Abstract
Previous studies have reported learning and navigation impairments in schizophrenia patients during virtual reality allocentric learning tasks. The neural bases of these deficits have not been explored using functional MRI despite well-explored anatomic characterization of these paradigms in non-human animals. Our objective was to characterize the differential distributed neural circuits involved in virtual Morris water task performance using independent component analysis (ICA) in schizophrenia patients and controls. Additionally, we present behavioral data in order to derive relationships between brain function and performance, and we have included a general linear model-based analysis in order to exemplify the incremental and differential results afforded by ICA. Thirty-four individuals with schizophrenia and twenty-eight healthy controls underwent fMRI scanning during a block design virtual Morris water task using hidden and visible platform conditions. Independent components analysis was used to deconstruct neural contributions to hidden and visible platform conditions for patients and controls. We also examined performance variables, voxel-based morphometry and hippocampal subparcellation, and regional BOLD signal variation. Independent component analysis identified five neural circuits. Mesial temporal lobe regions, including the hippocampus, were consistently task-related across conditions and groups. Frontal, striatal, and parietal circuits were recruited preferentially during the visible condition for patients, while frontal and temporal lobe regions were more saliently recruited by controls during the hidden platform condition. Gray matter concentrations and BOLD signal in hippocampal subregions were associated with task performance in controls but not patients. Patients exhibited impaired performance on the hidden and visible conditions of the task, related to negative symptom severity. While controls showed coupling between neural circuits, regional neuroanatomy, and behavior, patients activated different task-related neural circuits, not associated with appropriate regional neuroanatomy. GLM analysis elucidated several comparable regions, with the exception of the hippocampus. Inefficient allocentric learning and memory in patients may be related to an inability to recruit appropriate task-dependent neural circuits.
Keywords: allocentric, spatial learning, Morris Water Maze, hippocampus, independent component analysis, fMRI
Introduction
In characterizing cognitive and neural circuit deficits associated with schizophrenia (MacDonald and Carter 2002), utilizing translational cognitive paradigms that have been investigated thoroughly and directly in rodents and primates is useful. The Morris Water Task (MWT)(Morris 1981) is one such example, requiring subjects to navigate and to learn the spatial location of a platform hidden in a pool of water. When the hidden platform location is fixed, rats learn the hidden platform location rapidly despite minimal local navigation cues. In the MWT, subjects use distal contextual cues to map platform locations. Thus it is an excellent paradigm for measuring allocentric spatial navigation, learning, and memory. Hippocampal pathology is associated with severe place learning and memory deficits (Astur et al 2002; Broadbent et al 2006; Hort et al 2007). Likewise, superior place learning requires intact hippocampal function (Livingstone and Skelton 2007).
The MWT has been used extensively in the animal literature as a test of spatial navigation, learning, and memory (D'Hooge and De Deyn 2001). In rats, fornix/fimbra lesions have been shown to disrupt the allocentric component of navigation in the MWT (O'Keefe et al 1975). Accordingly, rats with bilateral hippocampal system lesions, or with unilateral damage to the dentate gyrus (Sutherland et al 1983) have significant difficulties finding a newly positioned platform following learning. The hippocampal lesion effect results in the most extensive behavioral disruption, as rats with parietal damage have difficulty with initial heading direction (Morris et al 1982), while diencephalic lesions disrupt spatial working memory components of the task (Mumby et al 1999). Selective dorsal lesions of the hippocampus may impair both allocentric and egocentric spatial processing (Czurkó et al 1997), arguing for its role in generalized spatial processing.
Other studies have observed behavioral deficits in navigation, acquisition, and memory following lesions to other brain regions, arguing for a more widespread network underlying allocentric spatial processing. The dorsomedial striatum also plays an important role in spatially guided behavior tested by conditioning in mazes, extending beyond motor difficulties induced by lesions in this region, and showing salient effects on response flexibility and motor learning (D'Hooge and De Deyn 2001). In particular, lesions in rat dorsal striatum (caudate and putamen) alter response and cue acquisition procedural learning (Devan et al 1996). Following successful training in a T-maze, rats with posterior dorsomedial striatum lesions switch from finding a reward in a specified place to learning the correct turn response (Yin and Knowlton 2004). However, there is evidence that lateral striatal lesions do not affect learning the platform location in the MWT (Devan and White 1999). Other lesioned regions that impair MWT performance include the basal forebrain (Waite et al 1994), cerebellum (Lalonde 1994), and prefrontal cortex (Mogensen et al 1995); however the specific functional contribution of these regions to task performance is unclear. The advent of virtual reality (VR) technology has allowed spatial learning paradigms to be more appropriately adapted for human allocentric functional neuroimaging studies (Maguire et al 1998; Maguire et al 1997; Pine et al 2002), and thus, will be more likely to clarify these issues in future studies.
Schizophrenia is associated with structural and functional brain changes in key regions (medial temporal lobe, prefrontal cortex, basal ganglia) putatively implicated in successful MWT performance (Antonova et al 2004). Recently, allocentric spatial (Hanlon et al 2006; Weniger and Irle 2008) and neuropsychological (Ku et al 2003) VR paradigms have been studied in schizophrenia. During allocentric VR investigations, patients were not helped by distal cues surrounding the “pool,” (Hanlon et al 2006), and on similar tasks (Weniger and Irle 2008) suggesting ineffective navigation and learning strategies; however impairments were not seen during egocentric processing (Weniger and Irle 2008). Although the neural bases of these behavioral differences were not investigated explicitly, data were consistent with hippocampal dependence on the virtual reality version of the Water Maze Task (vMWT) (Hanlon et al 2006; Hanlon et al 2005).
Astur developed a vMWT (Astur et al 1998) analogous to the original MWT. Using adaptations of this paradigm, investigators demonstrate better behavioral spatial learning performance for males (Astur et al 1998; Astur et al 2004), differences across developmental periods (Newhouse et al 2007), and deficiencies in patients with mesial temporal lobe damage (Astur et al 2002). The present investigation used a version of this task, applied to schizophrenia and healthy control subjects during fMRI scanning, to investigate the neural networks underlying place learning. A block design task was chosen because the paradigm does not lend itself well to the discrete phases needed in an event-related design, particularly because it would be difficult to equate behavior in such a punctuate task. In this version, subjects are presented with two conditions: one where the platform is hidden but distal stimuli are available for referencing outside of the pool, and another where the platform is visible, but where the distal room cues have been masked. The hidden condition is analgous to the original MWT in that subjects cannot see the platform beneath the water, but they can use distal room cues for navigation. The visible condition is a novel adaptation for human neuroimaging research, and as such, it is used as a control task so that participants can navigate to a visible platform without the need to rely on distal cues. Because the vMWT is cognitively complex (Hanlon et al 2006; Livingstone and Skelton 2007), it is difficult to study using traditional functional neuroimaging methods.
Recently, independent component analysis (ICA) has been shown to be a viable alternative to parametric analysis of fMRI data (Calhoun and Adali 2006; Calhoun et al 2001b), used to extract regional components directly (Calhoun et al 2004a) without relying on comparisons to an a priori model. ICA is a statistical and computational technique for revealing hidden factors that underlie sets of random variables, measurements, or signals, achieving blind source separation by decomposing a data set into statistically independent components (Calhoun et al 2004a; Calhoun et al 2004b). ICA demonstrates considerable promise for fMRI data analysis (Calhoun et al 2003). It has been applied to naturalistic behaviors (e.g. simulated driving) (Calhoun et al 2001a) and to differentiate between schizophrenia patients and healthy controls (Garrity et al 2007). Because spatial navigation and memory are cognitively complex tasks, we sought to investigate task-related and transiently task-related components, as ICA is known to capture temporally coherent activity that does not track smoothly with the task.
We sought to replicate impaired vMWT performance in schizophrenia and to understand the neural systems underlying successful and unsuccessful behavioral task performance employing an ICA of our fMRI data to examine the underlying distributed neural networks involved. Based on previous behavioral results, we expected healthy controls to show a greater degree of accuracy and efficiency in the hidden platform version of the task, related to selective recruitment of distributed circuits underlying mesial temporal lobe and hippocampal functioning. In patients, we expected decreased behavioral accuracy and efficiency, particularly in the hidden version, and we expected patients to show greater recruitment of extra-hippocampal regions that may be related to inefficient place learning. In addition, in order to compare ICA to more traditional methods of analysis, we also employed a GLM analysis of the time course.
Methods and Materials
Participants
Outpatients (N=34) met DSM-IV-TR criteria for schizophrenia with symptom severity gauged using the Positive and Negative Syndrome Scale ((PANSS)Kay et al 1987). Healthy controls (N=28) were recruited from the local community. Diagnoses were verified using the Structured Clinical Interviews for DSM-IV (American Psychiatric Association 2000). Exclusion criteria for controls included any present/past Axis I disorder and first-degree relatives with psychosis. We excluded potential subjects with significant medical/neurological illness, past major head injury, or substance abuse history within the prior 6 months. See Table 1.1 for demographic data. Groups were matched for age and right-handedness. Intelligence, estimated using the North American Adult Reading Test ((NAART)Blair and Spreen 1989), was higher among healthy controls. Controls were more educated and had fewer males than the schizophrenia group. After complete description of the study to the subjects, written informed, IRB-approved consent was obtained.
Table 1.
Demographic and Behavioral Variables of the Samples
1.1 shows demographic variables for subjects included in the fMRI investigation; 1.2 shows performance results for subjects completing the vMWT in the fMRI scanner; 1.3 shows behavioral data from an additional group of participants who were scanned using a different acquisition sequence. Thus, participants included in Table 1.3 were not included in the fMRI results here, but the results indicate equivalent behavioral performance in an additional group of subjects in order to verify group performance in general to account for the omitted fMRI group behavioral data.
| 1. Demographic Variable | p | HC Mean (SD) | SZ Mean (SD) | ||||
|---|---|---|---|---|---|---|---|
| N | - | 28 | 34 | ||||
| Age | ns | 33.2 (11.1) | 37.8 (11.3) | ||||
| Sex (M:F) | *** | 16:12 | 25:9 | ||||
| Education | ** | 10.8 (1.9) | 9.1 (2.9) | ||||
| NAART Estimated IQ | * | 106.9 (6.6) | 101.9 (10.4) | ||||
| Right Handedness | ns | 81.4 (15.6) | 86.1 (12.5) | ||||
| Total PANSS | - | - | 66.0 (23.6) | ||||
| 2. Behavioral (fMRI ) Variable | Condition | HC Mean (SD) | SZ Mean (SD) | †Condition F | †Group F | †Group X Condition F | HC vs. SZ Contrast t(41) |
| FOUND (# of times) | Hidden | 40.2 (11.9) | 23.6 (11.2) | 67.4*** | 21.4*** | 5.9* | 4.7*** |
| Visible | 46.7 (6.7) | 35.6 (11.1) | 3.9*** | ||||
| DISTANCE (a.u.) | Hidden | 761.8 (113.4) | 950.3 (220.5) | 17.1*** | 12.3** | 6.3* | 3.4** |
| Visible | 730.9 (53.1) | 822.9 (126.7) | 3.0** | ||||
| TIME (sec.) | Hidden | 9.1 (2.9) | 14.3 (5.0) | 66.3*** | 17.2*** | 11.1** | 4.0*** |
| Visible | 7.2 (1.1) | 9.7 (2.6) | 3.9*** | ||||
| ERROR (# of errors) | Hidden | .15 (.3) | .65 (.8) | 15.3*** | 8.4** | 5.6* | 2.7* |
| Visible | .02 (.1) | .09 (.1) | 2.5* | ||||
| 3. Behavioral (only) Variable | Condition | HC Mean (SD) | SZ Mean (SD) | ‡Condition F | ‡Group F | ‡Group X Condition F | HC vs. SZ Contrast t(35) |
| FOUND (# of times) | Hidden | 36.3 (11.0) | 20.4 (6.6) | 90.5*** | 30.4*** | 4.76* | 5.1*** |
| Visible | 46.5 (5.9) | 36.7 (7.8) | 4.4*** | ||||
| DISTANCE (a.u.) | Hidden | 784.1 (122.1) | 1178.4 (244.7) | 55.0*** | 35.5*** | 30.2*** | 6.4*** |
| Visible | 741.0 (56.0) | 888.5 (158.3) | 4.0*** | ||||
| TIME (sec.) | Hidden | 9.7 (2.3) | 15.8 (2.7) | 151.21*** | 67.04*** | 28.50*** | 7.5*** |
| Visible | 7.2 (0.7) | 9.4 (1.3) | 6.6*** | ||||
| ERROR (# of errors) | Hidden | 0.33 (0.6) | 1.6 (1.0) | 43.50*** | 25.91*** | 16.97*** | 4.7*** |
| Visible | 0.01 (0.0) | 0.20 (0.2) | 4.2*** | ||||
p<.001
p<.01
p<.05
df=1,41
df=1,35
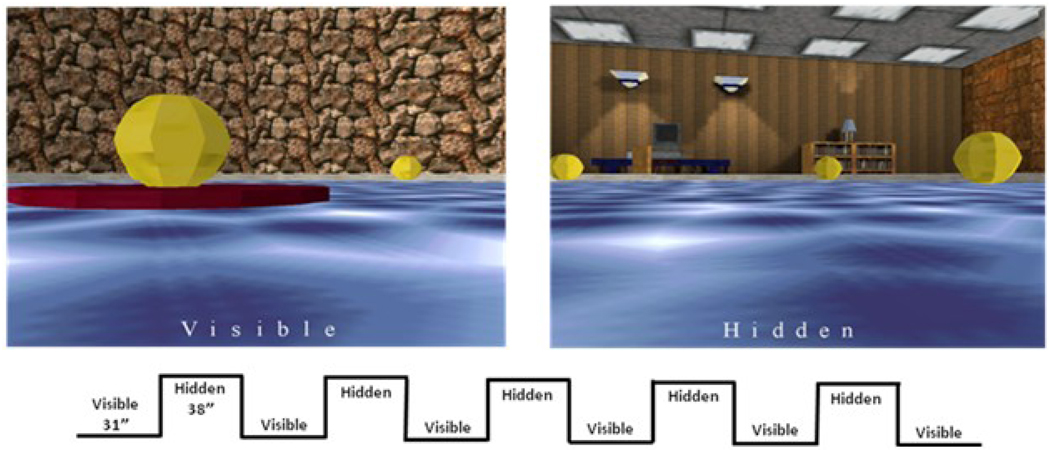
Virtual Morris Water Task Paradigm
We used a block fMRI design vMWT composed of VISIBLE and HIDDEN conditions (Figure 1). Subjects viewed a screen from the perspective of being placed in a pool of water within a surrounding room. In HIDDEN, furniture and objects were at fixed locations along the walls. In VISIBLE, a cylindrical wall masked these cues. In both, four equidistantly-spaced yellow balls hovered over the water surface as reference points to possible platform locations, with one being placed in the center of each of the four quadrants. The platform lay beneath one of these. Subjects maneuvered around the environment using a joystick until they found the platform. In VISIBLE, the platform could be easily seen between one ball and the water surface, and the platform was in the same location during each trial. In HIDDEN, the platform was ‘hidden’ beneath the surface of the water at the same ball location throughout the experiment. The platform was located identically in the HIDDEN and VISIBLE trials, however in VISIBLE there was a brick wall raised between the pool and the distal cues so that subjects were unaware that the locations were the same across trial types. For each trial, subjects began from a pseudorandomly determined North, South, East or West location, navigating the pool using a joystick. On successfully finding the platform, “Congratulations!” appeared on the screen. Each condition used a fixed time length, so that successful attempts resulted in additional platform searching trials until the subject ran out of time. During unsuccessful attempts, time spent during that condition would end, and the subject would be advanced to the next condition according to the block design timing.
Figure 1.
The Virtual Reality Pool Task. All participants completed 2 runs each of the task. Each run contained 6 visible and 5 hidden conditions. Participants navigated the virtual environment with a joystick. Successfully locating the platform prompted a “Congratulations!” message at the bottom of the screen, and additional navigation trials were successively presented until the end of each block.
Subjects completed two runs (7 min. 13 sec. each) of 6 visible (31 sec. each) and 5 hidden (38 sec. each) conditions. A three-second “Hidden” or “Visible” instruction screen preceded each condition and appeared on the bottom of trial screens. Before fMRI testing, subjects practiced 16 HIDDEN, one probe (where subjects navigated to a proposed platform when it was actually extracted from the pool), and 4 VISIBLE trials. Subjects who could not successfully complete the task following these trials were not permitted to continue with the fMRI task. Data from the practice trials, including the probe trials, were not collected, and are unavailable for analysis.
Behavior
We measured number of successful platform discoveries (FOUND), TIME it took (sec.), search DISTANCE, and ERRORs made while navigating to incorrect locations. We collected fMRI data on 62 subjects and corresponding behavioral data from 20 healthy controls and 23 patients. Due to a hardware failure, we were not able to include behavioral data from all subjects included in the neuroimaging results. Therefore, we collected behavioral data from 37 other schizophrenia patients and controls not included in the fMRI analysis because they were assessed using a different acquisition sequence. The behavioral data from the 43 subjects included in the fMRI results are shown in Table 1.2, and the additional behavioral data for verification of similar results are included in Table 1.3.
Neuroimaging
An Allegra 3 T scanner (Siemens, Erlangen, Germany) at the Olin Neuropsychiatry Research Center was used. The echo planar image (EPI) pulse sequence (TR =1.86 sec, TE =27 ms, Flip = 70°, 64×64, 3.4 × 3.4 mm in plane resolution, 3 mm slice thickness, 1 mm gap, 36 slices) covered the brain from the frontal pole to the parieto-occipital fissure. Each run consisted of 230 time points. A T1-weighted axial MPRAGE whole brain structural scan was acquired (TR = 2500, TE = 2.74, Flip = 8°, 1 mm, 0 gap, 176 slices).
fMRI Preprocessing
Data were preprocessed with SPM2 (Statistical Parametric Mapping, Wellcome Institute, London, UK) running in MATLAB 6.5 (The MathWorks, Natick, MA, USA), discarding the first five scans. Motion correction used the INRIAlign toolbox (Freire and Mangin 2001). Data were subsampled to 3 mm3 isotropic voxels. Anatomical images for each subject were coregistered to the MNI151 T1 image and functional images were coregistered to these, and normalization parameters were applied to each subject’s functional images. A linear interpolation corrected slice acquisition differences, data were smoothed (8-mm3 FWHM isotropic Gaussian kernel), and HRF convolved regressors were created for both conditions.
Independent Components Analysis
Preprocessed functional runs were imported into the Group ICA fMRI Toolbox (GIFT v. 1.3c: http://icatb.sourceforge.net) implemented in MATLAB 6.5 using a WFU Pick Atlas (Maldjian et al 2003) cerebral mask. Subsequent steps involved in GIFT ICA are detailed elsewhere (Calhoun et al 2001a; Calhoun et al 2001b). To select the five final component maps of interest from the final 20 maximally independent BOLD signal change spatial maps, we discarded non-MNI template-related components (Evans et al 1993), significant negative correlations to white matter, and head, eye, cardiac or respiratory motion components. We excluded non task-related component maps by examining the average R2 values of temporal association with the component time courses. Regions passing an FDR-corrected threshold (p<.0001) after a one-sample t-test in SPM2 are shown in Figure 2; corresponding MNI regions are listed in Table 3. GIFT calculated R2 values identifying associations between the design and component time course and standardized β-weights for subject-wise component time course to experimental condition associations which were averaged across sessions and analyzed using repeated-measures ANOVA.
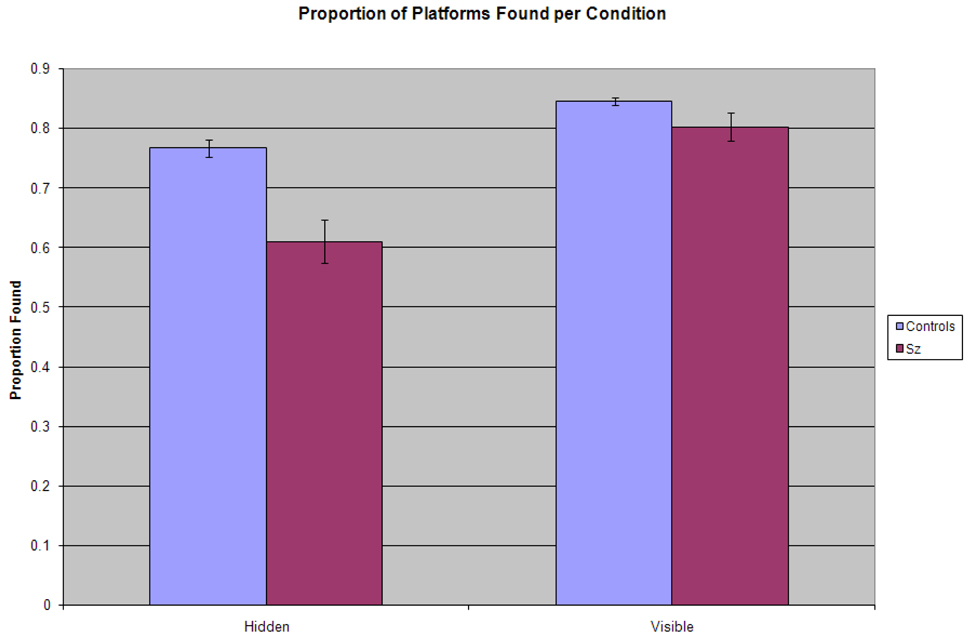
Figure 2.
Proportion of Platforms Found per Condition. Behavioral data for the number of times participants of each group found the platform correctly are shown as a proportion of the number of times they found the platform per total trials administered per condition. Although both groups found the visible platform at a similar proportion, patients were significantly deficient compared to healthy controls in the hidden condition.
Table 3.
Results from the repeated measures ANOVA on each component derived from the fMRI temporospatial ICA analysis
| β M(SD) | β M(SD) | F | F | F | ||||
|---|---|---|---|---|---|---|---|---|
| Component | Average R2 | Group | Hidden | Visible | Group | Condition | Group X Condition | |
| 1 | Yellow | 0.028 | HC | 0.080 (.34) | 0.081 (.36) | 3.89 | 4.74* | 5.06* |
| SZ | −0.051 (.29) | −0.103 (.28) | ||||||
| 2 | Blue | 0.032 | HC | −0.040 (.47) | −.036 (.48) | 1.29 | 3.67 | 5.13* |
| SZ | −0.123 (.29) | −0.174 (.29) | ||||||
| 3 | Green | 0.025 | HC | −0.004 (.38) | 0.042 (0.36) | 1.53 | 1.21 | 9.35** |
| SZ | −0.075 (.30) | −0.096 (.29) | ||||||
| 4 | Purple | 0.027 | HC | −0.098 (.40) | −0.061 (0.39) | 1.02 | 0.02 | 9.83** |
| SZ | −0.155 (.34) | −0.188 (.31) | ||||||
| 5 | Red | 0.018 | HC | 0.019 (.26) | 0.029 (.26) | 3.36 | 1.29 | 0.00 |
| SZ | −0.108 (.29) | −0.099 (.27) | ||||||
p <.01
p<.05
df=1,60
Structural MRI Data Analysis
All MPRAGE images were preprocessed and analyzed using SPM2 (http://www.fil.ion.ucl.ac.uk/spm/software/spm2/) software running in MATLAB 6.5.1. Control’s structural scans were stereotactically transformed(Good et al 2001) by registering each image to the ICBM152 template (Evans et al 1993). Voxels were interpolated to 1.5 mm3 and segmented into white matter (WM), gray matter (GM) and cerebrospinal fluid (CSF). Images were smoothed with an 8×8×8 mm3 FWHM Gaussian kernel and averaged across subjects to create T1, GM, WM, and CSF templates. To minimize mis-segmentation error, normalization was performed using the segmented GM and WM images. MRI images were segmented in native space and resulting GM and WM images spatially normalized to GM and WM templates to derive optimized normalization parameters and applied to the whole-brain structural images in native space prior to a new segmentation(Good et al 2001; Mechelli et al 2005). We examined both modulated (absolute GM volumes) and unmodulated (GM concentration) voxel values (Good et al 2001). GM, WM, and CSF maps were combined for total intracranial volume (TIV). We also applied an automated method of subparcellating the hippocampus (Amunts et al 2005) in individual VBM GM maps to obtain GM concentrations in the Cornu ammonis (CA), subicular complex (SUB), and dentate gyrus (Fascia dentata and CA4) (DG); the hippocampal-amygdaloid transition area (HATA), a component of the gyrus uncinatus and the uncal portion of the hippocampus (Rosene and van Hoesen 1987), and the entorhinal cortex (EC) using the SPM Anatomy Toolbox (Eickhoff et al 2005).
Analysis of BOLD Signal
To examine the specific contribution of regional neuroanatomy within three component circuits, we extracted BOLD signal variation by applying anatomically defined masks created in the WFU Pick Atlas to the t-maps created in GIFT. The particular regions selected were based on a priori assumptions (i.e. a procedural trial and error navigation strategy related to the caudate, a verbal rehearsal strategy related to the superior temporal gyrus, and a spatial navigational strategy related to the hippocampus). A small volume correction was applied to selected ROIs in SPM2 and BOLD signal was extracted from the maximum t-value for bilateral structures.
Blocked GLM Analysis
A GLM in SPM2 was calculated separately for each subject using HIDDEN and VISIBLE as regressors, resulting in a contrast map. To assess average group activation, the contrast maps were used in the second level random effects analysis to statistically compare the two groups using independent sample t- tests. Regions showing group differences were converted to standardized space (Talairach and Tournoux 1998) using the SPM tool box.
Results
Behavior
Table 1 shows mean (SD) results and repeated measures ANOVA tests for group, condition and their interactions. In both VISIBLE and HIDDEN conditions, controls FOUND the platform more often, while patients made more ERRORs, traveled a greater DISTANCE, and spent more TIME searching (see Table 1 for data and statistical results). We also investigated the effects of sex and group across experimental conditions on the behavioral variables, and there were no group differences. This effect was not significant for FOUND, F(1,38)=.816, p=.37, TIME, F(1,38)=.009, p=.93, DISTANCE, F(1,38)=.73, p=.39, or ERROR, F(1,38)=.37, p=.55. There were no main effects of sex for FOUND: F(1,40)=1.34, p=.25; TIME: F(1,40)=1.19, p=.28; DISTANCE: F(1,40)=.001, p=.98; or ERROR: F(1,40)=.06, p=.81., nor was sex-by-condition significant: FOUND: F(1,40)=.42, p=.52; TIME: F(1,40)=1.96, p=.17; DISTANCE: F(1,40)=.26, p=.61; and ERROR: F(1,40)=.45, p=.5. The resulting patterns (Table 1) are equivalent across the larger total sample (N=80), comprised of subjects who completed the behavioral task only and the larger sample of subjects who completed the fMRI task, indicating that these performance patterns are consistent across a larger sample. Subsequent analysis indicated that these two samples performed similarly on all behavioral variables from HIDDEN (Wilk’s Lambda=.92, F(4,75)=1.73, p=.15) and VISIBLE (Wilk’s Lambda=.94, F(4,75)=1.19, p=.32).
We also examined the proportion of the number of times participants found the platform out of the number of total trials they were presented in each condition. Analysis of these data using a repeated measures ANOVA identified a significant main effect of condition: F(1,40)=38.5, p<.001, where the platform was proportionately found more often overall in VISIBLE (M=82%, S.D.=8%) compared to HIDDEN (M=69%, S.D.=15%). The significant main effect for group, F(1,40)=16.5, p<.001) indicated that healthy controls (M=80.6%, S.D.=6.5%) proportionately found the platform a greater number of times than patients (M=70.7, S.D. =17.0%) overall. There was a condition X group interaction: F(1,40)=6.7, p=0.13 where controls (M=84.6%, S.D.=2.8%) and patients (M=80.3%, S.D.=10.7%) performed similarly in VISIBLE, however patients proportionately found the platform fewer times (M=61.1%, S.D.=16.9%) compared to controls (M=76.7%, S.D. = 6.8%) in HIDDEN. These data can be seen in Figure 2.
Total PANSS score was significantly inversely associated with the number of times patients found the platform during VISIBLE (rs= −.60, p<.01) and HIDDEN (rs= −.58, p<.05). Higher negative PANSS subscale scores predicted worse performance during VISIBLE (rs= −.66, p<.01) and HIDDEN (rs= −.70, p<.01).
ICA
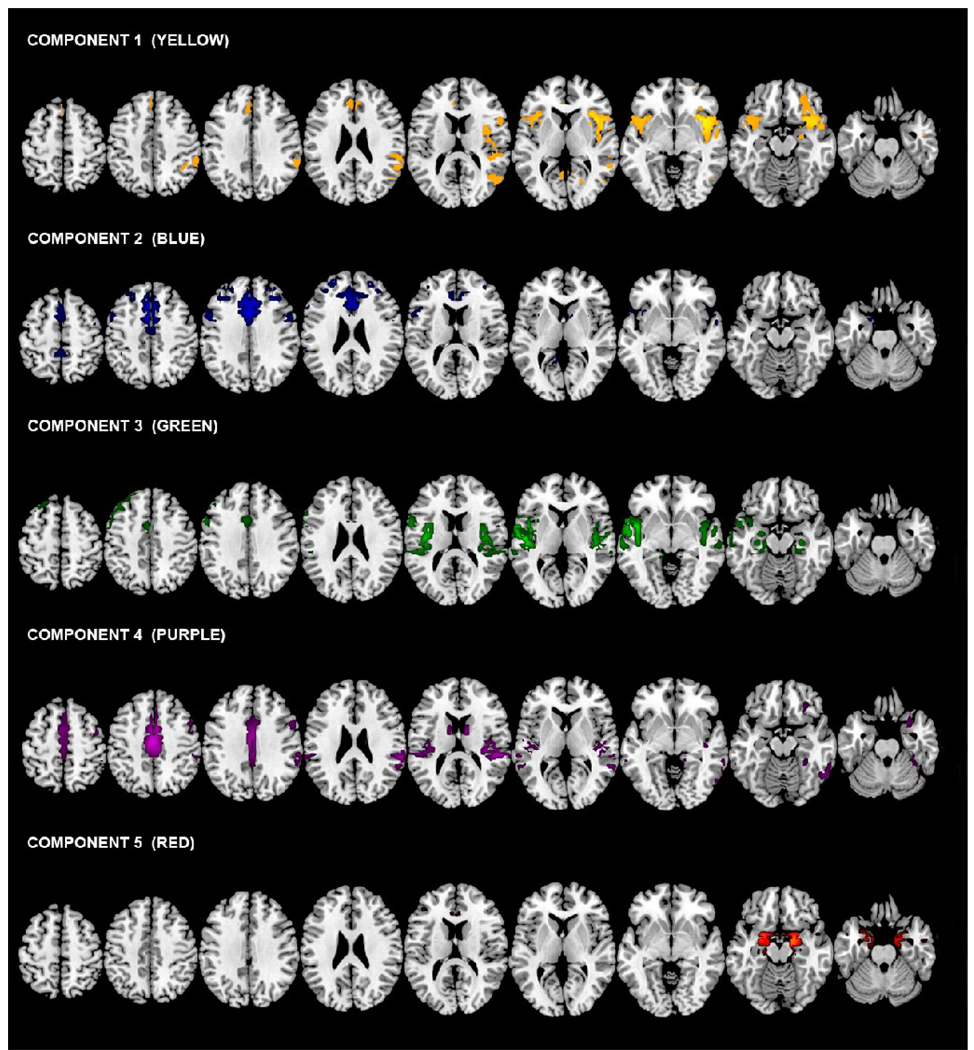
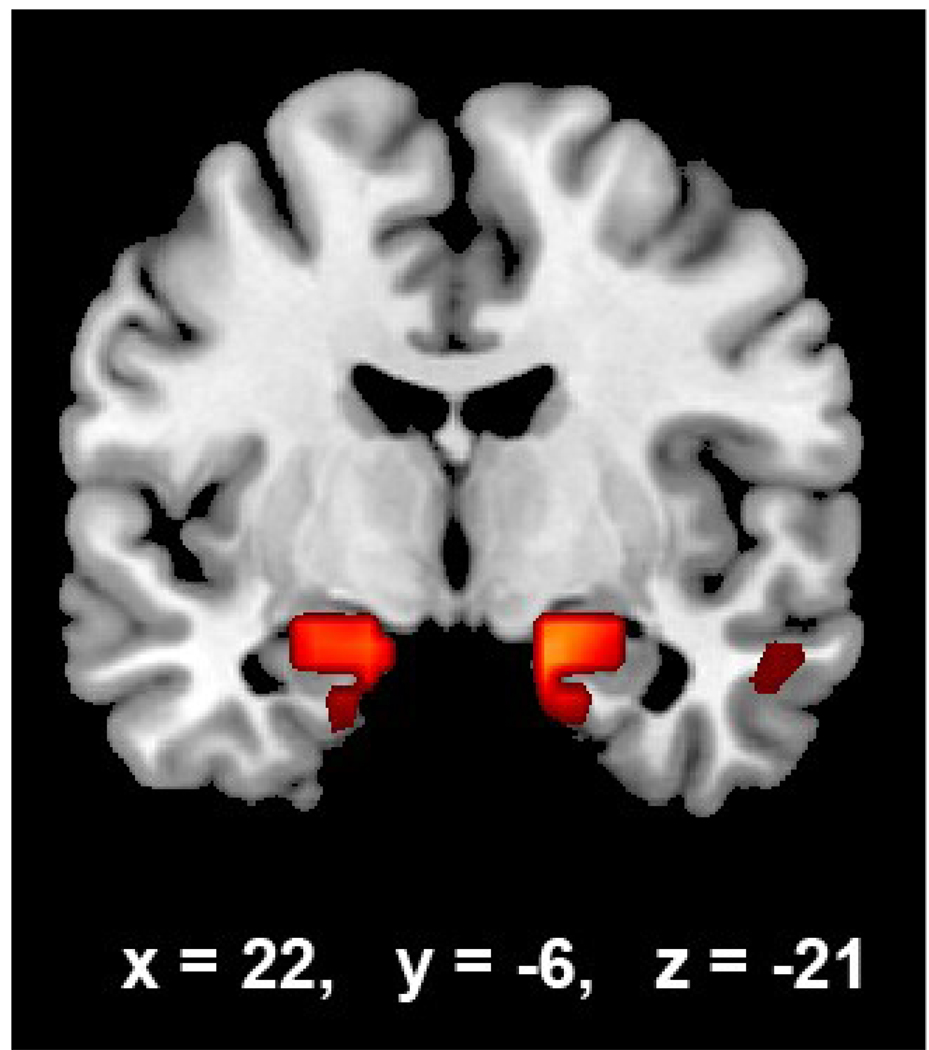
Regions for the five final components are listed in Table 2 and ANOVA results in Table 3. Spatial maps of the resulting components are given in Figure 3. We have included Figure 4 to more closely identify the mesial temporal and hippocampal region identified in Component 5.
Table 2.
MNI Regions from the ICA and associated t-values and Brodmann areas
| Random Effects max T (MNI x,y,z) | ||||
|---|---|---|---|---|
| Component | Region | Brodmann Area | L | R |
|
YELLOW 1 |
Insula | 13 47 22 40 | 25.0(39,9,−3) | 11.8(−39,15,2) |
| Inferior Frontal Gyrus | 47 13 45 44 11 | 22.3(42,14,−3) | 13.0(−45,17,−8) | |
| Superior Temporal Gyrus | 38 22 13 21 39 29 42 41 | 21.4(45,8,−8) | 13.1(−48,14,−8) | |
| Inferior Parietal Lobule | 40 | 11.5(56,−42,27) | NS | |
| Supramarginal Gyrus | 40 | 11.4(56,−39,30) | NS | |
| Middle Temporal Gyrus | 39 21 19 22 37 | 10.6(53,−6,−7) | 5.1(−50,−64,6) | |
| Posterior Cingulate | 31 30 23 | 8.1(15,−66,14) | 7.7(−3,−55,6) | |
| Parahippocampal Gyrus, Hippocampus, Amygdala | 28 34 | 8.0(27,−9,−12) | NS | |
| Medial Frontal Gyrus | 6 9 8 | 5.6(3,40,34) | 7.3(−3,40,34) | |
| Superior Frontal Gyrus | 11 8 6 10 | 6.7(27,40,−15) | 6.0(−3,37,48) | |
| Middle Frontal Gyrus | 11 47 10 46 | 6.6(27,37,−14) | NS | |
| Anterior Cingulate | 32 24 33 10 | 6.2(3,33,20) | 6.3(−6,36,15) | |
|
BLUE 2 |
Cingulate Gyrus | 9 31 | 20.8(6,22,29) | 20.6(0,13,32) |
| Anterior Cingulate | 33 9 | 19.5(6,24,24) | 18.5(−9,24,24) | |
| Medial Frontal Gyrus | 9 8 32 | 18.3(3,31,32) | 18.0(−3,31,32) | |
| Superior Frontal Gyrus | 10 8 6 | 15.1(30,42,26) | 12.0(−36,48,22) | |
| Middle Frontal Gyrus | 8 46 | 14.6(33,42,26) | 12.0(−27,33,29) | |
| Inferior Frontal Gyrus | 44 46 | 10.2(53,7,30) | 11.1(−50,10,33) | |
| Uncus | 36 34 20 | 6.1(15,−7,−22) | 9.9(−15,−4,−25) | |
| Paracentral Lobule | 31 | 8.4(3,−41,52) | 9.0(−3,−41,52) | |
| Superior Temporal Gyrus | 42 | 8.3(53,14,−11) | 8.7(−24,10,−26) | |
| Inferior Parietal Lobule | 40 | 5.3(−65,−25,34) | 6.4(−62,−31,29) | |
|
GREEN 3 |
Superior Temporal Gyrus | 22 38 13 21 41 42 29 | 14.4(50,−14,6) | 22.1(−45,3,−3) |
| Insula | 13 22 29 41 40 | 15.5(39,−11,6) | 21.0(−45,0,0) | |
| Middle Temporal Gyrus | 21 22 38 | 8.8(56,−6,−5) | 17.5(−56,−9,−5) | |
| Transverse Temporal Gyrus | 41 42 | 12.0(50,−17,12) | 17.4(−50,−17,12) | |
| Hippocampus | 21 | 9.8(45,−9,−7) | 14.6(−45,−6,−7) | |
| Inferior Frontal Gyrus | 44 9 45 13 47 | 7.3(53,1,19) | 11.1(−59,12,10) | |
| Parahippocampal Gyrus, Hippocampus, Amygdala | 28 35 36 27 34 30 37 | 10.8(27,−18,−12) | 10.3(−21,−12,−15) | |
| Middle Frontal Gyrus | 9 8 6 46 10 | 4.9(39,58,−5) | 9.4(−56,13,35) | |
| Cingulate Gyrus | 32 24 | 7.1(3,11,35) | 9.0(−3,11,35) | |
| Superior Frontal Gyrus | 8 9 6 | NS | 8.1(−21,43,45) | |
|
PURPLE 4 |
Cingulate Gyrus | 24 32 23 31 | 21.1(−3,−10,39) | 21.2(3,−10,39) |
| Paracentral Lobule | 31 6 5 | 17.8(−3,−9,45) | 15.4(3,−21,43) | |
| Medial Frontal Gyrus | 32 6 8 9 10 | 16.4(0,8,44) | 17.0(3,5,44) | |
| Insula | 13 40 41 29 22 | 9.5(−45,−25,15) | 16.4(53,−31,18) | |
| Superior Temporal Gyrus | 29 41 42 13 22 38 39 | 10.6(−56,−26,12) | 15.7(50,−28,15) | |
| Transverse Temporal Gyrus | 41 42 | 10.5(−53,−23,12) | 15.2(50,−26,12) | |
| Inferior Parietal Lobule | 40 | 9.5(−62,−25,26) | 15.2(59,−34,24) | |
| Superior Frontal Gyrus | 6 8 10 | 12.9(0,5,49) | 12.1(3,5,49) | |
| Caudate | 9.0(−12,1,14) | 12.7(12,1,14) | ||
| Inferior Temporal Gyrus | 20 37 21 | 4.4(−50,−56,−12) | 11.3(53,−56,−12) | |
| Middle Temporal Gyrus | 37 22 21 20 39 | 8.3(−59,−38,5) | 10.7(56,−56,−10) | |
| Fusiform Gyrus | 37 20 19 | 5.9(−50,−53,−15) | 9.6(50,−53,−15) | |
| Middle Frontal Gyrus | 9 6 11 8 46 10 | 5.1(−45,53,6) | 8.5(50,13,32) | |
| Inferior Frontal Gyrus | 9 47 11 44 46 10 45 | 6.4(−50,44,1) | 8.5(53,10,30) | |
| Parahippocampal Gyrus, Hippocampus, Amygdala | 34 30 37 36 35 27 | 6.0(−30,−21,−22) | 7.1(21,5,−18) | |
|
RED 5 |
Parahippocampal Gyrus, Hippocampus, Amygdala | 34 37 28 35 | 19.7(15,−7,−15) | 18.2(−15,−1,−15) |
| Uncus | 34 28 | 16.1(15,−7,−20) | 16.5(−24,−1,−20) | |
| Superior Temporal Gyrus | 38 21 22 | 8.6(27,5,−23) | 9.2(−27,5,−23) | |
| Anterior Cingulate | 24 25 32 | 6.6(3,3,−5) | 7.8(−3,2,−10) | |
| Cingulate Gyrus | 23 31 | 6.1(3,−28,32) | 5.7(0,−33,29) | |
All regions reported are significant at p<0.0001 FDR
Regions are reported for volume clusters that surpassed a .5 cm3 bilateral threshold
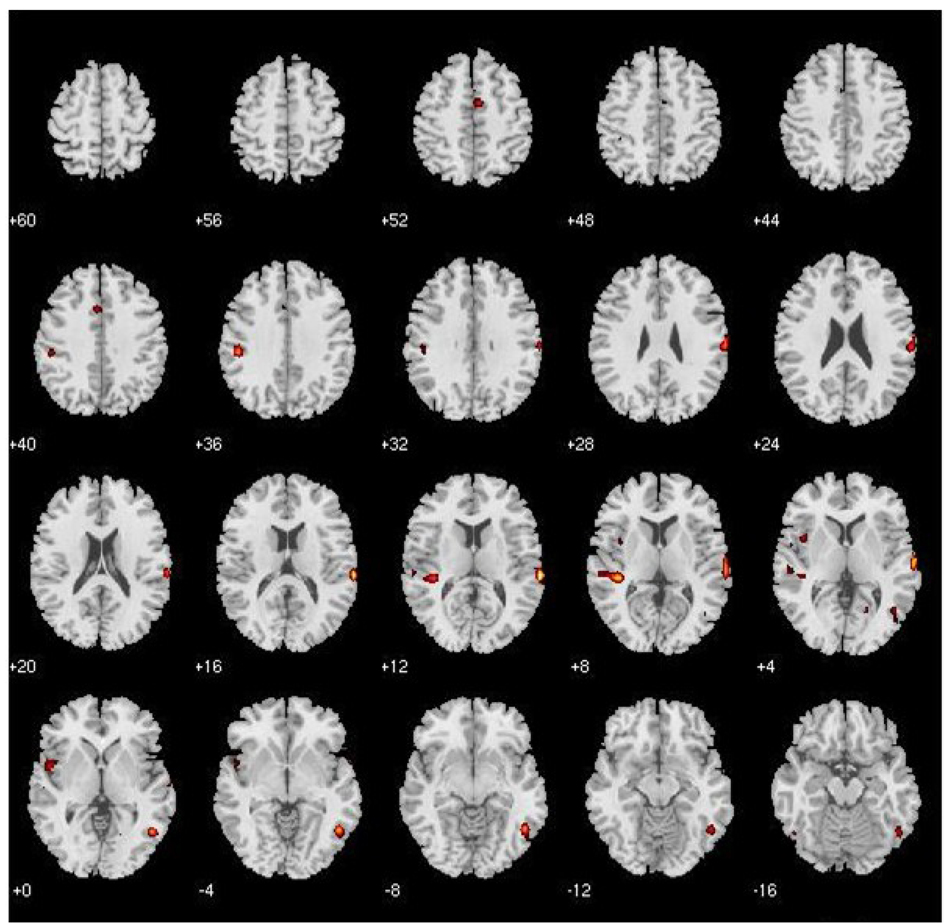
Figure 3.
Spatial maps of the resulting ICA components. The distributed functional neuroanatomy identified by each component from the ICA analysis is shown here. Each component represents a network of spatially independent brain regions sharing similar patterns of hemodynamic signal change over time.
Figure 4.
Component 5 in the coronal plane. In this figure, Component 5, which predominantly included regions from the mesial temporal lobe, including the hippocampus, is shown with the corresponding Talairach coordinates.
Component 1 (Yellow)
showed the strongest association with the vMWT, with a higher association with VISIBLE across all subjects (p=.03). The group effect was not significant (p=.053). For controls, this circuit was similarly associated with HIDDEN and VISIBLE, but for patients it had greater association with VISIBLE (p=.03).
Component 2 (Blue)
Neither effects of group (p=.26), nor condition (p=.06), were significant. For controls, this circuit was equally associated with HIDDEN and VISIBLE, but for patients it was more strongly associated with VISIBLE (p=.03).
Component 3 (Green)
The group (p=.22) and condition (p=.28) main effects were not significant. This component was associated equally with VISIBLE and HIDDEN in patients, but in controls the association was greater for VISIBLE (p=.003).
Component 4 (Purple)
The group (p=.32), and condition (p=.88) main effects were not significant. This circuit had a greater association with HIDDEN for controls and with VISIBLE in patients (p=.003).
Component 5 (Red)
The lack of significant group or condition effects from this predominantly mesial temporal lobe component (hippocampus, amygdala, uncus) indicates that this component was involved in all conditions of the task for both groups.
Overall, there were no group-specific components. Component 1 was associated preferentially with VISIBLE. Component 5 was a general processing component that was active across conditions and groups. While locating the visible platform, patients preferentially recruited Components 1, 2, and 4, comprising hippocampus, cingulate, insula, and basal ganglia. Controls preferentially recruited Component 3 during VISIBLE, comprising temporal, frontal, and mesial temporal regions. Component 4 was associated with strategies employed during HIDDEN for controls and VISIBLE for patients, including a prominent temporal lobe focus that extended to other frontal and subcortical regions.
Relationships between BOLD Signal Variation and Behavior
BOLD signal was extracted from bilateral superior temporal gyri (Component 3), bilateral caudate (Component 4), and bilateral hippocampus (Component 5), and correlations were computed between BOLD signal derived form these regions and behavioral variables (FOUND, TIME, DISTANCE, ERROR). See Table 4 for these associations (rs), which are described below.
Table 4.
Zero-order correlations between BOLD signal variation in key regions in selected components and behavioral performance
| Derived from Component |
Subregion | Behavioral Variable |
HC | SZ | ||
|---|---|---|---|---|---|---|
| rs Visible | rsHidden | rs Visible | rsHidden | |||
| 3 | Right Superior Temporal Gyrus | FOUND | 0.06 | 0.09 | −0.10 | −0.35 |
| TIME | 0.02 | −0.03 | 0.29 | 0.36 | ||
| DISTANCE | 0.12 | −0.03 | 0.01 | 0.24 | ||
| ERROR | 0.29 | −0.03 | 0.10 | 0.25 | ||
| 3 | Left Superior Temporal Gyrus | FOUND | −0.16 | −0.12 | −0.38 | −0.06 |
| TIME | 0.12 | 0.07 | 0.36 | 0.07 | ||
| DISTANCE | 0.06 | 0.12 | 0.32 | 0.27 | ||
| ERROR | 0.12 | 0.07 | −0.07 | 0.05 | ||
| 4 | Right Caudate | FOUND | 0.05 | 0.09 | −0.07 | 0.02 |
| TIME | −0.02 | −0.04 | 0.09 | 0.08 | ||
| DISTANCE | −0.02 | −0.12 | 0.26 | 0.38 | ||
| ERROR | 0.02 | −0.19 | 0.28 | −0.06 | ||
| 4 | Left Caudate | FOUND | 0.27 | 0.43 | −0.04 | 0.01 |
| TIME | −0.26 | −0.36 | −0.01 | 0.01 | ||
| DISTANCE | −0.24 | −0.16 | 0.11 | 0.02 | ||
| ERROR | 0.25 | −0.31 | 0.13 | −0.23 | ||
| 5 | Right Hippocampus | FOUND | 0.36 | 0.43 | −0.36 | −0.06 |
| TIME | *−0.46 | −0.40 | 0.00 | −0.13 | ||
| DISTANCE | *−0.46 | −0.25 | 0.05 | 0.19 | ||
| ERROR | 0.01 | −0.11 | −0.14 | −0.12 | ||
| 5 | Left Hippocampus | FOUND | *0.49 | ***0.68 | −0.09 | 0.04 |
| TIME | **−0.55 | **−0.63 | −0.11 | −0.08 | ||
| DISTANCE | −0.35 | *−0.47 | 0.00 | 0.09 | ||
| ERROR | −0.20 | **−0.52 | −0.07 | −0.14 | ||
p<.001
p<.01
p<.05
Superior Temporal Gyrus
Outside of the entire circuit comprising Component 3, there was no incremental contribution from superior temporal gyrus activity in patients or controls due to the lack of association between superior temporal gyrus BOLD signal and behavioral variables.
Dorsal Striatum
The contribution of Component 4 to the experimental paradigm in both groups was not caudate specific due to the lack of association between caudate BOLD signal and behavioral variables.
Hippocampus
For healthy controls, FOUND was associated with positive left hippocampal signal variation in both conditions and inversely associated with DISTANCE, TIME, and ERROR during HIDDEN and TIME during VISIBLE. Right hippocampus signal was inversely associated with TIME and DISTANCE during VISIBLE. For patients, BOLD signal was unassociated with any behavioral measure, indicating positive coupling between hippocampal activity and spatial navigation for controls.
Voxel Based Morphometry
Groups did not differ in overall TIV (t60= .22, p=.82). Covarying for TIV, they also had equivalent whole brain GM (F(1,59)= .65, p=.42), WM (F(1,59)= 2.41, p=.13), and CSF (F(1,59)= .14, p=.71) concentrations (Wilk’s Lambda=.95, F (2,58)=1.4, p=.25). Groups were matched for GM concentrations in hippocampus, covarying for TIV (Wilk's Lambda=.83, F (10,50)=1.02, p=.44), including: bilateral CA; bilateral EC; bilateral DG; bilateral HATA; and bilateral SUB. There were no group differences on total left (F(1,59)=1.37, p=.25) or right (F(1,59)=.49, p=.49) hippocampal volumes (mm3) from the modulated VBM analysis.
Association between VBM Derived GM and Behavior
The majority of associations (partial correlations controlling for TIV) were found in the hidden condition for healthy controls only, where FOUND was associated with right EC (r=.52, p=.01), right HATA (r =.47, p=.03), and left (r =.52, p=.02) and right (r =.53, p=.01) SUB. TIME was inversely associated with the same regions: Right EC (r =−.47, p=.03), right HATA (r =−.44, p=.05), and left (r =−.48, p=.03) and right (r =−.51, p=.02) SUB. ERROR was inversely associated with right EC (r =−.47, p=.03), left DG (r =−.46, p=.03), right HATA (r =−.51, p=.02), and right SUB (r =−.43, p=.05). During VISIBLE, FOUND was associated with left (r =.51, p=.02) and right SUB (r =.47, p=.03). None of the partial correlations between hippocampal subregions and behavior were statistically significant in the patient group. Thus, for controls only, there was significant coupling between behavior and GM concentrations in hippocampus subregions, indicating greater concentrations associated with efficient performance (finding the platform while covering less DISTANCE, spending less TIME, and making fewer ERRORs).
Association between Hippocampal BOLD Signal and GM Concentrations
Four of five components (1, 3, 4 and 5) included hippocampus and mesial temporal lobe structures, but the most prominent medial temporal lobe contribution arose within Component 5, a general processing circuit involved in both task conditions for all subjects. We examined the relationship between this region’s % signal change and VBM-derived GM concentrations from a hippocampus subparcellation separately for controls and patients controlling for TIV. There were no significant relationships for patients; however for controls, the left (r=.47, p=.013) and right (r=.57, p=.002) SUB and the right (r=.52, p=.005) HATA were associated with increased signal arising from the left (15,−7,−15) and right (15,−1,−15) hippocampus-identified MNI regions. Although analyses did not indicate a tighter coupling between the entire component (hippocampal and extra-hippocampal regions of the circuit) and any single group or condition, these associations suggest that when this component is deconstructed and time course signal from the mesial temporal region alone is investigated, controls show a tighter coupling between hippocampal anatomy and task-related BOLD signal than patients.
GLM Analysis
Table 5 lists all regions with greater BOLD signal for healthy controls compared to schizophrenia patients in HIDDEN compared to VISIBLE. Regions passing a statistical threshold of p <0.001 (uncorrected) are shown in Figure 5. Significant BOLD signal increases were evident in the superior and inferior temporal gyri bilaterally, the right transverse and left medial temporal gyri, bilateral postcentral, cingulate, middle occipital, gyri, left fusiform gyrus, right insula, and inferior parietal lobules. Many of these regions were also elucidated in the ICA, with the exception of mesial temporal lobe regions.
Table 5.
MNI Regions from the GLM analysis and associated t-values and Brodmann areas
| Random Effects Max T (MNI x,y,z) | |||
|---|---|---|---|
| Region | Brodmann Area | L | R |
| Superior Temporal Gyrus | 13, 22, 41, 42 | 4.3 (−62, −25, 15) | 3.8 (39, −29, 7) |
| Inferior Temporal Gyrus | 19, 37 | 4.1 (−48, −55, −2) | 3.3 (50, −70, −2) |
| Transverse Temporal Gyrus | 41 | 4.0 (42, −29, 10) | |
| Middle Temporal Gyrus | 37 | 4.0 (−48, −58, 3) | |
| Postcentral Gyrus | 1, 2, 3, 40 | 3.9 (−62, −19, 26) | 3.8 (48, −25, 34) |
| Cingulate Gyrus | 24, 32 | 3.7 (−6, −1, 47) | 3.6 (6, 16, 35) |
| Middle Occipital Gyrus | 19, 37 | 3.6 (−48, −59, −5) | 3.5 (50, −73, −4) |
| Inferior Parietal Lobule | 40 | 3.6 (−62, −22, 29) | 3.5 (48, −28, 32) |
| Fusiform Gyrus | 37 | 3.6 (−50, −59, −12) | |
| Insula | 13 | 3.6 (42, 9, 5) | |
All regions reported are significant at p<0.001 (uncorrected)
Regions are reported for volume clusters that surpassed a 0.1 cm3 threshold
Figure 5.
Spatial maps from the GLM analysis. In order to compare the spatiotemporal maps resulting from the ICA analysis to a more traditional approach for data analysis, we have included a GLM-based approach from the identical raw data. These data represent the group and condition contrasts (Healthy Controls – Patients) (Hidden – Visible). Corresponding coordinates can be observed in Table 4.
Discussion
We used ICA to deconstruct the neural components of a complex allocentric place learning and navigation task in schizophrenia patients and healthy controls hypothesizing that patients would show task performance deficits related to recruiting different neural circuits. Our behavioral results are consistent with a previous study finding relative impairments in schizophrenia (Hanlon et al 2006), suggesting an inefficient task strategy. PANSS negative symptoms in patients were associated with difficulty finding the correct platform in both conditions, possibly reflecting anhedonia, decreased motivation to perform (Brown and Pluck 2000), or executive and working memory deficits related to negative symptoms (Carter et al 1995; Szendi et al 2006).
In presenting our ICA data, we have attempted to organize the data into cognitively meaningful areas. Because resulting ICA components are known to capture temporally coherent data that do not track smoothly with the task (nodes subsumed by different cognitive processes would be expected to be apparent in different circuits rather than each component being tied to a single cognitive process), we have attempted to identify nodes in different components that likely subsume the different cognitive processes underlying a complex task. ICA identified 5 spatiotemporal components, each corresponding to a neural circuit involved in the vMWT. We propose the following relevant task-related cognitive functions included:
Place learning and navigation
Mesial temporal lobe structures were recruited in both groups when remembering and identifying platform locations. Thus, this region appeared as a general neural substrate to place learning and navigation. This finding is consistent with known functions of the hippocampus, parahippocampal gyrus, and anatomically related structures in place learning, navigation, and memory (Ekstrom et al 2003; Georges-Francois et al 1999; Hafting et al 2005; O'Keefe and Nadel 1978). The hippocampus and surrounding region is prominently involved in episodic memory (Squire 1987). Our paradigm, involving passive navigation through a virtual environment, required navigation, learning, and remembering spatial locations.
Verbal rehearsal
Superior temporal gyrus was prominent in Component 3, however it was also involved in the circuits comprising the remaining 4 components. This may be related to a verbal rehearsal strategy employed while navigating the pool and searching for the correct place, as subjects are likely involved in an internal monologue during both conditions. Previous investigations (Maguire et al 2003; Pine et al 2002) did not identify this region; however our results suggest its global contribution to the task.
Emotional reward/punishment
Components 2, 3 and 4 included insula as a circuit node. This region appears to have a role in craving, addiction (Naqvi et al 2007) and sensory-emotional integration (Ortigue et al 2007). Although our subjects were not asked to provide subjective ratings of their experiences, it is possible that repeated goal attainment (finding the platform) was rewarding and emotionally salient.
Attention and executive organization of information
Regions related to attention, cognitive control, and memory encoding and retrieval were nodes in many of the circuits including the cingulate, caudate, dorsal and medial frontal regions, and parietal regions. Although several of these regions were involved in Component 1, which showed a higher association with VISIBLE compared to HIDDEN, their general recruitment in the other circuits (including the predominantly hippocampal Component 5), indicates a generally subsumed neural system.
Working memory
This task required subjects to attend to, organize, rehearse, and update task information. Evidence of dorsolateral prefrontal cortex in particular, a prominent substrate of working memory (Courtney et al 1998; D'Esposito et al 1996; D'Esposito et al 1999; Sakai et al 2002), is evident in Components 1–4. For controls, and not for patients, a widely distributed circuit (Component 4) was preferentially recruited during place memory and learning during HIDDEN, indicating a neural substrate that may be partially responsible for group behavioral differences. Component 4 consisted primarily of frontal and temporal regions in addition to the insula and inferior parietal lobule. There is substantial evidence for disrupted frontotemporal connectivity in schizophrenia (Heckers et al 1998; Kubicki et al 2002; Ragland et al 2001), particularly related to memory, which may be modulated by aberrant cingulate activity(Fletcher et al 1999). The cingulate comprised the most statistically significant region of activity in this circuit, also composed of frontal and temporal cortices, caudate, insula and inferior parietal lobule. Our results do not support group differences related to mesial temporal structures alone, as there were no group differences in Component 5. Frontal-temporal disconnection in schizophrenia may then play a role in inefficient water task performance underscoring the importance of widely distributed networks that support place navigation, learning and memory in addition to mesial temporal structures.
For controls, increased hippocampal BOLD signal was associated with task efficiency. It is likely that patients failed to recruit mesial temporal structures comparably to controls. Component 5 was equally involved across conditions in both groups, however lacking associations between mesial temporal signal and behavior and evidence for disrupted frontal-temporal connectivity in schizophrenia may indicate inefficient hippocampal recruitment in circuits that would be required for optimal performance. More circumscribed hippocampal sub-regions may be involved in the reported anatomical and functional disruptions (Benes et al 1998; Heckers and Konradi 2002; Zhang and Reynolds 2002). This may be supported by our investigation of GM concentrations in hippocampal subregions and their associations with behavior. Controls showed significant coupling between behavior and GM concentrations. Our subparcellation was limited to anatomical variation in the anterior hippocampus, therefore the lack of association with behavior and hippocampal subregions in schizophrenia may indicate an (untested) association with more posterior regions, or that hippocampal involvement was more disorganized in schizophrenia. Hippocampal NMDA receptors are involved in place learning and memory, particularly in maintaining established place fields (Nakazawa et al 2004). There is also emerging evidence of glutamatergic NMDA receptor dysfunction in the pathophysiology of schizophrenia (Stone et al 2007). Thus, differences in behavior and neural circuitry between patients and controls may be related to an underlying failure to recruit necessary brain regions in consort and not to a circumscribed regional deficit.
The striatum is important for establishing stimulus reinforcer contingencies in spatial learning tasks, whereas the hippocampal system is involved in flexibly updating environmental conditions (Yin and Knowlton 2004). Connections between hippocampus and striatum support a shared system for spatially guided behavior (Brown and Sharp 1995). In the previously described PET study (1998), increased rCBF in the right caudate was associated with goal-directed navigation speed and egocentric navigation strategies (Livingstone and Skelton 2007). Based on Component 4, although controls may be recruiting the flexible, environmentally guided hippocampal component, and the more egocentric stimulus relationship-guided response strategy subsumed by the striatum during the more difficult, HIDDEN condition, patients were more likely to do this during VISIBLE. Thus, patients were less likely to efficiently recruit the navigational and procedural strategies during HIDDEN, where necessary recruitment of all pertinent strategies was important for finding the hidden platform.
These results also provide support for known disrupted frontal-temporal connectivity in schizophrenia (Fletcher et al 1999), particularly in regards to inefficient hippocampal recruitment (Heckers and Konradi 2002). Hippocampal/striatal connections also support a shared system for spatially-guided behavior (Brown and Sharp 1995; Livingstone and Skelton 2007), and Maguire (1998) has shown striatal activity during an allocentric task. Here, evidence from Component 4 suggests striatal and hippocampal activation for controls in HIDDEN and for patients in VISIBLE, likely reflecting inefficient strategy recruitment.
In the GLM, bilateral postcentral gyri activation is likely due to controls’ superior performance and increased joystick demands in HIDDEN. Right transverse and bilateral superior temporal gyri signal supports ICA-identified verbal mediation hypothesis that was likely greater for controls during HIDDEN. The ICA and GLM similarly produced cingulate and insula nodes. The GLM and ICA are consistent in that components that included these regions were differentially related to controls vs. patients during different task-related demands. The lack of relative signal in the hippocampal regions is not unexpected given the ICA results indicating hippocampal activation in both populations for both conditions. Thus, the comparative GLM analysis may have “subtracted out” this region, or there may have been attributes of the paradigm or subjects’ strategies that, in fact, decreased hippocampal-dependent activation in this task. For example, one recent study (Moses et al 2009) suggested that semantic or contextual meaningfulness of stimuli enhanced accuracy in a transitive inference (hippocampally-dependent) task. Thus, including more individually-referential or meaningful cues may enhance accuracy in the vMWT, however introducing these other elements was not consistent with our current goal, to identify the underlying neural circuits involved in MWT performance in patients and healthy controls. However, these results underscore the importance of ICA in elucidating applicable nodes in distributed neural networks that are masked by GLM.
In addressing caveats to the results, the statistically significant difference in intelligence between groups was not clinically meaningful, thus we do not believe this small difference to be relevant. Differences in educational attainment are commonly seen between patients and controls (Harvey et al 1998). Our sample is also biased towards males, particularly for patients. Sex differences have been investigated in spatially guided behavior, often identifying better performance in males compared to females (Crucian and Berenbaum 1998). In a similar vMWT (Astur et al 2004) males found the hidden platform more than females. However, presently, there were no significant behavioral sex effects, therefore neuroimaging sex effects were not examined parametrically. We could not collect corresponding behavioral data from all fMRI subjects, therefore we include data to show that the behavioral patterns observed in our fMRI participants seem generalizable to a larger sample showing similar behavior.
In addition, several versions of the original MWT have been used in the past in both animal and human research. Our development of a virtual reality paradigm was based on and informed by these previous studies, however one could argue that version was qualitatively different from others that have been used. For instance, our use of the floating balls, while not novel, is different from the original experiment, but necessary in the context of an fMRI experiment to be used with human subjects. We are also aware that healthy controls inherently receive more “training” during the longitudinal progression of the task because they achieve more platform attainments compared to the patients. Unfortunately, this is a necessary by-product of the current paradigm. Additionally, although we used a probe task in the training trials, the practice trials were used to assess task comprehension and compliance, and these data were not recorded for analysis. Thus, data form these trials, including those specifically from the probe trials are unavailable for analysis. We are developing event related versions of this paradigm for future studies that will employ more discrete versions of the two conditions along with a probe trial, but in this investigation of differences in distributed networks between healthy controls and schizophrenia patients, we wanted to use the most powerful block design paradigm available. The absence of probe trial data also makes it difficult to ascertain the precise strategy subjects used to locate the platform and to assert that this task is hippocampus-dependent. However, our group has previously shown this version of the task to activate the hippocampus (Shipman and Astur 2008; Astur et al 2006).
We report anomalous neural circuitry in schizophrenia during a vMWT relative to healthy controls. Although there was evidence for the expected hippocampally-related neural circuit in both groups, patients showed a different pattern of activation in four other, more widely distributed, neural circuits. Although task-related behavior was associated with BOLD signal activation and hippocampal gray matter concentrations in healthy controls, these associations were absent in schizophrenia and indicate that these relative differences may be modulated by negative symptoms. By using both analyses, we report anomalous neural circuitry in schizophrenia during a vMWT relative to healthy controls in an ecologically valid virtual environment that may allow translational cognitive paradigms to be used more widely in imaging research.
Acknowledgements
This work was supported by National Institute of Mental Health (2RO1 MH43775780 MERIT Award, 1RO1MH074797 and 1R01MH077945 to G.D.P. and 5T32MH18921 to B.S.F.) and the National Alliance for Research on Schizophrenia and Depression (Distinguished Investigator Award to G.D.P.). We also gratefully acknowledge the time and dedication given to our manuscript by the anonymous reviewers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures:
Dr. Folley reported no biomedical financial interests or potential conflicts of interest. Dr. Astur reported no biomedical financial interests or potential conflicts of interest. Ms. Jagannathan reported no biomedical financial interests or potential conflicts of interest. Dr. Calhoun reported no biomedical financial interests or potential conflicts of interest. Dr. Pearlson reported no biomedical financial interests or potential conflicts of interest.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR (Text Revision) Washington,D.C.: American Psychiatric Association Press; 2000. [Google Scholar]
- 2.Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- 3.Antonova E, Sharma T, Morris R, Kumari V. The relationship between brain structure and neurocognition in schizophrenia: a selective review. Schizophr Res. 2004;70:117–145. doi: 10.1016/j.schres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Astur RS, Ortiz ML, Sutherland RJ. A characterization of performance by men and women in a virtual Morris water task: a large and reliable sex difference. Behav Brain Res. 1998;93:185–190. doi: 10.1016/s0166-4328(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 5.Astur RS, Taylor LB, Mamelak AN, Philpott L, Sutherland RJ. Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behav Brain Res. 2002;132:77–84. doi: 10.1016/s0166-4328(01)00399-0. [DOI] [PubMed] [Google Scholar]
- 6.Astur RS, Tropp J, Sava S, Constable RT, Markus EJ. Sex differences and correlations in a virtual Morris water task, a virtual radial arm maze, and mental rotation. Behav Brain Res. 2004;151:103–115. doi: 10.1016/j.bbr.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 7.Astur R, Germain S, Tolin D, Ford J, Russell D, Stevens M. Hippocampus function predicts severity of post-traumatic stress disorder. CyberPsychology & Behavior. 2006;9:234–240. doi: 10.1089/cpb.2006.9.234. [DOI] [PubMed] [Google Scholar]
- 8.Benes FM, Kwok EW, Vincent SL, Todtenkopf MS. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry. 1998;44:88–97. doi: 10.1016/s0006-3223(98)00138-3. [DOI] [PubMed] [Google Scholar]
- 9.Blair JR, Spreen O. Predicting premorbid IQ: A revision of the National Adult Reading Test. The Clinical Neuropsychologist. 1989;3:129–136. [Google Scholar]
- 10.Broadbent NJ, Squire LR, Clark RE. Reversible hippocampal lesions disrupt water maze performance during both recent and remote memory tests. Learning & memory (Cold Spring Harbor, NY) 2006;13:187–191. doi: 10.1101/lm.134706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown MA, Sharp PE. Simulation of spatial learning in the Morris water maze by a neural network model of the hippocampal formation and nucleus accumbens. Hippocampus. 1995;5:171–188. doi: 10.1002/hipo.450050304. [DOI] [PubMed] [Google Scholar]
- 12.Brown RG, Pluck G. Negative symptoms: the 'pathology' of motivation and goal-directed behaviour. Trends Neurosci. 2000;23:412–417. doi: 10.1016/s0166-2236(00)01626-x. [DOI] [PubMed] [Google Scholar]
- 13.Calhoun VD, Adali T. Unmixing fMRI with independent component analysis. IEEE Eng Med Biol Mag. 2006;25:79–90. doi: 10.1109/memb.2006.1607672. [DOI] [PubMed] [Google Scholar]
- 14.Calhoun VD, Adali T, McGinty VB, Pekar JJ, Watson TD, Pearlson GD. fMRI activation in a visual-perception task: network of areas detected using the general linear model and independent components analysis. Neuroimage. 2001a;14:1080–1088. doi: 10.1006/nimg.2001.0921. [DOI] [PubMed] [Google Scholar]
- 15.Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001b;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calhoun VD, Adali T, Hansen LK, Larsen J, Pekar JJ. ICA of functional MRI data: an overview. Fourth International Symposium on Independent Component Analysis and Blind Signal Separation (ICA2003); April; Nara, Japan. 2003. pp. 281–288. [Google Scholar]
- 17.Calhoun VD, Kiehl KA, Liddle PF, Pearlson GD. Aberrant localization of synchronous hemodynamic activity in auditory cortex reliably characterizes schizophrenia. Biological Psychiatry. 2004a;55:842–849. doi: 10.1016/j.biopsych.2004.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calhoun VD, Pekar JJ, Pearlson GD. Alcohol intoxication effects on simulated driving: exploring alcohol-dose effects on brain activation using functional MRI. Neuropsychopharmacology. 2004b;29 doi: 10.1038/sj.npp.1300543. 2097-2017. [DOI] [PubMed] [Google Scholar]
- 19.Carter C, Robertson L, Nordahl T, Chaderjian M, Kraft L, O'Shora-Celaya L. Spatial working memory pathology and negative symptoms in schizophrenia. Biol Psychiatry. 1995;37:637–638. doi: 10.1016/S0006-3223(96)00350-2. [DOI] [PubMed] [Google Scholar]
- 20.Courtney SM, Petit L, Maisog JM, Ungerleider LG, Haxby JV. An area specialized for spatial working memory in human frontal cortex. Science (New York, NY. 1998;279:1347–1351. doi: 10.1126/science.279.5355.1347. [DOI] [PubMed] [Google Scholar]
- 21.Crucian GP, Berenbaum SA. Sex differences in right hemisphere tasks. Brain Cogn. 1998;36:377–389. doi: 10.1006/brcg.1998.0999. [DOI] [PubMed] [Google Scholar]
- 22.Czurkó A, Czéh B, Seress L, Nadel L, Bures J. Severe spatial navigation deficit in the Morris water maze after single high dose of neonatal x-ray irradiation in the rat. Proc Natl Acad Sci U S A. 1997;94:2766–2771. doi: 10.1073/pnas.94.6.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D'Esposito M, Alexander MP, Fischer R, McGlinchey-Berroth R, O'Connor M. Recovery of memory and executive function following anterior communicating artery aneurysm rupture. J Int Neuropsychol Soc. 1996;2:565–570. doi: 10.1017/s1355617700001740. [DOI] [PubMed] [Google Scholar]
- 24.D'Esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: an event-related fMRI study. Brain Cogn. 1999;41:66–86. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- 25.D'Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 26.Devan BD, Goad EH, Petri HL. Dissociation of hippocampal and striatal contributions to spatial navigation in the water maze. Neurobiol Learn Mem. 1996;66:305–323. doi: 10.1006/nlme.1996.0072. [DOI] [PubMed] [Google Scholar]
- 27.Devan BD, White NM. Parallel information processing in the dorsal striatum: relation to hippocampal function. J Neurosci. 1999;19:2789–2798. doi: 10.1523/JNEUROSCI.19-07-02789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 29.Ekstrom AD, Kahana MJ, Caplan JB, Fields TA, Isham EA, Newman EL, et al. Cellular networks underlying human spatial navigation. Nature. 2003;425:184–188. doi: 10.1038/nature01964. [DOI] [PubMed] [Google Scholar]
- 30.Evans AC, Collins DL, Mills SR, Brown ED, Kelly RL, Peters TM. 3D statistical neuroanatomical models from 305 MRI volumes; Proceedings of the IEEE Nuclear Science Symposium and Medical Imaging Conference; 1993. pp. 1813–1817. [Google Scholar]
- 31.Fletcher P, McKenna PJ, Friston KJ, Frith CD, Dolan RJ. Abnormal cingulate modulation of fronto-temporal connectivity in schizophrenia. Neuroimage. 1999;9:337–342. doi: 10.1006/nimg.1998.0411. [DOI] [PubMed] [Google Scholar]
- 32.Freire L, Mangin JF. Motion correction algorithms may create spurious brain activations in the absence of subject motion. Neuroimage. 2001;14:709–722. doi: 10.1006/nimg.2001.0869. [DOI] [PubMed] [Google Scholar]
- 33.Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant "default mode" functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- 34.Georges-Francois P, Rolls ET, Robertson RG. Spatial view cells in the primate hippocampus: allocentric view not head direction or eye position or place. Cerebral cortex (New York, NY. 1999;9:197–212. doi: 10.1093/cercor/9.3.197. [DOI] [PubMed] [Google Scholar]
- 35.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 36.Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- 37.Hanlon FM, Weisend MP, Hamilton DA, Jones AP, Thoma RJ, Huang M, et al. Impairment on the hippocampal-dependent virtual Morris water task in schizophrenia. Schizophr Res. 2006;87:67–80. doi: 10.1016/j.schres.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 38.Hanlon FM, Weisend MP, Yeo RA, Huang M, Lee RR, Thoma RJ, et al. A specific test of hippocampal deficit in schizophrenia. Behav Neurosci. 2005;119:863–875. doi: 10.1037/0735-7044.119.4.863. [DOI] [PubMed] [Google Scholar]
- 39.Harvey PD, Howanitz E, Parrella M, White L, Davidson M, Mohs RC, et al. Symptoms, cognitive functioning, and adaptive skills in geriatric patients with lifelong schizophrenia: a comparison across treatment sites. The American journal of psychiatry. 1998;155:1080–1086. doi: 10.1176/ajp.155.8.1080. [DOI] [PubMed] [Google Scholar]
- 40.Heckers S, Konradi C. Hippocampal neurons in schizophrenia. Journal of neural transmission (Vienna, Austria. 2002;109:891–905. doi: 10.1007/s007020200073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, et al. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci. 1998;1:318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- 42.Hort J, Laczo J, Vyhnalek M, Bojar M, Bures J, Vlcek K. Spatial navigation deficit in amnestic mild cognitive impairment. Proc Natl Acad Sci U S A. 2007;104:4042–4047. doi: 10.1073/pnas.0611314104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 44.Ku J, Cho W, Kim J, Peled A, Wiederhold BK, Wiederhold MD, et al. A virtual environment for investigating schizophrenic patients’ characteristics: assessment of cognitive and navigation ability. Cyberpsychol Behav. 2003;6:397–404. doi: 10.1089/109493103322278781. [DOI] [PubMed] [Google Scholar]
- 45.Kubicki M, Westin CF, Maier SE, Frumin M, Nestor PG, Salisbury DF, et al. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. The American journal of psychiatry. 2002;159:813–820. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lalonde R. Cerebellar contributions to instrumental learning. Neurosci Biobehav Rev. 1994;18:161–170. doi: 10.1016/0149-7634(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 47.Livingstone SA, Skelton RW. Virtual environment navigation tasks and the assessment of cognitive deficits in individuals with brain injury. Behav Brain Res. 2007;185:21–31. doi: 10.1016/j.bbr.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 48.MacDonald AW, 3rd, Carter CS. Cognitive experimental approaches to investigating impaired cognition in schizophrenia: a paradigm shift. Journal of Clinical & Experimental Neuropsychology. 2002;24:873–882. doi: 10.1076/jcen.24.7.873.8386. [DOI] [PubMed] [Google Scholar]
- 49.Maguire EA, Burgess N, Donnett JG, Frackowiak RS, Frith CD, O'Keefe J. Knowing where and getting there: a human navigation network. Science (Wash D C) 1998;280:921–924. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- 50.Maguire EA, Frackowiak RS, Frith CD. Recalling routes around london: activation of the right hippocampus in taxi drivers. J Neurosci. 1997;17:7103–7110. doi: 10.1523/JNEUROSCI.17-18-07103.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maguire EA, Spiers HJ, Good CD, Hartley T, Frackowiak RS, Burgess N. Navigation expertise and the human hippocampus: a structural brain imaging analysis. Hippocampus. 2003;13:250–259. doi: 10.1002/hipo.10087. [DOI] [PubMed] [Google Scholar]
- 52.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 53.Mechelli A, Price C, Friston K, Ashburner J. Voxel-Based Morphometry of the Human Brain: Methods and Applications. Current Medical Imaging Reviews. 2005;1:105–113. [Google Scholar]
- 54.Mogensen J, Pedersen TK, Holm S, Bang LE. Prefrontal cortical mediation of rats’ place learning in a modified water maze. Brain Res Bull. 1995;38:425–434. doi: 10.1016/0361-9230(95)02009-g. [DOI] [PubMed] [Google Scholar]
- 55.Morris RGM. Spatial localization does not require the presence of local cues. Learn Motiv. 1981;12:239–260. [Google Scholar]
- 56.Morris RGM, Garrud P, Rawlins J, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature (Lond) 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 57.Moses SN, Ryan JD, Bardouille T, Kovacevic N, Hanlon FM, McIntosh AR. Semantic information alters neural activation during transverse patterning performance. Neuroimage. 2009;46:863–873. doi: 10.1016/j.neuroimage.2009.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mumby DG, Cameli L, Glenn MJ. Impaired allocentric spatial memory and intact retrograde memory after thalamic damage caused by thiamine deficiency in rats. Behav Neurosci. 1999;113:42–50. [PubMed] [Google Scholar]
- 59.Nakazawa K, McHugh TJ, Wilson MA, Tonegawa S. NMDA receptors, place cells and hippocampal spatial memory. Nature Reviews. 2004;5:361–372. doi: 10.1038/nrn1385. [DOI] [PubMed] [Google Scholar]
- 60.Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science (New York, NY. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Newhouse P, Newhouse C, Astur RS. Sex differences in visual-spatial learning using a virtual water maze in pre-pubertal children. Behav Brain Res. 2007;183:1–7. doi: 10.1016/j.bbr.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 62.O'Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford: Clarendon Press; 1978. [Google Scholar]
- 63.O'Keefe J, Nadel L, Keightley S, Kill D. Fornix lesions selectively abolish place learning in the rat. Exp Neurol. 1975;48:152–166. doi: 10.1016/0014-4886(75)90230-7. [DOI] [PubMed] [Google Scholar]
- 64.Ortigue S, Grafton ST, Bianchi-Demicheli F. Correlation between insula activation and self-reported quality of orgasm in women. Neuroimage. 2007;37:551–560. doi: 10.1016/j.neuroimage.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 65.Pine DS, Grun J, Maguire EA, Burgess N, Zarahn E, Koda V, et al. Neurodevelopmental aspects of spatial navigation: a virtual reality fMRI study. Neuroimage. 2002;15:396–406. doi: 10.1006/nimg.2001.0988. [DOI] [PubMed] [Google Scholar]
- 66.Ragland JD, Gur RC, Raz J, Schroeder L, Kohler CG, Smith RJ, et al. Effect of schizophrenia on frontotemporal activity during word encoding and recognition: a PET cerebral blood flow study. The American Journal of Psychiatry. 2001;158:1114–1125. doi: 10.1176/appi.ajp.158.7.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosene D, van Hoesen G. The hippocampal formation of the primate brain. A review of some comparative aspects of cytoarchitecture and connections. In: Jones E, Peters A, editors. Further aspects of cortical function, including hippocampus. New York: Plenum; 1987. pp. 345–456. [Google Scholar]
- 68.Sakai K, Rowe JB, Passingham RE. Active maintenance in prefrontal area 46 creates distractor-resistant memory. Nat Neurosci. 2002;5:479–484. doi: 10.1038/nn846. [DOI] [PubMed] [Google Scholar]
- 69.Shipman S, Astur R. Factors affecting the hippocampal BOLD response during spatial memory. Behav Brain Res. 2008;187:433–441. doi: 10.1016/j.bbr.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 70.Squire LR. Memory and brain. New York ; Oxford: Oxford University Press; 1987. [Google Scholar]
- 71.Stone JM, Morrison PD, Pilowsky LS. Glutamate and dopamine dysregulation in schizophrenia--a synthesis and selective review. Journal of psychopharmacology (Oxford, England) 2007;21:440–452. doi: 10.1177/0269881106073126. [DOI] [PubMed] [Google Scholar]
- 72.Sutherland RJ, Whishaw IQ, Kolb B. A behavioural analysis of spatial localization following electrolytic, kainate- or colchicine-induced damage to the hippocampal formation in the rat. Behav Brain Res. 1983;7:133–153. doi: 10.1016/0166-4328(83)90188-2. [DOI] [PubMed] [Google Scholar]
- 73.Szendi I, Kiss M, Racsmany M, Boda K, Cimmer C, Voros E, et al. Correlations between clinical symptoms, working memory functions and structural brain abnormalities in men with schizophrenia. Psychiatry Res. 2006;147:47–55. doi: 10.1016/j.pscychresns.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 74.Waite JJ, Chen AD, Wardlow ML, Thal LJ. Behavioral and biochemical consequences of combined lesions of the medial septum/ diagonal band and nucleus basalis in the rat when ibotenic acid, quisqualic acid, and AMPA are used. Exp Neurol. 1994;130:214–229. doi: 10.1006/exnr.1994.1200. [DOI] [PubMed] [Google Scholar]
- 75.Weniger G, Irle E. Allocentric memory impaired and egocentric memory intact as assessed by virtual reality in recent-onset schizophrenia. Schizophr Res. 2008;101:201–209. doi: 10.1016/j.schres.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 76.Yin HH, Knowlton BJ. Contributions of striatal subregions to place and response learning. Learn Mem (Cold Spring Harb) 2004;11:459–463. doi: 10.1101/lm.81004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang ZJ, Reynolds GP. A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res. 2002;55:1–10. doi: 10.1016/s0920-9964(01)00188-8. [DOI] [PubMed] [Google Scholar]