Abstract
Background
Lymphotropic Polyomavirus (LPV) was isolated from a B-lymphoblastoid cell line of an African green monkey. This virus shares some characteristics with human polyomaviruses, but it is antigenically distinct from BK Virus (BKV) and JC Virus (JCV). Seroepidemiological studies revealed that human sera react in the presence of LPV antigens, and, recently, the viral genome was amplified in the peripheral blood from patients affected with HIV-related leukoencephalopathies.
Objectives
the aims of the study were to investigate and compare the presence of LPV DNA with that of JCV and BKV in different biological samples and patient groups.
Study Design
LPV, JCV and BKV DNA were searched and quantified in peripheral blood and CSF from HIV+ patients and in peripheral blood from healthy subjects.
Results
The LPV genome was detected in peripheral blood of both HIV+ patients and healthy subjects, with a prevalence of 7.2% and 4.7% respectively, but not in CSF. However, its presence was less frequent than that of JCV and BKV.
Conclusions
The amplification of LPV genome from human peripheral blood confirms the fact that LPV can infect the human population. LPV DNA was amplified from patients affected with HIV-related leukoencephalopathies but also from HIV patients without neurological disorders and from healthy subjects. Therefore, the results do not support the hypothesis of an association between LPV infection and any neurological disease. However, given their high similarity, it is possible that LPV, as well as BKV and JCV, could establish latency in humans and cause disease only in rare circumstances.
Keywords: LPV, Polyomavirus, peripheral blood, HIV patients, healthy subjects
1. Background
Lymphotropic Polyomavirus (LPV) was first isolated from a B-lymphoblastoid cell line of an African green monkey 1. This virus belongs to the Polyomaviridae family, that consists of small, non-enveloped DNA viruses. LPV shares morphologic features with the other known human polyomaviruses, BK Virus (BKV) and JC Virus (JCV), but it is antigenically distinct from them 2,3. Seroepidemiological studies revealed that human sera react in the presence of LPV antigens 4,5, and, recently, the viral genome was amplified in the peripheral blood from patients affected with HIV-related leukoencephalopathies 6. However, an extensive investigation of the presence of LPV genome in human clinical specimens from different clinical settings is still lacking, whereas the epidemiology of BKV and JCV has been widely described.
It is well known that BKV and JCV are widespread among the healthy human population, since about 90% and 80% of adults have antibody to BKV and JCV, respectively 5,7,8. The primary infection of both viruses is usually asymptomatic and is followed by a lifelong persistence, occurring mainly in the kidney 9. Lymphoid cells have been proposed as another site of latency, since polyomaviruses genome sequences have frequently been detected in blood cells from both immunocompromised and healthy subjects 10.
Reactivation of JCV in immunocompromised patients may cause a demyelinating disease of the Central Nervous System (CNS), named Progressive Multifocal Leukoencephalopathy (PML), whereas reactivation of BKV may cause haemorrhagic cystitis and Polyomavirus Associated Nephropathy (PVAN) in renal transplant patients 11,12.
The other Polyomaviruses that can infect the human population are the newly discovered WU and KI viruses, that have been mainly isolated from respiratory specimens13. To date, there are no published data regarding the relation between KI, WU and LPV, which, furthermore, has been never found in respiratory samples.
So far, the potential pathogenetic mechanism of LPV during the primary infection or during reactivation is unknown. Therefore, the distribution of LPV in the human population and its possible association with a specific disease need to be elucidated.
2. Objectives
The aims of the study were to examine and compare the presence of LPV, that was recently isolated from the human specimens, with that of JCV and BKV in the peripheral blood and CerebroSpinal Fluid (CSF) from HIV+ patients with and without neurological disorders and in the peripheral blood from healthy subjects.
3. Study Design
3.1 Study populations and samples
HIV+ patients
Peripheral Blood was collected from 83 HIV+ patients, referring to the Unit of General Neurology at the Mondino Hospital, Pavia, Italy and to the Department of Infectious Diseases at the Policlinico San Matteo, Pavia, Italy, for clinical care. The subjects included 61 males and 22 females, with a mean age of 49 years, which were enrolled into one of the four following disease groups: 11 patients were affected with PML, 16 patients were affected with Not Determined Leukoencephalopathy (NDLE), 11 patients were affected with Other Neurological Diseases (OND) and 45 patients did not suffer of any neurological disorders (NND).
CSF was also collected from all the patients affected with neurological diseases (PML, NDLE and OND) for diagnostic purpose and part of the CSF sample was stored at −80°C for research use. An informed consent form was signed by each patient at the time of collection of the clinical specimens.
Healthy subjects
Peripheral Blood was collected from 105 healthy subjects, matched for age (mean age: 42 years) and all tested HIV negative, referring to the Center for Translational Research, Saint Joseph Hospital, Milano, Italy, for routine blood testing and part of the sample was used for research purposes. The subjects included 50 males and 55 females, and were enrolled into one of the following age groups: 22 individuals in the <9 years group, 17 in the 9–19 years group, 26 in the 20–49 years group, 19 in the 50–69 years group and 21 in the over 70 years group. An informed consent form was signed by each patient at the time of collection of the clinical specimens.
3.2 Detection of the polyomaviruses genome
Human polyomaviruses loads were quantified after DNA extraction from 0.2 ml of peripheral blood with QIAmp blood mini kit (Qiagen, USA), and from 0.15 ml of CSF with Nucleospin RNA Virus kit (Macherey Nagels, Germany), following manufacturer’s instructions. To minimise the risk of contamination, DNA was isolated in a laminar air flow using aerosolresistant, sterile and disposable tips. In addition, a separate room was used to perform the Real Time PCR (Q-PCR) assays.
The Q-PCR protocol for the detection of JCV DNA has been described elsewhere 14, whereas the protocols for the detection of BKV and LPV DNA were newly designed. The primers and probes used are listed in table 1.
Table 1.
Primers and probes used for the detection of LPV, JCV and BKV in peripheral blood from HIV+ patients and healthy subjects
| VIRUS | TARGET REGION | PRIMERS SEQUENCE | PROBE SEQUENCE |
|---|---|---|---|
| JCV | LT-Ag | Forward: GAGTGTTGGGATCCTGTGTTTTC Reverse: GAGAAGTGGGATGAAGACCTGTTT |
6-FAM- TCATCACTGGCAAACATTTCTTCATGGC-MGB |
| BKV | VP1 | Forward: AGTGGATGGGCAGCCTATGTA Reverse: TCATATCTGGGTCCCCTGGA |
6-FAM-AGGTAGAAGAGGTTAGGGTGTTTGATGGCACAG-TAMRA |
| LPV | VP1 | Forward: TGGCCCTCAAAGAAAAAGG Reverse: GCGGGAATAGGGCATGTTT |
6-FAM-AGACGGAGCATGCAAA-MGB |
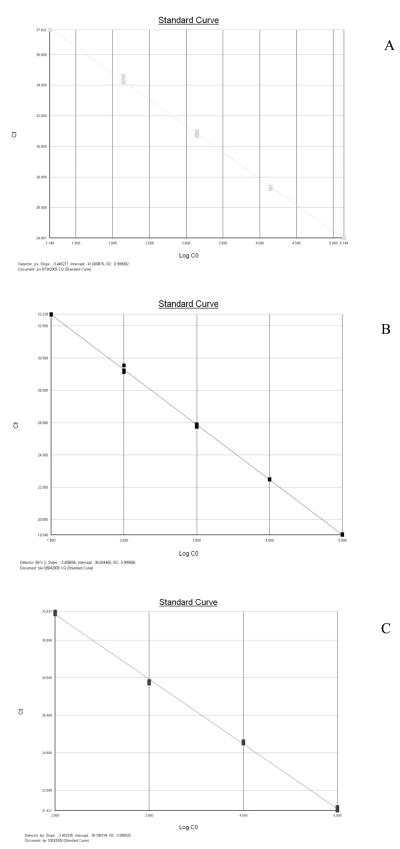
Briefly, the Q-PCR reactions were performed in a final volume of 25 μl containing 1X Taqman Universal PCR Master Mix (Applied Biosystems, USA), 0.4 μM primer Forward, 0.4 to 0.9 μM primer Reverse, 0.2 μM probe, and 250 ng of extracted nucleic acid. Thermal cycling was carried out according to the following steps: an initial denaturation at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min, at the end of which the fluorescence was read. The limit of detection was 2 copies/reaction for JCV assay, and 5 copies/reaction for BKV and LPV assays. Each sample was analyzed in triplicate, and each run contained a negative control constituted by the reaction mixture without the DNA template. Standard curves for quantification of viral genome were constructed using serial dilutions of a plasmid containing the entire JCV, BKV and LPV genome (range: 10 to 106 plasmid copies). As shown in figure 1, the three Q-PCR assays showed high efficiency, being the slope values −3.445 (JCV), −3.408 (BKV) and −3.453 (LPV) and the correlation coefficient 0.999 for all the three assays.
Fig.1.
Charts of the standard calibration curves of the Q-PCR assays for JCV, BKV and LPV (panels A, B and C). The slopes are −3.445, −3.408 and −3.453 respectively whereas the correlation coefficients (R2) are 0.999 for the three curves, showing the high efficiency of the three assays.
Viral loads were expressed as copies/ug of DNA extracted from the peripheral blood. In addition, the presence of beta-globin gene was tested in each sample, as a control to confirm the presence and quality of DNA, using primers and probe previously published15. Amplicons for the beta-globin control gene were detected in all the analyzed samples.
It should also be underlined that the laboratory successfully participated to the external quality control programs for the quantitative assays of JCV and BKV, provided by the Quality Control for Molecular Diagnostics (QCMD) organisation.
3.3 Data analysis
Statistical evaluations were performed using EpiInfo 6.0. Data were analyzed by using Fisher’s 2 sided exact test and p<0.05 was considered statistically significant.
4. Results
4.1 Presence of LPV genome
The prevalence of LPV DNA among the HIV+ population was 7.2% (6 out of 83), with no significant differences in the distribution among PML, NDLE and NND patients, whereas the prevalence of LPV DNA among the healthy subjects was 4.7% (5 out of 105). Among NND patients two were co-infected with LPV and BKV (Table 2, panels A and B). Among OND patients, none was infected with LPV (table 2). In addition, LPV was not detected in any of the CSF samples (data not shown).
Table 2.
panel A: Prevalence of LPV, JCV and BKV DNA sequences in peripheral blood from HIV+ patients, divided on the basis of the neurological disease.
Panel B: Prevalence of LPV, JCV and BKV DNA sequences in peripheral blood from healthy subjects, divided into ages groups.
| A | |||||
|---|---|---|---|---|---|
| HIV+ PATIENTS |
|||||
| PML | NDLE | OND | NND | TOTAL | |
| LPV | 1/11 (9.1%) |
2/16 (12.5%) |
0/11 | 3/45# (6.7%) |
6/83 (7.2%) |
| JCV | 2/11 (18.2%) |
2/16* (12.5%) |
3/11 (27.2%) |
6/45* (13.3%) |
13/83 (15.7%) |
| BKV | 1/7 (14.2%) |
3/11* (27.3%) |
2/5 (40%) |
11/45*# (24.4%) |
17/68 (25%) |
| TOTAL | 4/11 (36.4%) |
7/16 (43.7%) |
5/11 (45.4%) |
20/45 (44.4%) |
36/83 (43.4%) |
| B | ||||||
|---|---|---|---|---|---|---|
| HEALTHY SUBJECTS |
||||||
| < 9 | 9 – 19 | 20 – 49 | 50 – 69 | > 70 | TOTAL | |
| LPV | 1/22 (4.5%) |
0/17 | 1/26 (3.8%) |
1/19 (5.2%) |
2/21 (9.5%) |
5/105 (4.7%) |
| JCV | 0/22 | 0/17 | 0/26 | 0/19 | 2/21 (9.5%) |
2/105 (1.9%) |
| BKV | 2/22 (9.1%) |
1/17 (5.8%) |
3/26 (11.5%) |
3/19 (15.7%) |
3/21 (14.3%) |
12/105 (11.4%) |
| TOTAL | 3/22 (13.6%) |
1/17 (5.8%) |
4/26 (15.38%) |
4/19 (21.05%) |
7/21 (33.3%) |
19/105 (18.1%) |
: one patient had a double infection JCV+BKV;
: two patients had a double infection LPV+BKV
4.2 Presence of JCV and BKV genome
JCV DNA was found in 13 out of 83 HIV+ patients, with a significant higher prevalence (15.7%, p<0.0001) than in the healthy subjects (1.9%). JCV DNA was also found in all the CSF collected from the 11 PML patients (data not shown).
Only 68 peripheral blood samples from HIV+ patients were analyzed for the presence of BKV DNA, because the nucleic acid extracted from peripheral blood was not sufficient to perform BKV Q-PCR for the remaining 17 samples. It was observed that BKV was the most frequent detected human polyomavirus among both HIV+ patients and healthy subjects, with a prevalence of 25% (17 out of 68) and of 11.4% (12 out of 105), respectively. Among NDLE patients and NND patients, one was infected with both BKV and JCV. Both viruses were detected in all the subgroups of HIV+ patients without any significant difference (Table 2, panels A and B). BKV DNA was not detected in any of the CSF samples (data not shown).
As shown in table 2 (panels A and B), on the whole, the prevalence of the human polyomaviruses genomes among the HIV+ populations (43.4%) was much higher (p<0.0001) than among the healthy subjects (18.1%). In addition, the LPV distribution in both populations appeared to be slightly lower (5.8%) than that of JCV (7.9%) and BKV (16.8%).
4.3 Age dependent prevalence of human polyomaviruses in peripheral blood
When the viral presence was compared in the different age groups of healthy subjects, it was found that the rate of polyomaviruses slightly increased with increasing age, from 13.6% (3 out of 22) in the youngest’s group (aged 0–9 years) to 33.3% (7 out of 21) in the oldest’s group (aged over 70 years). In regard to the HIV+ patients, the rate of polyomaviruses did not differ with increasing age (data not shown), however the range of age in this group was too narrow (22–58 years) to draw any conclusion.
4.4 Viral load
The LPV median viral load was 1.41E+01 copies/ug of extracted DNA among the HIV+ patients and 4.70E+01 copies/ug of extracted DNA among the healthy subjects. The median viral loads of BKV and JCV did not significantly differ from the LPV median values among both the HIV+ population and the healthy subjects population (figure 2).
Figure 2.
Median Viral load of the human polyomaviruses genomes in the peripheral blood from HIV+ patients and from healthy subjects
5. Discussion
We have investigated the presence of LPV genome in different clinical specimens from immunocompromised and immunocompetent individuals and we have compared it with that of two well known human polyomaviruses (JCV and BKV), since, to date, the percentage of the human population exposed to LPV has not been determined.
A recent study has demonstrated that LPV seroprevalence in a wide human population was about 15% in both adult and pediatric subjects, but no evidence has been shown regarding the presence of the viral genome in biological samples 5.
The published reports about the detection of LPV DNA in the humans have shown contrasting results. Delbue and colleagues have observed, by means of nested-PCR, the presence of LPV genome in 6% of the studied populations (3 out of 50 peripheral blood samples), whereas, using a less sensitive standard PCR, Focosi and colleagues, whose case study included 2 peripheral blood and 7 CSF/brain tissues samples, did not found any positive samples 16.
In contrast to our previous study 6, in the current survey we could detect LPV genome not only in the biological samples from immunocompromised patients, but also in those from healthy subjects. The employment of the new designed Q-PCR, that has an higher sensitivity than the previously used nested-PCR, may account for the different results obtained. Thus, it could be possible that all the previously published data were underestimated and, consequently, an association between LPV and HIV-related leukoencephalopathies cannot be confirmed.
In addition, LPV was not detected in the studied CSF, confirming its strict lymphotropism1 and indicating that probably the virus is not directly involved in the pathogenesis of neurological diseases of immunocompromised patients 16.
The presence of polyomaviruses in the peripheral blood from immunocompromised patients was confirmed to be higher than that of healthy subjects17,18,19 and the overall prevalence of BKV DNA was confirmed to be higher if compared to that of JCV DNA 20.
It is not surprising that LPV DNA was detected with a lower prevalence than that of the other known human polyomaviruses, since LPV was characterized by a lower seroprevalence in humans than JCV and BKV 5. In addition, a wide distribution of LPV in the human population was not expected, because LPV has been so far considered a virus with a very narrow host range, limited to the primates.
In contrast with recent seroepidemiologic data 21, it could be observed that increasing age may not be a decisive and significant factor accounting for the presence of polyomaviruses DNA in the peripheral blood, since the observed differences in the prevalence were not significant among the groups of age.
To our knowledge, this is the first study that investigated the presence of LPV in a broad cohort of subjects and compared LPV distribution with that of JCV and BKV. This study demonstrates that LPV is present in peripheral blood from both immunocompromised and immunocompetent individuals, but not in the CSF collected from HIV+ patients with neurological diseases. Thus, these results are important because they support the serological evidence of LPV infection in the human population and reinforce the notion that some human viruses have eluded detection despite many years of virological research.
It is now clear that not all virus infections lead to illness and that confirmation of a virus association to a disease is difficult. However, since viruses are probably pathogens, their identification represents an urgent scientific task. It has been suggested that, rather than a single detection, longitudinal studies are essential to confirm causation.
In conclusion, our results do not support an association between LPV infection and the development of HIV-related leukoencephalopathies, but, given the high similarity between LPV and the other human polyomaviruses, JCV and BKV, it is possible that LPV could establish latency in humans, causing disease only in rare circumstances. Therefore, searching for a disease associated with LPV in humans would be challenging and could have important pathogenic implications.
Acknowledgments
Funding: This work was supported by the NIMH grant no. MH072528 and by a grant from the Italian Minister of Health (Ricerca Finalizzata 2007) to PF.
Abbreviations
- LPV
Lymphotropic Polyomavirus
- BKV
BK Virus
- JCV
JC Virus
- HIV
Human Immunodeficiency Virus
- CNS
Central Nervous System
- PML
Progressive Multifocal Leukoencephalopathy
- PVAN
PolyomaVirus Associated Nephropathy
- NDLE
Not Determined Leukoencephalopathy
- OND
Other Neurological Diseases
- NND
Not Neurological Disorders
- CSF
CerebroSpinal Fluid
- Q-PCR
real-time PCR
Footnotes
Competing interest: None declared
Ethical approval: the study received the approval of the ethics committee of Saint Jospeh Hospital
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zur Hausen H, Gissman L. Lymphotropic papovavirus isolated from African green monkey and human cells. Med Microbiol Immunol. 1979;167:137–53. doi: 10.1007/BF02121180. [DOI] [PubMed] [Google Scholar]
- 2.Takeomoto KK, Tadahito K. Lymphotropic Papovavirus Transformation of hamster embryo cells. J Virol. 1984;50:100–5. doi: 10.1128/jvi.50.1.100-105.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brade L, Muller-Lantzsch N, Kaiser S, Scharrer M. Biochemical studies on structural and non- structural proteins of African green monkey B-lymphtropic papovavirus (LPV) Virology. 1983;127:469–74. doi: 10.1016/0042-6822(83)90160-5. [DOI] [PubMed] [Google Scholar]
- 4.Viscidi RP, Clayman B. Serological cross reactivity between polyomavirus capsids. Adv Exp Med Biol. 1986;577:73–84. doi: 10.1007/0-387-32957-9_5. [DOI] [PubMed] [Google Scholar]
- 5.Kean JM, Rao S, Wang M, Garcea RL. Seroepidemiology of human Polyomaviruses. Plos Pathogens. 2009;5:e1000363. doi: 10.1371/journal.ppat.1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delbue S, Tremolada S, Branchetti E, Elia F, Gualco E, Marchioni E, et al. First identification and molecular characterization of Lymphotropic Polyomavirus in peripheral blood from patients with leukoencephalopathies. J Clin Microbiol. 2008;46:2461–2. doi: 10.1128/JCM.00381-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker DL, Padgett BL. The epidemiology of human polyomaviruses. In: Sever JL, Madden D, editors. Polyomaviruses and human neurological disease. New York: Alan R. Liss; 1983. pp. 99–106. [Google Scholar]
- 8.Knowles WA, Pipkin P, Andrews N, Vyse A, Minor P, Brown DW, et al. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol. 2003;71:115–23. doi: 10.1002/jmv.10450. [DOI] [PubMed] [Google Scholar]
- 9.Chesters PM, Heritage J, McCance DJ. Persistence of DNA sequences of BK virus and JC virus in normal human tissues and in diseases tissues. J Infect Dis. 1983;147:676–84. doi: 10.1093/infdis/147.4.676. [DOI] [PubMed] [Google Scholar]
- 10.Dorries K. Latent and persistent Polyomavirus infection. In: Kahlili K, Stoner GL, editors. Human Polyomaviruses: molecular and clinical perspectives. New York: Wiley-Liss; 2001. pp. 197–235. [Google Scholar]
- 11.Weber T, Major EO. PML: molecular biology, pathogenesis and clinical impact. Intevirology. 1997;40:98–111. doi: 10.1159/000150537. [DOI] [PubMed] [Google Scholar]
- 12.Celik B, Randhawa PS. Glomerular changes in BK virus nephropathy. Human Pathol. 2004;35:367–70. doi: 10.1016/j.humpath.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Dalianis T, Ramqvist T, Andreasson K, Kean JM, Garcea RL. KI, WU and Merkel cell polyomaviruses : a new era for human polyomavirus research. Semin Cancer Biol. 2009;19:270–5. doi: 10.1016/j.semcancer.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Delbue S, Sotgiu G, Fumagalli D, Valli M, Borghi E, Mancuso R, et al. A case of a PML patient with four different JC virus TCR rearrangements in CSF, blood, serum and urine. J Neurovirol. 2005;11:51–7. doi: 10.1080/13550280590900382. [DOI] [PubMed] [Google Scholar]
- 15.Lo YMD, Tein MSC, Lau TK, Haines CJ, Leung TN, Poon PMK, et al. Quantitative analysis of fetal DNA in maternal plasma and serum. Implications for noninvasive prenatal diagnosis. Am J Hum Genet. 1998;62:768–75. doi: 10.1086/301800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Focosi D, Maggi F, Andreoli E, Lanini L, Ceccherini-Nelli L, Petrini M. Polyomaviruses other than JCV are not detected in progressive multifocal leukoencephalopathy. J Clin Virol. 2009;45:161–2. doi: 10.1016/j.jcv.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Bialasiewicz S, Whiley DM, Lambert SB, Nissen MD, Sloots TP. Detection of BK, JC, WU, or KI polyomaviruses in faecal, urine, blood, cerebrospinal fluid and respiratory samples. J Clin Virol. 2009;45:249–54. doi: 10.1016/j.jcv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Dörries K, Vogel E, Günther S, Czub S. Infection of human polyomaviruses JC and BK in peripheral blood leukocytes from immunocompetent individuals. Virology. 1994;198:59–70. doi: 10.1006/viro.1994.1008. [DOI] [PubMed] [Google Scholar]
- 19.Ferrante P, Caldarelli-Stefano R, Omodeo-Zorini E, Cagni AE, Cocchi L, Suter F, et al. Comprehensive investigation of the presence of JC virus in AIDS patients with and without progressive multifocal leukoencephalopathy. J Med Virol. 1997;52:235–42. doi: 10.1002/(sici)1096-9071(199707)52:3<235::aid-jmv1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Gu ZY, Li Q, Si YL, Li X, Hao HJ, Song HJ. Prevalence of BK virus and JC virus in peripheral blood leukocytes and normal arterial walls in healthy individuals in China. J Med Virol. 2003;70:600–5. doi: 10.1002/jmv.10436. [DOI] [PubMed] [Google Scholar]
- 21.Egli A, Infanti L, Dumoulin A, Buser A, Samaridis J, Stebler C, Gosert R, Hirsch HH. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis. 2009;199:837–46. doi: 10.1086/597126. [DOI] [PubMed] [Google Scholar]