Abstract
Background
A systematic study of cortical surface parameters in adolescent offspring of schizophrenia subjects before clinical manifestation could clarify neurodevelopmental antecedents of increased genetic risk. We examined these measures obtained on structural magnetic resonance imaging (MRI) scans at baseline and one year on a series of offspring and healthy subjects.
Methods
We measured cortical surface area, curvature and thickness using BRAINS2 on structural MRI scans acquired using 1.5T GE whole body scanner on all subjects. We examined the differences between study groups at baseline using mixed effects models, and longitudinal trajectory of these measures using linear mixed effects models.
Results
At baseline, offspring of schizophrenia parents showed reduced gyral surface area in the fronto-parietal lobes along with increased sulcal curvature and parietal gyral cortical thinning compared to healthy subjects. Prospective follow up of these subjects for one year showed shrinking of the total surface area, especially in the bilateral frontal and occipital regions along with preservation of cortical thickness among offspring of schizophrenia parents whereas healthy subjects showed preserved or increased surface area and cortical thinning. Correlation of these measures with lobar volumes were not observed at baseline cross-sectional comparisons but were observed in longitudinal examinations.
Discussion
Our observations suggest that adolescents with genetically elevated risk for schizophrenia show altered cortical surface measures affecting cortical surface area and thickness differentially suggesting a divergent trajectory of neurodevelopment. Cortical surface measures appear to be more sensitive to genetic liability to schizophrenia compared to volumetric measures.
1. Introduction
Schizophrenia is proposed to be a neurodevelopmental disorder (Murray and Lewis 1987; Weinberger 1987). The genetic factors significantly contribute to its risk (Gottesman and Shields 1966). In majority of cases, it is proposed that the pathophysiological processes may begin much before the clinical manifestation, possibly in the prenatal life (Murray and Lewis 1987; Weinberger 1987). Examining the first-degree relatives of schizophrenia probands before the illness onset could potentially provide neurobiological data that may be relatively less affected by the clinical phenotype (Diwadkar et al. 2004; Gottesman and Shields 1973; Gottesman 1991). Such data could prove valuable to identify reliable intermediate phenotypes because first degree relatives have approximately 10 times higher risk for developing schizophrenia compared to the general population. Such reliable intermediate phenotypes may be used to conduct a prospective evaluation to delineate their developmental trajectory and possibly the pathophysiological pathways.
Altered regional brain volumes have been reported among the offspring subjects (Job et al. 2005; Keshavan et al. 1997; Lawrie et al. 1999). Volumetric measures may not reveal abnormalities in neurodevelopmentally relevant characteristics in the cortex, such as cortical thickness, curvature or surface area. Such alterations could better depict abnormalities in brain development that may have implications for its function. The importance of examining cortical surface maps among individuals genetically at an elevated risk for schizophrenia is supported by significant correlations of the entire cortex and global cortical surface area, especially in the association cortices with genetic factors (Schmitt et al. 2009). In a related study, the same group found substantial heterogeneity in heritability between the cortical regions; the strongest being the frontal region followed in a descending order by the temporal, parietal and occipital regions (Schmitt et al. 2008). Further, heritable reductions in cortical thickness were observed among schizophrenia subjects; however, the reduction in thickness among siblings of schizophrenia subjects did not reach statistical significance (Goldman et al. 2009). These authors suggest that the cortical thickness may not be a strong intermediate phenotype. Therefore, examining the cortical surface patterns that includes surface area, curvature and thickness together and their developmental trajectory could provide better insight into the brain development among these subjects.
Examination of cortical surface parameters using magnetic resonance imaging (MRI) offers the advantage of unravelling the dynamic changes in an in vivo environment. Thinning of the anterior, subcallosal, and rostral cingulate along with paracingulate regions predicted development of psychosis among the “ultra-high risk” subjects (Fornito et al. 2008). This suggests predictive ability of region-specific surface parameter changes in this population. Unaffected adult first degree relatives of schizophrenia demonstrated reversed asymmetry of sulcal thickness (Goghari et al. 2007a) and cortical surface area (Goghari et al. 2007b) in the cingulate and the superior temporal cortex. Another study on “ultra-high risk” subjects observed ‘contraction’ of the right dorsolateral prefrontal cortical surface area over 1 year among those who developed psychosis (converters) compared to those who did not (non-converters) (Sun et al. 2009.). There is paucity of both cross-sectional and longitudinal studies on unaffected adolescent offspring of schizophrenia subjects.
Normal cortical development appears to follow a predictable pattern and trajectory of changes. Deviations in this pattern and trajectory may suggest abnormal neurodevelopment. During the fetal development, cortical gyration and sulcation proceeds in stages where frontoparietal region near the central sulcus and medial occipital regions show the first gyral and sulcal formation followed by the parietal, occipital and posterior temporal, and then by the frontobasal, anterior frontal and anterior temporal regions (van der Knaap et al. 1996). Dynamic mapping using MRI scans showed that the cortical maturation progressed from lower order cortices to the higher order association areas, and from phylogenetically older areas to the newer ones among healthy subjects (Gogtay et al. 2004). Using the same method, schizophrenia subjects showed grey matter loss that spread from parietal to prefrontal and temporal regions compared to healthy subjects (Thompson et al. 2001b). Longitudinal imaging studies on adult schizophrenia subjects observed a progressive loss of grey matter in the frontal (Gur et al. 1998) and temporal (Lieberman et al. 2005; Nakamura et al. 2007) regions. Such observations among individuals at elevated risk for schizophrenia could provide useful data on the trajectory of in vivo neurobiological markers leading up to clinical manifestation.
Among the cortical surface measures, cortical surface area, thickness and curvature may comprehensively reflect the course of neurodevelopment. Brain development is a complex process involving several overlapping phases of neuronal proliferation, neuronal migration, differentiation, synaptogenesis, myelination, synaptic pruning, and plasticity that may last well into late adulthood. One proposal for the formation of cortical gyration posits that variations in axonal tension during the formation of cortico-cortical connections determine the number and shape of the neuronal cells (Hilgetag and Barbas 2005; Van Essen 1997). The human cerebral cortex is an extensively folded sheet of neuropil with more than 50% buried within the sulci. Disruption of corticocortical connectivity associated with variations in the gyrification pattern (Rakic 1988) may be reflected in the above measures. Further, neurons localized to the gyral and sulcal cortical layers may be morphologically different (Rakic 1988; Sisodiya et al. 2000); therefore, examining the gyral and sulcal measures may provide additional clues.
In this study, we examined cortical surface area, curvatures, and thickness in a well characterized cohort of offspring of probands with schizophrenia/schizoaffective disorder in comparison to matched healthy subjects. We tested the following hypotheses: (1) the cortical surface area, thickness and curvature will be reduced among offspring compared to healthy subjects, (2) such reductions will be more prominent in the frontal and temporal lobes, and (3) the study groups follow different trajectories of cortical development.
2. Methods
2.1. Clinical Evaluation
Thirty-one individuals between ages 10 and 20 (12 males and 19 females, mean age 16.26±2.76 years) with at least one parent diagnosed with schizophrenia/schizoaffective disorder were included in this study. Parents were administered the Structured Clinical Interview for DSM IV diagnoses (SCID-I) (First 1997) to confirm the diagnoses. We ascertained the diagnoses using DSM-IV criteria at consensus conference meetings attended by senior clinicians (MSK, KMP, DM). The participants were identified at the Western Psychiatric Institute and Clinic (WPIC), Pittsburgh. Offspring of schizophrenia parents with a lifetime history of DSM-IV diagnosis of a psychotic disorder (e.g. schizophrenia, schizophreniform illness or schizoaffective disorder), mental retardation, significant head injury, significant history of or current medical or neurological illness were excluded. Among the high risk subjects, 19 had no psychiatric diagnoses while others were diagnosed with axis I disorder (major depression 7, ADHD 2, PTSD 2 and cannabis abuse 1). The mean age at onset of psychotic episode among parents was 24.66±8.69 years after excluding 7 parents on whom we did not have the age of onset. Age of onset is defined as age at which first psychotic symptom was experienced. Of these parents 19 parents were diagnosed with schizophrenia and the rest had schizoaffective disorder. Thirty-three healthy comparison subjects (14 males and 19 females; mean age 17.20±3.81 years) were also recruited from the same neighborhoods as the offspring. The groups did not differ significantly in the age (t=1.13, p=0.26) or gender distribution (χ2=0.09, p=0.76). None of the healthy subjects had an axis I disorder. There were 15 Caucasians among offspring and 17 among healthy subjects (χ2=12.28, p=0.001). Subjects (both offspring and healthy subjects) with current substance use disorder were excluded from the study. Subjects from both groups were evaluated by using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) (First 1997), supplemented by the Behavioral Disorders sections of the K-SADS (Kaufman et al. 2000). All subjects provided written informed consent following full description of the studies. The parent or guardian also provided informed consent for subjects aged less than 18 years, and the subjects provided assent. All experimental protocols were approved by the University of Pittsburgh Institutional Review Board.
2.2. Imaging Methods
MRI scans were acquired on GE 1.5T whole body scanner. All scans were acquired using 3-dimension spoiled gradient recalled (3D SPGR) acquisition in the steady state pulse sequence. One hundred twenty four 1.5 mm thick contiguous images were acquired in the coronal section perpendicular to the AC-PC line (TE=5 m sec, TR=25 m sec, acquisition matrix=256 × 192). Scans with motion artifacts were not included in the analysis.
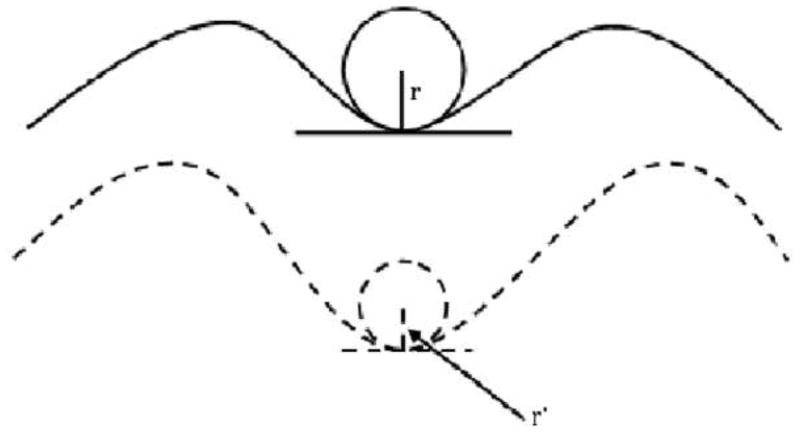
We used BRAINS2 package to conduct post-processing of the MRI scans (Magnotta et al. 2002). All images were resampled to 1 mm slices and realigned along the AC-PC line. T2 and proton density (PD) images were co-registered to the T1 images using the automatic image registration (AIR) algorithm. All images were segmented into grey, white and CSF (k>0.95). Using the built-in surface generation algorithm, cortical surface parameters were measured (Magnotta et al. 1999; Magnotta et al. 2002). This program generates surfaces for each hemisphere separately after removing cerebellum and brain stem. A triangle isosurface is generated similar to marching cubes algorithm. The program initially can place more than 300,000 triangles that are then decimated to about 100,000 using built-in algorithms. The surface program of the BRAINS2 package generates triangle isosurfaces to measure area at pixel intensity of 130 that represents pure grey matter – parametric center of grey matter intensity (Imaging Lab Iowa Neuroimaging Center 2006). This method erodes 50% of the grey matter that allows the measurements to reach the cortical depths and also eliminate the problem of “buried cortex” where the pixel from the one side of the gyral grey matter touches that from the other side. During the process of surface generation, curvature, area and thickness are computed. While running this program, each scan was visually inspected to insure that all lobes were parsed and separated with good quality. Specifically, we inspected the generated surfaces for intrusion into other hemispheres and cerebellum, poor midline alignment, traces missing in a slice, and misclassification of sulci and gyri. We also inspected the measured surface data for outliers and such scans were rerun to fix the problems. This method has been validated using phantom images of two simple geometrical structures (a sphere and a cube) with acceptable variations. Similarly, reliability has been tested by rescanning the same individual two weeks later (Magnotta et al. 1999; Magnotta et al. 2002). Although surface area and thickness can be intuitively conceived, the measurement of curvature merits more description. A curvature is defined as the reciprocal of the radius of the smallest imaginary circle that fits a given curvature. Therefore, as the radius of the ‘inserted circle’ increases, the curvature decreases and vice versa as shown in fig 1.
Fig 1.
Depiction of measurement of curvature and its relationship with area. Gyral surface areas were cortical laminae starting at half the distance between the deepest part of the sulcus to the tip of the gyrus and traversing the gyral peak. Similarly, sulcal surface areas started midpoint between the deepest part of the sulcus and the tip of the gyrus traversing the sulcus. Curvature (k) is the reciprocal of the radius of the smallest imaginary circle inserted into the sulcal or gyral curvature. Upper figure with an imaginary circle of a radius r has curvature k=1/r and the lower curvature k′=1/r′. Assuming r>r′, the curvature of the upper figure is decreased compared to that of the lower figure.
Lobar volumes were measured using lobar masks defined in the Wake Forest University PickAtlas (Maldjian et al. 2003) program within the Statistical Parametric Mapping, version 5 (SPM5) (http://www.fil.ion.ucl.ac.uk/spm/software/spm5/). This semi-automated method of measuring the volumes provides grey, white and cerebrospinal fluid (CSF) volumes of each lobe separately. We used the combined volumes of grey, white and CSF volumes to calculate the total volumes of each lobe, which was used in examining the convergence of changes in surface parameters with lobar volumes.
2.3. Statistical plan
We first examined the total surface area for the entire brain between the study groups. We did not examine the curvature and thickness for the entire brain because mean curvatures and thickness for the entire brain is difficult to interpret. Next, we examined the surface area, cortical curvature and thickness using mixed models and examined the group effect, group × type (gyral or sulcal), group by hemisphere and group × lobe × hemisphere interactions. To examine alterations in these cortical measures over the follow up period, we compared baseline and one year follow up data using linear mixed-effects models. Older individuals consistently had less brain curvature (all p < .070), with the exception of occipital lobe curvature. In addition, older individuals had consistently less brain density for all lobes examined (all p < 0.004). Sex effects were observed for frontal, parietal, and temporal surface area (all p < 0.076), with males demonstrating greater surface area in these regions. As such, all models included age and gender as covariates. We used both Cohen’s d and r to report effect sizes.
3. Results
The results are presented for baseline cross-sectional analyses followed by changes in longitudinal measures. The latter also includes differences observed cross-sectionally at year 1.
3.1 Overview of the results of baseline cross-sectional measures
The total surface area of the brain did not differ significantly between the two groups (Healthy Subjects, 1758.31 ± 164.40 cm2; offspring, 1729.88 ± 161.04 cm2; F(1, 64)=0.4, p=0.5, NS). The main effect of study group was observed on the parietal lobe gyral curvature (p=0.02). We observed group by type interaction at the frontal and parietal surface areas, and parietal cortical thickness. We did not observe significant differences in the group by hemisphere and group by lobe by hemisphere interactions on any of the surface measures (Table 1).
Table 1.
Differences in cortical surface measures between offspring of schizophrenia subjects and healthy comparison subjects
| High Risk | Control | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gyral | Sulcal | Gyral | Sulcal | Analysis | ||||||||
| L | R | L | R | L | R | L | R | Group Effect | Group × Type | Group × Hemi | Group × Type × Hemi | |
| Variable | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | p | p | p | p |
| Surface Area | ||||||||||||
| Frontal | 202.08 (21.35) | 208.40 (21.92) | 132.03 (18.43) | 137.98 (20.66) | 206.16 (18.41) | 214.04 (21.38) | 130.45 (16.61) | 137.22 (18.61) | .660 | .027 | .658 | .892 |
| Temporal | 112.49 (12.72) | 105.10 (12.52) | 86.28 (12.46) | 85.69 (12.64) | 115.58 (11.81) | 109.89 (13.00) | 90.39 (11.89) | 89.56 (12.03) | .120 | .977 | .696 | .606 |
| Parietal | 96.30 (13.70) | 99.87 (13.31) | 101.79 (14.55) | 105.47 (15.76) | 105.32 (14.67) | 109.32 (15.59) | 100.59 (16.15) | 104.12 (16.19) | .141 | .001 | .964 | .926 |
| Occipital | 67.42 (12.55) | 65.37 (11.61) | 68.01 (13.08) | 68.01 (11.82) | 64.68 (12.62) | 61.28 (9.66) | 65.13 (14.83) | 63.29 (11.68) | .146 | .876 | .518 | .920 |
| Curvature | ||||||||||||
| Frontal | .07 (.01) | .07 (.01) | .07 (.01) | .08 (.01) | .07 (.00) | .07 (.01) | .07 (.01) | .07 (.01) | .096 | .073 | .656 | .315 |
| Temporal | .08 (.01) | .08 (.01) | .07 (.01) | .08 (.01) | .08 (.01) | .08 (.01) | .08 (.01) | .08 (.01) | .914 | .006 | .286 | .457 |
| Parietal | .07 (.01) | .07 (.01) | .09 (.02) | .09 (.01) | .07 (.01) | .07 (.01) | .08 (.02) | .08 (.02) | .020 | < .001 | .982 | .827 |
| Occipital | .06 (.01) | .06 (.01) | .10 (.02) | .11 (.02) | .06 (.01) | .06 (.01) | .10 (.02) | .11 (.02) | .931 | .829 | .882 | .661 |
| Thickness | ||||||||||||
| Frontal | 2.58 (.35) | 2.58 (.37) | 1.88 (.25) | 1.90 (.25) | 2.59 (.33) | 2.62 (.33) | 1.86 (.22) | 1.89 (.21) | .947 | .276 | .639 | .589 |
| Temporal | 2.63 (.35) | 2.54 (.32) | 1.73 (.13) | 1.76 (.15) | 2.74 (.35) | 2.68 (.34) | 1.80 (.19) | 1.81 (.15) | .129 | .149 | .927 | .514 |
| Parietal | 2.21 (.34) | 2.23 (.35) | 1.81 (.21) | 1.80 (.23) | 2.29 (.34) | 2.36 (.32) | 1.79 (.22) | 1.81 (.20) | .330 | .005 | .310 | .740 |
| Occipital | 1.89 (.31) | 1.83 (.31) | 1.78 (.17) | 1.77 (.15) | 1.85 (.29) | 1.80 (.24) | 1.74 (.17) | 1.77 (.15) | .521 | .685 | .649 | .725 |
3.1.1 Frontal lobe
We observed a significant group by type interaction (p=0.027) for the surface area with a small effect size (Cohen’s d=0.24, r=0.11). Frontal gyral surface area was reduced among offspring of schizophrenia parents compared to healthy control subjects. Further the main effect of group, and group by type interaction on the curvature showed trend toward significance. Frontal curvature was increased (p=0.096) at the sulcal regions (p=0.073) among offspring compared to healthy subjects. There were no differences in the frontal lobe cortical thickness. The reduction in the gyral surface area was approximately 2.5% among the offspring compared to controls at baseline (Table 1). No significant differences were observed in the frontal grey matter volume between the groups at baseline.
3.1.2. Parietal lobe
We observed significant group by type interactions on all three surface parameters surface area p=0.001; curvature p<0.001; thickness p=0.005). Gyral surface area and gyral cortical thickness were reduced by 8.6% and 4.5%, respectively among offspring compared to controls. Sulcal curvature was increased by 3.75% among the offspring compared to controls. The effect sizes were medium for the surface area (Cohen’s d=0.64, r=0.31) and curvature (Cohen’s d=0.57, r=0.27) whereas it was small for the thickness (Cohen’s d=0.31, r=0.15). We did not observe a significant main effect of study groups, group by hemisphere or group by hemisphere by type interactions (Table 1). No differences were observed between groups in parietal gray matter volume at baseline.
3.1.3. Temporal lobe
We observed a significant group by type interaction at the sulcal curvature (p=0.006) with a reduced sulcal curvature (6.25%) among offspring compared to controls. The effect size was medium (Cohen’s d=0.50, r=0.24). We did not observe a main effect of study groups, or significant group by hemisphere or group by type by hemisphere interactions (Table 1). In addition, no baseline differences were observed between groups in temporal gray matter volume.
3.1.4 Occipital lobe
We did not observe main effect of group or significant effect of interactions on any of the baseline surface or grey matter volume measurements (Table 1).
3.2. Longitudinal trajectory of cortical measures
Of the 64 subjects at baseline, 31 subjects were followed up at one year (offspring=16, healthy subjects=15). We did not observe differences in demographic, clinical and cortical surface parameters between those who were followed up and those who were not. The total brain surface area across the four lobes showed a significant change over time between the two groups, with high risk subjects showing greater reductions in total surface area by 1.4% compared to healthy subjects (F=5.40, p=0.021) after adjusting for age and gender. The declining trajectory in surface area resulted in significantly less cortical surface area in high risk subjects at follow-up (F=7.34, p=0.012). No interactions with brain hemisphere or other changes were observed in surface area between high risk and healthy subjects.
3.2.1 Frontal lobe
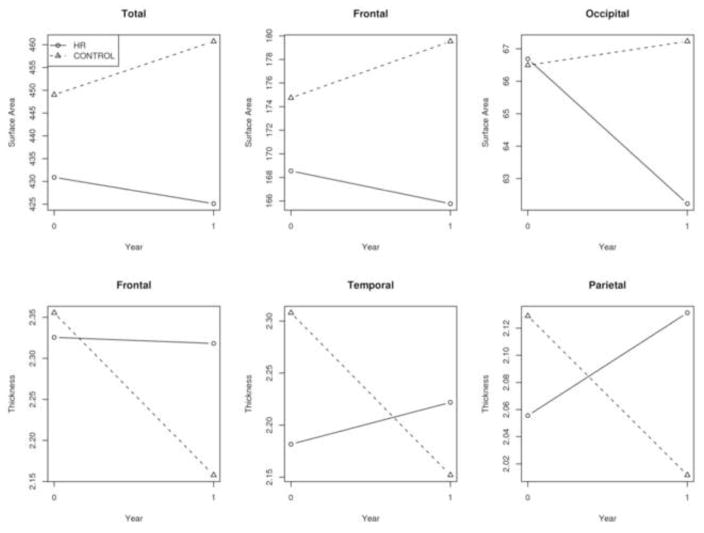
We observed bilateral frontal lobe surface area loss (F=7.26, p=0.008) among offspring of schizophrenia subjects compared to healthy subjects across both sulcal and gyral measures, resulting in smaller surface area by 8% in offspring compared to healthy subjects at follow-up (F=5.36, p=0.028) (Fig 2). Similarly, bilateral frontal lobe cortical thickness (F=18.75, p<0.001) showed highly significant differential changes across gyral and sulcal measurements, with offspring of schizophrenia parents demonstrating a maintenance or slight increase in thickness and healthy subjects demonstrating slightly decreased cortical thickness by 8.4% over time (Fig. 1). No significant interactions with hemisphere were observed. No significant difference in cortical thickness was observed at follow-up in these lobes.
Fig 2.
Progression of cortical surface areas over one year follow up among offspring and healthy subjects. Graphs show that the healthy subjects show increase or preservation of surface area while offspring consistently show a decrease in the frontal and occipital lobes.
3.2.2 Parietal lobe
showed thinning of cortex bilaterally (F=15.28, p<0.001) across both gyral and sulcal measurements, with offspring demonstrating a maintenance or slight increase in thickness and healthy subjects demonstrating decreases in cortical thickness by 5% over 1 year (Fig 1). No significant interactions with hemisphere or significant differences at follow-up were observed.
3.2.3 Temporal lobe
Bilateral temporal lobe cortical thickness (F=12.80, p<0.001) showed highly significant differential changes across gyral and sulcal measurements, with high risk subjects demonstrating a maintenance or slight increase in thickness and healthy subjects demonstrating decreases in cortical thickness by 6.8% in these regions over time (Fig 1). No significant interactions with hemisphere were observed. While significant differential changes were observed in thickness between high risk and healthy subjects, no significant differences at follow-up were observed. Only the left temporal sulcal mean curvature demonstrated differential change over time, with high risk subjects showing increased curvature during the year of study (F=4.16, p=0.043). In addition, at follow-up, high risk subjects demonstrated overall greater bilateral curvature in the temporal lobe compared to controls (F=5.14, p=0.032).
3.2.4 Occipital lobe
The occipital lobe surface (F=4.10, p=0.045) showed significant longitudinal surface area loss by 6.7% in high risk subjects compared to healthy subjects across both sulcal and gyral measures, resulting in smaller surface area by 7.9% in high risk subjects compared to healthy subjects at follow-up (F=4.51, p=0.043) (Fig 1). No interactions with brain hemisphere or other changes were observed in surface area between high risk and healthy subjects.
With regard to longitudinal volumetric changes, high risk subjects demonstrated significant differential loss of gray matter in all lobes examined compared to healthy subjects in the frontal (F=5.44, p=0.022), parietal (F=10.67, p=0.002), temporal (F=13.98, p<0.001) and occipital (F=9.14, p=0.003) lobes. However, cross-sectionally at follow-up, only occipital (F=6.91, p=0.014) and temporal (F=6.47, p=0.014) lobes demonstrated significant cross-sectional reductions in controls but not high risk subjects.
4. Discussion
The main findings of our study are a reduction in surface area along with preservation or slight increase in cortical thickness among high risk subjects compared to healthy subjects. Reduction in cortical surface area among high risk subjects was more prominently seen in the fronto-parietal lobes whereas thickness was preserved in the frontal, parietal and temporal lobes. Similar observations among schizophrenia subjects (Giedd et al. 1999; Gogtay et al. 2004) suggest that such reductions may predate the illness. These findings provide clues to detectable developmental brain alterations and their short term progression before the onset of the illness among those at genetically elevated risk for schizophrenia/schizoaffective disorder. Interestingly, correlations of surface parameters with volumes was observed for longitudinal changes but not for cross-sectional comparisons suggesting that the cortical surface parameters may be more sensitive indicators of genetic susceptibility to schizophrenia.
Examination of cortical surface parameters in a neurodevelopmental disorder such as schizophrenia provides important clues to the cortical development. We observed significant changes in all three surface parameters; cross-sectionally there were more widespread changes in the surface area and curvature at baseline whereas longitudinally shrinking of surface area associated with relative preservation of thickness among high risk subjects was noted. This could be because cortex tends to grow tangentially as opposed to radially. Evolutionary data suggests that the human cortex is estimated to have increased by more than 1000 times whereas the thickness has grown by 2–3 times (Price 2004). Such observations are important because cortical gyration is an intrinsic property of the growing brain and not a result of non-specific mechanical constraints of a rigid skull box. This is supported by studies that noted folding of the cortex in cultured hemispheres of mice in the absence of mechanical constraints of skull with the application of lipophosphatidic acid (LPA) to embryonic mouse hemispheres (Kingsbury et al. 2003). Expression of a stabilized form of β-catenin in CNS progenitor cells of transgenic mice increased the cortical surface area and induced cortical folding (Chenn and Walsh 2002, 2003). Differential growth of inner and outer cortical layers has also been associated with cortical gyration (Richman et al., 1975). Besides, folding of the cortex may be heritable because individual variations of several gyri and sulci are minimal, and does not appear to be random (Cheverud et al. 1990; Eckert et al. 2002). Thus, individuals genetically at an elevated risk for schizophrenia may be more vulnerable to preservation or expansion of the surface area.
Our observations of reduced gyral and sulcal surface area and increased curvatures in different lobes at baseline are intriguing. Although changes in surface area are relatively easier to conceptualize, changes in curvature merit description (Fig 1). In this study, with decreased gyral surface area, one would expect a decreased sulcal curvature if the pathology were to affect the cortical layers uniformly. Further, relatively preserved gyral curvature along with significant changes in sulcal curvature suggests that the pathology may affect gyral and sulcal layers differentially. Systematic studies examining variations of neuronal receptor density and morphology along the sulcogyral extent are sparse in the literature. One study that examined the variations in the distribution of glutamatergic NMDA receptors along the sulcogyral regions reported an increased immunoreactivity in lamina II and III in the gyral crown and gyral lips whereas it was barely detectable in the sulcus (Sisodiya et al. 2000). The same study observed that some individuals showed immunoreactivity in layers V and VI of the sulcus but not the gyral crowns. These authors rule out that these observations may be due to the section orientation, edge effects or other methodological differences. This differential distribution of NMDA receptors along the sulcogyral extent may assume significance during neurodevelopment because glutamate transmission has been associated with proper neuronal migration and axonal guidance (Rio et al. 1997; Yau et al. 2003). Taken together, altered neuronal migration possibly mediated by glutamate transmission may underlie such observations.
The progression of cortical surface parameters during adolescence could provide additional clues to the trajectory of neurobiological changes leading up to the clinical manifestation of schizophrenia. Fetal developmental data reviewed earlier (van der Knaap et al. 1996), dynamic mapping of healthy subjects (Gogtay et al. 2004) and longitudinal imaging of schizophrenia subjects (Gur et al. 1998; Lieberman et al. 2005; Nakamura et al. 2007) suggest a relatively predictable pattern of neurodevelopment. Our observations of differing trajectories in the surface area and curvature over a relatively short period of follow up among offspring and healthy subjects suggest an altered trajectory of neurodevelopment in the study groups (Fig 2). Taking the observations at baseline and follow up together, there were differences in the progression of cortical surface characteristics. Frontal and parietal surface areas showed reductions at baseline but only frontal lobe continued to show decreases at follow up. This could suggest that the fronto-parietal surface areas may start to show reduction earlier than 16 years age, and only frontal lobe continues to show ‘shrinking’ of surface area. In contrast, surface measures of the occipital lobe were not different at baseline but started to show progressive reduction over 1 year.
Compared to this pattern, healthy subjects showed either an increase or preservation of surface characteristics in these regions suggesting different trajectories of neurodevelopment in the groups. In contrast, we observed cortical thinning only in the parietal gyral regions at baseline. However, the longitudinal trajectory showed that the thickness remained almost the same whereas healthy subjects were showing thinning. These observations suggest that while healthy adolescents show increasing neuropil endowments through expanding the cortical surface area along with cortical thinning offspring show shrinking of surface area while maintaining the cortical thickness. Regardless of the mechanism, these observations suggest a divergent trajectory of neurodevelopment.
MRI volumetric and post-mortem studies support this. MRI volumetric studies on healthy children and adolescents have noted that the grey matter volume peaked in the frontal and parietal lobes at about 12 years of age and temporal lobe at 16 years among healthy children and adolescents after which a reduction was observed (Giedd et al. 1999). Another MRI study noted similar pattern of grey matter loss starting from the dorsal parietal and sensorimotor regions that spread laterally and caudally to the temporal cortices and anteriorly to the dorsolateral prefrontal regions (Gogtay et al. 2004). Phosphorus (31P) magnetic resonance spectroscopy (MRS) - a non-invasive technique that provides in vivo data on synaptic/dendritic dynamics - studies on young relatives of subjects with schizophrenia have reported decreased freely-mobile phosphomonoesters (free-PME) and increased freely-mobile phosphodiesters (free-PDE) (Keshavan et al. 2003). Subjects with schizophrenia showed similar abnormalities in membrane phospholipid metabolism in the prefrontal cortex (Pettegrew et al. 1991). PMEs reflect the membrane phospholipid precursors and PDEs represent the breakdown products. Higher free-PME are observed during the time and site of neuritic sprouting whereas elevated free-PDEs are observed at the site and time of neuronal membrane breakdown (Geddes et al. 1997). A longitudinal developmental study noted a positive correlation of the PME/PDE ratio with grey matter volumes in healthy children and adolescents (Goldstein et al. 2009) supporting these observations. Thus, the pattern observed in the above 31P spectroscopy studies suggests an increased breakdown and decreased synthesis of membrane phospholipid that may suggest increased synaptic pruning; however, the contribution of other neuronal events such as decreased neuronal size cannot be ruled out. Additional support for such a possibility comes from postmortem studies that have reported a phase of net synaptic elimination to start in the auditory cortex that ends by 12 years of age whereas in the prefrontal cortex, it was observed to extend into mid-adolescence (Huttenlocher 1979; Huttenlocher and Dabholkar 1997). Furthermore, decreased spine density (Garey et al. 1998; Glantz and Lewis 2000), increased neuronal density in area 9 (Selemon et al. 1995) and 46 (Selemon et al. 1998) associated with smaller neuronal somal size in 9 (Selemon et al. 1995) and area 46 (Rajkowska and Goldman-Rakic 1995a, b) have been noted in subjects with schizophrenia. Taking the MRI and post-mortem studies together, our observations suggest that subjects at an elevated risk for schizophrenia show significant reduction in neuropil density in certain regions of the cortex over a 1-year follow up. Given the mean age of offspring and healthy subjects in our sample, such reductions may be due to an early start of synaptic pruning or increased pruning among the offspring of schizophrenia/schizoaffective disorder probands compared to healthy subjects.
Our observations on the correlation of surface area measures with lobar volumes merit discussion. While the lobar volumes showed reductions, cortical surface parameters showed more extensive alterations that correlated with the pattern observed in previous studies (Gogtay et al. 2004; Schmitt et al. 2008; Thompson et al. 2001a). This may suggest that the extent of subtler neurodevelopmental changes may not be detectable by gross volumetric changes supporting our earlier premise that the cortical surface measurements may be more useful.
Some of the strengths of our study are the use of a comprehensive set of cortical surface parameters in young individuals genetically at an elevated risk for schizophrenia before the clinical manifestation of psychosis. We have examined the baseline differences as well as the longitudinal trajectory of these measures that show convergent results. Some weaknesses of the study include a relatively smaller sample size for longitudinal comparisons that was mainly due to the fact that this is an ongoing study. In addition, we have used lobar measures that may not reveal differences at key brain regions involved in processing specific cognitive functions.
In summary, we report that offspring of schizophrenia parents show systematic differences in cortical surface area and thickness compared to healthy subjects. In addition, the trajectory of cortical development was different among offspring and healthy subjects during a short term follow up. Examining associations between neurobiological changes, and neuropsychological and behavioral data could provide better insights into the contribution of these neurobiological changes to the clinical manifestation of schizophrenia. Incorporating neuropsychological and behavioral data along with cortical measures could help in formulating a data-driven “close-in” approach to identify ultra-high risk subjects who develop schizophrenia later in their lives. Such an approach could potentially provide clues to develop preventive interventions. One of our preliminary studies that incorporated neuropsychological, psychopathological and MRI data demonstrates such an attempt (Eack et al. 2008). Longer term follow-up of these cortical changes along with neuropsychological performance and prodromal symptoms could clarify the bio-behavioral evolution of schizophrenia.
Acknowledgments
Authors wish to sincerely acknowledge the help of following individuals: Ms. Karol Rosengarth for her assistance in quality assurance of the data collection, Dr. Debra Montrose, Ms. Diana Dworakowski, Mr. Govner Thorp and Dr. Liz Radomsky for their efforts in characterizing the study subjects, and Ms. Jean Miewald for database management.
5. 1.Role of Funding Source
This work was funded by MH64023 and NARSAD Established Investigator Award (MSK), and MH72995 (KMP). The funding agencies did not have any further role in the design or execution of the study, analyses and preparation of the manuscript.
Footnotes
5.2. Contributors
KMP and MSK designed this study, and did the literature search. DG, MR, TM and JN conducted the imaging analyses under the supervision of KMP and MSK. KMP, SE and MSK did the statistical analyses and interpreted the results of the analyses. KMP wrote the manuscript that was edited, modified and revised by MSK, RR, and SE. All authors contributed to and approved the final manuscript.
5.3. Conflict of Interest
All authors declare no conflict of interest with the design, collection, analyses and interpretation of the results of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297(5580):365–9. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- Chenn A, Walsh CA. Increased neuronal production, enlarged forebrains and cytoarchitectural distortions in beta-catenin overexpressing transgenic mice. Cereb Cortex. 2003;13(6):599–606. doi: 10.1093/cercor/13.6.599. [DOI] [PubMed] [Google Scholar]
- Cheverud JM, Falk D, Vannier M, Konigsberg L, Helmkamp RC, Hildebolt C. Heritability of brain size and surface features in rhesus macaques (Macaca mulatta) The Journal of heredity. 1990;81(1):51–7. doi: 10.1093/oxfordjournals.jhered.a110924. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA, Prasad KM, Keshavan MS. Approaches for adolescents with an affected family member with schizophrenia. Curr Psychiatry Rep. 2004;6(4):296–302. doi: 10.1007/s11920-004-0080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Prasad KM, Montrose DM, Goradia DD, Dworakowski D, Miewald J, Keshavan MS. An integrated psychobiological predictive model of emergent psychopathology among young relatives at risk for schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008 doi: 10.1016/j.pnpbp.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Molloy EA, Blumenthal J, Zijdenbos A, Giedd JN. The epigenesis of planum temporale asymmetry in twins. Cereb Cortex. 2002;12(7):749–55. doi: 10.1093/cercor/12.7.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB. The Structured Clinical Interview for DSM-IV for Axis I disorders: Clinical Version, Administration Booklet. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- Fornito A, Yung AR, Wood SJ, Phillips LJ, Nelson B, Cotton S, Velakoulis D, McGorry PD, Pantelis C, Yucel M. Anatomic Abnormalities of the Anterior Cingulate Cortex Before Psychosis Onset: An MRI Study of Ultra-High-Risk Individuals. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.05.032. [DOI] [PubMed] [Google Scholar]
- Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, Barnes TR, Hirsch SR. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65(4):446–53. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes JW, Panchalingam K, Keller JN, Pettegrew JW. Elevated phosphocholine and phosphatidylcholine following rat entorhinal cortex lesions. Neurobiol Aging. 1997;18(3):305–8. doi: 10.1016/s0197-4580(97)80312-0. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57(1):65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Goghari VM, Rehm K, Carter CS, MacDonald AW. Sulcal thickness as a vulnerability indicator for schizophrenia. Br J Psychiatry. 2007a;191:229–33. doi: 10.1192/bjp.bp.106.034595. [DOI] [PubMed] [Google Scholar]
- Goghari VM, Rehm K, Carter CS, MacDonald AW., 3rd Regionally specific cortical thinning and gray matter abnormalities in the healthy relatives of schizophrenia patients. Cereb Cortex. 2007b;17(2):415–24. doi: 10.1093/cercor/bhj158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101(21):8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman AL, Pezawas L, Mattay VS, Fischl B, Verchinski BA, Chen Q, Weinberger DR, Meyer-Lindenberg A. Widespread reductions of cortical thickness in schizophrenia and spectrum disorders and evidence of heritability. Arch Gen Psychiatry. 2009;66(5):467–77. doi: 10.1001/archgenpsychiatry.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein G, Panchalingam K, McClure RJ, Stanley JA, Calhoun VD, Pearlson GD, Pettegrew JW. Molecular neurodevelopment: an in vivo 31P-1H MRSI study. J Int Neuropsychol Soc. 2009;15(5):671–83. doi: 10.1017/S1355617709990233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Shields J. Contributions of twin studies to perspectives on schizophrenia. Prog Exp Pers Res. 1966;3:1–84. [PubMed] [Google Scholar]
- Gottesman II, Shields J. Genetic theorizing and schizophrenia. Br J Psychiatry. 1973;122(566):15–30. doi: 10.1192/bjp.122.1.15. [DOI] [PubMed] [Google Scholar]
- Gottesman II. Schizophrenia genesis: the origins of madness. New York: W. H. Freeman; 1991. [Google Scholar]
- Gur RE, Cowell P, Turetsky BI, Gallacher F, Cannon T, Bilker W, Gur RC. A follow-up magnetic resonance imaging study of schizophrenia. Relationship of neuroanatomical changes to clinical and neurobehavioral measures. Arch Gen Psychiatry. 1998;55(2):145–52. doi: 10.1001/archpsyc.55.2.145. [DOI] [PubMed] [Google Scholar]
- Hilgetag CC, Barbas H. Developmental mechanics of the primate cerebral cortex. Anat Embryol (Berl) 2005;210(5–6):411–7. doi: 10.1007/s00429-005-0041-5. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163(2):195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387(2):167–78. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Imaging Lab Iowa Neuroimaging Center. BRAINS2 Standard Workup Manual. Iowa City, IA: University of Iowa Hospitals and Clinics; 2006. [Google Scholar]
- Job DE, Whalley H, Johnstone EC, Lawrence E. Grey matter changes over time in high risk subjects developing schizophrenia. Neuroimage. 2005;25(4):1023–1030. doi: 10.1016/j.neuroimage.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent DA, Ryan ND, Rao U. K-Sads-Pl. J Am Acad Child Adolesc Psychiatry. 2000;39(10):1208. doi: 10.1097/00004583-200010000-00002. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Montrose DM, Pierri JN, Dick EL, Rosenberg D, Talagala L, Sweeney JA. Magnetic resonance imaging and spectroscopy in offspring at risk for schizophrenia: preliminary studies. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21(8):1285–95. doi: 10.1016/s0278-5846(97)00164-4. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Stanley JA, Montrose DM, Minshew NJ, Pettegrew JW. Prefrontal membrane phospholipid metabolism of child and adolescent offspring at risk for schizophrenia or schizoaffective disorder: an in vivo (31)P MRS Study. Mol Psychiatry. 2003;8(3):316–323. doi: 10.1038/sj.mp.4001325. [DOI] [PubMed] [Google Scholar]
- Kingsbury MA, Rehen SK, Contos JJ, Higgins CM, Chun J. Non-proliferative effects of lysophosphatidic acid enhance cortical growth and folding. Nat Neurosci. 2003;6(12):1292–9. doi: 10.1038/nn1157. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Whalley H, Kestelman JN, Abukmeil SS, Byrne M, Hodges A, Rimmington JE, Best JJ, Owens DG, Johnstone EC. Magnetic resonance imaging of brain in people at high risk of developing schizophrenia. Lancet. 1999;353(9146):30–3. doi: 10.1016/S0140-6736(98)06244-8. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Tollefson GD, Charles C, Zipursky R, Sharma T, Kahn RS, Keefe RS, Green AI, Gur RE, McEvoy J, Perkins D, Hamer RM, Gu H, Tohen M. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry. 2005;62(4):361–70. doi: 10.1001/archpsyc.62.4.361. [DOI] [PubMed] [Google Scholar]
- Magnotta VA, Andreasen NC, Schultz SK, Harris G, Cizadlo T, Heckel D, Nopoulos P, Flaum M. Quantitative in vivo measurement of gyrification in the human brain: changes associated with aging. Cereb Cortex. 1999;9(2):151–60. doi: 10.1093/cercor/9.2.151. [DOI] [PubMed] [Google Scholar]
- Magnotta VA, Harris G, Andreasen NC, O’Leary DS, Yuh WT, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Comput Med Imaging Graph. 2002;26(4):251–64. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Murray RM, Lewis SW. Is schizophrenia a neurodevelopmental disorder? Br Med J (Clin Res Ed) 1987;295(6600):681–2. doi: 10.1136/bmj.295.6600.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Salisbury DF, Hirayasu Y, Bouix S, Pohl KM, Yoshida T, Koo MS, Shenton ME, McCarley RW. Neocortical gray matter volume in first-episode schizophrenia and first-episode affective psychosis: a cross-sectional and longitudinal MRI study. Biol Psychiatry. 2007;62(7):773–83. doi: 10.1016/j.biopsych.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettegrew JW, Keshavan MS, Panchalingam K, Strychor S, Kaplan DB, Tretta MG, Allen M. Alterations in brain high-energy phosphate and membrane phospholipid metabolism in first-episode, drug-naive schizophrenics. A pilot study of the dorsal prefrontal cortex by in vivo phosphorus 31 nuclear magnetic resonance spectroscopy. Arch Gen Psychiatry. 1991;48(6):563–8. doi: 10.1001/archpsyc.1991.01810300075011. [DOI] [PubMed] [Google Scholar]
- Price DJ. Lipids make smooth brains gyrate. Trends Neurosci. 2004;27(7):362–4. doi: 10.1016/j.tins.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. Remapping of areas 9 and 46 using quantitative criteria. Cereb Cortex. 1995a;5(4):307–22. doi: 10.1093/cercor/5.4.307. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the Talairach Coordinate System. Cereb Cortex. 1995b;5(4):323–37. doi: 10.1093/cercor/5.4.323. [DOI] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241(4862):170–6. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Rio C, Rieff HI, Qi P, Khurana TS, Corfas G. Neuregulin and erbB receptors play a critical role in neuronal migration. Neuron. 1997;19(1):39–50. doi: 10.1016/s0896-6273(00)80346-3. [DOI] [PubMed] [Google Scholar]
- Schmitt JE, Lenroot RK, Ordaz SE, Wallace GL, Lerch JP, Evans AC, Prom EC, Kendler KS, Neale MC, Giedd JN. Variance decomposition of MRI-based covariance maps using genetically informative samples and structural equation modeling. Neuroimage. 2009;47(1):56–64. doi: 10.1016/j.neuroimage.2008.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt JE, Lenroot RK, Wallace GL, Ordaz S, Taylor KN, Kabani N, Greenstein D, Lerch JP, Kendler KS, Neale MC, Giedd JN. Identification of Genetically Mediated Cortical Networks: A Multivariate Study of Pediatric Twins and Siblings. Cereb Cortex. 2008;18(8):1737–1747. doi: 10.1093/cercor/bhm211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex. A morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry. 1995;52(10):805–18. doi: 10.1001/archpsyc.1995.03950220015005. discussion 819–20. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Elevated neuronal density in prefrontal area 46 in brains from schizophrenic patients: application of a three-dimensional, stereologic counting method. J Comp Neurol. 1998;392(3):402–12. [PubMed] [Google Scholar]
- Sisodiya SM, Heffernan J, Harrison PJ, Squier MV, Thom M. Sulcogyral variation in NMDA receptor 2A/B subunit immunoreactivity in human brain. Neuroreport. 2000;11(11):2601–6. doi: 10.1097/00001756-200008030-00050. [DOI] [PubMed] [Google Scholar]
- Sun D, Phillips L, Velakoulis D, Yung A, McGorry PD, Wood SJ, van Erp TG, Thompson PM, Toga AW, Cannon TD, Pantelis C. Progressive brain structural changes mapped as psychosis develops in ‘at risk’ individuals. Schizophr Res. 2009;108(1–3):85–92. doi: 10.1016/j.schres.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen VP, Huttunen M, Lonnqvist J, Standertskjold-Nordenstam CG, Kaprio J, Khaledy M, Dail R, Zoumalan CI, Toga AW. Genetic influences on brain structure. Nat Neurosci. 2001a;4(12):1253–8. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Vidal C, Giedd JN, Gochman P, Blumenthal J, Nicolson R, Toga AW, Rapoport JL. From the Cover: Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. PNAS. 2001b;98(20):11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Knaap MS, van Wezel-Meijler G, Barth PG, Barkhof F, Ader HJ, Valk J. Normal gyration and sulcation in preterm and term neonates: appearance on MR images. Radiology. 1996;200(2):389–96. doi: 10.1148/radiology.200.2.8685331. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385(6614):313–8. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44(7):660–9. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Yau HJ, Wang HF, Lai C, Liu FC. Neural development of the neuregulin receptor ErbB4 in the cerebral cortex and the hippocampus: preferential expression by interneurons tangentially migrating from the ganglionic eminences. Cereb Cortex. 2003;13(3):252–64. doi: 10.1093/cercor/13.3.252. [DOI] [PubMed] [Google Scholar]