Abstract
BACKGROUND AND PURPOSE: The application of volumetric techniques to preterm infants has revealed brain volume reductions. Such quantitative data are not available in routine neonatal radiologic care. The objective of this study was to develop simple brain metrics to compare brain size in preterm and term infants and to correlate these metrics with brain volumes from volumetric MR imaging techniques.
MATERIALS AND METHODS: MR images from 189 preterm infants <30 weeks’ gestational age or <1250 g birthweight scanned at term-equivalent age and 36 term infants were studied. Fifteen tissue and fluid measures were systematically evaluated on 4 selected sections. The results were correlated with total brain, gray matter, white matter, and CSF volumes.
RESULTS: The mean bifrontal, biparietal, and transverse cerebellar diameters were reduced (−11.6%, 95% confidence interval [CI], −13.8% to −9.3%; −12%, 95% CI, −14% to −9.8%; and −8.7%, 95% CI, −10.5% to −7% respectively) and the mean left ventricle diameter was increased (+22.3%, 95% CI, 2.9%–41.6%) in preterm infants (P < .01). Strong correlations were found between the bifrontal and biparietal measures with total brain tissue volume, whereas the size of the ventricles and the interhemispheric measure correlated with CSF volume. Intraobserver reliability was high (intraclass correlation coefficients [ICC], >0.7), where interobserver agreement was acceptable for tissue measures (ICC, >0.6) but lower for fluid measures (ICC, <0.4)
CONCLUSIONS: Simple brain metrics at term-equivalent age showed smaller brain diameters and increased ventricle size in preterm infants compared with full-term infants. These measures represent a reliable and easily applicable method to quantify brain growth and assess brain atrophy in this at-risk population.
The survival rates for preterm infants have increased steadily over the past 2 decades.1,2 Alongside increased survival rates, there is continuing recognition of the high prevalence of motor, cognitive, and behavioral disabilities observed in survivors of preterm birth.3 The major neuropathologies recognized on cranial sonography in the preterm infant, such as intraventricular hemorrhage and cystic periventricular leukomalacia (PVL), have remained stable or declined in prevalence during this period,4 without accompanying improvements in outcomes. It is now increasingly recognized that the “cerebral lesions” responsible for these broad ranges of disabilities may not be visible on cranial sonography and may involve altered cerebral development as well as injury.
Quantitative evaluation of cerebral structures in at-risk preterm infants may assist in defining the impact of preterm birth on cerebral development. Previous approaches to this have included quantitative volumetric MR imaging techniques as well as cranial sonography measures. Cranial sonography has shown a decrease in the rate of growth of the corpus callosum in preterm infants, which has correlated with later outcomes.5 MR imaging techniques have evaluated total and regional cerebral volumes by tissue segmentation and voxel-based morphometry. These studies have strongly suggested a reduction in total cerebral volumes with regional variation that is apparent at term-equivalent age6 and persists into childhood.7
An alternative biometric approach to cerebral growth has been developed in fetal brain imaging by applying a set of measurements to the qualitative MR image, including the biparietal diameter or the transverse cerebellar diameter.8 These measures showed a close correlation with maturity.9 Thus, we adapted this methodology for a simple quantitative determinant of brain size in preterm infants approaching term age. The primary objectives of this study were the following: 1) to develop a simple metric for the evaluation of brain size in preterm infants scanned at term-corrected age and to compare the results obtained between preterm and full-term infants, and 2) to compare the results with the quantitative volumetric MR imaging data on the same cohort.
Materials and Methods
Subjects
Our study was conducted on existing MR imaging datasets collected on a prospective longitudinal cohort study of preterm infants born <1250 g or <30 weeks’ gestational age and term-born control infants. The gestational age was based on maternal last menstrual date or dating by earliest sonogram. The preterm infants were admitted between April 2001 and December 2003 to the Royal Women's Hospital in Melbourne, Australia. Perinatal data (ie, sociodemographic and obstetric data, birth characteristics, neonatal therapies, and morbidities) were obtained by chart review. For an early detection of brain lesions including intraventricular hemorrhage (IVH), cranial sonograms were obtained serially throughout the neonatal intensive care course in all infants within the first 48 hours and at ages 4–7 days and 4–6 weeks.
During the study period, 348 eligible preterm infants were admitted and 236 (64%) were recruited. There were no significant differences in obstetric or birth data between infants recruited compared with those not recruited (data not shown). Infants with known or suggested brain malformation or congenital abnormalities (n = 4) were excluded. Infants were also excluded if there was insufficient or a suboptimal quality of MR imaging (n = 39) or perinatal data (n = 4). Thus, MR images were studied for 189 preterm infants. Fifty-one term controls were also enrolled; 13 were excluded for technical issues related to the acquisition of the MR images, and 2, for proved neonatal sepsis. Thus MR images were analyzed for 36 term controls.
MR Imaging
All infants were scanned at term or term-equivalent age without sedation. Infants were fed, swaddled, outfitted with earphones, and placed in a vacuum-fixation bean bag. Sleeping infants were scanned in a 1.5T Sigma System MR imaging scanner with a pediatric quadrature head coil (GE Healthcare, Milwaukee, Wis), located at the Royal Children's Hospital, Melbourne. The 2 different imaging modes applied were the following: 3D T1-weighted spoiled gradient-recalled (1.2-mm coronal sections; flip angle, 45°; TR, 35 ms; TE, 9 ms; FOV, 21 × 15 cm2; matrix, 256 × 192) and T2-weighted dual-echo (interleaved acquisition) fast-recovery fast spin-echo sequences (2-mm coronal; TR, 4000 ms; TE, 60/160 ms; FOV, 22 × 16 cm2; matrix, 256 × 192; interpolated, 512 × 512). Coronal T2-weighted sections were available for 189 preterm infants and 36 term controls, axial T2-weighted sections were available for 50 preterm infants and 8 term controls, and sagittal T1-weighted sections were available for 74 preterm infants and 13 term controls. Due to the small number of axial T1-weighted sections, only the coronal T2-weighted MR images and the sagittal T1-weighted images were studied.
MR Imaging Analysis
Brain Metrics.
The MR images were displayed by using a DICOM browser (DicomWorks, http://dicom.online.fr/). Fifteen parameters divided into “tissue” measures (ie, bifrontal diameter, left and right frontal height, brain and bone biparietal diameter, frontal-occipital diameter, length of the corpus callosum, surface of the vermis, and transverse cerebellar diameter), “fluid” measures of the pericerebral space (interhemispheric distance, craniocaudal left and right interopercular distances), and the intracerebral spaces (diameters of the left and right lateral ventricles and the third ventricle) were manually measured on 4 selected sections. Sections and landmarks are detailed in On-line Table 1 and Fig 1.
Fig 1.
A and B, Selected sections and landmarks (see On-line Table 1) used to calculate the coronal brain metrics with examples of a full-term (A) and a preterm infant (B)
The metrics on the coronal sections were tested for reliability. We calculated the interobserver correlation from repeating measurements on 7 scans by 3 different observers. The intraobserver correlation was calculated from 3 scans measured twice by each of the 3 observers. A screen copy of each section selected for measurement was produced by the observers. They were checked to search for the source of variability relating to the section selection.
The brain metrics were compared with the growth curves of normative data produced from fetal MR imaging brain assessment (biparietal diameter, fronto-occipital diameter, and transverse cerebellar diameter).
Qualitative MR Imaging Analysis.
MR images were scored by using a standardized scoring system.10 White matter abnormality was graded according to 5 scales, which assessed 1) the nature and extent of white matter signal intensity abnormality, 2) the loss of periventricular white matter, 3) the presence of cysts, 4) the degree of ventricular dilation, and 5) the thinning of the corpus callosum. The scores from individual scales were then combined to give an overall white matter abnormality score categorized as normal, mild, moderate, or severe.
Quantitative Volumetric MR Imaging Analysis.
A previous volumetric analysis by using Sun Microsystems workstations (Palo Alto, Calif) has been reported.6 The parameters retained for analysis in the current study were the volumes of the total brain tissue, the cortical gray matter, the myelinated and unmyelinated white matter, the deep nuclear gray matter, and the CSF.
Statistical Analyses
Statistical analysis was performed by using the Statistical Package for the Social Sciences, Version 15 (SPSS, Chicago, Ill). Study group characteristics were evaluated by using t tests for continuous variables and χ2 tests for categoric variables. Exploratory analysis indicated that brain tissue metrics were approximately normally distributed and that the distribution of fluid measures was left skewed. For univariate analysis, a t test or a Kruskal-Wallis nonparametric test was used as appropriate to study the effect of the group (preterm versus full-term) and the sex on brain metrics. The Pearson correlation coefficient was used to study the effects of gestational age at MR imaging on brain metrics. A general linear model approach was used to model the most relevant brain metrics as a function of the study group and sex, with gestational age at MR imaging as a covariate. The relationships between these brain metrics and brain volumes were studied by the Pearson correlation coefficient. The intraclass correlation coefficients (ICC) for consistency (2-way random model, single measure) were calculated to analyze the inter- and intraobserver agreement.
Results
Subjects
The characteristics of the cohort are presented in On-Line Table 2. The rate of multiple pregnancies was relatively high (42%). More than 80% of MR imaging findings were considered as qualitatively normal or mildly abnormal. The mean gestational age at MR imaging was 40.1 ± 1.3 weeks (range, 36–44 weeks), though the preterm infants were younger than the term infants at MR imaging (t = −2.4, P = .02). The head circumference and the weight at MR imaging were significantly smaller in preterm infants, before and after adjustment for gestational age at MR imaging (t = −4.2, P < .001 for weight; t = −2.4, P = .016 for head circumference).
Reproducibility
The intrarater and inter-rater consistencies were high for all the tissue measures, with ICC coefficients >0.8 (On-line Table 3). For the fluid measures, the intrarater consistency was acceptable but the inter-rater consistency was low due to some discrepancies in the choice between adjacent sections.
Brain Metrics
Results of brain metrics are presented in On-line Table 4.
The measures obtained in the full-term infants were compared visually with those previously published with the same methodology on fetal MR images (Fig 2) and were found to be in accordance with these values. The term infants seemed to display a slightly larger transverse cerebellar diameter than the prenatal cohort scanned at 37–38 weeks’ gestational age.
Fig 2.
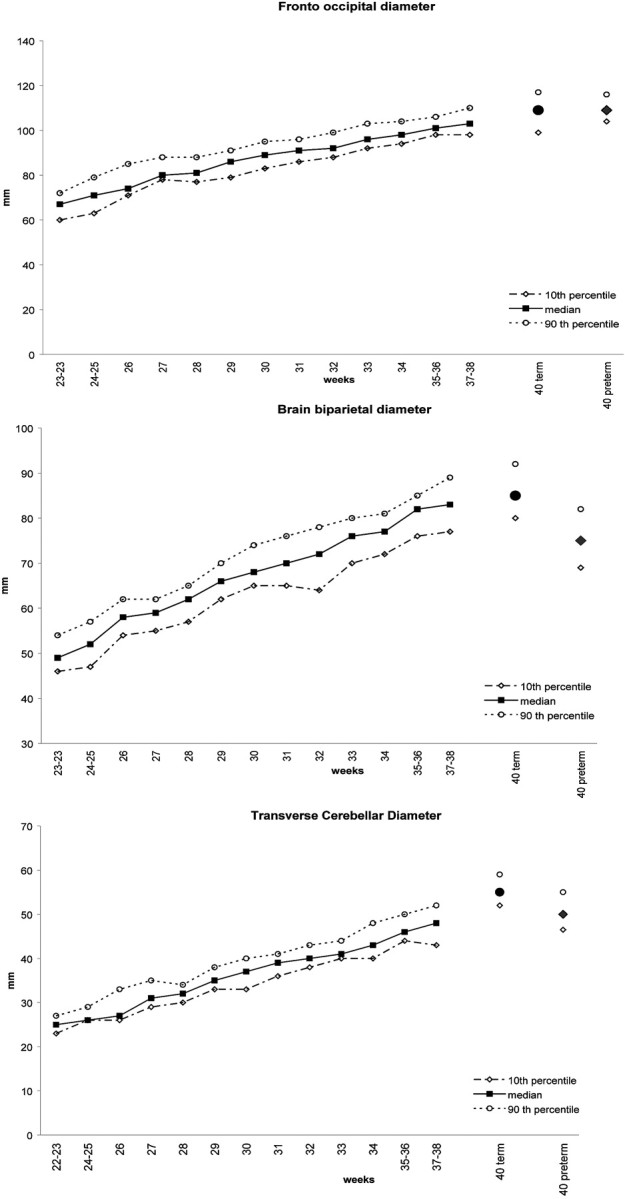
Comparison (median, 10th–90th percentiles) of the fronto-occipital, biparietal, and transverse cerebellar diameters by weeks of gestational age measured in term infants (circle) and in the preterm infants (diamond), with the results obtained during pregnancy by measuring fetal brains (square) with the same methodology. Adapted with permission from Garel.9
Three tissue measures were markedly decreased in preterm infants compared with full-term infants (Fig 3). These included the bifrontal diameter, the brain and bone biparietal diameters, and the transverse cerebellar diameter. A smaller reduction of the frontal heights was also observed, significant only for the left side. The fronto-occipital diameter, the surface of the vermis, and the length of the corpus callosum did not differ between the 2 groups. For fluid measures, the pericerebral space was larger in preterm infants in both the interhemispheric distance and extra-axial space. Preterm infants also had larger lateral ventricles than term infants with the difference being significant only for the left side.
Fig 3.
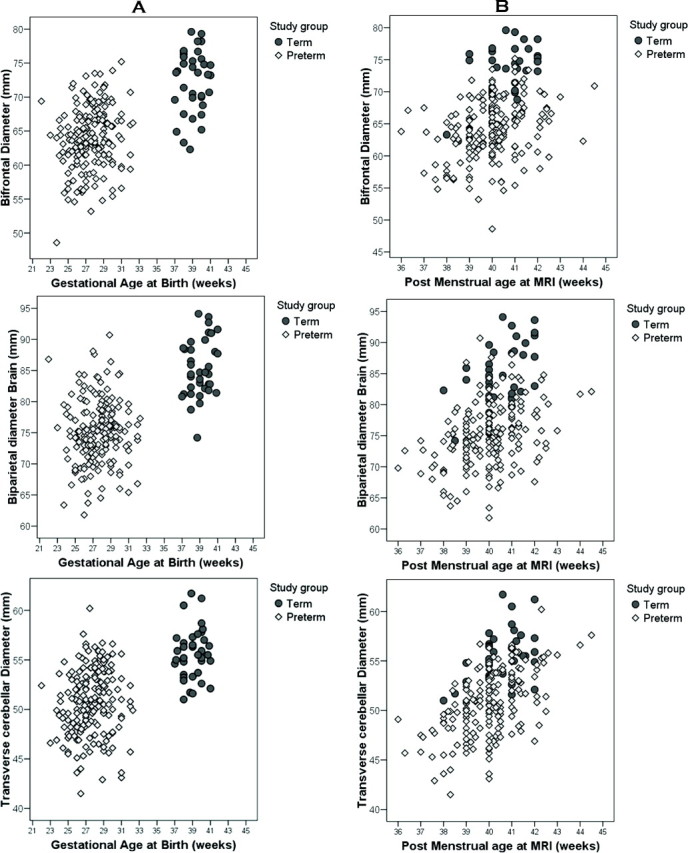
A and B, Comparison of bifrontal, biparietal, and transverse cerebellar diameters in preterm infants (diamond) and full-term infants (circle) by their gestational age at birth (A) and by their postmenstrual age at the time of MR imaging (B), demonstrating the lower values obtained in the preterm cohort.
In both groups, there was a significant correlation between gestational age at MR imaging and tissue brain metrics (Fig 3B), with no interaction between this variable and the study group. There was no effect of the gestational age at MR imaging on the fluid measures.
Examination of sex differences demonstrated that the bifrontal and the biparietal diameters were larger in boys (+4.2%; 95% confidence interval [CI], 2%–6.4%, P < .001; and +2.5%, 95% CI, 0.43%–4.68%, P = .019, respectively). Sex differences were not observed for the transverse cerebellar diameter and the frontal heights or for any of the fluid measures.
A multivariate analysis by linear regression was undertaken for the tissue measures demonstrating differences in preterm infants, with a relatively narrow CI in the bifrontal diameter, the biparietal diameter, and the transverse cerebellar diameter. The results are presented in On-line Table 5, along with the same analysis for the fronto-occipital diameter and the length of corpus callosum. There was an effect of group, associated with the effects of gestational age and sex, for the bifrontal and biparietal diameters with no interaction between these variables. The transverse cerebellar diameter showed equivalent effects of group and gestational age at MR imaging, but no gender difference. For the fronto-occipital diameter and the length of the corpus callosum, only the gestational age at MR imaging had an effect (significant only for the fronto-occipital diameter).
Correlations with Brain Volumes and Head Circumference
Quantitative volumetric data were available for 178 preterm infants and 29 term control infants. Correlations between brain metrics and volumes are reported in On-line Table 6. The tissue measurements (ie, bifrontal diameter, frontal heights, biparietal diameter, and transverse cerebellar diameter) were well correlated with the brain tissues volumes (total brain tissue, cortical gray matter, unmyelinated white matter, and deep nuclear gray matter) except for the myelinated white matter. The strongest relationships were seen between the transverse cerebellar, bifrontal, and biparietal diameters and the total brain tissue and cortical gray matter volumes.
The fluid measures correlated well with the total volume of CSF (r > 0.4, P < .01). There was also a negative correlation between the lateral ventricle measures and the volume of the deep nuclear gray matter (On-line Table 6).
Head circumference was measured at MR imaging for 188 preterm infants and 35 controls. Head circumference was significantly correlated with the brain metrics, though the correlation coefficients were lower than those obtained between the brain metrics and the brain volumes (On-line Table 6).
Discussion
This study demonstrates that measuring the diameters of the cerebral structures and the cerebral cavity on raw MR images at term may represent a simple and reliable approach for the evaluation of brain size and hence brain growth in the at-risk infant. Our results show striking differences between preterm and full-term infants. The bifrontal cerebral, biparietal cerebral, and transverse cerebellar diameters were clearly reduced in preterm infants, without an increase in the fronto-occipital diameter. The ventricular size was larger in preterm infants; the difference was significant for only the left side. In preterm infants as in full-term infants, the gestational age at MR imaging was correlated with the cerebral diameters, which continued to increase during the last weeks of gestation. The ventricular size and the extra-axial space dimensions were not affected by the gestational age at MR imaging. Sex influenced some parameters, with males having larger bifrontal and biparietal diameters. Brain metrics, as 1D measures on qualitative MR images, were well correlated to the 3D volumetric data previously calculated in this cohort and were also correlated, to a lesser degree, with the head circumference.
The measures obtained in our full-term infants appear in accordance with those previously published with the same methodology on fetal MR images.9 The transverse cerebellar diameter observed in the term infants scanned at 40 weeks postmenstrual age was slightly larger than the fetal brain results obtained at 37–38 weeks gestational age. This finding is likely to be explained by the rapid growth rate of the cerebellum during the last trimester.11 Less data exist about such MR imaging measures used postnatally. In 1 study comparing sonography and MR imaging measures in 26 preterm and 8 term infants scanned after 37 weeks, measures for the length of the corpus callosum and transverse cerebellum similar to those in our study were documented.12
Mewes et al13 also observed a similar length for the corpus callosum, splenium of the corpus callosum, the bitemporal diameter, and the fronto-occipital diameter in 20 term infants. This study then compared these measures with 24 low-risk preterm infants at term after reconstruction of the sagittal and axial sections and alignment on the Talairach atlas. The authors found results similar to ours for the length of the corpus callosum (no difference) and the bitemporal diameter (decreased in preterm infants). However, they observed a significant increase in the fronto-occipital diameter, which they hypothesized reflected the nonsynostotic dolichocephaly commonly observed in preterm infants,14 due to the sideways head position required by the respiratory support. In contrast, in our study, we did not find any difference in the fronto-occipital diameter in our preterm infants. This may have differed because many of the infants in our cohort had water pillows, a method known to prevent head deformation15,16 and were nursed supine from the first days of life because early endotracheal extubation to nasal continuous positive airway pressure was the common practice in the neonatal intensive care unit (NICU).
Smaller brain volumes in children and adolescents born preterm have been described in several studies, along with ventricular enlargement and an increased volume of CSF. Brain metrics, similar to those used in this study have been applied in evaluating adolescents born preterm with reductions noted in coronal and sagittal diameters and length of corpus callosum.17 Using manual measurement in 66 preterm and 48 full-term individuals scanned at 15 years, Nosarti et al7 also demonstrated a decreased whole-brain volume (−6%), reduced cortical gray matter (−11.8%), and enlarged ventricles (+42%), which persisted after controlling for brain volume. Semiautomated volumetric techniques have explored both regional and tissue-specific alterations in brain structure in preterm adolescents. A recent large study demonstrated decreased gray matter volume in the temporal, frontal, and occipital cortices as well as in the cerebellum, putamen, and thalamus.18 In this study, loss in white matter volume was observed in the brain stem, the internal capsule, and the temporal and frontal lobes. Most interesting, some cerebral regions demonstrated increased gray matter and white matter volumes. These increases were most marked in adolescents who had major neonatal neuropathologies and suggest that a compensatory abnormal cytoarchitectural organization may have developed secondary to cerebral injury. Consistent with the hypothesis of abnormal organization, alterations in orbitofrontal sulcal formation have been shown in ex-preterm adolescents.19
Most important, reduced volume and/or altered cerebral structure has been linked to suboptimal functional outcomes. For example, lower intelligence quotients have been shown to be associated with smaller corpus callosums,20,21 bilateral reduction of the parieto-occipital volumes,22 and reduced cerebellar volumes in very preterm infants.23
Computational morphometric volumetric techniques have notable variation in the findings in preterm infants at term equivalent. There is a consensus across the studies documenting increased CSF and reduced deep gray matter volumes.24,25 However, variation exists in the delineation of reductions in cerebellar volumes, which were found universally in 1 study11 or only in the presence of injury in others.26,27 Variation also exists in relation to total brain-tissue volumes. Previous data in this same cohort identified a reduction in total brain tissue volume6 in both low- and high-risk preterm infants compared with term infants in a fashion similar to that in another previous smaller cohort study.28 However, other studies with a smaller number of subjects have not demonstrated this reduction in brain volume in preterm infants.29 In 1 larger study (89 preterm compared with 20 full-term infants), Boardman et al30 described increased ventricular CSF volume with similar total brain volumes. In contrast to our study, the Boardman cohort consisted of more-mature low-risk preterm infants (mean gestational age at birth, 29.9 weeks; mean birthweight, 1290 g) whose head circumferences did not differ from those of the control population. Such discrepancies had raised the question of the timing of the alterations in brain structure—either during the neonatal period or later in childhood. Our results, using simple measurements, confirm that impairment in brain growth or brain atrophy may be present at term-equivalent age. This emphasizes the need for a better understanding of the factors influencing brain growth during the stay within the NICU. The apparent discordant results within this field may be related to differences in population characteristics because large variations in prenatal and postnatal standards of care between countries have been demonstrated.31
There are limitations to our approach. The imaging protocol and sequence acquisition varied throughout the study period due to considerations regarding volumetric analyses. The small size of the control cohort further weakened the statistical analysis. In particular, the small number of control infants with sagittal sections lowered the statistical power for the group comparisons for the fronto-occiptal diameter and the length of corpus callosum. The positions of the landmarks were not precisely defined because the images were not realigned in anterior circulation–posterior circulation space. Because the principal aim was to develop a simple and widely applicable method for standard clinical practice, we chose to use the raw MR images, displayed with a DICOM browser without reconstructions. This choice led us to eliminate some MR images from the study because of an inaccurate orientation of the sections. However, the landmarks chosen allowed good reproducibility, with the exception of ventricular size.
There was a high rate of multiple pregnancies (42%) in our preterm cohort. Twin pregnancies are associated with lower birthweight32 and higher risk of neurologic impairment.33 Even though the proportion of intrauterine growth restriction (11%) in our population was in the range of the rates previously published in similar cohorts,34,35 these factors—associated with an enhanced role of genetic factors on brain size—may affect our results.
We did not adjust the brain metrics for head circumference because we considered that the skull growth in our population may reflect directly the cerebral tissue growth or CSF expansion, and not external factors. We chose to study the whole cohort, including the infants with cerebral injury and IVH. The number of infants with severe cerebral injury (cystic PVL and IVH, grade 4) was low, representing <10% of the population. Moreover, it appears that such neuropathologies may represent only the visible component of a much more diffuse lesion that could be detected by brain metrics.
Conclusions
This study has defined a reliable reproducible set of brain metrics, which can be easily applied in the clinical setting. These measures have good comparability with those of brain volumes. Finally, the measures identify alterations in brain structure in preterm infants in 3 key brain diameters—the bifrontal, biparietal, and transverse cerebellum. Further investigations are in progress to identify the relationships between brain metrics with neonatal factors and neurodevelopmental outcome. These measures may assist in both the research and clinical settings to delineate better the impact of preterm birth on brain development.
Acknowledgments
We appreciate the hard work of Merilyn Bear and the Vibes research team and the wonderful ongoing support of the families and children of the Vibes cohort.
Footnotes
This work was supported by the National Health and Medical Research Council of Australia (237117).
Paper previously presented in part at: Joint Annual Meeting of the Pediatric Academic Societies and Asian Society for Pediatric Research, May 2–6, 2008; Honolulu, Hawaii.
Indicates article with supplemental on-line tables.
References
- 1.Horbar JD, Badger GJ, Carpenter JH, et al. Trends in mortality and morbidity for very low birth weight infants, 1991–1999. Pediatrics 2002;110 (1 pt 1):143–51 [DOI] [PubMed] [Google Scholar]
- 2.Wilson-Costello D, Friedman H, Minich N, et al. Improved neurodevelopmental outcomes for extremely low birth weight infants in 2000–2002. Pediatrics 2007;119:37–45 [DOI] [PubMed] [Google Scholar]
- 3.Larroque B, Ancel PY, Marret S, et al. Neurodevelopmental disabilities and special care of 5-year-old children born before 33 weeks of gestation (the EPIPAGE study): a longitudinal cohort study. Lancet 2008;371:813–20 [DOI] [PubMed] [Google Scholar]
- 4.Hamrick SE, Miller SP, Leonard C, et al. Trends in severe brain injury and neurodevelopmental outcome in premature newborn infants: the role of cystic periventricular leukomalacia. J Pediatr 2004;145:593–99 [DOI] [PubMed] [Google Scholar]
- 5.Anderson NG, Laurent I, Cook N, et al. Growth rate of corpus callosum in very premature infants. AJNR Am J Neuroradiol 2005;26:2685–90 [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson DK, Warfield SK, Carlin JB, et al. Perinatal risk factors altering regional brain structure in the preterm infant. Brain 2007;130 (pt 3::667–77. Epub 2006 Sep 28. [DOI] [PubMed] [Google Scholar]
- 7.Nosarti C, Al-Asady MH, Frangou S, et al. Adolescents who were born very preterm have decreased brain volumes. Brain 2002;125:1616–23 [DOI] [PubMed] [Google Scholar]
- 8.Garel C. The role of MRI in the evaluation of the fetal brain with an emphasis on biometry, gyration and parenchyma. Pediatr Radiol 2004;34:694–99. Epub 2004 Jul 28 [DOI] [PubMed] [Google Scholar]
- 9.Garel C. MRI of the Fetal Brain. Berlin, Germany: Springer-Verlag;2004
- 10.Woodward LJ, Anderson PJ, Austin NC, et al. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med 2006;355:685–94 [DOI] [PubMed] [Google Scholar]
- 11.Limperopoulos C, Soul JS, Gauvreau K, et al. Late gestation cerebellar growth is rapid and impeded by premature birth. Pediatrics 2005;115:688–95 [DOI] [PubMed] [Google Scholar]
- 12.Leijser LM, Srinivasan L, Rutherford MA, et al. Structural linear measurements in the newborn brain: accuracy of cranial ultrasound compared to MRI. Pediatr Radiol 2007;37:640–48 [DOI] [PubMed] [Google Scholar]
- 13.Mewes AU, Zöllei L, Hüppi PS, et al. Displacement of brain regions in preterm infants with non-synostotic dolichocephaly investigated by MRI. Neuroimage 2007;36:1074–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutchison BL, Thompson JM, Mitchell EA. Determinants of nonsynostotic plagiocephaly: a case-control study. Pediatrics 2003;112:e316. [DOI] [PubMed] [Google Scholar]
- 15.Marsden DJ. Reduction of head flattening in preterm infants. Dev Med Child Neurol 1980;22:507–09 [DOI] [PubMed] [Google Scholar]
- 16.Schwirian PM, Eesley T, Cuellar L. Use of water pillows in reducing head shape distortion in preterm infants. Res Nurs Health 1986;9:203–07 [DOI] [PubMed] [Google Scholar]
- 17.Cooke RW, Abernethy LJ. Cranial magnetic resonance imaging and school performance in very low birth weight infants in adolescence. Arch Dis Child Fetal Neonatal Ed 1999;81:F116–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nosarti C, Giouroukou E, Healy E, et al. Grey and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome. Brain 2008;131:205–17 [DOI] [PubMed] [Google Scholar]
- 19.Giménez M, Junqué C, Vendrell P, et al. Abnormal orbitofrontal development due to prematurity. Neurology 2006;28:67:1818–22 [DOI] [PubMed] [Google Scholar]
- 20.Nosarti C, Rushe TM, Woodruff PW, et al. Corpus callosum size and very preterm birth: relationship to neuropsychological outcome. Brain 2004;127:2080–89 [DOI] [PubMed] [Google Scholar]
- 21.Narberhaus A, Segarra D, Caldú X, et al. Corpus callosum and prefrontal functions in adolescents with history of very preterm birth. Neuropsychologia 2008;46:111–16. Epub 2007 Aug 10 [DOI] [PubMed] [Google Scholar]
- 22.Peterson BS, Vohr B, Staib LH, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. Pediatrics 2003;111:939–48 [DOI] [PubMed] [Google Scholar]
- 23.Allin MP, Salaria S, Nosarti C, et al. Vermis and lateral lobes of the cerebellum in adolescents born very preterm. Neuroreport 2005;16:1821–24 [DOI] [PubMed] [Google Scholar]
- 24.Boardman JP, Counsell SJ, Rueckert D, et al. Abnormal deep grey matter development following preterm birth detected using deformation-based morphometry. Neuroimage 2006;32:70–78 [DOI] [PubMed] [Google Scholar]
- 25.Srinivasan L, Dutta R, Counsell SJ, et al. Quantification of deep gray matter in preterm infants at term-equivalent age using manual volumetry of 3-Tesla magnetic resonance images. Pediatrics 2007;119:759–65 [DOI] [PubMed] [Google Scholar]
- 26.Shah DK, Anderson PJ, Carlin JB, et al. Reduction in cerebellar volumes in preterm infants: relationship to white matter injury and neurodevelopment at two years of age. Pediatr Res 2006;60:97–102 [DOI] [PubMed] [Google Scholar]
- 27.Srinivasan L, Allsop J, Counsell SJ, et al. Smaller cerebellar volumes in very preterm infants at term-equivalent age are associated with the presence of supratentorial lesions. AJNR Am J Neuroradiol 2006;27:573–79 [PMC free article] [PubMed] [Google Scholar]
- 28.Inder TE, Warfield SK, Wang H, et al. Abnormal cerebral structure is present at term in premature infants. Pediatrics 2005;115:286–94 [DOI] [PubMed] [Google Scholar]
- 29.Zacharia A, Zimine S, Lovblad KO, et al. Early assessment of brain maturation by MR imaging segmentation in neonates and premature infants. AJNR Am J Neuroradiol 2006;27:972–77 [PMC free article] [PubMed] [Google Scholar]
- 30.Boardman JP, Counsell SJ, Rueckert D, et al. Early growth in brain volume is preserved in the majority of preterm infants. Ann Neurol 2007;62:185–92 [DOI] [PubMed] [Google Scholar]
- 31.Zeitlin J, Draper ES, Kollée L, et al, for the MOSAIC research group. Differences in rates and short-term outcome of live births before 32 weeks of gestation in Europe in 2003: results from the MOSAIC cohort. Pediatrics 2008;121:e936–44. Epub 2008 Mar 31 [DOI] [PubMed] [Google Scholar]
- 32.Sebire NJ, Snijders RJ, Hughes K, et al. The hidden mortality of monochorionic twin pregnancies. Br J Obstet Gynaecol 1997;104:1203–07 [DOI] [PubMed] [Google Scholar]
- 33.Pharoah PO. Neurological outcome in twins. Semin Neonatol 2002;7:223–30 [DOI] [PubMed] [Google Scholar]
- 34.Anderson PJ, Doyle LW, for the Victorian Infant Collaborative Study Group. Executive functioning in school-aged children who were born very preterm or with extremely low birth weight in the 1990s. Pediatrics 2004. :114:50–57 [DOI] [PubMed] [Google Scholar]
- 35.Stoelhorst GM, Rijken M, Martens SE, et al, for the Leiden Follow-Up Project on Prematurity. Changes in neonatology: comparison of two cohorts of very preterm infants (gestational age <32 weeks): the Project On Preterm and Small for Gestational Age Infants 1983 and the Leiden Follow-Up Project on Prematurity 1996–1997. Pediatrics 2005;115:396–405 [DOI] [PubMed] [Google Scholar]



