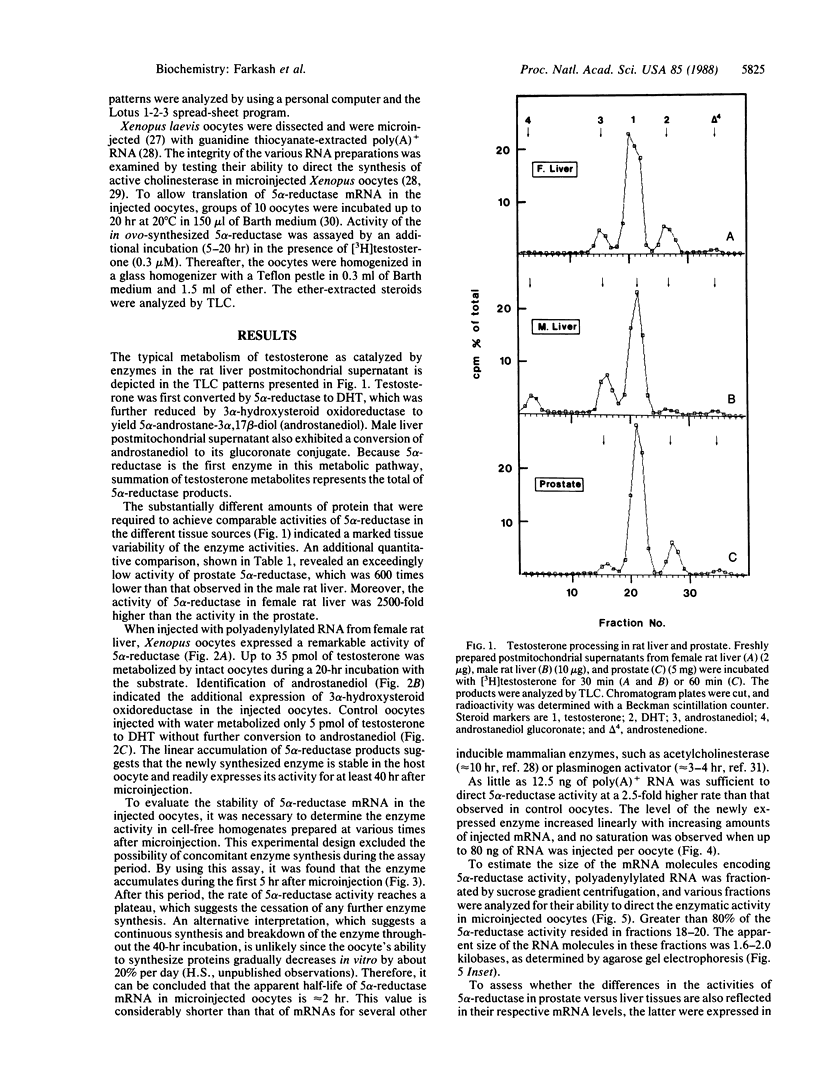
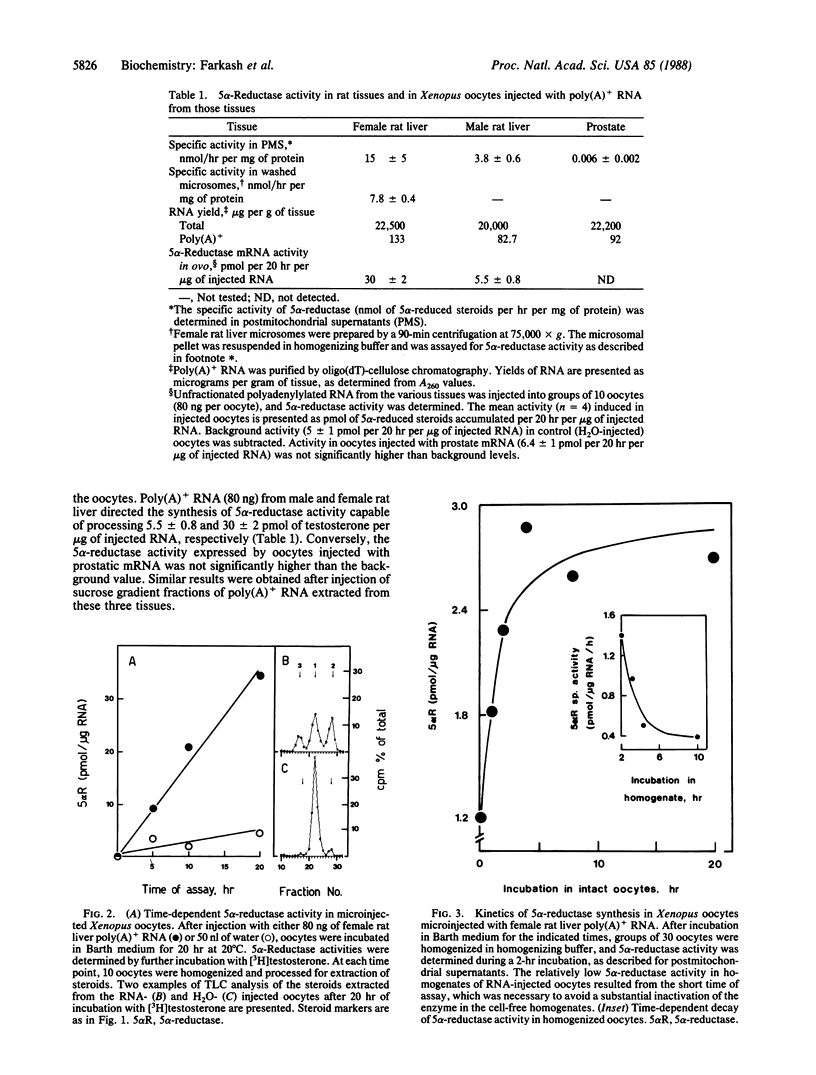
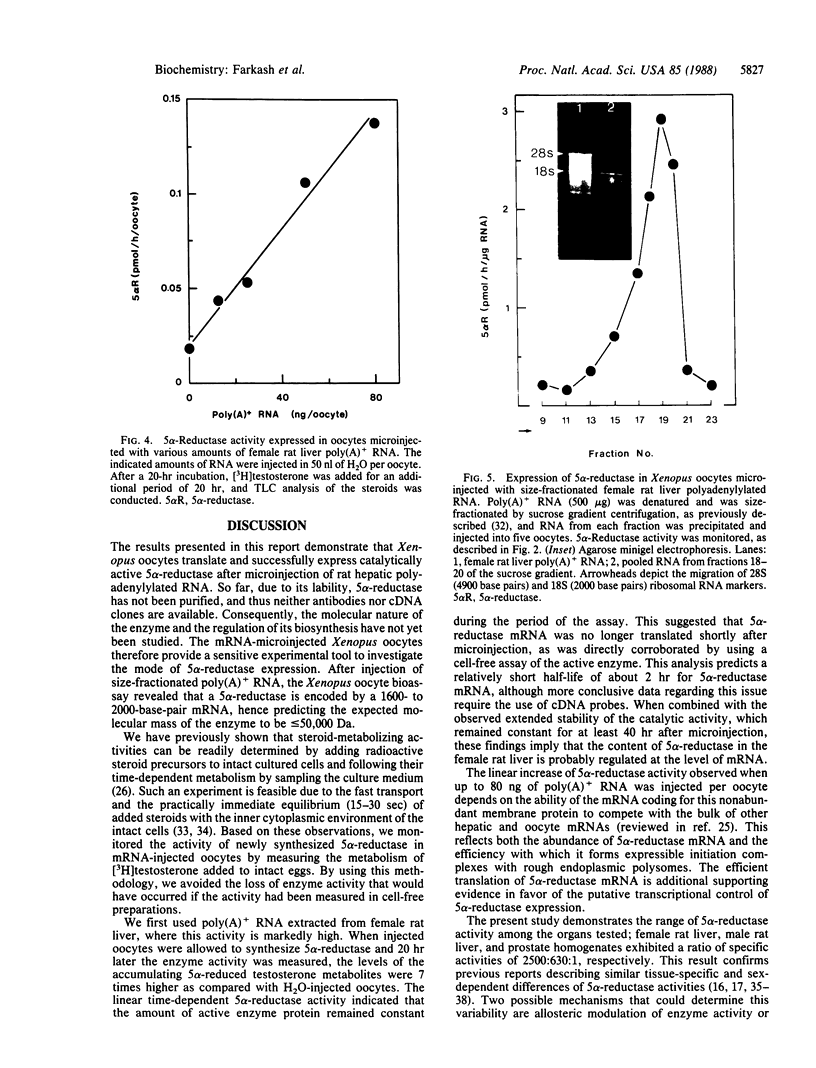

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. M., Liao S. Selective retention of dihydrotestosterone by prostatic nuclei. Nature. 1968 Jul 20;219(5151):277–279. doi: 10.1038/219277a0. [DOI] [PubMed] [Google Scholar]
- Baulieu E. E., Lasnizki I., Robel P. Metabolism of testosterone and action of metabolites on prostate glands grown in organ culture. Nature. 1968 Sep 14;219(5159):1155–1156. doi: 10.1038/2191155a0. [DOI] [PubMed] [Google Scholar]
- Bertics P. J., Karavolas H. J. Pituitary progesterone 5 alpha-reductase: solubilization and partial characterization. J Steroid Biochem. 1985 Jun;22(6):795–802. doi: 10.1016/0022-4731(85)90288-2. [DOI] [PubMed] [Google Scholar]
- Bingham K. D., Shaw D. A. The metabolism of testosterone by human male scalp skin. J Endocrinol. 1973 Apr;57(1):111–121. doi: 10.1677/joe.0.0570111. [DOI] [PubMed] [Google Scholar]
- Bruchovsky N., Wilson J. D. The conversion of testosterone to 5-alpha-androstan-17-beta-ol-3-one by rat prostate in vivo and in vitro. J Biol Chem. 1968 Apr 25;243(8):2012–2021. [PubMed] [Google Scholar]
- Burmeister M., Avivi A., Schlessinger J., Soreq H. Production of EGF-containing polypeptides in Xenopus oocytes microinjected with submaxillary gland mRNA. EMBO J. 1984 Jul;3(7):1499–1505. doi: 10.1002/j.1460-2075.1984.tb02002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. S., Bertics P. J., Karavolas H. J. The kinetic mechanism of the anterior pituitary progesterone 5 alpha-reductase. J Steroid Biochem. 1986 Apr;24(4):801–806. doi: 10.1016/0022-4731(86)90440-1. [DOI] [PubMed] [Google Scholar]
- Denef C., Magnus C., McEwen B. S. Sex-dependent changes in pituitary 5alpha-dihydrotestosterone and 3alpha-androstanediol formation during postnatal development and puberty in the rat. Endocrinology. 1974 May;94(5):1265–1274. doi: 10.1210/endo-94-5-1265. [DOI] [PubMed] [Google Scholar]
- Geller J., Albert J., Lopez D., Geller S., Niwayama G. Comparison of androgen metabolites in benign prostatic hypertrophy (BPH) and normal prostate. J Clin Endocrinol Metab. 1976 Sep;43(3):686–688. doi: 10.1210/jcem-43-3-686. [DOI] [PubMed] [Google Scholar]
- Giorgi E. P., Stein W. D. The transport of steroids into animal cells in culture. Endocrinology. 1981 Feb;108(2):688–697. doi: 10.1210/endo-108-2-688. [DOI] [PubMed] [Google Scholar]
- Giorgi E. P. The transport of steroid hormones into animal cells. Int Rev Cytol. 1980;65:49–115. doi: 10.1016/s0074-7696(08)61959-2. [DOI] [PubMed] [Google Scholar]
- Goldring N. B., Orly J. Concerted metabolism of steroid hormones produced by cocultured ovarian cell types. J Biol Chem. 1985 Jan 25;260(2):913–921. [PubMed] [Google Scholar]
- Gurdon J. B., Lane C. D., Woodland H. R., Marbaix G. Use of frog eggs and oocytes for the study of messenger RNA and its translation in living cells. Nature. 1971 Sep 17;233(5316):177–182. doi: 10.1038/233177a0. [DOI] [PubMed] [Google Scholar]
- Gustafsson J. A., Pousette A. Properties of nuclear 5 alpha-reductase in rat liver. Biochemistry. 1974 Feb 26;13(5):875–881. doi: 10.1021/bi00702a007. [DOI] [PubMed] [Google Scholar]
- Hanukoglu I., Karavolas H. J., Goy R. W. Progesterone metabolism in the pineal, brain stem, thalamus and corpus callosum of the female rat. Brain Res. 1977 Apr 15;125(2):313–324. doi: 10.1016/0006-8993(77)90624-2. [DOI] [PubMed] [Google Scholar]
- Imperato-McGinley J., Peterson R. E., Gautier T., Sturla E. Male pseudohermaphroditism secondary to 5 alpha-reductase deficiency--a model for the role of androgens in both the development of the male phenotype and the evolution of a male gender identity. J Steroid Biochem. 1979 Jul;11(1B):637–645. doi: 10.1016/0022-4731(79)90093-1. [DOI] [PubMed] [Google Scholar]
- Kuttenn F., Mowszowicz I., Schaison G., Mauvais-Jarvis P. Androgen production and skin metabolism in hirsutism. J Endocrinol. 1977 Oct;75(1):83–91. doi: 10.1677/joe.0.0750083. [DOI] [PubMed] [Google Scholar]
- Liang T., Heiss C. E., Ostrove S., Rasmusson G. H., Cheung A. Binding of a 4-methyl-4-aza-steroid to 5 alpha-reductase of rat liver and prostate microsomes. Endocrinology. 1983 Apr;112(4):1460–1468. doi: 10.1210/endo-112-4-1460. [DOI] [PubMed] [Google Scholar]
- MacIndoe J. H., Woods G. R. Steroid-metabolizing enzymes in human breast cancer cells. II. 5 alpha-Reductase, 3 alpha-hydroxysteroid oxidoreductase, and 17 beta-hydroxysteroid oxidoreductase. Endocrinology. 1981 Apr;108(4):1407–1413. doi: 10.1210/endo-108-4-1407. [DOI] [PubMed] [Google Scholar]
- Martini L. The 5alpha-reduction of testosterone in the neuroendocrine structures. Biochemical and physiological implications. Endocr Rev. 1982 Winter;3(1):1–25. doi: 10.1210/edrv-3-1-1. [DOI] [PubMed] [Google Scholar]
- Massa R., Justo S., Martini L. Conversion of testerone into 5alpha-reduced metabolites in the anterior pituitary and in the brain of maturing rats. J Steroid Biochem. 1975 May;6(5):567–571. doi: 10.1016/0022-4731(75)90036-9. [DOI] [PubMed] [Google Scholar]
- Masu Y., Nakayama K., Tamaki H., Harada Y., Kuno M., Nakanishi S. cDNA cloning of bovine substance-K receptor through oocyte expression system. 1987 Oct 29-Nov 4Nature. 329(6142):836–838. doi: 10.1038/329836a0. [DOI] [PubMed] [Google Scholar]
- Meikle A. W., Collier E. S., Middleton R. G., Fang S. M. Supranormal nuclear content of 5 alpha-dihydrotestosterone in benign hyperplastic prostate of humans. J Clin Endocrinol Metab. 1980 Oct;51(4):945–947. doi: 10.1210/jcem-51-4-945. [DOI] [PubMed] [Google Scholar]
- Miskin R., Soreq H. Microinjected Xenopus oocytes synthesize active human plasminogen activator. Nucleic Acids Res. 1981 Jul 24;9(14):3355–3363. doi: 10.1093/nar/9.14.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. J., Wilson J. D. Extraction of the reduced nicotinamide adenine dinucleotide phosphate:delta 4-3-ketosteroid-5-alpha oxidoreductase of rat prostate with digitonin and potassium chloride. Biochemistry. 1974 Jan 29;13(3):450–456. doi: 10.1021/bi00700a009. [DOI] [PubMed] [Google Scholar]
- Prody C., Zevin-Sonkin D., Gnatt A., Koch R., Zisling R., Goldberg O., Soreq H. Use of synthetic oligodeoxynucleotide probes for the isolation of a human cholinesterase cDNA clone. J Neurosci Res. 1986;16(1):25–35. doi: 10.1002/jnr.490160105. [DOI] [PubMed] [Google Scholar]
- Sansone G., Reisner R. M. Differential rates of conversion of testosterone to dihydrotestosterone in acne and in normal human skin--a possible pathogenic factor in acne. J Invest Dermatol. 1971 May;56(5):366–372. doi: 10.1111/1523-1747.ep12261252. [DOI] [PubMed] [Google Scholar]
- Scheer H., Robaire B. Solubilization and partial characterization of rat epididymal delta 4-steroid 5 alpha-reductase (cholestenone 5 alpha-reductase). Biochem J. 1983 Apr 1;211(1):65–74. doi: 10.1042/bj2110065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H., Noack I., Voigt K. D. Metabolism and mode of action of androgens in target tissues of male rats. II. Mode of action of testosterone and 5 -dihydrotestosterone at a cellular level on seminal vesicles and prostates of rats. Acta Endocrinol (Copenh) 1972 Jan;69(1):165–173. doi: 10.1530/acta.0.0690165. [DOI] [PubMed] [Google Scholar]
- Soreq H., Parvari R., Silman I. Biosynthesis and secretion of catalytically active acetylcholinesterase in Xenopus oocytes microinjected with mRNA from rat brain and from Torpedo electric organ. Proc Natl Acad Sci U S A. 1982 Feb;79(3):830–834. doi: 10.1073/pnas.79.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreq H. The biosynthesis of biologically active proteins in mRNA-microinjected Xenopus oocytes. CRC Crit Rev Biochem. 1985;18(3):199–238. doi: 10.3109/10409238509085134. [DOI] [PubMed] [Google Scholar]
- Soreq H., Zevin-Sonkin D., Razon N. Expression of cholinesterase gene(s) in human brain tissues: translational evidence for multiple mRNA species. EMBO J. 1984 Jun;3(6):1371–1375. doi: 10.1002/j.1460-2075.1984.tb01979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven G., Heyns W., De Moor P. Interconversion between 17 beta-hydroxy-5alpha-androstan-3-one (5alpha-dihydrotestosterone) and 5alpha-androstane-3alpha, 17 beta-diol in rat kidney: heterogeneity of 3alpha-hydroxysteroid oxidoreductases. Eur J Biochem. 1976 Jun 1;65(2):565–576. doi: 10.1111/j.1432-1033.1976.tb10374.x. [DOI] [PubMed] [Google Scholar]
- Wilson J. D., Griffin J. E., George F. W. The mechanism of phenotypic sex differentiation. Arthritis Rheum. 1979 Nov;22(11):1275–1283. doi: 10.1002/art.1780221116. [DOI] [PubMed] [Google Scholar]
- Wilson J. D. The pathogenesis of benign prostatic hyperplasia. Am J Med. 1980 May;68(5):745–756. doi: 10.1016/0002-9343(80)90267-3. [DOI] [PubMed] [Google Scholar]
- YATES F. E., HERBST A. L., URQUHART J. Sex difference in rate of ring A reduction of delta 4-3-keto-steroids in vitro by rat liver. Endocrinology. 1958 Dec;63(6):887–902. doi: 10.1210/endo-63-6-887. [DOI] [PubMed] [Google Scholar]