Abstract
Rationale
The opioid peptide β-endorphin (β-E) is synthesized by the pro-opiomelanocortin gene in response to environmental stressors and alcohol administration and is implicated in the behavioral sequelae associated with these stimuli.
Objectives
We sought to determine the influence of β-E on the stress response by evaluating basal measures of anxiety as well as on EtOH-induced anxiolytic behavior using transgenic mice that differ with respect to β-E.
Methods
Anxious behavior was evaluated for male and female heterozygous, wild type, and β-E knockout mice using the Light-Dark Box and Plus Maze assays. Subsequent tests evaluated behavior 20 min after administration of intraperitoneal saline or EtOH (0.5, 1.0 and 1.5 g/kg).
Results
We observed an inverse relationship between β-E levels and the percentage of entries into open arms of the Plus Maze as well as the time spent in either the open arms or the light compartment of the Light-Dark box during basal conditions, suggesting that this peptide normally inhibits anxious behavior. However, mice lacking β-E demonstrated an exaggerated anxiolytic response to EtOH in these assays.
Conclusions
These data suggest that β-E moderates the response to stressful stimuli and supports the hypothesis that this peptide influences the behavioral effects of EtOH.
Keywords: Addiction, Ethanol, Anxiety, Opioids, Mice, Transgenic, Self-Medication
β-endorphin (β-E) is a 31 amino-acid peptide that is cleaved from the carboxyl terminus of the Proopiomelanocortin (POMC) gene. As a member of the large family of opioid peptides that are widely and differentially distributed throughout the nervous system, it has been implicated in a variety of behaviors including the regulation of pain and reward, as well as in modulating neurocircuitry involved in learning and memory, motivation and processes associated with stress, fear or anxiety (e.g. Bloom 1980). The response to stressors involves a complex cascade of endocrine, autonomic and behavioral changes that seem to be generally aimed at maintaining or restoring homeostasis. At least in part because of its complexity, neither a precise definition nor thorough knowledge of the neurobiological underpinnings of the stress response has been elucidated (Pacak and Palkovitis 2001). Nonetheless, nearly any operational understanding includes activation of the hypothalamic-pituitary-adrenal (HPA) axis. This reaction involves secretion of corticotropin releasing hormone (CRH) from the hypothalamus leading to adrenocorticotropic hormone (ACTH) release into the bloodstream and subsequent glucocortiocid secretion from the adrenal glands, contributing to the behavioral fight/flight response. Corticotropin releasing hormone in both the hypothalamus and pituitary stimulates POMC gene expression which is initially cleaved into two subunits, one of which eventually gives rise to ACTH and the other which may yield β-E (Pfaff et. al 2004).
The most well-studied effect of β-E is its ability to modulate pain, but an early report by Fratta et al. (1981) suggested that central administration of β-E produced opposite effects to that of ACTH administration (“reciprocal antagonism”) on several behaviors in rats including cataplexy, rigidity and analgesia, and supported the notion that β-E is directly implicated in the homeostatic regulation of ACTH response. Indeed, β-E synthesis and release is precipitated by stressful stimuli (Guillemin et al. 1977; Oltras et al. 1987) and low doses of β-E have been shown to decrease stress responding (Panksepp 2003). Modulation of the behavioral response to stressful stimuli by β-E is discussed in a recent review by Ribiero et al. (2005) and previously by Yamada and Nabeshima (1995).
Corticotropin Releasing Hormone is the primary regulator of POMC peptides, and ethanol (EtOH) exposure leads to CRH release in vivo (Redei et al. 1988) and in vitro (deWaele and Gianoulakis 1993). Thus, acute EtOH, like exposure to classic stressors, increases the synthesis and release of ACTH and β-E (Froehlich et al 1995 and 2000; Gianoulakis 1990; Marinelli et al. 2004; Millan 1981; Olive et al. 2001; Rivier 1996; Sarkar et al. 2007; Scanlon et al 1992; Schulz et al 1980; Thiagarajan et al. 1988, 1989). Paradoxically though, one of the primary factors influencing alcohol ingestion is thought to be stress reduction, particularly in genetically or environmentally prone individuals (see Cappell and Herman 1972; Pohorecky, 1991 for reviews).
Although the relationship between EtOH consumption and the sequelae associated with stress is naturally complex (as each, independently, are highly multidimensional) there is a large body of empirical research evincing the anxiolytic effects of EtOH (Eckardt et al. 1998; Gianoulakis et al. 2003; Grobin et al. 1998; LaBuda and Fuchs 2001; Lack et al. 2005). Furthermore, some stressors have been shown to increase self-administration of EtOH (e.g. Eckardt et al. 1998; Mollenauer et al. 1993; Wolffgramm 1990) and inhibiting the stress axis via CRH antagonism reduces EtOH consumption (Funk et al. 2006). In the clinic, alcoholism and anxiety disorders are frequently co-morbid (Kushner et al. 1990; Bradizza et al. 2006; Conway et al. 2006; Fein et al. 2006; Vendruscolo et al. 2006) and a significant proportion of alcohol-dependent individuals report that alcohol is reinforcing because it reduces anxiety (Barrenha and Chester 2007; Hill and Angel 2005; Koven et al. 2005; Lawyer et al. 2002). Thus, the stress attenuating properties of EtOH likely contribute to its reinforcing effects, and these may be mediated by β-E.
We chose to directly assess the impact of β-E on behavior associated with anxiety, as well as the anxiolytic effects of EtOH, by evaluating transgenic mice that have been engineered to lack β-E. These mice were developed over a decade ago in the laboratory of Malcolm Low (Rubinstein et al. 1996) and have been evaluated for differences in pain responses and energy balance (Appleyard et al. 2003; Hayward et al. 2006; Mogil et al. 2000) as well as sensitivity to cocaine and morphine (Marquez et al. 2008). In addition, we have looked at oral self-administration of EtOH (Grisel et al. 1999 and Williams et al. 2007) where heterozygote mice, possessing half of the normal levels of β-E, generally drink slightly more than either wildtype controls or mice totally lacking this peptide. Beside the direct correlation between gene and peptide concentration, no differences have been found in other POMC peptides, or in opioid receptor levels across genotypes (Mogil et al 2000; Rubinstein et al. 1996). In order to further evaluate the effects of β-E on behavior and pharmacology, we studied three lines of mice with differing levels of β-E in the Plus Maze and Light Dark tests, both under basal conditions and following EtOH administration.
Method
Subjects were adult male and female wild type controls (C57BL/6J; B6), β-E deficient (KO), and heterozygous (HT) mice bred in-house from progenitors obtained from the Jackson Laboratories (Bar Harbor, ME). The gene mutation has been fully backcrossed to the C57BL/6J strain (> 20 generations). HT mice were bred from KO males and B6 females; others were bred under identical conditions from genotype-matched pairs. These subjects were housed 2–5 per Plexiglas cage following weaning at 20 to 21 days with sex-matched siblings. The ventilated colony room maintained a constant light schedule (12 hour reverse light/dark cycle with lights on 1900 hr) and a temperature of 22° ± 2°C. Water and food (Lab Diet 5015) were available ad libitum. All subjects were between 50 and 75 days of age at the time of testing, and each subject was tested only one time. Throughout experimentation, subject order was counterbalanced with regard to genotype, sex and drug. Behavioral assessment of genotypic differences may be facilitated by testing during the animal’s active phase (Hossain, et al. 2004) and so occurred between 9 a.m. and 6 p.m. In all experiments, data was recorded by an experimenter blind to drug condition and genotype. In addition, all procedures were carried out in accordance with the National Institutes of Health guidelines and were approved by the Animal Care and Use Committee of Furman University.
Experimental Apparatus
The elevated Plus Maze, made from Plexiglas, was raised approximately 45 cm from ground level. The perimeter of the base of the maze was enclosed with a clear, 15 cm tall wall; the area of the base was filled with wood shavings. The floor of the elevated portion of the maze was black. Two opposite arms (30 × 5 cm each) of the maze were enclosed by a clear, 10cm high Plexiglas wall, and the remaining two arms were “open”, with a very short (2 mm) wall. A center space (5 cm2) between these four arms was also not enclosed. The elevated portion of the apparatus was cleaned with a dilute detergenet between each session.
The Light-Dark Box was composed of two Plexiglas chambers of equal size (25 cm3) that shared a common wall. The bottom of the entire apparatus was constructed of black, opaque Plexiglas, as were the four walls of the dark chamber. A 27 cm2 panel of the same black Plexiglas served as a top for the dark side of the apparatus. The three walls of the light side were constructed of clear Plexiglas, as was the top panel; the fourth wall of the light chamber was black, as it was shared with the dark chamber. A square hole (2.5 cm2) in the bottom center of the wall shared by both chambers allowed subjects to pass freely between light and dark chambers. Between each subject’s test session, the apparatus was cleaned using a sponge filled with a dilute, low-residue detergent.
Experiment 1A
Animals (KO n=10, HT n=11, B6 n=11) were removed from the colony room, marked and weighed, and allowed 45–60 min to acclimate to conditions in an experimental room before testing. This room was lit with fluorescent ceiling lights as well as natural light from a single window, and thus contrasted with the colony room (dark phase). Though this abrupt transition may have altered chemical or behavioral function, we wanted to test subjects during their active phase, and plus maze behavior is routinely assessed in well lit conditions, as results may depend upon lighting (Costall et al. 1989). Experiment 1A subjects received no injections but were placed in individual housing for 20 min after habituation and before testing (to parallel methods used in experiments involving drug administration; because saline and EtOH-injected animals are generally taken from the same cage, we wanted to avoid possible confounds from differential interaction between cage mates following injection). After this 20 min period, they were lifted by the tail and placed gently into a closed arm facing the center of the maze. For the following 5 min, the number of open arm entries, closed arm entries, time spent in open arms, closed arms, and center area were recorded by a blind observer seated about 1M from the maze. Entry into an arm was defined as all four feet crossing into the region. From these data, the percentage of arm entries in to open arms was calculated as a measure of anxious behavior corrected for general locomotor activity.
Experiment 1B
The procedure for this experiment mimicked that of Experiment 1A with the exception that animals received intraperitoneal (IP) injections of either saline, 0.5, 1.0, or 1.5 g/kg 20% EtOH v/v (KO n=11, 10, 11, and 9 respectively, B6 n=9, 9, 7, and 7) after about 45 min of acclimation. Immediately following injection, subjects were individually housed for 20 min and then placed onto the Plus Maze where behavior was evaluated, as in experiment 1A, for 5 min. Because the primary purpose of this study was to evaluate a range of EtOH doses to inform subsequent testing (Exp 1C and 2B), heterozygote mice were not assessed.
Experiment 1C
The procedure for this experiment paralleled those used in Experiment 1B with the exception that each of the three genotypes was tested following IP injections of 1.5 g/kg 20% EtOH v/v (KO n=13, HT n=10, B6 n=14) or equivolume saline (KO n=13, HT n=12, B6 n=14) after 45 min of acclimation and before 20 min of individual housing. This dose was chosen based on the results of experiment 1B.
Experiment 2A
Experiment 2A was similar to Experiment 1A except that behavioral testing took place in the Light-Dark box. Subjects (KO n=10, HT n=8, B6 n=7) were removed from the colony room, marked and weighed, and allowed 45 min to acclimate to experimental conditions before testing. The testing room was lit with fluorescent ceiling lights as well as natural light from a single window, and thus contrasted with the colony room (dark phase). A 60 Watt halogen lamp was also affixed above the light side of the apparatus to ensure optimal contrast between light and dark chambers (to facilitate discrimination between the two chambers). Again, we wanted to test subjects during their active phase, and this test is conventionally done in bright conditions. Experiment 2A subjects received no injections but were individually housed for 20 min before testing. After this period, they were placed into the dark side of the Light-Dark Box facing the wall opposite the opening into the light chamber. The dark chamber was immediately covered and behavior was recorded over a five-min period by an experimentally blind, silent observer seated approximately 1M from the experimental apparatus. Latency to emerge from the dark chamber, number of crossings between chambers (defined as all four feet across the boundary), and total time spent in the light chamber were recorded.
Experiment 2B
Procedure for this experiment was as described for Experiment 2A with the exception that all subjects received intraperitoneal (IP) injections of either 1.5 g/kg 20% EtOH v/v (KO n=9, HT n=11, B6 n=11) or equivolume saline (KO n=12, HT n=14, B6 n=11) before being placed into individual housing for 20 minutes. A dose of .75 g/kg EtOH was also evaluated but produced no effects substantially differing from saline; those data are not included.
Experiment 3
As an additional measure of stress reactivity, we assessed adrenal gland weight in mice of each genetic line. Briefly, mice were sacrificed by cervical dislocation, and both adrenal glands were located, removed and weighed (together). The experimenter was blind to genotype in all cases. There was a total N of 143: 56 KOs, 59B6s, and 28 HTs. Many of the adrenal glands were taken after experimental manipulation (at least 1–2 weeks post manipulation) but about 30 percent were experimentally naïve (approximately equally divided across genotypes).
Statistical Analysis
Data were analyzed separately for each experiment by Factorial Analysis of Variance (ANOVA) in SYSTAT: first by genotype, sex and drug (where appropriate), and then, in the absence of interactions with sex, collapsing across this factor. Significant main effects and interactions were investigated further using Tukey’s HSD test for post hoc comparisons. In all cases the criterion for significance (α level) was set at p ≤ 0.05.
Results
There were no significant interactions with sex in any of the data analyses, and so this factor was excluded from subsequent analysis and results.
Experiment 1A
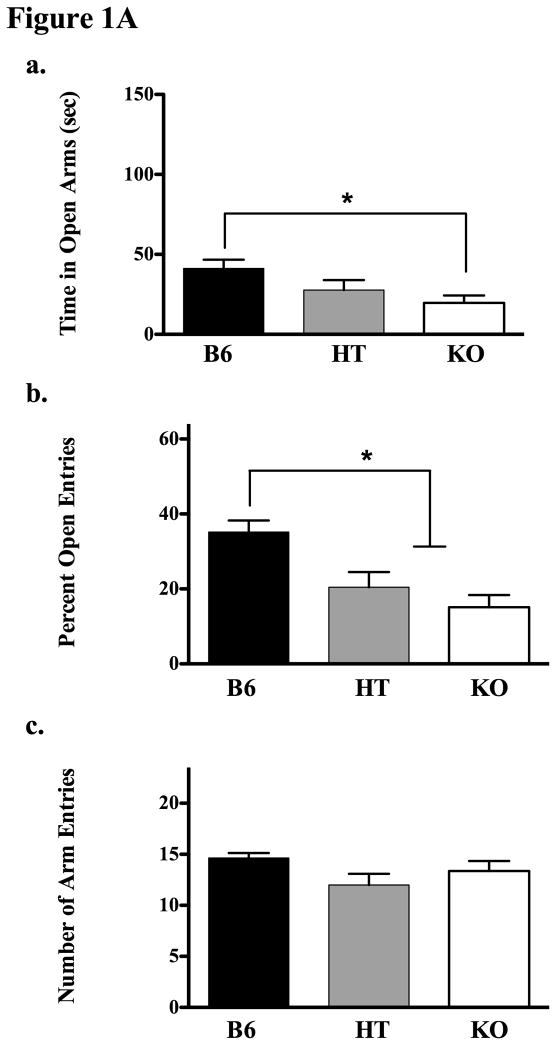
Experiment 1A, using the Plus Maze, found a main effect of genotype on the time spent in open arms (see Fig 1Aa for means and standard error; F(2,29) = 4.481, p < 0.05). Subsequent post hoc analysis (Tukey’s HSD) indicated that the KO mice were less likely to be in open arms than B6s. Another way of looking at this data is to evaluate the percentage of time spent in open arms as a function of total arm time (in other words, controlling for time spent in the center of the maze). In our analysis, the F statistic was virtually identical in both cases (for percentage of open time, F(2,29) = 4.917, p < 0.05), so the more conservative raw data are depicted in Figure 1Aa. KOs also evidenced a smaller percentage of entries into open arms than B6s (as did HTs; Fig 1Ab, F(2,29) = 10.416, p < 0.05), but there were no significant genotypic differences in total arm entries (Fig 1Ac, p = 0.136) a measure of general locomotor activity.
Figure 1.
Figure 1A. Experiment 1A evaluated behavior of B6, HT and KO mice on the plus maze. The upper panel (a) shows time spent in the open arms of the maze over a 5 min period (data show means ± SE). The middle panel (b) shows the percentage of open arm entries during this period in each genotype, and panel c shows the total number of arm entries (c). Significant group differences were determined following ANOVA by post-hoc analysis (Tukey’s HSD test) and are designated by an asterisk (all p’s ≤ 0.05).
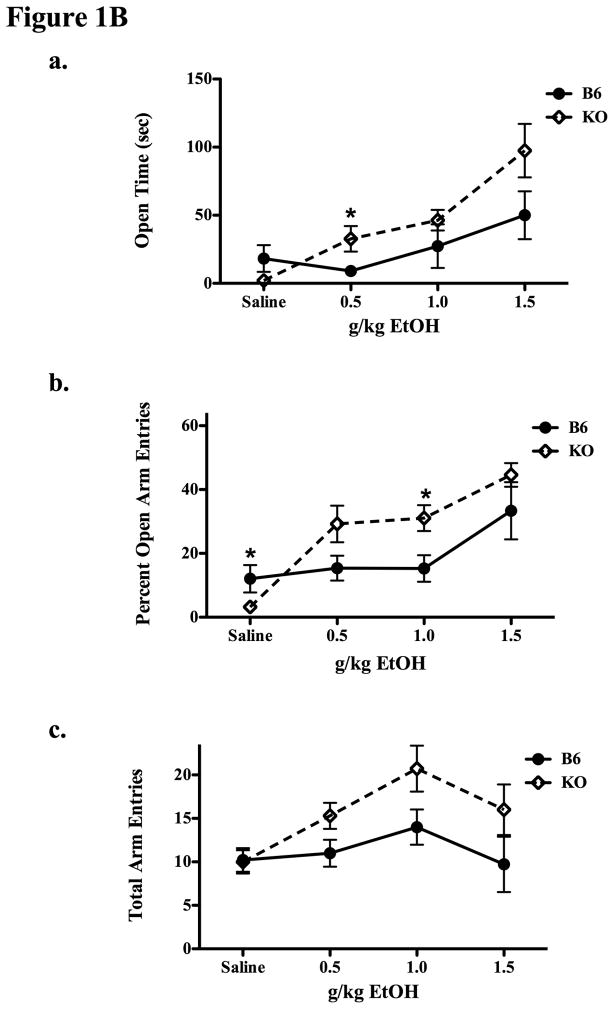
Figure 1B. Experiment 1B compared B6 and β-E KO mice on the plus maze 20 min after saline, 0.5, 1.0, or 1.5 g/kg IP EtOH. Figures 1Ba and b show the amount of time spent in open arms (a) and the percentage of open arm entries (b) and 1Bc shows the total number of arm entries in each genotype and condition. Data are means ± SE. Significant differences between relevant groups were determined by post-hoc analysis (Tukey’s HSD test) and are designated by an asterisk (all p’s ≤ 0.05).
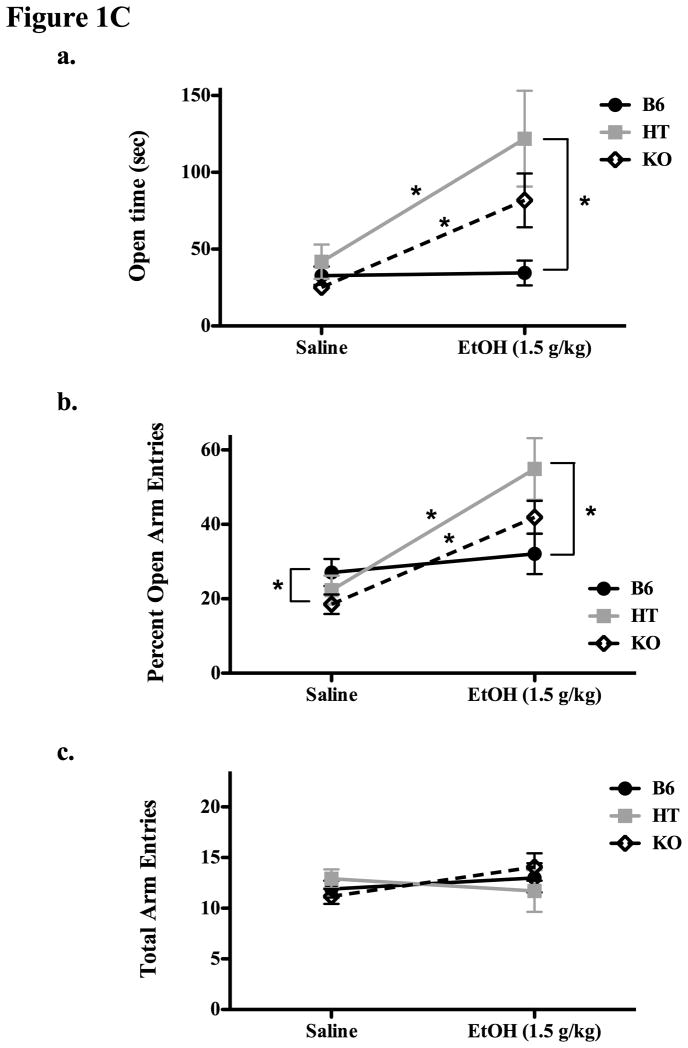
Figure 1C. Results of Experiment 1C depict plus maze behavior (means ± SE) in β-E deficient mice (KOs and HTs) as well as controls (B6) 20 min following either saline or 1.5 g/kg EtOH. The top panel shows (means ± SE) the amount of time spent in open arms, the middle panel (b) shows the percentage of open arm entries, and Panel c shows the total number of arm entries. Significant (p ≤ 0.05) within and between group differences are designated by an asterisk.
Experiment 1B
Results were quite different following EtOH administration (Experiment 1B), in which mice lacking β-E demonstrated an exaggerated anxiolytic and locomotor response to the drug. In general, β-E deficiency resulted in a shift of the dose-response curve for EtOH to the left, indicative of increased sensitivity to the effects of EtOH on the Plus Maze. Overall, KO mice spent more time in the open arms than wildtypes (Figure 1Ba; F(1,65) = 5.382, p < 0.05). There was also a dose effect as open time increased with increasing EtOH dose (F(3,65) = 11.578, p < 0.001). Notably, the interaction term was also significant (F(3,65) = 2.735, p = 0.051) indicative of an increased sensitivity to the anxiolytic effects of EtOH for β-E deficient mice in this behavioral measure. Post hoc analysis showed a tendency for the KO mice to spend less time in the open arms than B6s after saline (p = 0.08) but more time after 0.5 g/kg EtOH (p = 0.039). Genotypes did not differ significantly on this measure after either 1.0 or 1.5 g/kg EtOH. The results were virtually identical when looking at the percentage of time spent in open arms, and so we chose to depict only the raw data.
In terms of the percentage of open arm entries in this dose response analysis, we found the same pattern: a significant effect of genotype (F(1,65) = 5.906, p < 0.05) and of dose (F(3,65) = 14.921, p < 0.001) as well as a significant interaction (F(3,65) = 3.205, p < 0.05) reflecting the fact that mice deficient in β-E were especially likely to increase the percentage of entries they made into open arms following EtOH (see Figure 1Bb). Post hoc analysis indicated significant genotypic effects after saline (p = 0.05; KOs < B6s) a trend following 0.5 g/kg (p = 0.067) and significant differences again after 1.0 g/kg (p = 0.020; KOs > B6) in that EtOH reversed the decreased likelihood of entering open arms seen in β-E deficient mice.
Finally, there were genotypic differences in locomotor activity as measured by the total number of arm entries on the Plus Maze: (Figure 1Bc; F(1,65) = 7.843, p < 0.01). There were also significant effects of EtOH dose on this measure (F(3,65) = 3.929, p < 0.05), but not a significant interaction between genotype and dose (F(3,65) = 1.137, p = 0.34).
Experiment 1C
In Experiment 1C we compared B6, HT and KO mice on the Plus Maze after either saline or 1.5 g/kg EtOH and continued to find that low or absent β-E leads to greater EtOH-induced anxiolysis. This dose of EtOH was selected based on the results of Experiment 1B where there were differences in behavior on the plus maze indicative of differential sensitivity to EtOH-induced anxiolysis, without differences in the locomotor response to EtOH. Indeed, these data were replicated and extended as both HTs and KOs spent more time in open arms and an increased their percentage of open entries following EtOH administration, while B6s were relatively insensitive to the anxiolytic effects of this dose of EtOH in our test (Figure 1C). A significant main effect of genotype on open time (Fig 1B(a); F(2,69) = 4.644, p < 0.05) was observed as well as an effect of drug (F(1,69) = 15.564, p < 0.001) and significant interaction between genotype and drug (F(2,69) = 5.045, p < 0.01). The same pattern was evident with respect to the percentage of open time (controlling for the time spent in the center of the maze) as both main effects showed significant differences: genotype, F(2,69) = 3.665, p < 0.05 and drug, F(1,69) = 16.462, p < 0.001. The interaction of genotype and dose was also significant: F(2,69) = 5.031, p < 0.01, but only the raw data are presented in Figure 1Ca. Post hoc analysis of open time showed that HT and KO mice had a significant increase in this measure after EtOH (as compared to saline), and that HT mice were also spent more time in the open arms after EtOH than did B6 mice (all ps < 0.05). Again, β-E deficient mice evidenced an exaggerated response to EtOH with respect to the percentage of open arm entries (1Cb). There was a significant effect of genotype (F(2,69) = 3.665, p < 0.05), an overall effect of drug (F(1,69) = 16.462, p < 0.001) as well as an interaction between genotype and EtOH F(2,69) = 5.031, p < 0.01). Here too, post hoc analysis indicated significant effects of EtOH in HT and KO mice, a difference in the percentage of open arm entries following EtOH between B6 and HT mice, and, in addition, a significant difference between B6 and KO mice following saline (KOs had a lower percentage of open arm entries in this case). As shown in Fig 1Cc, there were no genotypic differences in locomotor activity as measured by the total number of arm entries, nor was there any effect of 1.5 g/kg EtOH compared to saline administration on this measure, or interaction between EtOH and line (all ps > 0.05).
Experiment 2A
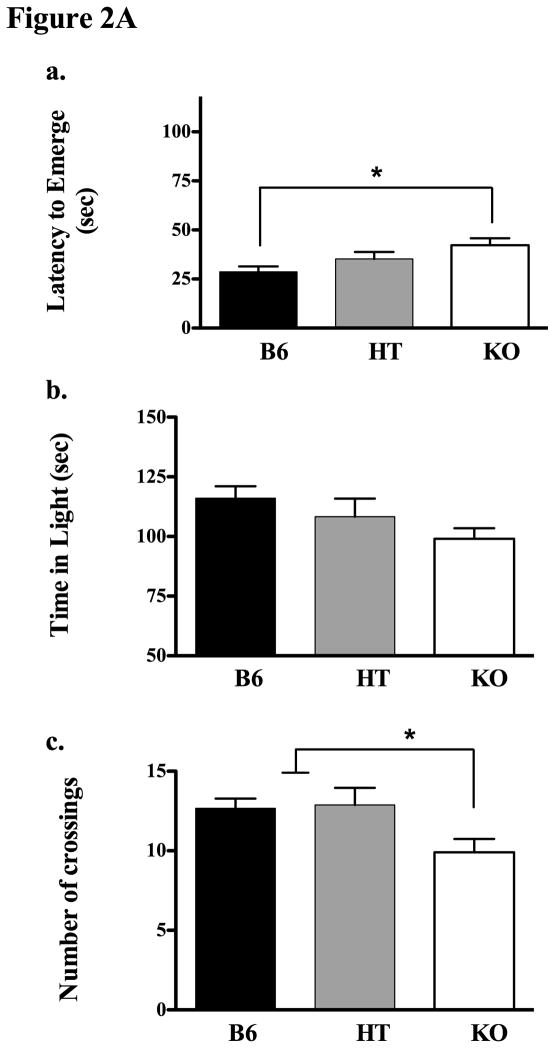
In Experiment 2A we evaluated behavior in the Light-Dark box and again found a genotype effect in which KOs took longest to emerge and B6s emerged most quickly (see Figure 2A(a) for means and standard errors, F(2,22) = 3.673, p < 0.05). Similarly, KOs demonstrated fewer crossings into the light than B6s and HTs (2Ac, F(2,22) = 3.673, p < 0.05). The same general trend was apparent in the amount of time each genotype spent in the light side of the box, however these data were not significant (2Ab, p = 0.146).
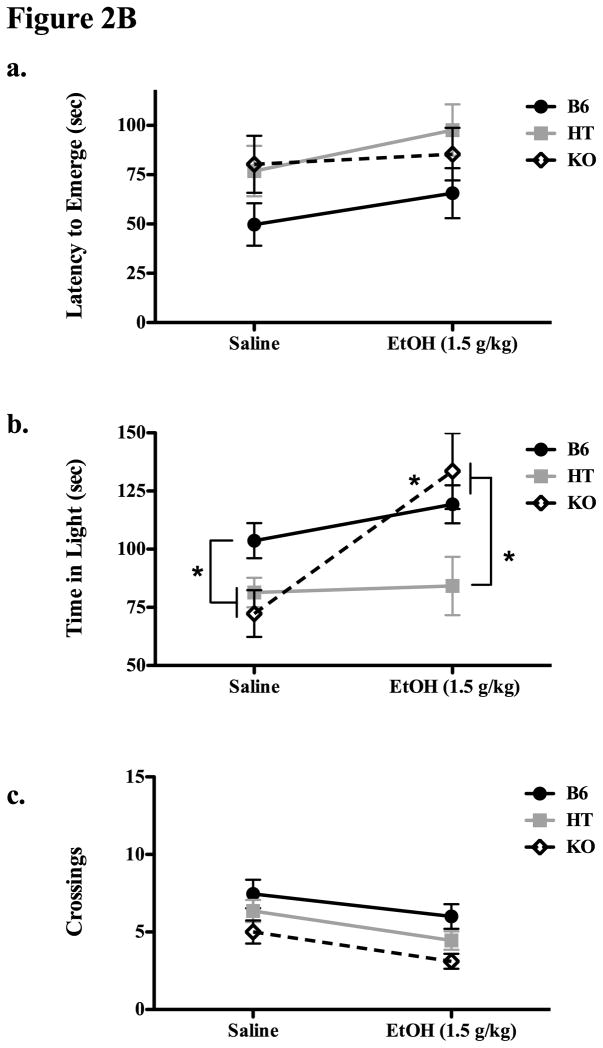
Figure 2.
Figure 2A. Experiment 2A evaluated behavior in the light-dark box in B6, HT and KO mice. The top panel (a) shows the latency to emerge from the dark side of the box in all three genotypes. The middle panel (b) shows time spent in the light, and panel c depicts the total number of light-dark crossings in each line. Asterisks indicate significant (p ≤ 0.05) genotypic differences; see text for methodological details or specific ANOVA results.
Figure 2B. Experiment 2B evaluated behavior in the light dark box in B6, HT and KO mice following either saline or 1.5 g/kg EtOH (IP). The top panel shows the latency to emerge from the dark compartment in each genotype (means ± SE), the middle panel shows the total time spent in the light compartment over the 5 min test period, and the lower panel shows the total number of light-dark crossings. Significant (p ≤ 0.05) group differences (both within and between groups) are signified by an asterisk.
Experiment 2B
In Experiment 2B, there was a tendency for B6 mice to emerge more quickly overall (F(2,62) = 2.976, p = 0.058) but no significant effects of EtOH on latency to emerge or interaction between genotype and drug (p’s > 0.05 Figure 2Ba). However, despite the fact that KO mice spent relatively little time in the light side of the Light-Dark box in Experiment 2A and after saline injections here, they had the largest increase in this measure following IP injection of 1.5 g/kg EtOH (Figure 2Bb). For time spent in the light, there were significant main effects of both drug and of genotype (F(2,62) = 4.479, p < 0.05 and F(1,62) = 10.358, p < 0.05, respectively) as well as a significant interaction (F(2,62) = 4.507, p < 0.05) indicative of this large change in behavior following EtOH in KO mice but not HT or B6 mice (also evidenced by significant post hoc testing where KO mice receiving saline or EtOH differed from each other). Significant main effects of drug and genotype were observed on the variable crossings into light (F(2,62) = 6.1664, p < 0.05 and F(1,62) = 8.3028, p < 0.05, respectively) though the interaction was not significant, indicative of the fact that KOs were generally less active than B6 mice in this test, and EtOH had a nonspecific tendency to decrease activity in this test.
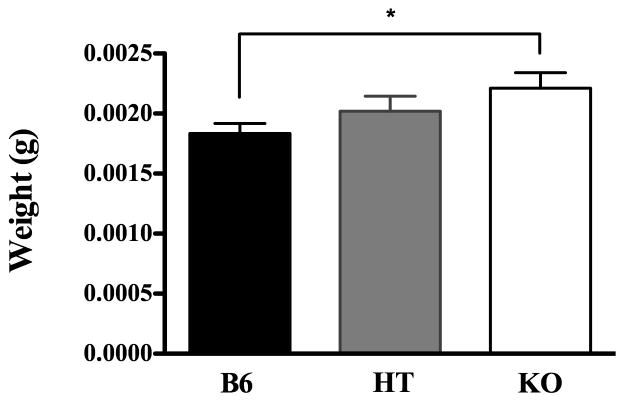
Experiment 3
As an attempt to corroborate the behavioral data gleaned especially in Experiments 1A and 2A, adrenal gland weights were assessed in Experiment 3. A one-way ANOVA on strain indicated a significant effect of genotype F(2,140) = 3.247, p < 0.05, and post hoc analysis revealed significant group differences between B6 and KO mice (Figure 3). Because some animals in each group had been manipulated experimentally, we conducted a separate ANOVA including this factor along with genotype. Because there was no significant effect of prior manipulation or interaction with this factor, data presented and analyzed are collapsed across prior experience.
Figure 3.
Adrenal gland weights (means±SE) in B6, HT and β-E deficient KO mice.
Discussion
Employing two animal models of anxiety, our studies suggest that the opioid peptide β-E modulates the stress response. The elevated Plus Maze and the Light-Dark box assays were used to study the relationship between β-E and behavior, under basal conditions and following EtOH administration. In the absence of any manipulation, measures of anxious behavior (e.g., risk assessment, avoidance of open/lit areas, fewer entries into open areas) were higher in β-E deficient mice, suggesting that β-E normally attenuates the behavioral response to stress. Notably, the “hyper-anxious” state evident in mice lacking β-E was completely ameliorated following EtOH administration so that deficient mice generally displayed lower levels of anxious behavior than wildtype counterparts following EtOH administration.
The primary neural site of β-E synthesis is the ventromedial arcuate nucleus of the hypothalamus. Exposure to stressful stimuli increases β-E synthesis and release here (e.g., Constanopoulos et al. 1995; Schedlowski et al. 1995) via activation of CRH (Bronstein & Akil 1990; Poplawski et al. 2005). Moreover, stimulation of hypothalamic β-E neurons inhibits CRH secretion from the paraventricular nucleus (Buckingham 1986; Plotsky 1991). Because β-E circuitry in the hypothalamus is localized to cells that are responsible for stimulating CRH mediated cortisol release from adrenal glands following exposure to a stressor (Brown 1986) this peptide is well situated to moderate activity of the HPA axis. Also, functional genetic variation in the μ-opioid receptor predicts the response to stressful stimuli (Chong et al. 2006). This circuitry fits well with the present results indicating that low or absent levels of β-E lead to an exaggerated stress response in the Plus Maze and Light-Dark assays. The fact that β-E deficient mice also have enlarged adrenal glands further supports the contention that the stress axis is persistently over-activated in these mice (see Amario 2007 for a recent and comprehensive review of how the HPA axis transduces stressful stimuli and how this process is modified by chronic exposure). Thus, in terms of behavior and gross anatomy, our data support the notion that β-E normally functions to attenuate the stress response and that a lack of negative feedback amplifies stress activity (e.g. Sarkar et al. 2007).
Though alcohol abuse and dependence are mediated by a complex set of environmental and genetic factors, variation in β-E is also thought to contribute to differential sensitivity to the reinforcing effects of EtOH. The opioid-deficiency hypothesis argues that individuals at high risk for developing alcoholism may be so in part because of modified endorphinergic circuits (Gianoulakis 2004; Oswald and Wand 2004; Zalewska-Kaszubska & Czarnecka, 2005). For instance, some studies have demonstrated lower endorphin levels in animals or humans prone to high levels of EtOH intake (Dai et al. 2005; Grisel et al. 1999; Marinelli et al. 2003; Williams et al. 2007). However, the mechanisms underlying the relationship between EtOH and β-E have not been clarified. One theory holds that a deficit in β-E leads to hypoactivity in the mesolimbic reward pathways and that this can be ameliorated by EtOH administration (Sher 2003; Herz 1997). Another model emphasizes the role of the HPA axis in modifying EtOH reward. For instance, HPA functioning has been found to differ in nonalcoholic subjects with either a positive or negative family history for the disease (Hernandez-Avila et al. 2001; Wand et al. 2001). In these studies, genetically prone individuals showed an exaggerated HPA response to challenge by opiate antagonists. This relationship was also found in a recent laboratory study in which two lines of mice selectively bred for high alcohol preference demonstrated exaggerated fear potentiated startle relative to their low alcohol preferring counterparts (Barrenha and Chester 2007). Furthermore, the withdrawal state experienced following chronic EtOH exposure, which is associated with high anxiety, is thought to result in part from down regulation of β-E (Aguirre et al. 1995; Diana et al. 1993; Scanlon et al. 1992; Valdez et al. 2004). Although these theories are not mutually exclusive, the present data especially support the latter idea by suggesting that β-E modifies sensitivity to both stress and EtOH. Specifically, low β-E leads to increased activity of the HPA axis, and by some unknown mechanism, this deficit facilitates EtOH-mediated anxiolysis.
Although transgenic animals like the ones used in this study can provide useful insight into neural mechanisms underlying behavior, the differences evident in our mouse lines may not be directly related to an effect of missing β-E. For example, in a study of μ-receptor knockout mice (a primary site of β-E action) no differences were found in EtOH-mediated anxiolysis on the plus maze (LaBuda & Fuchs 2001). Furthermore, a review of the literature failed to find precedent for the notion that absent β-E should exaggerate EtOH effects, and the most straightforward interpretation seems unlikely (that β-E acts as a functional EtOH antagonist). It is plausible however, that the enhanced sensitivity to EtOH’s anxiolytic effect seen in β-E deficient mice reflects compensatory changes in related neural systems that occur as a result of absent β-E throughout development. Especially in the case of inbred strains, gene knockout studies can help elucidate the functional interplay between contributing neural factors (e.g. Mogil and Grisel 1998) and epistatic interactions between genes and their products now seems to be the norm rather than the exception in neurobiology. Thus, consequent adaptation of the nervous system to experimental transgenic manipulation may provide a window into the complex systems underlying behavioral states such as anxiety and alcoholism (e.g. Bowers et al. 1999).
Alcoholism and anxiety disorders are frequently co morbid and a significant percentage of alcoholics report that they ingest alcohol to reduce anxiety (Compton et al. 2007, Zimmerman et al. 2003). The fact that low β-E levels have been linked to a higher risk for excessive alcohol intake and alcoholism (Dai et al. 2005; Gianoulakis 1996; Wand et al. 1998) and, in our study, to predict higher levels of anxious behavior, suggest that β-E may be a contributing factor to this relationship. Future efforts employing these mice may be useful in elucidating the neural substrates mediating these relationships.
Acknowledgments
This publication was made possible by NIH Grant Numbers P20 RR-016461 from the National Center for Research Resources, AA13259 (through the INIA Stress Consortium), AA13641 from the National Institute on Alcohol Abuse and Alcoholism and P20 RR-016461 / SC INBRE.
Personal Acknowledgements:
The authors would like to acknowledge the foresight of Dr. John C. Crabbe at Oregon Health Sciences University that lead to this series of studies, the Psychology Department at Reed College, in Portland OR where the studies began, and Christopher Smith, Gregory Cloonan, Amanda Lee and Carmen Sanchez who assisted with experiments at Furman University.
References
- Aguirre JC, del Arbol JL, Rico J, Raya J, Mirand MT. Classification of alcoholics on the basis of plasma b-endorphin concentration. Alcohol. 1995;12:531–534. doi: 10.1016/0741-8329(95)00039-9. [DOI] [PubMed] [Google Scholar]
- Appleyard SM, Hayward M, Young JI, Butler AA, Cone RD, Rubinstein M, Low MJ. A role for the endogenous opioid beta-endorphin in energy homeostasis. Endocrinology. 2003;144:1753–1760. doi: 10.1210/en.2002-221096. [DOI] [PubMed] [Google Scholar]
- Amario A. The hypothalamic-pituitary-adrenal axis: what can it tell us about stressors? CNS Neurol Disord Drug Targets. 2006;5:485–501. doi: 10.2174/187152706778559336. [DOI] [PubMed] [Google Scholar]
- Barrenha GD, Chester JA. Genetic correlation between innate alcohol preference and fear-potentiated startle in selected mouse lines. Alcohol Clin Exp Res. 2007;31:1081–1088. doi: 10.1111/j.1530-0277.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- Bloom FE. The endorphins: natural peptides for pain, pleasure, and other purposes. Psychopharmacol Bull. 1980;16:51–52. [PubMed] [Google Scholar]
- Boyadjieva NI, Sarkar DK. The role of cAMP in ethanol-regulated beta-endorphin release from hypothalamic neurons. Alcohol Clin Exp Res. 1997;21:728–31. [PubMed] [Google Scholar]
- Bowers BJ, Owen EH, Collins AC, Abeliovich A, Tonegawa S, Wehner JM. Decreased ethanol sensitivity and tolerance development in gamma-protein kinase C null mutant mice is dependent on genetic background. Alcohol Clin Exp Res. 1999;23:387–397. [PubMed] [Google Scholar]
- Bradizza CM, Stasiewicz PR, Paas ND. Relapse to alcohol and drug use among individuals diagnosed with co-occuring mental health and substance use disorders: a review. Clin Psychol Rev. 2006;26:162–178. doi: 10.1016/j.cpr.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Bronstein DM, Akil H. In vitro release of hypothalamic beta-endorphin (beta E) by arginine vasopressin, corticotropin-releasing hormone and 5-hydroxytryptamine: evidence for release of opioid active and inactive beta E forms. Neuropeptides. 1990;16:33–40. doi: 10.1016/0143-4179(90)90027-v. [DOI] [PubMed] [Google Scholar]
- Brown M. Corticotropin releasing factor: Central nervous system sites of action. Brain Res. 1986;99:10–14. doi: 10.1016/0006-8993(86)90595-0. [DOI] [PubMed] [Google Scholar]
- Buckingham JC. Stimulation and inhibition of corticotrophin releasing factor secretion by endorphin. Neuroendocrinology. 1986;42:148–152. doi: 10.1159/000124266. [DOI] [PubMed] [Google Scholar]
- Chong RY, Oswald L, Yang X, Uhart M, Lin PI, Wand GS. The Micro-opioid receptor polymorphism A118G predicts cortisol responses to naloxone and stress. Neuropsychopharmacology. 2006;31:204–211. doi: 10.1038/sj.npp.1300856. [DOI] [PubMed] [Google Scholar]
- Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2007;64:566–576. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- Constantopoulos A, Papadaki-Papandreou U, Papaconstantinou E. Increased beta-endorphin but not Leuenkephalin in plasma due to preoperative stress. Experientia. 1995;51:16–18. [PubMed] [Google Scholar]
- Conway KP, Compton W, Stinson FS, Grant BF. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2006;67:247–57. doi: 10.4088/jcp.v67n0211. [DOI] [PubMed] [Google Scholar]
- Costall B, Jones BJ, Kelly ME, Naylor RJ, Tomkins DM. Exploration of mice in a black and white test box: validation as a model of anxiety. Pharmacol, Biochem Behav. 1989;32:777–785. doi: 10.1016/0091-3057(89)90033-6. [DOI] [PubMed] [Google Scholar]
- Cowen MS, Chen F, Lawrence AJ. Neuropeptides: implications for alcoholism. J Neurochem. 2004;89:273–285. doi: 10.1111/j.1471-4159.2004.02394.x. [DOI] [PubMed] [Google Scholar]
- Dai X, Thavundayil J, Gianoulakis C. Differences in the peripheral levels of b-E in response to alcohol and stress as a function of alcohol dependence and family history of alcoholism. Alcohol Clin Exp Res. 2005;29:1965–1975. doi: 10.1097/01.alc.0000187599.17786.4a. [DOI] [PubMed] [Google Scholar]
- De Waele JP, Gianoulakis C. Effects of single and repeated exposures to ethanol on hypothalamic beta-endorphin and CRH release by the C57BL/6 and DBA/2 strains of mice. Neuroendocrinology. 1993;57:700–709. doi: 10.1159/000126428. [DOI] [PubMed] [Google Scholar]
- Diana M, Pistis M, Carboni S, Gessa GL, Rossetti ZL. Profound decrement of mesolimbic dopaminergic neuronal activity during ethanol withdrawal syndrome in rats: Electrophysiological and biochemical evidence. Proc Natl Acad Sci U S A. 1993;90:7966–7969. doi: 10.1073/pnas.90.17.7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, Kalant H, Koob Gf, Li TK, Tabakoff B. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Finn P, Scheiner DL. Sub-diagnostic psychiatric comorbidity in alcoholics. Drug Alcohol Depend 2007. 2006;87(2–3):139–145. doi: 10.1016/j.drugalcdep.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratta W, Rossetti ZI, Poggioli R, Gessa GL. Reciprocal antagonism between ACTH1–24 and b-endorphin in rats. Neurosci Lett. 1981;24:71–74. doi: 10.1016/0304-3940(81)90361-x. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Harts J, Lument L, Li T-K. Naloxone attenuates voluntary ethanol intake in rats selectively bred for high ethanol preference. Pharmacol Biochem Behav. 1990;35:385–390. doi: 10.1016/0091-3057(90)90174-g. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Zink RW, Li TK, Christian JC. Analysis of heritability of hormonal responses to alcohol in twins: beta-endorphin as a potential biomarker of genetic risk for alcoholism. Alcohol Clin Exp Res. 2000;24:265–277. [PubMed] [Google Scholar]
- Gianoulakis C. Characterization of the effects of acute ethanol administration on the release of beta-endorphin peptides by the rat hypothalamus. Eur J Pharmacol. 1990;180:21–29. doi: 10.1016/0014-2999(90)90588-w. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C. Implications of endogenous opioids and dopamine in alcoholism: human and basic science studies. Alcohol. 1996;1:33–42. [PubMed] [Google Scholar]
- Gianoulakis C, Dai X, Brown T. Effect of chronic alcohol consumption on the activity of the hypothalamic-pituitary-adrenal axis and pituitary b-E as a function of alcohol intake, age, and gender. Alcohol Clin Exp Res. 2003;27:410–423. doi: 10.1097/01.ALC.0000056614.96137.B8. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C. Endogenous opioids and addiction to alcohol and other drugs of abuse. Current Topics in Medicinal Chemistry. 2004;4:39–50. doi: 10.2174/1568026043451573. [DOI] [PubMed] [Google Scholar]
- Grisel JE, Mogil JS, Grahame NJ, Rubinstein M, Belknap JK, Crabbe JC, Low MJ. Ethanol oral self-administration is increased in mutant mice with decreased b-E expression. Brain Res. 1999;835:62–7. doi: 10.1016/s0006-8993(99)01384-0. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol. Psychopharmacology. 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- Guillemin R, Vargo T, Rossier J, Minick S, Ling N, Rivier C, Vale W, Bloom F. beta-Endorphin and adrenocorticotropin are selected concomitantly by the pituitary gland. Science. 1977;197:1367–1369. doi: 10.1126/science.197601. [DOI] [PubMed] [Google Scholar]
- Hayward MD, Schaich-Borg A, Pintar JE, Low MJ. Differential involvement of endogenous opioids in sucrose consumption and food reinforcement. Pharmacol Biochem Behav. 2006;85:601–611. doi: 10.1016/j.pbb.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology. 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- Hill TD, Angel RJ. Neighborhood disorder, psychological distress, and heavy drinking. Soc Sci Med. 2005;61:965–75. doi: 10.1016/j.socscimed.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Hossain SM, Wong BK, Simpson EM. The dark phase improves genetic discrimination for some high throughput mouse behavioral phenotyping. Genes Brain Behav. 2004;3:167–177. doi: 10.1111/j.1601-183x.2004.00069.x. [DOI] [PubMed] [Google Scholar]
- Koven NS, Heller W, Miller GA. The unique relationship between fear of cognitive dyscontrol and self-reports of problematic drinking. Addict Behav. 2005;30:489–499. doi: 10.1016/j.addbeh.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Sher KJ, Beitman BD. The relation between alcohol problems and anxiety disorders. Am J Psychiatry. 1990;147:685–695. doi: 10.1176/ajp.147.6.685. [DOI] [PubMed] [Google Scholar]
- LaBuda, Christopher J, Fuchs, Perry N. The anxiolytic effect of acute ethanol or Diazepam exposure is unaltered in u-opioid receptor knockout mice. Brain Res Bull. 2001;55:755–760. doi: 10.1016/s0361-9230(01)00569-x. [DOI] [PubMed] [Google Scholar]
- Lawyer SR, Karg RS, Murphy JG, McGlynn FD. Heavy drinking among college students is influenced by anxiety sensitivity, gender, and contexts for alcohol use. J Anxiety Disord. 2002;16:165–173. doi: 10.1016/s0887-6185(02)00092-0. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Quirion R, Gianoulakis C. A microdialysis profile of beta-endorphin and catecholamines in the rat nucleus accumbens following alcohol administration. Psychopharmacology. 2003;169:60–67. doi: 10.1007/s00213-003-1490-2. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Quirion R, Gianoulakis C. An in vivo profile of beta-endorphin release in the arcuate nucleus and nucleus accumbens following exposure to stress or alcohol. Neuroscience. 2004;127:777–784. doi: 10.1016/j.neuroscience.2004.05.047. [DOI] [PubMed] [Google Scholar]
- Marquez P, Baliram R, Dabaja I, Gajawada N, Lufty K. The role of beta-endorphin in the acute motor stimulatory and rewarding actions of cocaine in mice. Psychopharmacology. 2008;197:443–448. doi: 10.1007/s00213-007-1053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ. Stress and endogenous opioid peptides: a review. Mod Probl Pharmacopsychiatry. 1981;17:49–67. doi: 10.1159/000402406. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Grisel JE. Transgenic studies of pain. Pain. 1998;77:107–28. doi: 10.1016/S0304-3959(98)00093-1. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Grisel JE, Hayward MD, Bales JR, Rubinstein M, Belknap JK, Low MJ. Disparate spinal and supraspinal opioid antinociceptive responses in beta-endorphin-deficient mutant mice. Neuroscience. 2000;101:709–717. doi: 10.1016/s0306-4522(00)00422-x. [DOI] [PubMed] [Google Scholar]
- Mollenauer S, Bryson R, Robison M, Sardo J, Coleman C. EtOH self-administration in anticipation of noise stress in C57BL/6J mice. Pharmacol Biochem Behav. 1993;46:35–38. doi: 10.1016/0091-3057(93)90313-i. [DOI] [PubMed] [Google Scholar]
- Oroszi G, Goldman D. Alcoholism: genes and mechanisms. Pharmacogenomics. 2004;5:1037–1048. doi: 10.1517/14622416.5.8.1037. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Wand GS. Opioids and alcoholism. Physiol Behav. 2004;81:339–58. doi: 10.1016/j.physbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Pacak K, Palkovits M. Stressor specificity of central neuroendocrine responses: Implications for stress-related disorders. Endocrine Reviews. 2001;22:502–548. doi: 10.1210/edrv.22.4.0436. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Feeling the Pain of Social Loss. Science. 2003;302:237–239. doi: 10.1126/science.1091062. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Phillips MI, Rubin RT. Principles of Hormone/Behavior Relations. Elsevier Press; Amsterdam Boston Heidelberg London New York Oxford Paris San Diego San Francisco Singapore Sydney Tokyo: 2004. pp. 55–56. [Google Scholar]
- Plotsky PM. Pathways to the secretion of adrenocorticotropin: a review from the portal. J Endocrinol. 1991;3:1–18. doi: 10.1111/j.1365-2826.1991.tb00231.x. [DOI] [PubMed] [Google Scholar]
- Poplawski Poplawski MM, Boyadjieva N, Sarkar DK. Vasoactive intestinal peptide and corticotropin-releasing hormone increase beta-endorphin release and proopiomelanocortin messenger RNA levels in primary cultures of hypothalamic cells: effects of acute and chronic ethanol treatment. Alcohol Clin Exp Res. 2005;29:648–655. doi: 10.1097/01.alc.0000158834.11252.2e. [DOI] [PubMed] [Google Scholar]
- Ribeiro SC, Kennedy SE, Smith YR, Stohler CS, Zubieta JK. Interface of physical and emotional stress regulation through the endogenous opioid system and mu-opioid receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1264–1280. doi: 10.1016/j.pnpbp.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Rivier C. Alcohol stimulates ACTH secretion in the rat: mechanisms of action and interactions with other stimuli. Alcohol Clin Exp Res. 1996;20:240–254. doi: 10.1111/j.1530-0277.1996.tb01636.x. [DOI] [PubMed] [Google Scholar]
- Sarkar DK, Kuhn P, Marano J, Chen C, Boyadjieva N. Alcohol exposure during the developmental period induces beta-endorphin neuronal death and causes alteration in the opioid control of stress axis function. Endocrinology. 2007;148:2828–2834. doi: 10.1210/en.2006-1606. [DOI] [PubMed] [Google Scholar]
- Scanlon MN, Lazar-WEGrant KA, Kunos G. Proopiomelanocortin messenger RNA is decreased in medio-basal hypothalamus of rats made dependent upon ethanol. Alc Clin Exp Res. 1992;16:1147–1151. doi: 10.1111/j.1530-0277.1992.tb00711.x. [DOI] [PubMed] [Google Scholar]
- Schedlowski M, Flüge T, Richter S, Tewes U, Schmidt RE, Wagner TO. Beta-endorphin, but not substance-P, is increased by acute stress in humans. Psychoneuroendocrinology. 1995;20:103–110. doi: 10.1016/0306-4530(94)00048-4. [DOI] [PubMed] [Google Scholar]
- Schulz R, Wuster M, Duka T, Herz A. Acute and chronic ethanol treatment changes endorphin level in brain and pituitary. Psychopharmacology. 1980;68:221–227. doi: 10.1007/BF00428107. [DOI] [PubMed] [Google Scholar]
- Sher L. Alcoholism, anxiety, and opioid-dopaminergic interactions. Psychopharmacology. 2003;165:202–203. doi: 10.1007/s00213-002-1308-7. [DOI] [PubMed] [Google Scholar]
- Thiagarajan AB, Mefford IN, Eskay RL. Acute effect of intragastric ethanol administration on plasma levels of stress hormones. Adv Alcohol Subst Abuse. 1988;7:227–130. doi: 10.1300/J251v07n03_29. [DOI] [PubMed] [Google Scholar]
- Thiagarajan AB, Mefford IN, Eskay RL. Single-dose ethanol administration activates the hypothalamic-pituitary-adrenal axis: exploration of the mechanism of action. Neuroendocrinology. 1989;50:427–432. doi: 10.1159/000125259. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Sabino V, Koob GF. Increased anxiety-like behavior and ethanol self-adminstration in dependent rats: reversal via corticotrophin-releasing factor-2 receptor activation. Alcohol Clin Exp Res. 2004;28:865–872. doi: 10.1097/01.alc.0000128222.29875.40. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Terenina-Rigaldie E, Raba F, Ramos A, Takahashi RN, Mormede P. Evidence for a female-specific effect of a chromosome 4 locus on anxiety-related behaviors and ethanol drinking in rats. Genes Brain Behav. 2006;5:441–450. doi: 10.1111/j.1601-183X.2005.00177.x. [DOI] [PubMed] [Google Scholar]
- Wand GS, Mangold D, El Deiry S, McCaul ME, Hoover D. Family history of alcoholism and hypothalamic opioidergic activity. Arch Gen Psychiatry. 1998;55:1114–1119. doi: 10.1001/archpsyc.55.12.1114. [DOI] [PubMed] [Google Scholar]
- Williams SB, Holloway A, Karwan K, Allen SA, Grisel JE. Oral self-administration of Ethanol in transgenic mice lacking b-endorphin. 2007 Published online, Impulse, http://impulse.schc.sc.edu/articles/2007-9-14-Williams.pdf.
- Wolffgramm J. Free choice ethanol intake of laboratory rats under different social conditions. Psychopharmacology. 1990;101:233–239. doi: 10.1007/BF02244132. [DOI] [PubMed] [Google Scholar]
- Yamada K, Nabeshima T. Stress-induced behavioral responses and multiple opioid systems in the brain. Behav Brain Res. 1995;67:133–145. doi: 10.1016/0166-4328(94)00150-e. [DOI] [PubMed] [Google Scholar]
- Zalenska-Kaszubska J, Czarnecka E. Deficit in beta-endorphin peptide and tendency to alcohol abuse. Peptides. 2005;26:701–705. doi: 10.1016/j.peptides.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Zimmerman P, Wittchen H–U, Hofler M, Pfister H, Kessler RC, Lieb R. Primary anxiety disorders and the development of subsequent alcohol use disorders: a 4-year community study of adolescents and young adults. Psychological Medicine. 2003;33:1211–1222. doi: 10.1017/s0033291703008158. [DOI] [PubMed] [Google Scholar]