Abstract
Interleukin-15 (IL-15) stimulates the differentiation and proliferation of T, B and NK cells, enhances CD8+ cytolytic T cell activity, helps maintain CD44hiCD8+ memory T cells, and stimulates immunoglobulin synthesis by B cells. IL-15 is trans-presented to effector cells by its receptor, IL-15Rα, expressed on dendritic cells (DC) and monocytes. We examined the anti-tumor effect of adenoviral-mediated gene transfer of IL-15 and IL-15Rα to augment a DC vaccine directed against the NEU (ErbB2) oncoprotein. Transgenic BALB-neuT mice vaccinated in late stage tumor development with a DC vaccine expressing a truncated NEU antigen, IL-15 and its receptor (DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα) were protected from mammary carcinomas with 70% of animals tumor-free at 30 weeks compared to none of the animals vaccinated with NEU alone (DCAd.Neu). The combination of neu, IL-15 and IL-15Rα gene transfer lead to a significantly greater anti-NEU antibody response compared to mice treated with DCAd.Neu, or DCAd.Neu combined with either IL-15 (DCAd.Neu+Ad.mIL-15), or IL-15Rα (DCAd.Neu+Ad.mIL-15Rα). The anti-tumor effect was antibody mediated and involved modulation of NEU expression and signaling. Depletion of CD4+ cells did not abrogate the anti-tumor effect of the vaccine, nor did it inhibit the induction of anti-NEU antibodies. Co-expression of IL-15 and IL-15Rα in an anticancer vaccine enhanced immune responses against the NEU antigen and may overcome impaired CD4+ T-helper function.
Keywords: Cancer vaccine, interleukin-15, dendritic cells, CD4 help, breast cancer
Introduction
Interleukin-15 (IL-15) is a pleiotropic cytokine that was identified for its ability to stimulate T cell proliferation (1–3). It was further shown to induce the proliferation of NK cells, B cells and interferon-producing killer dendritic cells (4–6). IL-15 is essential for the differentiation and maintenance of memory CD8+ T cells, NK/T and NK cells (7). It also stimulates immunoglobulin synthesis by B cells (5), promotes development of DC (8), and stimulates the production of proinflammatory cytokines by macrophages (9). IL-15 mRNA is expressed by a wide variety of cells including monocytes, macrophages, DC, fibroblasts, epithelial cells of various origins, and skeletal muscle (10); however, IL-15 itself has a short half-life and its expression is tightly regulated at the translational level (11).
IL-15 signals through a heterotrimeric receptor composed the cytokine-specific IL-15Rα, IL-2R/IL-15Rβ (CD122) shared with the IL-2 receptor, and the common cytokine receptor γ-chain (γc, CD132) (12, 13). Unlike IL-2, that requires all three receptor subunits for high affinity binding, IL-15 is highly bound by IL-15Rα alone. Trans-presentation of IL-15 by IL-15Rα expressed on activated DC and monocytes to the IL-2R/IL-15Rβ and γc on effector T, B and NK cells is thought to be the dominant mechanism for IL-15 action (14). Burkett et al, demonstrated the requirement for co-expression of IL-15 and IL-15Rα by non-lymphoid cells such as DC to support IL-15 function (15). IL-15Rα has been shown to stabilize IL-15 and increase the half-life of the cytokine (16–19). The similar pathology of IL-15−/− and IL-15Rα−/− mice, and the ability of complexes of IL-15 with soluble IL-15Rα–IgFc to correct the immune defects in these mice is evidence for the role of IL-15Rα in IL-15 action (16).
We examined the anti-tumor effect of a DC vaccine expressing IL-15, IL-15Rα and the tumor antigen, NEU, in a transgenic mouse breast cancer model. Co-expression of IL-15 and IL-15Rα prevented or delayed development of mammary carcinomas in this aggressive late stage tumorigenesis model. Furthermore, we demonstrated that mice vaccinated with DC co-expressing IL-15 and IL-15Rα generated significantly greater levels of anti-NEU antibodies compared to DC expressing the tumor antigen alone, or expressing the NEU antigen along with either IL-15 or IL-15Rα. Co-expression of IL-15 and IL-15Rα allowed for the induction of humoral immunity largely independent of CD4-help that may be an added benefit in the setting of reduced or dysfunctional CD4+ T cells as in patients with cancer or HIV infection.
Materials and Methods
Cell lines
The NEU-expressing cell lines TUBO and N202.1A derived from mammary cancers from a BALB-neuT and FVB-neuN mouse, respectively, were gifts from Dr. Patrizia Nanni (20, 21) (University of Bologna, Bologna, Italy) and were grown in Dulbecco’s modified Eagle’s medium (DMEM; BioSource, Rockville, MD) with 10% fetal bovine serum (FBS, Gemini, Woodland, CA) and 10 μg/mL gentamicin sulfate (BioSource, Inc). Human embryonic kidney (HEK-293) cells were grown in DMEM with 10% FBS and 10 μg/mL gentamicin sulfate (BioSource, Inc) and were purchased from American Type Culture Collection(ATCC; Manassas, VA).
Peptides
Synthetic peptides p66 (TYVPANASL), a dominant rat NEU epitope (22), HEX486–494 (KYSPSNVKI) from adenovirus hexon (23) and OVA257–264 (SIINFEKL), from hen ovalbumin (23), were purchased from Genscript (Piscataway, NJ).
Adenoviruses
The cDNA encoding the extracellular and transmembrane (ECM-tm) domains of the rat neu oncogene was provided by Dr. Augusto Amici (20) (University of Camerino, Camerino, Italy). The murine IL-15 and IL-15Rα cDNAs (14) were provided by Dr. Yutaka Tagaya (National Cancer Institute, Bethesda, MD). Ad.Neu, Ad.mIL-15 and Ad.mIL-15Rα are E1, E3-deleted recombinant adenoviruses (rAd) expressing the neu ECM-tm domains, murine IL-15 or IL-15Rα, respectively. Ad.null is an E1, E3-deleted rAd expressing no transgene. All vectors were generated using the AdMax system (Microbix, Toronto, Canada) (24), were plaque-isolated, expanded on HEK-293 cells, purified on two-step and continuous CsCl gradients, or anion-exchange column (Sartorius Stedim, Edgewood, NY), titered as plaque forming units (pfu)/mL and stored at −70°C.
Animals
Animal studies were approved by the Animal Care and Use Committee of the National Cancer Institute (NCI). Female BALB-neuT mice, transgenic for a transforming rat neu oncogene under the control of a chimeric mouse mammary tumor virus promoter (25) were from a breeding colony established at the NCI. Female BALB/c mice were obtained from the Division of Cancer Treatment, NCI (Frederick, MD). CD4−/− (H-2d) mice (26) were obtained from Dr. Jay A. Berzofsky (Vaccine Branch, NCI, Bethesda, MD).
Dendritic cells
DC were generated as previously described (27). Briefly, bone marrow was harvested from the tibias and femurs of 8 to 10 week-old BALB/c mice. Erythrocytes were lysed with ACK buffer (BioWhittaker, Walkersville, MD) and the cells plated in plastic bacteriological dishes in RPMI 1640 (Life Technologies-Invitrogen, Inc., Grand Island, NY) with 10% heat-inactivated FBS (Life Technologies-Invitrogen, Inc.) and 20 ng/mL murine granulocyte/macrophage-colony stimulating factor (mGM-CSF; PeproTec Inc., Rocky Hill, NJ). Cultures were refreshed with 20 ng/mL mGM-CSF on days 3, 6, and 8. On day 8, DC were collected and transduced with rAd. On day 10, DC were collected, washed three times and used for vaccination.
Flow cytometry
DC were incubated with fluorescein isothiocyanate (FITC) or phycoerythrin (PE)-labeled anti-mouse CD40, CD80, CD86, or H-2Kd (BD-PharMingen, San Diego, CA), and analyzed on a FACSort (Becton Dickinson, San Jose, CA). Forty-eight hours after transfection with Ad.null, Ad.Neu, Ad.mIL-15 and/or Ad.mIL-15Rα, DC were incubated with anti-rat NEU (Oncogene Research, La Jolla, CA) or anti-mouse IL-15 (MBL International, Woburn, MA) followed by incubation with FITC-labeled rabbit anti-mouse immunoglobulin. IL-15Rα was detected by FITC-labeled anti-mouse IL-15Rα polyclonal antibody (R&D Systems Minneapolis, MN) and analyzed using FlowJo software (Tree Star, Inc., Ashland, OR).
Vaccinations
Groups of 12-week-old female BALB-neuT mice received four weekly subcutaneous injections of 1 × 106 DC transduced at a multiplicity of infection (MOI) of 10 pfu/cell with Ad.Neu (DCAd.Neu), Ad.Neu and Ad.mIL-15 (DCAd.Neu+Ad.mIL-15), Ad.Neu and Ad.mIL-15Rα (DCAd.Neu+Ad.mIL-15Rα), Ad.Neu, Ad.mIL-15 and Ad.mIL-15Rα (DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα), Ad.mIL-15 and Ad.mIL-15Rα (DCAd.mIL-15+Ad.mIL-15Rα), or Ad.null (DCAd.null) as a control. Mice were examined twice weekly for the development of tumors.
In another set of experiments, groups of 5 to 6 week-old BALB/c mice were given subcutaneous injections of 1 × 106 DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα or PBS (100 μL) weekly for two weeks. On the day of the final vaccination, the mice were subcutaneously injected with 1 × 106 TUBO cells and were then monitored for tumor growth. Tumor volumes were calculated using the formula: V= l × w2/2.
Immune cell depletion
Groups of BALB/c mice were depleted of specific immune cell populations (28). Briefly, CD4+ or CD8+ cells were depleted with anti-CD4 or anti-CD8 antibodies purified from the supernatants of hybridomas GK1.5 (ATCC) and 2.43 (ATCC), respectively. Five days prior to vaccination with DCAd.Neu or DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα, mice were intraperitoneally injected with 200 μg of the appropriate antibody for 3 consecutive days, and continued every 3 days thereafter for the duration of the experiment. To deplete NK cells, anti-asialo-GM1 (WAKO, Richmond, VA) 50 μg was administered beginning 5 days prior to tumor implantation for 3 consecutive days, and then continued every 3 days thereafter. Greater than 95% depletion of specific lymphocyte populations was confirmed by peripheral blood flow cytometry.
Serum anti-NEU antibodies
Whole blood was drawn prior to vaccination and one week after the fourth vaccination. Serum was separated and stored at −20°C. N202.1A cells (neu+) were used to quantify anti-NEU antibodies as previously described (28, 29). Briefly, 2 × 105 N202.1A cells were incubated with test sera diluted 1:10 in 1% FBS in PBS at 4°C for 1 h. Cells were washed and incubated with FITC-labeled rabbit anti-mouse immunoglobulin antibody (DAKO, Carpinteria, CA) and mean fluorescence intensity measured by flow cytometry.
Cellular response assays
To detect cytolytic responses, splenocytes were isolated one week after the last vaccination with either DCAd.Neu, DCAd.Neu+Ad.mIL-15, DCAd.Neu+Ad.mIL-15Rα, DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα, or DCAd.null. Effector cells were re-stimulated by co-culturing 3 × 106 splenocytes with mitomycin C treated TUBO stimulator cells in RPMI 1640 supplemented with 20 U/mL IL-2 (PeproTech, Inc.) for 5 days. Effector cells were assayed for their ability to lyse TUBO cells at effector:target ratios of 100:1, 10:1 and 1:1. Cytotoxicity was quantified by lactate dehydrogenase (LDH) release (CytoTo×96® Non-Radioactive Cytotoxicity Assay, Promega, Madison, WI) as per the manufacturer’s protocol. The percent cytotoxicity was calculated as: 100 × ([experimental release] – [effector spontaneous release] – [target spontaneous release])/([target maximum release] – [target spontaneous release]).
To detect a CD8+ response against the NEU antigen, splenocytes were assayed for interferon-gamma (IFN-γ) secretion. Splenocytes from groups of animals (n = 3) vaccinated with DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα, or DCAd.null were pooled and plated at 2 × 106 cells per well in 24-well plates in triplicate. Splenocytes were co-cultured with 10 μg/mL of the CD8 dominant peptides p66 (NEU), OVA257–264, or HEX486–494 for 72 h. Supernatants were collected and IFN-γ was measured by ELISA (R&D Systems, Minneapolis, MN) according to manufacturer’s instructions. All samples were tested in triplicate.
Adoptive serum transfer
Serum was collected from BALB/c mice immunized with DCAd.Neu+Ad.mIL-15+Ad.mIL- 15Rα or injected with PBS was pooled, diluted 3-fold, titered and stored at 4°C. Groups of naïve BALB/c mice were intraperitoneally injected with 0.3 mL of the diluted serum every 3 days from day 5 to 17 after subcutaneous injection of 1 × 106 TUBO cells. Mice were examined twice weekly for tumor growth.
Effects of immunized serum on NEU signaling and apoptosis
Alteration of NEU (ErbB2), AKT and p38 expression and phosphorylation following treatment with sera from DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα vaccinated mice was examined using a cell-based ELISA (30). TUBO cells were seeded at 1 × 104 cells per well in a 96-well plate. The following day, the media was exchanged for media containing 5% FBS + 5% sera from DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα vaccinated mice, or 5% FBS + 5% sera from PBS injected mice. The cells were incubated for 0, 1, or 4 h and NEU, AKT and p38 phospho protein and total protein levels were quantitated using the Cellular Activation of Signaling ELISA (CASE Kit, Super Array Bioscience. Frederick, MD) following the manufacturer’s instructions.
Apoptosis was detected using an ssDNA apoptosis ELISA kit (Chemicon International, Temecula, CA). TUBO cells were seeded at 1 × 104 cells per well in a 96-well plate. The following day, media was exchanged for media containing 5% FBS + 5% sera from DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα vaccinated mice, or 5% FBS + 5% sera from PBS injected mice and incubated for 24 h. Apoptosis was detected according to manufacturer’s protocol.
Statistical analysis
Statistical analysis was performed using JMP Statistical Software version 5.1 (SAS Institute Inc., Cary, NC). Kaplan-Meier non-parametric regression analyses were performed for tumor prevention experiments with significance determined by the log-rank test. The comparison of the effect of vaccination on antibody titers among different groups was analyzed by one-way analysis using Tukey-Kramer HSD and non-parametric Wilcoxon/Kruskal-Wallis tests. A p-value <0.05 was considered significant.
Results
DC transduced with Ad.Neu, Ad.mIL-15 and Ad.mIL-15Rα expressed NEU (ErbB2) oncoprotein, IL-15, IL-15Rα, and exhibit maturation
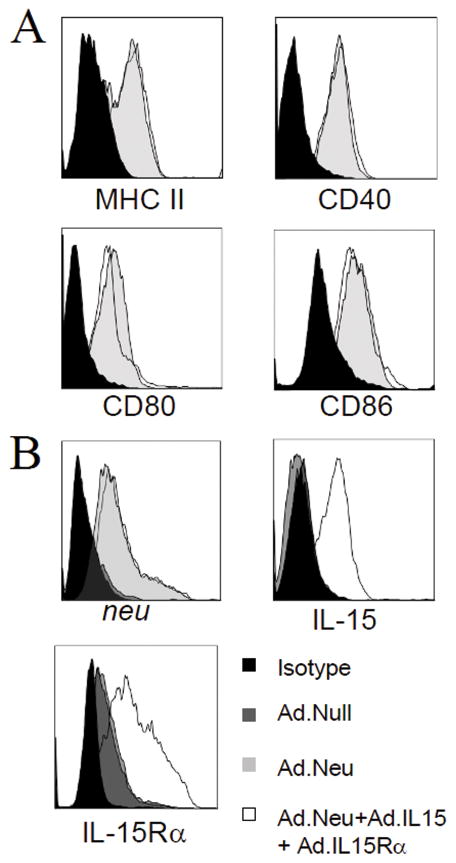
DC from 10 day bone marrow cultures expressed CD80, CD86, CD40, and MHC class II. Compared with unmodified DC, DC transduced with Ad.Neu, Ad.mIL-15 and Ad.mIL-15Rα (DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα) or Ad.Neu alone (DCAd.Neu) expressed higher levels of CD80, CD86, CD40, and MHC class II indicating maturation (Fig. 1A).
Figure 1.
Dendritic cells (DC) transduced with Ad.Neu, Ad.mIL15 and Ad.mIL15Rα exhibit a mature phenotype and express NEU, IL-15 and IL-15Rα. Bone marrow derived DC transduced with Ad.Neu together with Ad.mIL-15 and Ad.mIL-15Rα; Ad.Neu; or Ad.null at an MOI of 10 pfu/cell were examined by flow cytometry for: A; DC maturation markers; CD40, CD80, CD86 and MHC class II B; Surface expression of NEU, IL-15 and IL-15Rα.
Forty-eight hours after transduction with Ad.Neu, or the combination of Ad.Neu, Ad.mIL-15 and Ad.mIL-15Rα, DC were examined for the expression of NEU, IL-15 and IL-15Rα by flow cytometry (Fig. 1B). NEU protein was detected in DC transduced with Ad.Neu at comparable levels to those transduced with the combination of Ad.Neu, Ad.mIL-15 and Ad.mIL-15Rα. Expression was not observed in DC transduced with Ad.null. IL-15 was detected in DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα, but not on DCAd.Neu or DCAd.null. IL-15Rα was detected on all DC; however, greater expression levels were observed in DC transduced with Ad.Neu in combination with Ad.mIL-15 and Ad.mIL-15Rα.
Vaccination of BALB-neuT mice in late stage tumorigenesis with DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα prevented autochthonous mammary cancers
Mice treated with DCAd.Neu or DCAd.mIL-15+Ad.mIL-15Rα showed no survival advantage over mice treated with the control DCAd.null vaccine, with median survivals of 17 and 18 weeks, respectively (P=0.47, P=0.313) (Fig 2). All animals treated with DCAd.null, DCAd.Neu, or DCAd.mIL-15+Ad.mIL-15Rα developed tumors by 23 weeks of age. In contrast, mice treated with DCAd.Neu+Ad.mIL-15 or DCAd.Neu+Ad.mIL-15Rα demonstrated improved tumor-free survival compared to DCAd.Neu (P=0.014, P=0.005), possibly due to the effect of endogenous IL-15 and IL-15Rα interacting with the transferred receptor or cytokine, respectively. Mice receiving DCAd.Neu+Ad.mIL-15 had a median tumor-free survival of 22.5 weeks and at 30 weeks 10% of mice were free of tumor, while mice treated with DCAd.Neu+Ad.mIL-15Rα survived a median of 23 weeks with 20% of mice tumor-free at 30 weeks. Mice treated with the triple combination, DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα exhibited significantly greater tumor-free survival when compared with mice treated with DCAd.Neu (P<0.001), DCAd.Neu+Ad.mIL-15 (P=0.001), DCAd.Neu+Ad.mIL-15Rα (P=0.004), or DCAd.null (P<0.001). Furthermore, in mice treated with the Ad.null modified DC or DCAd.Neu, the onset of the first tumor occurred at 14 weeks, and all of the mice developed at least one mammary cancer by 23 weeks. In mice vaccinated with DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα, the first tumor occurred at 22 weeks and 70% of mice remained free of tumor at 30 weeks.
Figure 2.
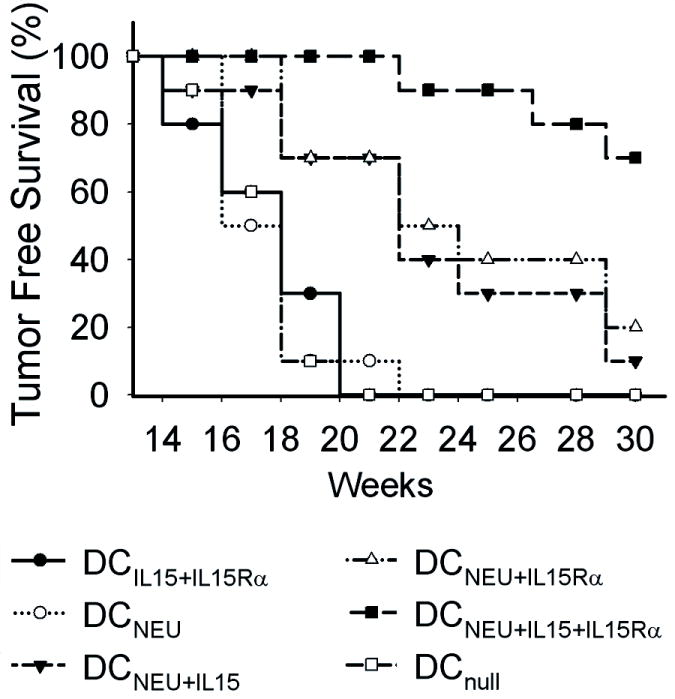
DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα vaccination of BALB-neuT mice significantly delayed or prevented the onset of mammary tumors. Groups (n =10) of 12-week-old female BALB-neuT mice received four weekly subcutaneous injections of 1 × 106 DCAd.Neu, DCAd.Neu+Ad.mIL-15, DCAd.Neu+Ad.mIL-15Rα, DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα, DCAd.mIL-15+Ad.mIL-15Rα or DCAd.null. Mice were examined twice weekly for the formation of mammary tumors.
DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα vaccination induced serum anti-NEU antibodies, but not a cytolytic T-lymphocyte (CTL) response
To determine the ability of the triple vaccination to induce cell and antibody mediated immune responses, we examined the induction of anti-NEU antibodies and tumor-specific CTL. Vaccination with DCAd.null or DCAd.mIL-15+Ad.mIL-15Rα failed to induce significant increases of anti-NEU antibodies compared to Ad.null vaccinated mice (Fig. 3A). In contrast, mice treated with DCAd.Neu, DCAd.Neu+Ad.mIL-15, DCAd.Neu+Ad.mIL-15Rα or DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα showed significant increases in serum anti-NEU antibodies when compared to DCAd.null vaccination (P<0.05) (Fig. 3A). Furthermore, vaccination with DCAd.Neu+Ad.mIL-15, DCAd.Neu+Ad.mIL-15Rα or DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα induced on average 63%, 53% and 102% greater levels of anti-NEU antibodies compared to mice treated with DC transduced with Ad.Neu alone. Compared to all other groups, mice vaccinated with DC transduced with the combination of Ad.Neu, Ad.mIL-15 and Ad.mIL-15Rα generated the highest titers of anti-NEU antibodies (P<0.05). To investigate the functionality of the anti-NEU antibodies we examined their ability to induce apoptosis of the neu+ TUBO cell line in vitro (Fig 3B.). Serum from DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα vaccinated mice induced apoptosis of TUBO cells. This effect was significantly greater than that seen with serum from PBS treated mice. The induction of tumor cell apoptosis by anti-NEU antibodies may be a mechanism for the reduction in tumor formation in the BALB-neuT mice.
Figure 3.
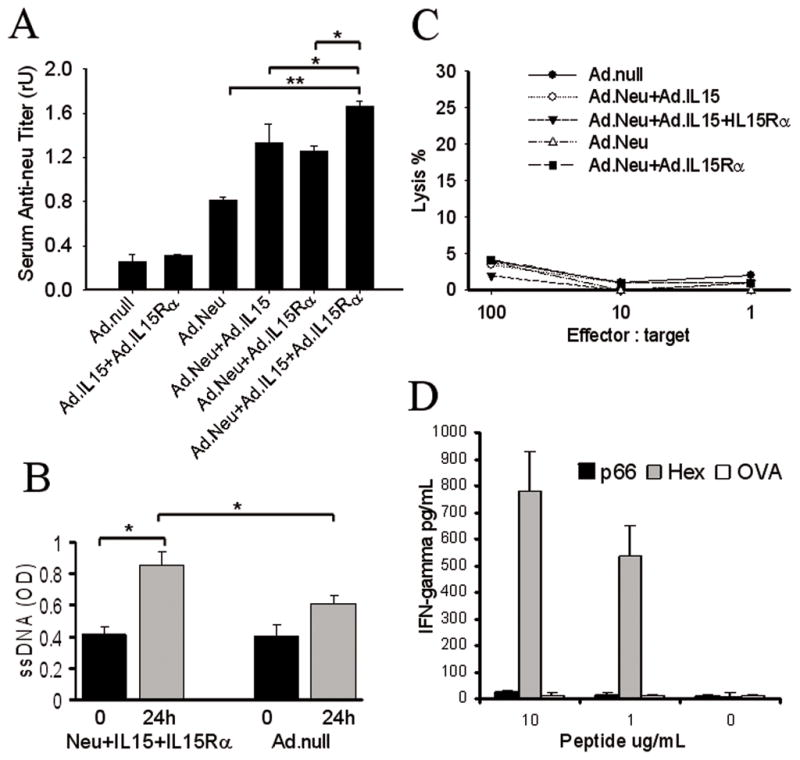
Vaccination with DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα induced a humoral immune response, but did not stimulate a CTL response against the NEU-expressing tumor. BALB-neuT mice were treated with four weekly subcutaneous injections of 1 × 106 DCAd.Neu, DCAd.Neu+Ad.mIL-15, DCAd.Neu+Ad.mIL-15Rα, DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα, DCAd.mIL-15+Ad.mIL-15Rα or DCAd.Null (control). A; The animals were bled two weeks after the last vaccination and anti-NEU antibodies measured. The results represent the mean titers in each group (n = 5 per group). Error bars, ±SD. * P<0.05, ** P<0.001. B; Apoptosis in TUBO cells after 24 h incubation in serum from DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα or DCAd.Null vaccinated mice. The results represent the mean values (n = 5 per group). Error bars, ±SD. * P<0.05. C; Two weeks after the last vaccination, splenocytes were harvested and assayed for CTL response and, D; IFN-γ secretion. Two independent experiments showed similar results, and the data presented are the means for a triplicate treatment in one experiment.
Tumor-specific CTL could not be demonstrated in BALB-neuT mice vaccinated with DC expressing neu alone, or in combination with IL-15 and/or IL-15Rα (Fig. 3C). When looking at IFN-γ secretion by the splenocytes of mice vaccinated with DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα versus DCAd.null re-stimulated with CD8-specific peptides for NEU, ovalbumin or adenovirus hexon, we found little IFN-γ production in the NEU vaccinated mice stimulated with the NEU specific peptide (Fig. 3D). In contrast, when these splenocytes were stimulated with the adenovirus specific peptide, there was a strong IFN-γ response detected. No IFN-γ secretion was detected when they were stimulated with the irrelevant OVA257–264 peptide.
Serum from DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα vaccinated mice inhibited tumor growth in vivo and decreased NEU (ErbB2) signaling in vitro
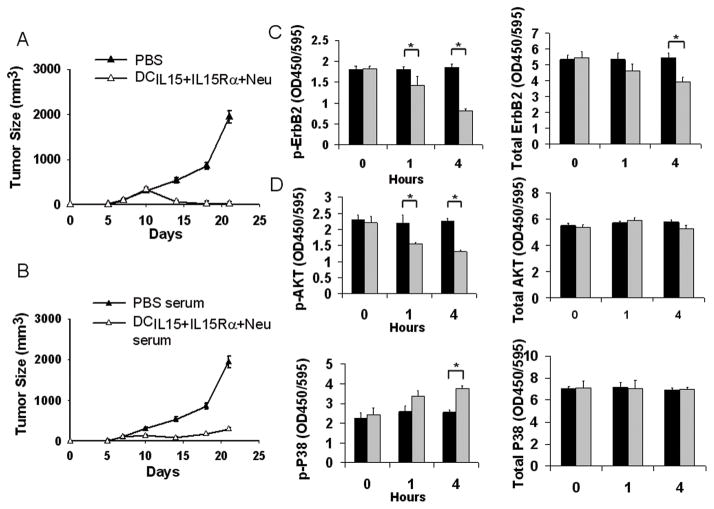
To explore whether NEU-specific antibodies played a role, we looked at whether vaccination would protect mice from tumor challenge with the neu-expressing TUBO tumor cell line. All the animals treated with DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα or PBS developed tumors within 7 days of challenge; however, in mice vaccinated with DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα the tumors regressed, while in the PBS treated mice, the tumors continued to grow (Fig 4A).
Figure 4.
Serum from DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα vaccinated mice inhibits tumor growth and modulates ErbB2/neu, AKT and p38 phosphorylation. A; Groups of 5- to 6-week-old BALB/c mice received subcutaneous injections of DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα (1 × 106 cells) or PBS (100μL), weekly for two weeks. On the day of the final vaccination, the mice were subcutaneously challenged with 1 × 106 TUBO cells and were then monitored for tumor growth. B; Serum was collected on day 14 from DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα immunized mice. Tumor-bearing mice (n = 5) were injected with immune sera every 3 days from day 5 to day 17 after TUBO cell injection. PBS treated mouse serum was used as negative control. The mice were monitored for tumor growth twice weekly. Expression of phosphorylated and total C; ErbB2 or D; AKT and p38 in TUBO cells following incubation in sera of DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα (black bar) or PBS (grey bar) vaccinated mice was determined. *P< 0.05.
Next we examined if serum from DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα vaccinated mice could inhibit tumor in naïve mice challenged with TUBO cells. Serum from animals vaccinated with DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα inhibited the growth of TUBO tumors compared to mice receiving serum from the PBS treated animals (P<0.01) (Fig. 4B). This indicates that specific anti-NEU antibodies play a protective role following vaccination.
To examine how anti-NEU antibodies inhibited tumor growth, we looked at NEU protein expression and signaling in TUBO cells following exposure to serum from immunized mice. Serum from DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα vaccinated mice induced time-dependent inhibition of NEU phosphorylation as well as reduced total levels of NEU protein. After 4 hours incubation with DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα serum, the levels of phosphorylated NEU in TUBO cells were significantly lower than that detected at time zero, or when compared to incubation in PBS treated mouse serum at any time point (P<0.05). Total NEU levels were also reduced after 4 hours of incubation with DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα vaccinated serum with average NEU levels 28% lower than that detected at time zero (P<0.05) (Fig 4C). After showing that the DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα vaccine induced antibodies that can inhibit phosphorylation of NEU as well as down-modulate its overall level, we examined the effect on signaling in pathways downstream of NEU. Four hours after incubation with serum from mice vaccinated with DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα, TUBO cells demonstrated reduced levels of phospho-AKT and increased phosphorylated p38 protein. At 4 hours, phospho-AKT was 43% lower than at time zero, while the level of phosphorylated p38 was 51% higher than at time zero, or when compared to the PBS treated serum (Fig 4D). Reduction in phospho-AKT expression and increased p38 phosphylation are indicative of decreased NEU signaling (31).
CD4+ T cells are required for antitumor immunity with DCAd.Neu vaccination, but not DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα vaccination
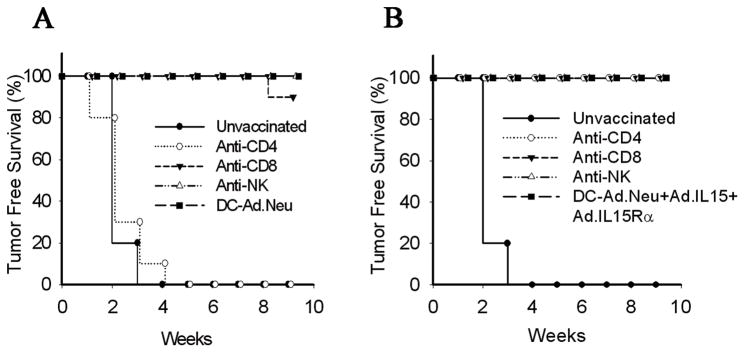
To further explore the effect of the combination of IL-15 and its receptor on anti-tumor vaccination, immune cell subpopulations in groups of BALB/c mice were depleted All mice receiving DCAd.null developed palpable tumors by 21-days post-implantation (Fig. 5A & B). Animals vaccinated with DCAd.Neu, or DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα were protected from tumor (Fig. 5A & B). When mice were depleted of CD8+, CD4+ or NK cells after vaccination with DCAd.Neu, or DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα they also failed to form tumors. However, mice depleted of CD4+ cells prior to DCAd.Neu vaccination all developed tumors indicating a need for CD4+ T cell help for an effective response. In contrast, mice depleted of CD4+ cells before DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα vaccination were fully protected from tumors indicating that CD4+ T cell help may not be required when IL-15 and IL-15Rα was included in the vaccine (Fig. 5B).
Figure 5.
CD4+ T cells are required for generation of antitumor responses following vaccination with DCAd.Neu, but not DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα CD4+, CD8+ and NK cell were depleted from groups of BALB/c mice vaccinated with A; DCAd.Neu or B; DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα, using injections of 200 μg of anti-CD4 (GK1.5) or anti-CD8 (2.43), or 50 μg anti-NK (anti-asialo GM1) antibodies as described in Materials and Methods. Two weeks after the final vaccination mice were injected with 1 × 106 TUBO cells and followed for tumor free survival (10 mice per group).
DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα vaccination induced anti-NEU antibodies in CD4-depleted mice, but not in CD4 nullmice
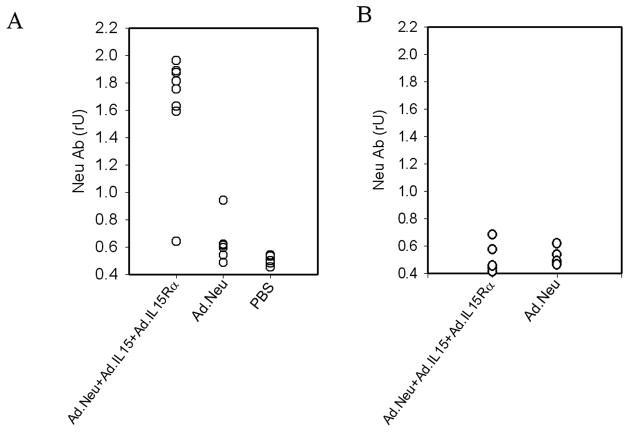
To determine if the triple vaccine was able to prime B-lymphocytes to produce NEU-specific antibodies in the absence of CD4+ T-help, we depleted CD4+ cells prior to vaccination and examined anti-NEU antibody levels. Significant levels of antibody were detected in the sera of DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα vaccinated mice with a normal CD4 component as well as those depleted of CD4 cells (Fig. 6A). In contrast, mice vaccinated with DCAd.Neu demonstrated high levels of antibody when CD4+ cells were present; however, when CD4+ cells were depleted, DCAd.Neu treated mice produced little antibody with vaccination. These data indicate that exogenous IL-15 and IL-15Rα incorporated into DC vaccines may compensate for the need for CD4 help in priming B cells to produce antibody.
Figure 6.
DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα vaccination induced anti-neu antibodies in antibody mediated CD4-depleted mice, but not in CD4 −/−mice. A; CD4-depleted BALB/c mice (n = 8), or B; BALB/c CD4 −/− mice (n = 5) were vaccinated with four weekly subcutaneous injections of 1 × 106 DCAd.Neu or DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα. Two weeks after the final vaccination, the animals were bled and the serum anti-neu antibodies measured.
We further examined this effect using CD4−/− knockout mice. Unlike the antibody-mediated CD4 depletion model, CD4−/− mice vaccinated with either the DCAd.Neu or DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα demonstrated only low levels of anti-NEU antibodies after vaccination (Fig. 6B). There was no difference in the levels of antibody produced whether the mice were given DCAd.Neu or DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα. In addition, there was no protection afforded by either vaccine from the challenge with TUBO cells.
Discussion
IL-15 has demonstrated the ability to increase the effectiveness of vaccines through enhancement of both innate and adaptive immune responses. Co-expression of its receptor, IL-15Rα, additionally enhances the biological activity of IL-15 through improved trans-presentation of IL-15 to the signaling β and common γ-receptors (15, 17–19). In late stage (12-week-old) BALB-neuT transgenic mice, vaccination with genetically-modified DC expressing IL-15, IL-15Rα and a truncated NEU antigen, prevented or significantly delayed of the onset of breast tumors (Fig. 2). At this age, the mice already demonstrate advanced microscopic mammary lesions (32) and immunization with DCAd.Neu alone offered little or no benefit.
Further examining the effects of vaccination with IL-15, IL-15Rα and NEU, we found that this triple combination enhanced humoral immune responses to the NEU antigen compared to vaccination with NEU-expressing DC alone, or when singularly combined with IL-15 or IL-15Rα (Fig. 3A). This is consistent with increases in antibody titers reported by others using IL-15 as an adjuvant combined with smallpox vaccination, and with that observed with an experimental HIV vaccine in mice (33, 34). The isotypes observed following vaccination with DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα was largely IgG1 and IgG2a, with lesser amounts of IgG2b and IgM (data not shown). This pattern is consistent with other reports vaccinating with Ad.neu alone (29). The addition of IL-15 and IL-15Rα did not change the relative predominance of isotypes, but rather resulted in overall increased antibody levels. This increase may result from the ability of IL-15 to enhance proliferation, differentiation, and immunoglobulin synthesis by B cells (5, 35, 36). In addition, IL-15 also inhibits B cell apoptosis (37), and may play a role in generating long-term serological memory (36).
Subcutaneous vaccination with either DCAd.Neu or directly with Ad.Neu did not result in a measurable anti-NEU CTL response in our model (28, 29). While IL-15 has been shown to increase CTL responses (33), we were unable to demonstrate classical CTL or large increases in IFN-γ secretion (Fig. 3C & D). The antitumor response appeared to be largely due to induction of antibody. This was confirmed by the observation that serum transferred from Ad.Neu hyper vaccinated mice protected naïve mice from tumor challenge indicating an inhibitory effect of NEU-specific antibodies (29, 38). Transfer of serum from DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα immunized mice into naïve TUBO bearing mice inhibited tumor growth (Fig. 4B). Furthermore, IL-15 itself was undetectable in the serum of DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα vaccinated mice (data not shown). As the anti-tumor effect occurred in the absence of IL-15, we concluded that these anti-NEU antibodies alone were sufficient to inhibit tumor growth. Passive immunization was not as effective as active vaccination with DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα (Fig. 4A), likely due to limitations on the amount of antibody that could be transferred.
In vitro, serum from DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα vaccinated mice was able to inhibit phosphorylation of NEU, as well as down-modulate NEU expression in TUBO cells (Fig. 4C). Serum from DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα vaccinated mice also affected downstream NEU signaling pathways decreasing activation of the pro-mitotic AKT pathway and increasing p38 MAPK expression (Fig. 4D) that ultimately enhances apoptosis (Fig 3B.). These findings are consistent with previous studies that demonstrated that anti-NEU antibodies from the serum of Ad.Neu vaccinated mice (38) or the direct administration of monoclonal antibodies inhibited NEU phosphorylation and signaling (39). The anti-HER-2/neu monoclonal antibodies, trastuzumab and pertuzumab used in the clinic inhibit breast cancer growth, in part, through activation of p38 MAPK and inhibition of AKT (40, 41).
CD4+ T cells are required for induction of anti-tumor responses in BALB-neuT mice (28, 29). Indeed, when DCAd.Neu alone was used to vaccinate mice, depletion of CD4+ cells prior to vaccination abrogated the anti-tumor effect (Fig. 5A). Depletion of CD4+ cells 72–96 h after vaccination had no effect on the anti-tumor response (data not shown). This is consistent with a requirement for CD4+ help at the time of vaccination to facilitate B cell priming (29). Depletion of CD8+ cells and NK cells prior to vaccination with either DCAd.Neu or DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα vaccination did not affect tumor-free survival indicating that these cells do not play a critical role in anti-tumor immunity in this model (Fig. 5). Remarkably, mice depleted of CD4+ cells and vaccinated with DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα demonstrated no loss of anti-tumor response. Induction of an effective anti-tumor response in light of CD4+ cell depletion suggests that a lack of a normal CD4+ T cell component may be overcome by the addition of IL-15 and IL-15Rα (Fig. 5B). Protective levels of antibody were produced with DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα despite the depletion of CD4+ cells, while mice vaccinated with DC lacking IL-15 and IL-15Rα did not produce adequate antibodies (Fig. 6A).
IL-15 increases proliferation and immunoglobulin secretion by B cells as well as up-regulates expression of co-stimulatory molecules such as CD40L (42). In a study examining an experimental Pneumocystis vaccine in which CD40L was co-expressed, it was reported that antibodies to Pneumocystis were induced independent of CD4+ status (43).
While the induction of antibodies in the absence of CD4+ help has not been reported for IL-15, the induction of CD8+ T cell responses has (44, 45). Kutzler et al showed that when co-administing a vaccine with an optimized IL-15 expression plasmid, the resulting CD8+ T cells demonstrated enhanced function and longevity that was largely independent of CD4+ help (44). Incorporation of CD40L into a vaccine was also found to enhance CD8+ T cell responses in the absence of CD4+ help (46). In another study, Oh and coworkers demonstrated that in the absence of CD4+ helper-T cells, antigen-specific T cells are short-lived and exhibit defective secondary CD8+ T cell responses because of tumor necrosis factor-related apoptosis inducing ligand (TRAIL)-mediated apoptosis (45). Further, IL-15 co-delivered with vaccines can largely overcome CD4+ T-cell deficiency of cytotoxic T cells by promoting longevity of antigen-specific CD8+ T cells and avoidance of TRAIL-mediated apoptosis by modulating Bax and Bcl-xL expression (45). Similar up-regulation of Bcl-xL and inhibition of apoptosis in B-cells has also been reported following treatment with IL-15 (47). The ability of IL-15 to substitute for CD4+ help in the generation of a CD8+ T cell response parallels the present study that demonstrated that IL-15 can largely substitute for CD4+ T-cell help in the generation of antibody.
The ability of IL-15 with IL-15Rα to overcome a deficiency of CD4+ help is not absolute. The antibody response in CD4-depleted mice was not mirrored in CD4 knockout mice. CD4−/− mice vaccinated with either DCAd.Neu+Ad.mIL-15+Ad.mIL-15Rα or DCAd.Neu did not produce significant levels of anti-NEU antibodies (Fig. 6B) and were not protected from challenge with tumor. These results are similar to that reported the effects of IL-15 on CD8+ cells in the absence of CD4+ cells (44). The disparity in antibody production in animals depleted of CD4+ cells using antibody and CD4−/− mice suggests a fundamental difference in the CD4+ cell component of these models. While the depletion model had CD4+ cell numbers reduced by greater than 95%, it did retain a small population of CD4+ positive cells, whereas CD4−/− mice had no detectable CD4+ cells suggesting the combination of IL-15 and its receptor does require some, albeit, a reduced level of CD4+ help for B cell priming during vaccination.
The co-expression of IL-15 and IL-15Rα along with the NEU antigen in a genetically-modified DC vaccine enhanced anti-tumor activity in late stage mammary carcinogenesis in neu transgenic mice. The addition of IL-15 and IL-15Rα allowed induction of a protective antibody response against NEU in the setting of a severe deficiency of CD4+ T cells. This strategy may be valuable in patients deficient in CD4+ T cell number or function such as patients undergoing lymphocyte-depleting chemotherapy or suffering from advanced cancer (48), or those infected with HIV.
Acknowledgments
This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
References
- 1.Grabstein KH, Eisenman J, Shanebeck K, et al. Cloning of a T cell growth factor that interacts with the βchain of the interleukin-2 receptor. Science. 1994;264:965–8. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 2.Bamford RN, Grant AJ, Burton JD, et al. The interleukin (IL)-2 receptor-β chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer-cells. Proc Natl Acad Sci USA. 1994;91:4940–4. doi: 10.1073/pnas.91.11.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burton JD, Bamford RN, Peters C, et al. A lymphokine, provisionally designated interleukin-T and produced by a human adult T-cell leukemia line, stimulates T-cell proliferation and the induction of lymphokine-activated killer-cells. Proc Natl Acad Sci USA. 1994;91:4935–9. doi: 10.1073/pnas.91.11.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carson WE, Giri JG, Lindemann MJ, et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180(4):1395–403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armitage RG, Macduff BM, Eisenman J, Paxton R, Grabstein KH. IL-15 has stimulatory activity for the induction of B cell proliferation and differentiation. J Immunol. 1995;154:483–90. [PubMed] [Google Scholar]
- 6.Arina A, Murillo O, Dubrot J, et al. Interleukin-15 liver gene transfer increases the number and function of IKDCs and NK cells. Gene Ther. 2008;15(7):473–83. doi: 10.1038/gt.2008.4. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy MK, Glaccum M, Brown SN, et al. Reversible defects in natural killer and memory CD8T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–80. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pulendran B, Dillon S, Joseph C, Curiel T, Banchereau J, Mohamadzadeh M. Dendritic cells generated in the presence of GM-CSF plus IL-15 prime potent CD8 Tc1 responses in vivo. Euro J Immunol. 2004;34 (1):66–73. doi: 10.1002/eji.200324567. [DOI] [PubMed] [Google Scholar]
- 9.Krutzik SR, Hewison M, Liu PT, et al. IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway. J Immunol. 2008;181(10):7115–20. doi: 10.4049/jimmunol.181.10.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 11.Budagiana V, Bulanovaa E, Pausb R, Bulfone-Paus S. IL-15/IL-15 receptor biology: A guided tour through an expanding universe. Cytokine Growth Factor Rev. 2006;17:259–80. doi: 10.1016/j.cytogfr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Giri JG, Ahdieh M, Eisenman J, et al. Utilization of the β and γ chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 1994;13:2822–30. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giri JG, Kumaki S, Ahdieh M, et al. Identification and cloning of a novel IL-15 binding protein that is structurally related to the αchain of the IL-2 receptor. EMBO J. 1995;15:3654–63. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Rα Recycles and Presents IL-15 In trans to Neighboring Cells. Immunity. 2002;17:537–47. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 15.Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A. Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J Exp Med. 2004;200(7):825–34. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubois S, Patel HJ, Zhang M, Waldmann TA, Müller JR. Preassociation of IL-15 with IL-15R alpha-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action. J Immunol. 2008;180(4):2099–106. doi: 10.4049/jimmunol.180.4.2099. [DOI] [PubMed] [Google Scholar]
- 17.Bergamaschi C, Rosati M, Jalah R, et al. Intracellular interaction of interleukin-15 with its receptor alpha during production leads to mutual stabilization and increased bioactivity. J Biol Chem. 2008;283 (7):4189–99. doi: 10.1074/jbc.M705725200. [DOI] [PubMed] [Google Scholar]
- 18.Stoklasek TA, Schluns KS, Lefrancois L. Combined IL-15/IL-15Rα immunotherapy maximizes IL-15 activity in vivo. J Immunol. 2006;177:6072–80. doi: 10.4049/jimmunol.177.9.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubinstein MP, Kovar M, Purton JF, et al. IL-15 to a superagonist by binding to soluble IL-15Rα. Proc Natl Acad Sci USA. 2006;103(24):9166–71. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rovero S, Amici A, Carlo ED, et al. DNA vaccination against rat her-2/Neu p185 more effectively inhibits carcinogenesis than transplantable carcinomas in transgenic BALB/c mice. J Immunol. 2000;165:5133–42. doi: 10.4049/jimmunol.165.9.5133. [DOI] [PubMed] [Google Scholar]
- 21.Nanni P, Pupa SM, Nicoletti G, et al. p185(neu) protein is required for tumor and anchorage-independent growth, not for cell proliferation of transgenic mammary carcinoma. Int J Cancer. 2000;87:186–94. [PubMed] [Google Scholar]
- 22.Nava-Parada P, Forni G, Knutson KL, Pease LR, Celis E. Peptide vaccine given with a Toll-like receptor agonist is effective for the treatment and prevention of spontaneous breast tumors. Cancer Res. 2007;67:1326–34. doi: 10.1158/0008-5472.CAN-06-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schirmbeck R, Reimann J, Kochanek S, Kreppel F. The immunogenicity of adenovirus vectors limits the multispecificity of CD8 T-cell responses to vector-encoded transgenic antigens. Mol Ther. 2008;28:1609–16. doi: 10.1038/mt.2008.141. [DOI] [PubMed] [Google Scholar]
- 24.Ng P, Parks RJ, Cummings DT, Evelegh CM, Graham FL. An enhanced system for construction of adenoviral vectors by the two-plasmid rescue method. Human Gene Ther. 2000;11:693–9. doi: 10.1089/10430340050015590. [DOI] [PubMed] [Google Scholar]
- 25.Di Carlo E, Diodoro MG, Boggio K, et al. Analysis of mammary carcinoma onset and progression in HER-2/neu oncogene transgenic mice reveals a lobular origin. Lab Investig. 1999;79:1261–9. [PubMed] [Google Scholar]
- 26.Killen N, Sawada S, Littman DR. Regulated expression of human CD4 rescues helper T cell development in mice lacking expression of endogenous CD4. EMBO J. 1993;12:1547–53. doi: 10.1002/j.1460-2075.1993.tb05798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lutz MB, Kukutsch N, Ogilvie AL, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 28.Sakai Y, Morrison BJ, Burke JD, et al. Vaccination by genetically modified dendritic cells expressing a truncated neu oncogene prevents development of breast cancer in transgenic mice. Cancer Res. 2004;64:8022–8. doi: 10.1158/0008-5472.CAN-03-3442. [DOI] [PubMed] [Google Scholar]
- 29.Park JM, Terabe M, Sakai Y, et al. Early role of CD4+ Th1 cells and antibodies in HER-2 adenovirus-vaccine protection against autochthonous mammary carcinomas. J Immunol. 2005;174:4228–36. doi: 10.4049/jimmunol.174.7.4228. [DOI] [PubMed] [Google Scholar]
- 30.Versteeg HH, Nijhuis E, van den Brink GR, et al. A new phosphospecific cell-based ELISA for p42/p44 mitogen-activated protein kinase (MAPK), p38 MAPK, protein kinase B and cAMP-response-element-binding protein. Biochem J. 2000;350(Pt 3):717–22. [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson JM, Fry DW. Akt, MAPK (Erk1/2), and p38 act in concert to promote apoptosis in response to ErbB receptor family inhibition. J Biol Chem. 2001;276(18):14842–7. doi: 10.1074/jbc.M008786200. [DOI] [PubMed] [Google Scholar]
- 32.Pannellini T, Forni G, Musiani P. Immunobiology of Her-2/neu transgenic mice. Breast Dis. 2004;20:33–42. doi: 10.3233/bd-2004-20105. [DOI] [PubMed] [Google Scholar]
- 33.Perera LP, Waldmann TA, Mosca JD, et al. Development of smallpox vaccine candidates with integrated interleukin-15 that demonstrate superior immunogenicity, efficacy, and safety in mice. J Virol. 2007;81(16):8774–83. doi: 10.1128/JVI.00538-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh S, Berzofsky JA, Burke DS, Waldmann TA, Perera LP. Coadministration of HIV vaccine vectors with vaccinia viruses expressing IL-15 but not IL-2 induces long-lasting cellular immunity. Proc Natl Acad Sci USA. 2003;100(6):3392–7. doi: 10.1073/pnas.0630592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kacani L, Sprinzl GM, Erdei A, Dierich MP. Interleukin-15 enhances HIV-1-driven polyclonal B-cell response in vitro. Exp Clin Immunogenet. 1999;16:162–72. doi: 10.1159/000019108. [DOI] [PubMed] [Google Scholar]
- 36.Hiroi T, Yanagita M, Ohta N, Sakaue G, Kiyono H. IL-15 and IL-15 receptor selectively regulate differentiation of common mucosal immune system-independent B-1 cells for IgA responses. J Immunol. 2000;165:4329–37. doi: 10.4049/jimmunol.165.8.4329. [DOI] [PubMed] [Google Scholar]
- 37.Bulfone-Paus SD, Ungureanu D, Pohl T, et al. Interleukin-15 protects from lethal apoptosis in vivo. Nat Med. 1997;3:1124–8. doi: 10.1038/nm1097-1124. [DOI] [PubMed] [Google Scholar]
- 38.Park JM, Terabe M, Steel JC, et al. Therapy of advanced established murine breast cancer with a recombinant adenoviral ErbB-2/neu vaccine. Cancer Res. 2008;68(6):1979–87. doi: 10.1158/0008-5472.CAN-07-5688. [DOI] [PubMed] [Google Scholar]
- 39.Whittington PJ, Piechocki MP, Heng HH, et al. DNA vaccination controls Her-2+ tumors that are refractory to targeted therapies. Cancer Res. 2008;68(18):7502–11. doi: 10.1158/0008-5472.CAN-08-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wen XF, Yang G, Mao W, et al. HER2 signaling modulates the equilibrium between pro- and antiangiogenic factors via distinct pathways: implications for HER2-targeted antibody therapy. Oncogene. 2006;25(52):6986–96. doi: 10.1038/sj.onc.1209685. [DOI] [PubMed] [Google Scholar]
- 41.Nahta R, Hung MC, Esteva FJ. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 2004;64(7):2343–6. doi: 10.1158/0008-5472.can-03-3856. [DOI] [PubMed] [Google Scholar]
- 42.Ramsborg CG, Papoutsakis ET. Global transcriptional analysis delineates the differential inflammatory response interleukin-15 elicits from cultured human T cells. Experimental Hematology. 2007;35 (3):454–64. doi: 10.1016/j.exphem.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng M, Ramsay AJ, Robichaux MB, et al. CD4+ T cell-independent DNA vaccination against opportunistic infections. J Clin Invest. 2005;115(12):3536–44. doi: 10.1172/JCI26306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kutzler MA, Robinson TM, Chattergoon MA, et al. Coimmunization with an optimized IL-15 plasmid results in enhanced function and longevity of CD8 T cells that are partially independent of CD4 T cell help. J Immunol. 2005;175(1):112–23. doi: 10.4049/jimmunol.175.1.112. [DOI] [PubMed] [Google Scholar]
- 45.Oh S, Perera LP, Terabe M, Ni L, Waldmann TA, Berzofsky JA. IL-15 as a mediator of CD4+ help for CD8+ T cell longevity and avoidance of TRAIL-mediated apoptosis. Proc Natl Acad Sci USA. 2008;105(13):5201–6. doi: 10.1073/pnas.0801003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Assudani D, Cho HI, DeVito N, Bradley N, Celis E. In vivo expansion, persistence, and function of peptide vaccine-induced CD8 T cells occur independently of CD4 T cells. Cancer Res. 2008;68(23):9892–9. doi: 10.1158/0008-5472.CAN-08-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Demirci G, Chang XC. IL-2 and IL-15 exhibit opposing effects on Fas mediated apoptosis. Cell Mol Immunol. 2004;1(2):123–8. [PubMed] [Google Scholar]
- 48.Mackall CL. T-cell immunodeficiency following cytotoxic antineoplastic therapy: a review. Stem Cells. 2000;18:10–8. doi: 10.1634/stemcells.18-1-10. [DOI] [PubMed] [Google Scholar]