Abstract
Isoliquiritigenin (2′,4′,4-trihydroxychalcone, ILG), a chalcone found in licorice root and many other plants, has shown potential chemoprevention activity through induction of phase II enzymes such as quinone reductase-1 in murine hepatoma cells. In this study, the in vivo metabolism of ILG was investigated in rats. In addition, ILG glucuronides and ILG-glutathione adducts were observed in human hepatocytes and in livers from rats treated with ILG. ILG glucuronides were detected in both plasma and rat liver tissues. Also, in a full-term cancer chemoprevention study conducted with 7,12-dimethylbenz(a)anthracene (DMBA)-treated female Sprague-Dawley rats, dietary administration of ILG slightly increased tumor latency but had a negative effect on the incidence of mammary tumors starting at approximately 65 days post DMBA. Further, no significant induction of phase II enzymes was found in mammary glands, which is consistent with the low level of ILG observed in these tissues. However, ILG significantly induced quinone reductase-1 activity in the colon, and glutathione as well as glutathione S-transferase in the liver. Analysis of mRNA expression in tissues of rats treated with ILG supported these findings. These results suggest ILG should be tested for chemopreventive efficacy in non-mammary models of cancer.
Keywords: isoliquiritigenin, metabolism, cancer prevention, rat mammary tumor model
In our program for the discovery of novel plant-derived chemopreventive agents, induction of quinone reductase-1 (QR-1) has been used as one marker of activity (1). As a result, isoliquiritigenin (2′,4′,4-trihydroxychalcone, ILG), a flavonoid found in licorice (Glycyrrhiza uralensis), shallot, Sinofranchetia chinensis, Dalbergia odorifera and soybean (2–6), was shown to mediate significant chemopreventive activities, including the inhibition of carcinogen-induced lesion formation in a mouse mammary organ culture (MMOC) assay, and an increase of chemically-induced mammary tumor latency in rats (7, 8). Subsequently, ILG was demonstrated to induce QR-1 in two mutant cell lines less responsive to bifunctional inducers, indicating that ILG was devoid of cytochrome P450-activating properties. This induction was found to be regulated at the transcriptional level by interaction with the antioxidant response element (7). In addition, ILG has demonstrated potent antioxidant (9), anti-inflammatory (10), phytoestrogenic (11, 12), tyrosinase inhibition (13), and cardiac properties (14), and exhibited significant inhibitory effects of carcinogenesis in various tumor models. It was also reported to induce cell cycle arrest and up-regulate p21 expression in lung cancer cells (15), suppress pulmonary metastasis of mouse renal cell carcinoma through activation of immune system (16), and induce apoptosis in human gastric cancer cells (17).
Although ILG is a promising anti-tumor agent, to date, no published report has described in vivo metabolism. However, phase I metabolites of ILG formed in vitro using human liver microsomes have been reported to include aromatic hydroxylation on the A-ring to form 2′,4,4′,5′-tetrahydroxychalcone, aromatic hydroxylation on the B-ring to form butein, reduction of the carbon-carbon double bond of the α,β-unsaturated ketone to form 2′,4,4′-trihydroxydihydrochalcone, and oxidation and cyclization to form (Z/E)-6,4′-dihydroxyaurone (18). Some of these phase I metabolites might have pharmacological activities distinct from ILG. In addition, phase II in vitro metabolism of ILG has been investigated, and ILG was found to form five monoglucuronides including the two most abundant metabolites ILG 4′-O-glucuronide and ILG 2′-O-glucuronide (19). Here, we studied the metabolism of ILG and the conjugation of ILG with glutathione (GSH), with rats and in vitro models. In addition, following dietary administration of ILG to rats, the induction of phase II metabolizing enzymes was determined in liver, colon and mammary glands through enzyme assays and RT-PCR analysis, and the effect on 7,12-dimethylbenz(a)anthracene (DMBA)-induced tumor formation was investigated. Some comparative data are presented from studies conducted with sulforamate (a monofunctional enzyme inducer) and 4′- bromoflavone (4′BF) (a bifunctional enzyme inducer).
Materials and Methods
Chemicals and biochemicals
ILG and authentic standards of the ILG metabolites butein and sulfarein were purchased from Indofine (Hillsborough, NJ). HPLC-grade solvents were purchased from Fisher Scientific (Pittsburgh, PA). Sulforamate (20) and 4’BF (21) were prepared as described previously. All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Rat liver microsomes and cryopreserved human hepatocytes were purchased from In Vitro Technologies (Baltimore, MD).
To prepare a GSH conjugate of ILG, GSH (0.4 mmol) in methanol was added dropwise to a mixture containing ILG (1 mM) and triethylamine (0.1 mL) in methanol (30 mL), and the reaction mixture was stirred at room temperature for 3 h. The solution was purified by flash chromatography on Sephadex LH-20 using methanol as the eluent, yielding a light yellow powder (39 mg, 20.7%) that was identified spectroscopically as an ILG-GSH adduct. NMR spectra were obtained on a Bruker Avance 360 MHz NMR spectrometer. The structure of the ILG-GSH adduct was determined from proton and carbon one-dimensional NMR as well as 1H-1H COSY, 1H-13C 2D gradient heteronuclear multiple quantum coherence (HMQC), and long range 1H-13C 2D gradient heteronuclear multiple bond correlation (HMBC) (data not shown).
Cell culture
Rat hepatoma H4IIE cells were obtained from the American Type Culture Collection (Rockville, MD). Cells were cultured in MEM medium (Invitrogen, Carlsbad, CA) containing 10% heat-inactivated fetal bovine serum, non-essential amino acids, 1 mM sodium pyruvate (BioWhittaker, Walkersville, MD), 100 units of penicillin/mL, and 100 µg streptomycin/mL (Gibco Invitrogen, Grand Island, NY). The cells were maintained in a 5% CO2 atmosphere at 37 °C and were tested routinely for mycoplasma contamination.
To determine changes in GSH levels in the H4IIE cells in response to ILG exposure, 3 × 106 cells were treated with various concentrations of ILG and harvested after 24 h. Whole-cell pellets were lysed with 200 µL of buffer (100 mM potassium phosphate buffer, pH 7.0 and 2 mM EDTA) to obtain protein lysates, and protein concentrations were quantified using the Bradford method. GSH concentrations of the lysates were determined using a Cayman Chemical (Ann Arbor, MI) GSH assay kit. Briefly, GSH was measured using an enzymatic recycling procedure developed for the sensitive determination of total GSH levels in cells cultured in 96-well plates. The manufacturer’s protocol was followed using 1 µg of protein. A GSH standard curve was constructed, and GSH levels were expressed as µM.
Inhibition of DMBA-induced mammary carcinogenesis in rats
Virgin female Sprague-Dawley rats were received from Harlan (Indianapolis, IN) at 35 days of age and placed on a diet of Teklad 4% rat/mouse chow. After 1 week (42 days of age), animals were randomized by weight into five groups and placed on experimental diets containing 7.5 or 10.0 g ILG/kg diet. At 50 days of age, the animals received a single i.g. dose of DMBA (15 mg) in sesame oil (or vehicle only) following an overnight fast. The rats were maintained on the ILG diets until the end of the study (85 days post-DMBA). During the experimental period, all animals were weighed weekly. Palpation for mammary tumors began 3 weeks after the animals received DMBA and continued until termination of the study. The dates of appearance and locations of all tumors were recorded. Animals were observed twice daily to assess their general health. Moribund animals were sacrificed by CO2 asphyxiation. Moribund animals or animals found dead were necropsied immediately. At the end of the study, tissues (liver, colon, and mammary gland) were collected, rinsed with saline, frozen in liquid nitrogen, and stored at −80 °C until analysis. Blood samples were also collected, and the concentration of ILG in plasma was determined by using LC-MS.
Analysis of quinone reductase-1 (QR-1) activity, glutathione S-transferase (GST) activity and GSH level in rat tissues
Samples of liver and colon from the treated rats were homogenized in 0.25 M sucrose and centrifuged at 15,000×g for 30 min at 4 °C. The supernatant fractions were collected, and 0.1 M CaCl2 in 0.25 M sucrose was added (20% by volume total). After incubation at 0 °C for 30 min and further centrifugation at 15,000×g for 30 min at 4 °C, a clear cytosolic fraction was obtained for enzyme assays. Mammary glands of each animal were pooled, homogenized in 1 mL of ice-cold 0.1 M phosphate buffer (pH 6.5) and centrifuged (15,000×g, 30 min at 4 °C) to yield clear supernatant fractions suitable for enzyme assays. Protein concentrations were determined using the Bradford method. QR-1 activity was measured in 50 µL of a suitable dilution of the tissue supernatant as described previously (20). GST activity was determined using the Cayman GST assay kit as described for the cellular system.
Microsomal incubations
Incubations with rat liver microsomes contained 0.5 mg/mL of microsomal protein, 10 µM ILG, and 1 mM NADPH in 50 mM phosphate buffer, pH 7.4, in a total volume of 0.4 mL. The reactions were initiated by addition of NADPH (1 mM) after a 5 min preincubation and were carried out at 37 °C for 40 min. The incubations were terminated by adding 1.6 mL of an ice-cold mixture of acetonitrile/ethanol (1:1, v/v) and chilling the resulting mixture on ice. After centrifugation to remove precipitated proteins, the supernatant was evaporated to dryness in vacuo. Each residue was reconstituted in 150 µL of HPLC mobile phase immediately prior to analysis using LC-MS-MS.
Glucuronidation of ILG by rat liver microsomes
In vitro glucuronidation of ILG was carried out using rat liver enzymes as described previously with some minor modifications (19). In brief, 0.4 mg of rat liver microsomes, 25 µg/mL of alamethicin, and 146 µL of 0.1 mM phosphate buffer (pH 7.4) were mixed and placed on ice for 15 min. Next, MgCl2 (8 mM), 5 mM saccharic acid and 10 µM ILG were added to the mixture and preincubated at 37 °C for 3 min. Reactions were initiated by adding uridine diphosphate glucuronic acid (UDPGA; 5 mM final concentration) in a total volume of 200 µL. After 20 min, reactions were terminated by the addition of 0.8 mL ice-cold methanol/acetonitrile (1:1, v/v). After centrifugation to remove precipitated proteins, the supernatant was evaporated to dryness in vacuo. Each residue was reconstituted in 150 µL of HPLC mobile phase immediately prior to analysis using LC-MS-MS. All incubations were carried out at least three times.
Metabolism of isoliquiritigenin by human hepatocytes
Cryopreserved human hepatocytes were thawed according to the supplier’s instructions, and approximately 1 × 106 cells in a 1-mL suspension were incubated with ILG (10 µM) per well of a 6-well plate. Control experiments were identical except for the substitution of heat-inactivated hepatocytes. The plate was placed in an incubator at 37 °C with 5% CO2 and 90% relative humidity and gently shaken at 50 rpm for 4 h. Incubations were terminated by addition of 3 mL of ice-cold methanol/acetonitrile (1:1, v/v). The cell suspensions were centrifuged, and aliquots of the supernatants were analyzed directly using LC-MS-MS.
Quantification of isoliquiritigenin in rat plasma and tissue samples
Rat blood was centrifuged for 15 min at 3,000×g and 4 °C, and plasma was collected. Ice-cold methanol (125 µL) containing 0.5 µM naringenin as internal standard was added to rat plasma (25 µL) to precipitate protein. After centrifugation at 10,000×g for 10 min, the supernatant was removed and analyzed using LC-MS-MS. Blank rat plasma, spiked with concentrations of ILG from 1.0 to 1000 ng/mL, was used for calibration curves and quality control analyses.
Each rat liver or mammary gland was weighted accurately and homogenized in phosphate buffer (0.1 M, pH 7.4). Then, naringenin (2 µL, 2.5 µM) was added as internal standard. Ice-cold methanol (125 µL) was added to each homogenate (25 µL) to precipitate the proteins. After centrifugation at 10,000×g for 10 min, the supernatant was removed and analyzed using LC-MS-MS as described below. Blank rat liver homogenate, spiked with concentrations of ILG from 1–500 ng/mL, was used for calibration curves and quality control analyses.
RT-PCR analysis of mRNA expression
At 42 days of age, female Sprague-Dawley rats (n=3) were treated by gavage with 0.2 mL of vehicle (ETOH: PEG400; 10/90, v/v) or test compound (ILG, 800 mg/kg; 4’BF, 400 mg/kg; sulforamate, 200 mg/kg) for four days. Animals were weighed and sacrificed by cervical dislocation. Tissues (liver, mammary gland, and colon) were collected, rinsed with saline, frozen in liquid nitrogen, and stored at −80 °C until analyzed. Mammary glands from each animal were pooled, and all tissues were homogenized in 1 ml of TRIzol reagent (Invitrogen). RNA was isolated via a phenol/chloroform extraction, further purified using a RT2 qPCR-grade RNA isolation kit (SA Biosciences), and quantified by UV absorbance. Reverse transcription was performed to obtain cDNA via an RT2 First Strand kit (SA Biosciences). One part of appropriately diluted cDNA was added to 12 parts master mix and 11 parts deionized water. This mixture was pipetted into an SA Biosciences rat drug metabolism PCR array, cycled in an Applied Biosystems 7300 Real-time PCR apparatus using primers for β-actin as an internal control, and Ct and fold change values were obtained. Each tissue was analyzed individually. The results of individual tissues agreed within 10%. The values for each dosage group were averaged to allow for statistical analysis. Fold change values reported as significant have a p-value less than 0.05 when they vary from one (unity) by 0.5 or more.
LC-MS and LC-MS-MS
High resolution accurate mass measurements were obtained using LC-MS and LC-MS/MS with a Micromass (Manchester, UK) Q-TOF2 quadrupole time-of-flight hybrid mass spectrometer or a Thermo Finnigan (San Jose, CA) LTQ FT-ICR hybrid mass spectrometer equipped with either a Waters 2690 HPLC system or a Shimadzu (Columbia, MD) HPLC system incorporating LC-10ADvp pumps and a LC PAL (CTC Analytics, Switzerland) autosampler, respectively. Both positive and negative ion electrospray mass spectra were recorded. ILG and its metabolites were separated using reversed-phase HPLC on an Agilent (Santa Clara, CA) ZORBAX SB 2.1 × 100 mm C18 column (3.5 µm particle size) with a linear solvent gradient system from 0.1% formic acid in water to methanol as follows: 20% to 70% methanol over 25 min and then 70% to 95% methanol over an additional 10 min. The flow rate was 0.2 mL/min, the column temperature was 30 °C, and the autosampler was maintained at 4 °C. After determining the elemental compositions of each ILG metabolite, product ion tandem mass spectra were obtained for structural characterization. Specifically, the LTQ FT-ICR mass spectrometer was used for data-dependent MS/MS analysis in which the most abundant ions in each mass spectrum were selected for collision-induced dissociation.
Based on high resolution accurate mass measurements, tandem mass spectrometric analyses, comparison with standards, and the studies of ILG metabolism reported previously by Guo et al. (18, 19), several ILG metabolites were identified in incubations with rat liver microsomes. These ILG metabolites were then profiled using an Applied Biosystems (Foster City, CA) API 4000 triple quadruple mass spectrometer with negative ion electrospray ionization and selective reaction monitoring (SRM). The SRM transitions used for LC-MS-MS included m/z 255 to 119 for ILG and liquiritigenin (M1), m/z 271 to 119 for 2′,4,4′,5′-tetrahydroxychalcone (M2), m/z 269 to 133 for sulfuretin (M3), m/z 271 to 135 for butein (M4), m/z 257 to 151 for davidigenin (M5), m/z 353 to 135 for 6,4′-dihydroxyaurone (M6 or M7), m/z 431 to 255 for ILG monoglucuronides, and m/z 335 to 255 for ILG sulfate conjugates.
For LC-MS-MS quantitative analysis of ILG, the HPLC separation was optimized to consist of a 10 min linear gradient from 60–90% methanol in 0.1% aqueous formic acid followed by 90% methanol for 5 min. The flow rate was 0.3 mL/min and the injection volume was 10 µL. The SRM transitions of m/z 255-119 and m/z 253-151 were measured for ILG and naringenin (internal standard), respectively. The ILG LC-MS-MS standard curve (using spiked rat plasma) was linear (r2 > 0.999) over the concentration range 1.0 to 1000 ng/mL. The limit of detection of ILG was 2 pg (7.8 fmol) injected on-column (200 pg/mL, 10 µL injection volume), and the limit of quantitation was 5 pg (20 fmol, 500 pg/mL, 10 µL injection volume). In addition to LC-MS-MS with SRM, precursor ion scanning was used on a triple quadrupole mass spectrometer to detect GSH conjugates that fragmented to form characteristic GSH ions of m/z of 308 (22).
Statistical analysis
Data were expressed as means ± SD and analyzed through one-way analysis of variance (ANOVA) followed by pair-wise comparisons made with Dunnett’s test using the SAS statistical package (SAS Institute, Cary, NC). All of the tests were two-sided, and a p value of < 0.05 was considered to be significant.
Results
Induction of GSH levels in rat hepatoma cells
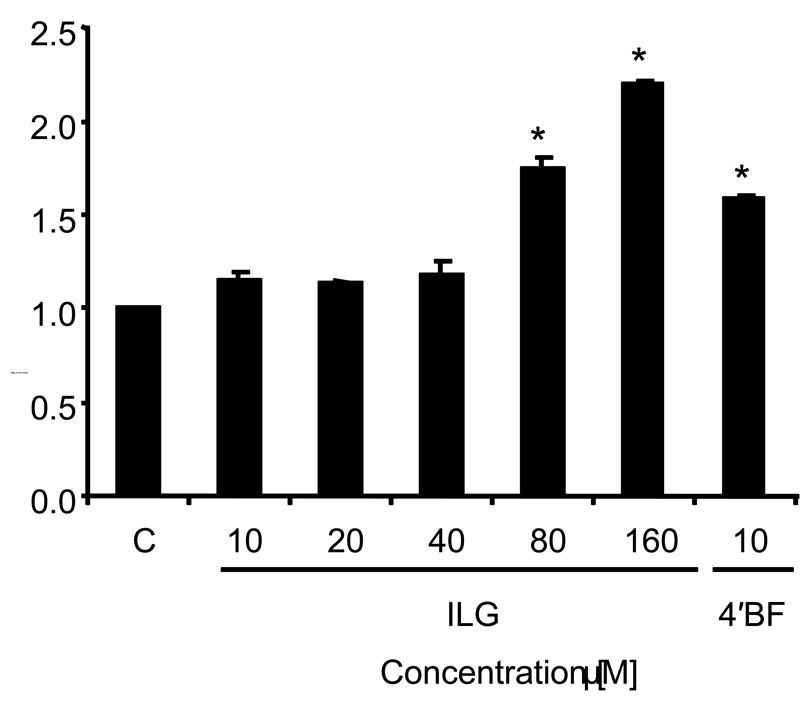
Since ILG had been determined previously to induce QR-1 activity in mouse hepatoma cells (7), we investigated the potential of ILG to induce GSH levels in cultured H4IIE rat hepatoma cells. The induction profile, shown in Figure 1, indicates that ILG induces GSH levels in a dose-dependent manner in the concentration range of 40–160 µM with a maximum of 2.5-fold induction at the highest concentration tested. 4'-Bromoflavone (10 µM) was used as a positive control and showed a significant increase in GSH levels.
Figure 1.
ILG induces GSH in H4IIE cells. Cells were treated with 10–160 µM ILG, 10 µM 4'-bromoflavone (4'BF), or DMSO (0.5% final concentration) as control (C) for 24 h and then analyzed for GSH. Results are shown as fold-induction relative to the level observed in the control. Results are the means of three determinations ± SD.
* Significantly different from control values (p < 0.001).
In vitro metabolism of ILG
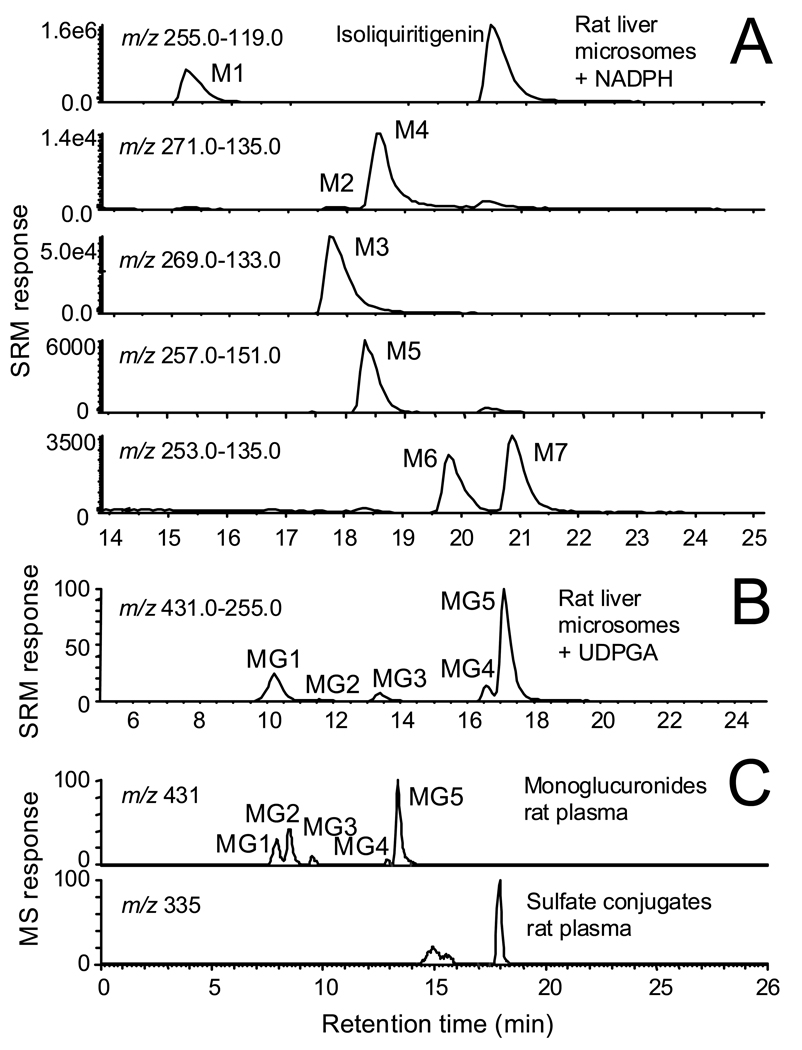
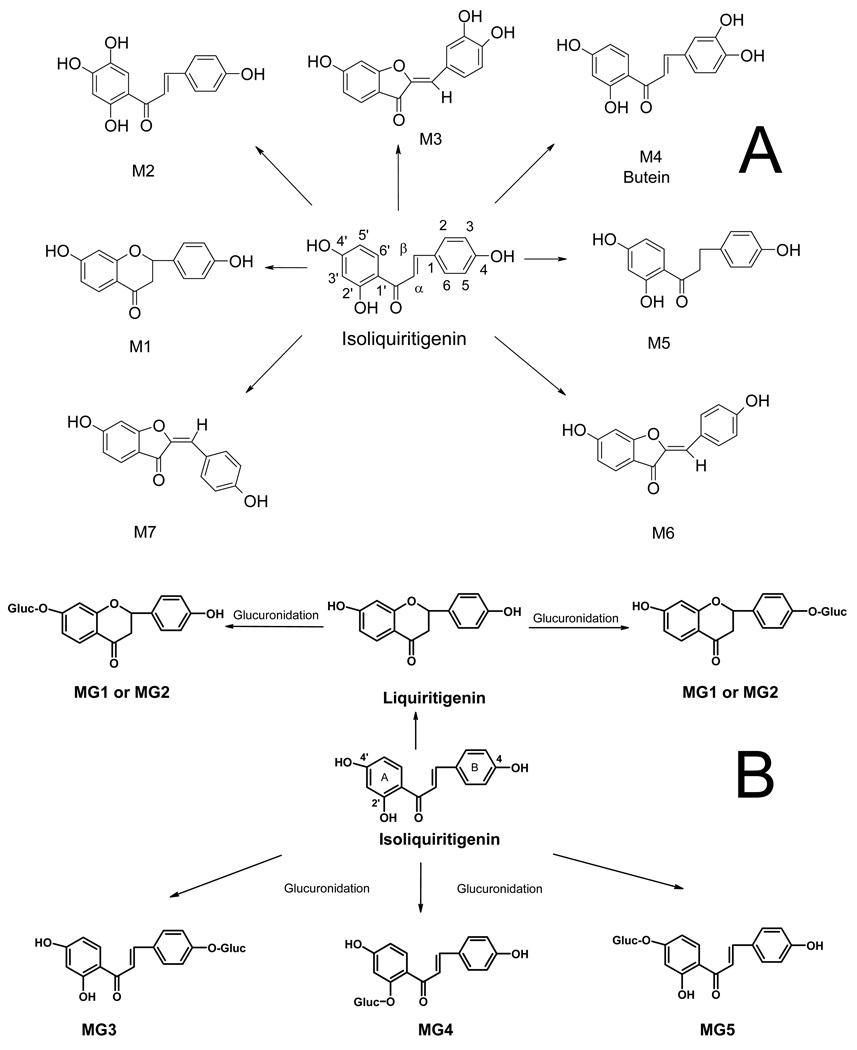
Selected reaction mass chromatograms for the negative ion electrospray LC-MS-MS analysis of an incubation of ILG with rat liver microsomes in the presence of NADPH are shown in Figure 2A. Four major (M1, M3, M4, M6) and three minor (M2, M5, M7) phase I metabolites were detected. By comparison of the HPLC retention times, elemental compositions (based on accurate mass measurements), and tandem mass spectra of these ILG metabolites with those reported previously for incubations with human liver microsomes (18), the structures of these seven metabolites were assigned as liquiritigenin (M1), 2′,4,4′,5′-tetrahydroxychalcone (M2), sulfuretin (M3), butein (M4), davidigenin (M5), trans-6,4’-dihydroxyaurone (M6), and cis-6,4’-dihydroxyaurone (M7). The structures of these ILG phase I metabolites are shown in Figure 3.
Figure 2.
A) Negative ion electrospray selected reaction monitoring (SRM) LC-MS-MS chromatograms showing detection of phase I metabolites M1–M7 of ILG after incubation with rat liver microsomes in the presence of NADPH. B) Mass chromatograms of ILG phase II glucuronide conjugates formed by rat liver microsomes in the presence of UDPGA. Metabolites MG3, MG4, and MG5 correspond to 4-glucuronosylisoliquiritigenin, 2′-glucuronosylisoliquiritigenin, and 4′-glucuronosylisoliquiritigenin, respectively, and MG1 and MG2 are monoglucuronides of liquiritigenin (see structures in Figure 3). C) Computer-reconstructed selected ion chromatograms of ILG glucuronides and sulfate conjugates detected in rat plasma using high resolution LC-MS with negative ion electrospray. Although different HPLC systems were used for parts B and C, the same five glucuronide conjugates were detected in rats as were observed with in vitro systems.
Figure 3.
A) Phase I metabolites of ILG formed during incubation with rat liver microsomes and NADPH. Based on accurate mass measurements, HPLC retention times, MS/MS analyses, and comparison to data reported by Guo et al. (29), the structures of metabolites M1, M2, M3, M4, M5, M6, and M7 were assigned as liquiritigenin, 7,8,4′-trihydroxychalcone, sulfuretin, 7,3′,4′-trihydroxychalcone, davidigenin, trans-6,4′-dihydroxyaurone, and cis-6,4′-dihydroxyaurone, respectively. B) Structures of ILG glucuronide conjugates formed by rat liver microsomes in the presence of UDPGA.
The LC-MS-MS analysis of ILG metabolites formed during incubation with rat liver microsomes and UDPGA are shown in Figure 2B. The structures of these glucuronides (Figure 3) were determined by comparison of their elemental compositions, HPLC retention times, and tandem mass spectra to those reported previously (19). Metabolites MG3, MG4, and MG5 corresponded to monoglucuronides with conjugation at one of the three hydroxyl groups of ILG to form 4-glucuronosylisoliquiritigenin, 2′-glucuronosylisoliquiritigenin, and 4′-glucuronosylisoliquiritigenin, respectively. After cyclization of ILG during incubation to form liquiritigenin, monoglucuronidation on liquiritigenin occurred to form MG1 and MG2. However, which liquiritigenin glucuronide corresponded to MG1 or MG2 was not determined.
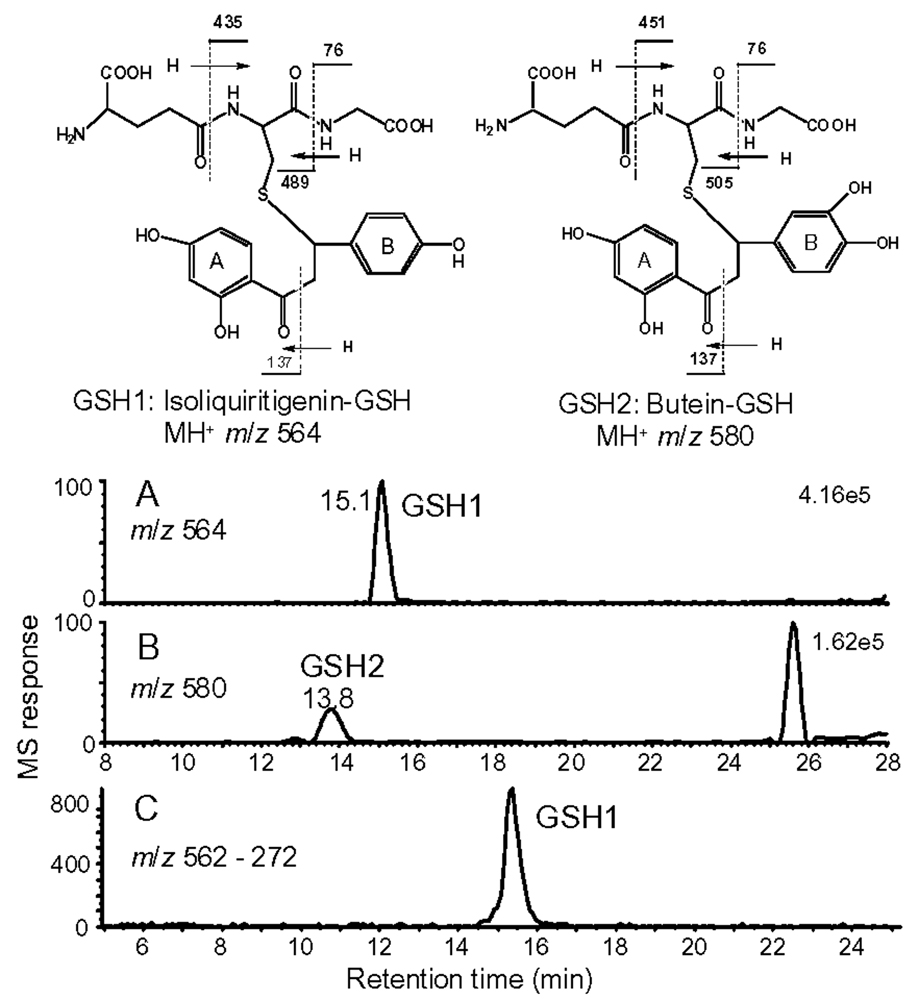
Since ILG contains an electrophilic α,β-unsaturated ketone, it might react with intracellular GSH to form conjugates. To investigate this possibility, ILG was incubated with human hepatocytes, and then positive ion electrospray LC-MS-MS was used with precursor ion scanning to detect GSH conjugates that fragmented to form protonated GSH at m/z 308. During LC-MS-MS, two abundant GSH conjugates (designated GSH1 and GSH2) were detected, eluting at 15.1 and 13.8 min, with protonated molecules of m/z 564 and 580, respectively (Figure 4). Based on accurate mass measurements, GSH2 was found to contain one oxygen atom more than GSH1. The product ion tandem mass spectra of GSH1 and GSH2 (data not shown) showed base peaks at m/z 308.1 corresponding to protonated GSH. Ions of lower abundance were detected in both tandem mass spectra at m/z 179 and 233 corresponding to losses of 129 or 75 from protonated GSH. In addition, losses of 129 or 75 from protonated GSH1 and GSH2 were also observed at m/z 489 or 505 and m/z 435 or 451, respectively (see structures in Figure 4). These fragment ions are class characteristic of GSH conjugates (23). Combined with accurate mass measurements, these fragmentation patterns confirmed that GSH1 and GSH2 were GSH conjugates.
Figure 4.
(A and B) Positive ion electrospray LC-MS-MS chromatograms showing the detection of two abundant GSH adducts, GSH1 and GSH2, in lysates of human hepatocytes that had been incubated with ILG. Precursor ion scanning was used to detect ions that fragmented to form the characteristic GSH product ion of m/z 308. (C) Negative ion electrospray MS-MS with collision-induced dissociation and SRM was used to detect GSH1 in rat liver following administration of ILG. The structures of GSH1 and GSH 2 were determined by comparison with synthetic standards, and the fragmentation patterns are based on high resolution product ion tandem mass spectrometry with accurate mass measurement.
The GSH1 product ion of m/z 257 (data not shown) corresponded to the protonated molecule of ILG after loss of GSH. A similar ion of m/z 273 in the tandem mass spectrum of GSH2 indicated that this compound was a GSH conjugate of a monooxygenated metabolite of ILG. The product ion of m/z 137 in both tandem mass spectra, which corresponded to the A-ring (Figure 4), showed that the A-ring of the ILG precursor was unchanged and that the site of monooxygenation was probably on the B-ring. The structure of GSH1 was confirmed to be a GSH conjugate of ILG (see the structure in Figure 4) by comparing the elemental compositions, LC-MS retention times and MS-MS fragmentation patterns with those of a synthetic standard. Since butein is the most abundant monohydroxylated metabolite of ILG (18), GSH2 was probably a GSH conjugate of butein (see structure in Figure 4), although no synthetic standard was available for confirmation.
Metabolism of ILG in the rat
After administration of ILG to rats, plasma samples were obtained and then analyzed using high resolution LC-MS. In rat plasma, unconjugated ILG (data not shown) and five monoglucuronide derivatives were detected (Figure 2C). Among the five glucuronides, 4′-glucuronosylisoliquiritigenin (MG5) was the most abundant. These findings are similar to those obtained from the in vitro studies in which the same five monoglucuronides were observed in similar proportions (Figure 2B). In addition to the ILG glucuronides, at least two partially resolved ILG sulfate conjugates were observed eluting at 14.9 and 15.6, min, and a third and more abundant sulfate conjugate was detected at a retention time of 17.9 min (Figure 2C). Isoliquiritigenin sulfate conjugates were not observed in the incubations with human hepatocytes, perhaps due to species differences. Since standards were not available for the sulfate conjugates, the specific sites of sulfation of ILG were not determined.
Finally, plasma and liver homogenates from rats treated with ILG were analyzed for GSH conjugates of isoliquiritigenin. Although no GSH conjugates of ILG were detected in plasma, one GSH conjugate of ILG of m/z 562 was detected in an extract of homogenized rat liver using LC-MS-MS in negative ion mode (see Figure 4C). The elemental composition of this peak was determined to be identical to GSH1 using high resolution accurate mass measurement mass spectrometry, and the structure of this metabolite was confirmed to be GSH1 by comparison of LC-MS-MS retention time and fragmentation pattern to the synthetic standard. The GSH2 that was observed in human hepatocyte incubations was not detected in rat liver.
Quantitative analysis of isoliquiritigenin in rat plasma and tissues
The concentrations of ILG in rat plasma and tissue samples were determined using LC-MS-MS (Table 1). The levels of ILG in plasma varied according to dosage. ILG was detected in plasma from rats receiving both dosages of ILG, and no ILG was detected in the control group receiving rat chow without ILG. The highest plasma concentration of ILG, 134.6 ± 47.2 ng/mL, corresponded to a dosage of 10.0 g ILG/kg diet, and the lowest ILG concentration of 57.81 ± 19.65 ng/mL corresponded to a dosage of 7.5 g ILG/kg diet (Table 1). There was no statistical difference with respect to ILG levels in plasma between the group of rats administered DMBA and 10.0 g ILG/kg diet and the group administered only 10.0 g ILG/kg diet, indicating that DMBA exposure had no effect on the bioavailability of ILG.
Table 1.
Concentrations of ILG in rat plasma, liver and mammary tissue.
| ILG Dosage | Plasma (ng/mL) |
Liver tissue (µg/g tissue) |
Mammary tissue (µg/g tissue) |
|---|---|---|---|
| Basal diet + DMBA | 0 | 0 | 0 |
| 7.50 g/kg diet + DMBA | 57.8 ± 19.6 | 2.56 ± 0.96 | 0.044 ± 0.014 |
| 10.0 g/kg diet + DMBA | 134.6 ± 47.2 | 4.71 ± 1.64 | 0.084 ± 0.040 |
| 10.0 g/kg diet | 142.2 ± 35.9 | 3.25 ± 1.38 | 0.094 ± 0.043 |
| Basal diet | 0 | 0 | 0 |
Like plasma, ILG was detected in liver and mammary tissue from rats treated with either dosage of ILG, and the levels of ILG in the tissue samples showed dose dependence (Table 1). Although ILG was detected in rat mammary tissue, the ILG level in the mammary tissue was significantly lower than that found in liver tissue. This result indicated that ILG was not concentrated in rat mammary tissue.
Effect of ILG on DMBA-induced mammary carcinogenesis
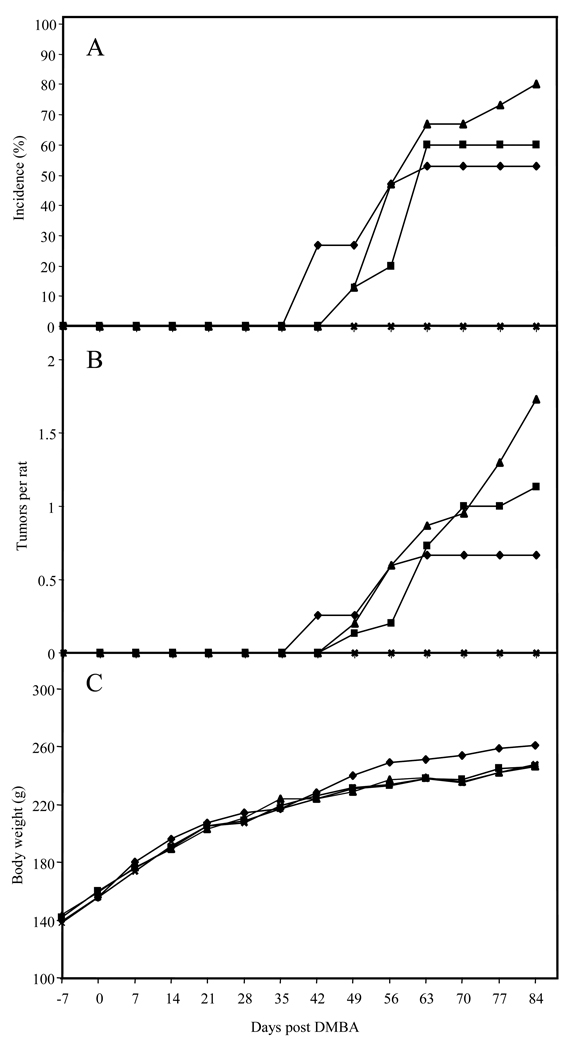
In a preliminary study, administration of ILG (5.0 g/kg diet) for one week prior and then one week after a single dose of DMBA increased tumor latency in Sprague-Dawley rats, but had little effect on the incidence of mammary tumors after 120 days (7). Based on these early data, a full-term carcinogenesis study with chronic administration schedules was performed. Since no adverse effects were detected at the 5.0 g/kg diet level, even higher doses were selected (7.5 and 10 g/kg diet) in order to assess efficacy. As shown in Figures 5A an 5B, administration of ILG during the entire study increased tumor latency, which confirmed the previous results (7). However, administration of ILG increased the overall tumor incidence and multiplicity in Sprague-Dawley rats after 84 days. There were no significant differences in body weight between the different groups (Figure 5C).
Figure 5.
Effect of dietary ILG on percent incidence in rats of observable mammary tumors (A), number of tumors (B), and body weight (C). Female Sprague-Dawley rats were given a single i.g. dose of 7,12-dimethylbenz(a)anthracene (DMBA) on day 0. ILG was included in the rat chow from 7 days prior to DMBA administration (−7) to the end of the study. The rat treatment groups were as follows: ◆ DMBA in sesame oil; ■ DMBA and 7.5 g/kg diet of ILG; ▲ DMBA and 10.0 g/kg diet of ILG; × 10.0 g/kg diet of ILG.
In vivo induction of drug-metabolizing enzymes
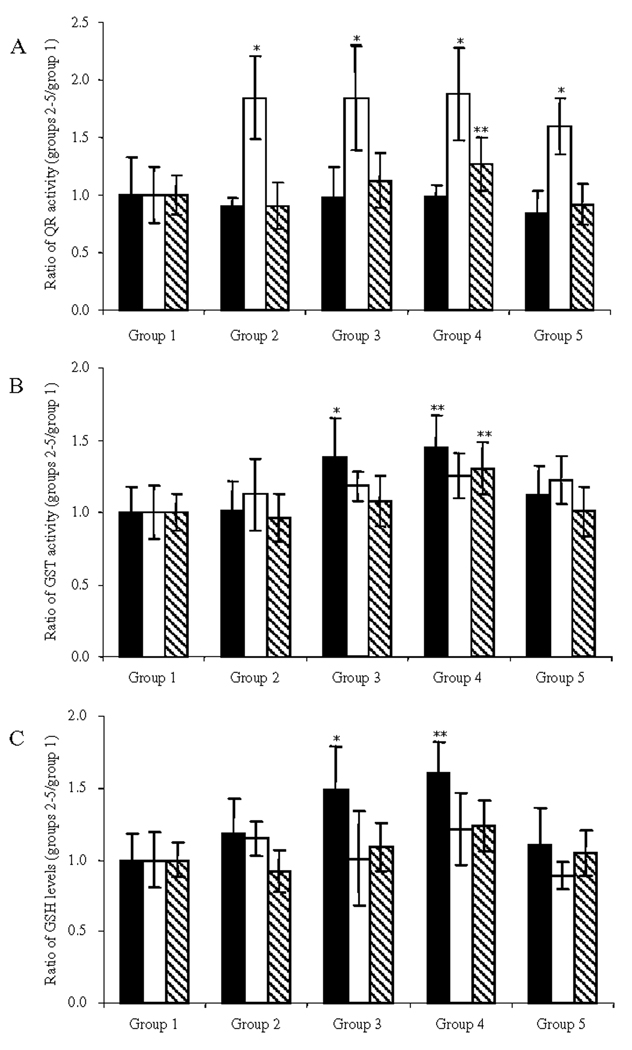
On the basis of the in vitro activities, we investigated the potential of ILG to induce higher steady-state levels of QR-1, GST and GSH in rat liver, colon and mammary gland tissues. Tissues of 6 rats per group were taken at the end of the in vivo study. In the colon, QR-1 activity was significantly elevated as a result of treatment with ILG, whereas no significant induction of QR-1 activity was found in the liver or mammary gland tissues (Figure 6A). At a dose of 10.0 g/kg diet, GST activity and GSH levels were found to be significantly elevated only in the liver (Figures 6B and 6C).
Figure 6.
Effect of dietary ILG on QR-1 induction (A), GST induction (B), and GSH levels (C) in rat liver (■), colon (□) and mammary gland ( ). Induction was calculated by comparing group 2 (DMBA and 7.5 g/kg diet of ILG) and group 3 (DMBA and 10.0 g/kg diet of ILG) with group 1 (DMBA in sesame oil), and group 4 (10.0 g/kg diet of ILG) with group 5 (basal diet). Treatment groups were significantly different (p<0.05) from the DMBA only control group 1 (*) or from the basal diet control group 5 (**) with n = 6.
). Induction was calculated by comparing group 2 (DMBA and 7.5 g/kg diet of ILG) and group 3 (DMBA and 10.0 g/kg diet of ILG) with group 1 (DMBA in sesame oil), and group 4 (10.0 g/kg diet of ILG) with group 5 (basal diet). Treatment groups were significantly different (p<0.05) from the DMBA only control group 1 (*) or from the basal diet control group 5 (**) with n = 6.
Analysis of mRNA expression
Real time PCR (RT-PCR) was performed to compare mRNA expression in liver, colon, and mammary tissues collected from Sprague-Dawley rats treated with either ILG, 4’BF, or sulforamate, with tissues from vehicle-treated rats (Table 2). Sulforamate (20, 21) and 4’BF (24, 25) were selected as for comparison based on their potential to serve as mono- or bifunctional enzyme inducers, respectively. Up-regulation was seen in genes for several phase II detoxifying enzymes with ILG treatment, particularly Nqo1 (quinone reductase-1), which was induced in all three tissues examined. Especially in the colon, a 54.8-fold increase compares favorably with the 49.0-fold increase induced by 4’BF. The fold change of Gstp1 (glutathione S-transferase π1) of 2.62 in colon tissue is slightly lower than tissue treated with 4’BF (4.06), but comparable to tissues treated with sulforamate (2.52).
Table 2.
RNA expression in rat tissues treated with isoliquiritigenin, 4’-bromoflavone, or sulforamate, expressed as fold change of expression relative to tissues from vehicle-treated animals.a
| Isoliquiritigenin | 4'-Bromoflavone | Sulforamate | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Name | Liver | Mammary | Colon | Liver | Mammary | Colon | Liver | Mammary | Colon | |
| A01 | ATP-binding cassette B1B | 2.64 | 0.857 | 829 | 1.28 | 0.701 | NS | 2.69 | 0.579 | 0.759 |
| A09 | Aminolevulinate, delta-, dehydratase | 1.04 | 5.43 | 272 | 0.484 | 9.48 | 36.1 | 0.553 | 9.85 | 3.45 |
| B02 | Aryl hydrocarbon receptor nuclear translocator | 1.09 | 6.30 | 73.4 | 0.351 | 11.5 | 12.0 | 0.586 | 11.1 | 2.52 |
| B08 | Carbohydrate sulfotransferase 1 | 1.35 | 5.75 | 472 | 0.622 | 10.7 | 56.7 | 0.546 | 14.9 | 2.96 |
| B12 | Cytochrome P450 19a1 | 0.631 | 41.5 | 58.1 | 0.314 | 4.95 | NS | 0.413 | 2.34 | 1.23 |
| C01 | Cytochrome P450 1a1 | 5.14 | 6.84 | NS | 3760 | 2460 | 6330 | 2.97 | 6.24 | NS |
| C05 | Cytochrome P450 2b15 | 0.699 | 0.113 | NS | 1.52 | 0.870 | NS | 2.05 | 0.095 | 1.15 |
| C06 | Cytochrome P450IIB3 | 0.728 | 0.209 | NS | 1.89 | 1.14 | NS | 2.09 | 0.169 | 0.992 |
| C08 | Cytochrome P450, subfamily IIC6 | 1.18 | 0.108 | 175 | 0.831 | 0.085 | 1.69 | 1.12 | 0.031 | 0.198 |
| C09 | Cytochrome P450 2c7 | 1.29 | 0.146 | NS | 0.194 | 0.106 | 23.0 | 0.774 | 0.045 | NS |
| C10 | Cytochrome P450 2e1 | 0.859 | 0.116 | 2.63 | 0.594 | 0.401 | 0.637 | 0.902 | 0.120 | 0.292 |
| C11 | Cytochrome P450 3a23/1 | 1.07 | 0.031 | NS | 2.25 | 0.020 | 8.90 | 2.28 | 0.013 | 0.121 |
| D05 | Glutamate decarboxylase 2 | NS | 2.56 | 5380 | 1.49 | 4.55 | 254 | 3.54 | 5.85 | 9.18 |
| D06 | Glucokinase regulatory protein | 0.597 | 1.12 | 1090 | 0.639 | 1.64 | 91.7 | 1.71 | 1.89 | 6.82 |
| D12 | Glutathione peroxidase 5 | 0.931 | 2.33 | 863 | 0.352 | 2.51 | NS | 0.528 | 2.59 | 1.35 |
| E10 | Glutathione-S-transferase, pi 1 | 2.79 | 3.57 | 2.62 | 96.4 | 5.86 | 4.06 | 1.61 | 5.97 | 2.52 |
| F05 | Myristoylated alanine rich protein kinase C substrate |
0.918 | 5.48 | 1.19 | 0.587 | 9.96 | 1.23 | 0.691 | 11.6 | 6.89 |
| G01 | Nitric oxide synthase 2, inducible | 1.23 | 5.44 | 8.56 | 0.676 | 4.70 | 7.25 | 0.658 | 2.97 | 2.32 |
| G03 | NAD(P)H dehydrogenase, quinone 1 | 1.81 | 2.68 | 54.8 | 15.4 | 15.8 | 49.0 | 1.26 | 3.92 | 2.23 |
| G06 | Paraoxonase 1 | 0.990 | 0.082 | 67.7 | 1.30 | 0.132 | 2.60 | 1.13 | 0.059 | 0.313 |
Numbers in boldface type indicate a significant increase in expression; those in italics indicate a decrease. Values shown are the average of tissues derived from three animals and analyzed individually. Results for each tissue at each dosage agreed within 10%. A value of NS (not significant) is assigned to data which differ from 1.0 by more than 0.5 and have a p-value greater than 0.05.
Increased expression of another phase II gene, Gpx5 (glutathione peroxidase 5), was seen in mammary tissue with a fold increase of 2.33, similar to 4’BF and sulforamate, with 2.51- and 2.59-fold increases, respectively. Also, six cytochrome P450 (CYP) genes from subfamilies 2 and 3 were down-regulated. This trend was also seen in the expression of CYP genes in tissues treated with the two positive controls. The CYP19a1 gene was significantly up-regulated in tissues treated with ILG, demonstrating a 41.5-fold increase, as compared with 4.95- and 2.34-fold increases with 4’BF and sulforamate, respectively. In liver tissue from animals treated with ILG, CYP1a1 was up-regulated by a 5.14-fold increase, slightly higher than the 2.97-fold increase produced by treatment with sulforamate, but much lower than the 3760-fold increase produced by treatment with 4’BF.
Discussion
In our search for novel cancer chemopreventive agents, ILG, a compound isolated from Dipteryx odorata (Aubl.) Willd. (tonka bean), but also present in licorice and shallots, was found to significantly induce QR-1 activity in mouse Hepa 1c1c7 cells (CD: 2 µM) (7). Identical results in two mutant cell lines less responsive to bifunctional inducers, which induce phase I as well as phase II drug-metabolizing enzymes, indicated that ILG was devoid of cytochrome P450-activating properties. Also, treatment with ILG significantly induced luciferase expression via interaction with the ARE in a dose-dependent manner and did not show any cytotoxicity (7). In addition, ILG exhibited a significant response in a carcinogen-treated mouse mammary organ culture assay (76% inhibition at 10 µg/mL) (8), and inhibited azoxymethane-induced murine colon carcinogenesis and azoxymethane-induced murine colon aberrant crypt focus formation (26). This compound has also been found to suppress metastasis in a pulmonary metastasis model of mouse renal cell carcinoma and to prevent severe 5-fluorouracil-induced leukocytopenia in this model (16). Based on these results, as well as an increase in tumor latency in rats using the DMBA-induced mammary tumorigenesis model, studies of the metabolism of ILG and a full-term carcinogenesis study with chronic administration schedules were pursued.
Studies of in vitro cytotoxicity of hydroxychalcones have suggested they deplete intracellular GSH levels (27). Whereas most hydroxychalcones tested significantly depleted GSH in hepatocytes, ILG reduced GSH concentrations only slightly. In our study, measurements of total GSH in rat hepatoma cells showed a significant increase at doses of 80 µM ILG or higher (Figure 1). Furthermore, dietary ILG significantly enhanced GSH levels in rat liver (Figure 6). These observations indicate that ILG might be less toxic than most other hydroxychalcones.
In the in vitro metabolic profiling studies, seven phase I metabolites and five glucuronic acid conjugates of ILG were detected in the rat liver microsomal incubation. ILG and abundant monoglucuronides (MG1–MG5) were also detected in liver and plasma of rats treated with ILG. In addition, significant conjugation of ILG with sulfate was observed to occur in rats, even though sulfation was not predicted by previous human hepatocytes studies (19), perhaps due to interspecies differences. Therefore, rapid conjugation of ILG with glucuronic acid might explain its relatively low in vivo chemopreventive activity compared with in vitro studies. Moreover, the low level of ILG in rat mammary gland tissue indicates that ILG does not concentrate in target organ, which is another explanation for the low chemopreventive activity of ILG in the rat mammary gland tumorigenesis model.
Although ILG was reported to deplete GSH in isolated rat hepatocytes (28), there had been no evidence that GSH conjugates of ILG were formed in these cells. In the present studies, ILG-GSH was shown to be formed both in isolated hepatocytes and in the liver of rats. Previously, ILG had only been reported to form GSH adducts in wheat (23).
ILG was evaluated in the DMBA-induced rat mammary tumorigenesis model with chronic administration schedules. Administration of ILG slightly increased tumor latency in Sprague-Dawley rats, but had a negative effect on the incidence of mammary tumors, starting approximately 65 days post DMBA. The reason for this enhanced response is not known. However, the estrogenic activity of ILG has been reported in various systems (12, 28). In MCF-7 cells, for example, low and intermediate ILG concentrations showed estrogen receptor-dependent growth promoting effects; higher doses were cytotoxic (12). In the present studies, the concentrations of ILG observed in rat plasma and tissue samples were dose-dependent. Thus, the activity of ILG and the balance between risk or chemoprevention for estrogen-dependent breast cancer might depend on dietary intake or the rate of elimination. The fact that ILG had little effect on phase II enzyme levels in rat mammary glands might be due to insufficient amounts of unmetabolized ILG reaching this site. However, significant induction of QR-1 was found in the colon, and significant increases of GST and GSH were observed in the liver, which were probably the result of exposure of these organs to much higher levels of ILG.
Analysis of mRNA expression in tissues collected from Sprague-Dawley rats treated with either ILG, 4’BF, or sulforamate supported data from cell culture studies. The up-regulation of genes encoding phase II detoxification enzymes (QR1, GSTp1) in liver, colon, and mammary tissues of rats treated with isoliquiritigenin indicates that ILG treatment may increase detoxification and help to prevent carcinogen formation. The fold increases in expression of these genes with ILG treatment compare favorably to those resulting from sulforamate or 4’BF treatment. Increased QR1 expression, in particular, points to chemopreventive potential in the colon.
There are several additional indications that ILG could have a chemopreventive effect in mammary tissue. These include increased expression of glutathione peroxidase 5 and down-regulation of several cytochrome P450 (CYP) genes. Decreased expression of CYP genes, which encode phase I enzymes that can activate procarcinogens, suggests chemopreventive potential. CYP genes from subfamilies 2 and 3 were also down-regulated in mammary tissue treated with 4’BF or sulforamate, indicating this trend may be related to the activity of these compounds. However, the significant up-regulation of the CYP19a1 gene with ILG treatment may counteract these positive effects. CYP19 encodes the aromatase enzyme which is responsible for the final stages of estrogen biosynthesis and has been related to breast cancer, so its over-expression may account for the negative effect on the incidence of mammary tumors. In addition, as noted above, phytoestrogenic activity has been associated with ILG (11, 12).
ILG was found to be a monofunctional inducer of phase II enzymes through a QR1 assay in two mutant cell lines, and it was determined that induction by ILG was regulated by the antioxidant response element (ARE) using stably transfected HepG2 cells. The expression of CYP1a1 is slightly elevated in liver tissue from rats treated with ILG. A lesser, but similar increase, was observed in liver from animals treated with sulforamate, another monofunctional inducer. However, treatment with 4’BF, a bifunctional inducer, results in a 3760-fold increase, three orders of magnitude greater than that caused by ILG or sulforamate. Clearly, AHH activity can be induced to a much greater degree by the bifunctional compound. CYP1a1 is regulated by the aryl hydrocarbon receptor (Ahr). Although the Ahr gene is not significantly affected by ILG, expression of Arnt (aryl hydrocarbon receptor nuclear translocator) is increased in mammary and colon tissue. Overall, these results support the already comprehensive data demonstrating the activity of ILG is monofunctional.
In summary, multiple phase I and phase II metabolites of ILG were formed by rat liver microsomes and in human hepatocytes. Some of these metabolites are clearly of relevance since analogous observations were obtained with the intact rat. The liver produced a high rate of biotransformation. However, rat mammary gland exposure to ILG was low. This may explain why the in vivo study using a rat mammary tumorigenesis model showed predominantly negative results. In fact, the trend toward increasing tumor number suggests caution, possibly due to the estrogenic activity of ILG, as well as increased expression of CYP19a1. On the other hand, based on tissue distribution and modulation of biomarkers, ILG could be effective in the chemoprevention of cancer types such as liver or colon, so further investigation is required.
Acknowledgments
Grant support: NIH award P01 CA48112
References
- 1.Kang Y, Pezzuto J. Induction of quinone reductase as a primary screen for natural product anticarcinogens. Methods Enzymol. 2004;382:380–414. doi: 10.1016/S0076-6879(04)82021-4. [DOI] [PubMed] [Google Scholar]
- 2.Kape R, Parniske M, Brandt S, D DW. Isoliquiritigenin, a strong nod gene- and glyceollin resistance-inducing flavonoid from soybean root exudate. Appl Environ Microbiol. 1992;58:1705–1710. doi: 10.1128/aem.58.5.1705-1710.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao Y, Wang Y, Ji C, Ye J. Determination of liquiritigenin and isoliquiritigenin in Glycyrrhiza uralensis and its medicinal preparations by capillary electrophoresis with electrochemical detection. J Chromatogr A. 2004;1042:203–209. doi: 10.1016/j.chroma.2004.05.049. [DOI] [PubMed] [Google Scholar]
- 4.Ramadan M, Kamel M, Ohtani K, Kasai R, Yamasaki K. Minor phenolics from Crinum bulbispermum bulbs. Phytochemistry. 2000;54:891–896. doi: 10.1016/s0031-9422(00)00184-9. [DOI] [PubMed] [Google Scholar]
- 5.Pan X, Kong L, Zhang Y, Cheng C, Tan R. In vitro inhibition of rat monoamine oxidase by liquiritigenin and isoliquiritigenin isolated from Sinofranchetia chinensis. Acta Pharmacol Sin. 2000;21:949–953. [PubMed] [Google Scholar]
- 6.Kong L, Zhang Y, Pan X, Tan R, Cheng C. Inhibition of xanthine oxidase by liquiritigenin and isoliquiritigenin isolated from Sinofranchetia chinensis. Cell Mol Life Sci. 2000;57:500–505. doi: 10.1007/PL00000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuendet M, Oteham C, Moon R, Pezzuto J. Quinone reductase induction as a biomarker for cancer chemoprevention. J Nat Prod. 2006;69:460–463. doi: 10.1021/np050362q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jang D, Park E, Hawthorne M, et al. Potential cancer chemopreventive constituents of the seeds of Dipteryx odorata (tonka bean) J Nat Prod. 2003;66:583–587. doi: 10.1021/np020522n. [DOI] [PubMed] [Google Scholar]
- 9.Vaya J, Belinky P, Aviram M. Antioxidant constituents from licorice roots: isolation, structure elucidation and antioxidative capacity toward LDL oxidation. Free Radic Biol Med. 1997;23:302–313. doi: 10.1016/s0891-5849(97)00089-0. [DOI] [PubMed] [Google Scholar]
- 10.Chan S, Chang Y, Wang J, Chen S, Kuo S. Three new flavonoids and antiallergic, anti-inflammatory constituents from the heartwood of Dalbergia odorifera. Planta Med. 1998;64:153–158. doi: 10.1055/s-2006-957394. [DOI] [PubMed] [Google Scholar]
- 11.Tamir S, Eizenberg M, Somjen D, Izrael S, Vaya J. Estrogen-like activity of glabrene and other constituents isolated from licorice root. J Steroid Biochem Mol Biol. 2001;78:291–298. doi: 10.1016/s0960-0760(01)00093-0. [DOI] [PubMed] [Google Scholar]
- 12.Maggiolini M, Statti G, Vivacqua A, et al. Estrogenic and antiproliferative activities of isoliquiritigenin in MCF7 breast cancer cells. J Steroid Biochem Mol Biol. 2002;82:315–322. doi: 10.1016/s0960-0760(02)00230-3. [DOI] [PubMed] [Google Scholar]
- 13.Nerya O, Musa R, Khatib S, Tamir S, Vaya J. Chalcones as potent tyrosinase inhibitors: The effect of hydroxyl positions and numbers. Phytochemistry. 2004;65:1389–1395. doi: 10.1016/j.phytochem.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 14.Wegener J, Nawrath H. Cardiac effects of isoliquiritigenin. Eur J Pharmacol. 1997;326:37–44. doi: 10.1016/s0014-2999(97)00134-9. [DOI] [PubMed] [Google Scholar]
- 15.Ii T, Satomi Y, Katoh D, et al. Induction of cell cycle arrest and p21(CIP1/WAF1) expression in human lung cancer cells by isoliquiritigenin. Cancer Lett. 2004;207:27–35. doi: 10.1016/j.canlet.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 16.Yamazaki S, Morita T, Endo H, et al. Isoliquiritigenin suppresses pulmonary metastasis of mouse renal cell carcinoma. Cancer Lett. 2002;183:23–30. doi: 10.1016/s0304-3835(02)00113-1. [DOI] [PubMed] [Google Scholar]
- 17.Ma J, Fu N, Pang D, Wu W, AL AX. Apoptosis induced by isoliquiritigenin in human gastric cancer MGC-803 cells. Planta Med. 2001;67:754–757. doi: 10.1055/s-2001-18361. [DOI] [PubMed] [Google Scholar]
- 18.Guo J, Liu D, Nikolic D, Zhu D, Pezzuto J, Breemen Rv. In vitro metabolism of isoliquiritigenin by human liver microsomes. Drug Metab Dispos. 2008;36:461–468. doi: 10.1124/dmd.107.018721. [DOI] [PubMed] [Google Scholar]
- 19.Guo J, Liu A, Cao H, Luo Y, Pezzuto J, Breemen Rv. Biotransformation of the chemopreventive agent isoliquiritigenin by UDP-glucuronosyltransferases. Drug Metab Dispos. 2008;36:2104–2112. doi: 10.1124/dmd.108.021857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerhäuser C, You M, Liu J, et al. Cancer chemopreventive potential of sulforamate, a novel analog of sulforaphane that induces phase 2 drug-metabolizing enzymes. Cancer Res. 1997;57:272–278. [PubMed] [Google Scholar]
- 21.Moriarty R, Naithani R, Kosmeder J, Prakash O. Cancer chemopreventive activity of sulforamate derivatives. Eur J Med Chem. 2006;41:121–124. doi: 10.1016/j.ejmech.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Nikolic D, Fan P, Bolton J, RB RvB. Screening for xenobiotic electrophilic metabolites using pulsed ultrafiltration-mass spectrometry. Comb Chem High Throughput Screen. 1999;2:165–175. [PubMed] [Google Scholar]
- 23.Cummins I, O'Hagan D, Jablonkai I, et al. Cloning, characterization and regulation of a family of phi class glutathione transferases from wheat. Plant Mol Biol. 2003;52:591–603. doi: 10.1023/a:1024858218804. [DOI] [PubMed] [Google Scholar]
- 24.Song L, Kosmeder J, Lee S, et al. Cancer chemopreventive activity mediated by 4’-bromoflavone, a potent inducer of phase II detoxification enzymes. Cancer Res. 1999;59:578–585. [PubMed] [Google Scholar]
- 25.Li Y, Grubjesic S, Nikolic D, et al. In vitro assessment of intestinal permeability and hepatic metabolism of 4’-bromoflavone, a promising cancer chemopreventive agent. Xenobiotica. 2004;34:535–547. doi: 10.1080/00498250410001713159. [DOI] [PubMed] [Google Scholar]
- 26.Baba M, Asano R, Takigami I, et al. Studies on cancer chemoprevention by traditional folk medicines XXV. Inhibitory effect of isoliquiritigenin on azoxymethane-induced murine colon aberrant crypt focus formation and carcinogenesis. Biol Pharm Bull. 2002;25:247–250. doi: 10.1248/bpb.25.247. [DOI] [PubMed] [Google Scholar]
- 27.Sabzevari O, Galati G, Moridani M, Siraki A, PJ POB. Molecular cytotoxic mechanisms of anticancer hydroxychalcones. Chem Biol Interact. 2004;148:57–67. doi: 10.1016/j.cbi.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Hillerns P, Zu Y, Fu Y, Wink M. Binding of phytoestrogens to rat uterine estrogen receptors and human sex hormone-binding globulins. Z Naturforsch [C] 2005;60:649–656. doi: 10.1515/znc-2005-7-823. [DOI] [PubMed] [Google Scholar]