Abstract
Systemic ethanol (EtOH) administration activates the hypothalamic-pituitary-adrenal (HPA) axis of rats in a sexually dimorphic manner. The present studies tested the role played by the CNS in this phenomenon. In order to localize the effects of the drug to the brain, we utilized an EtOH administration paradigm whereby a small, non-toxic amount of the drug was delivered intracerebroventricularly (icv). Icv EtOH rapidly diffuses throughout the CSF and brain, and does not cause neuronal damage or have any long-term physiological or behavioral effects. Experimental groups included intact males, intact cycling females, and ovariectomized (OVX) animals with or without replacement estradiol (E2). Icv EtOH- induced HPA hormonal activation was determined by measuring plasma adrenocorticotropin (ACTH) levels. Activation of brain areas that both regulate HPA function and are responsive to gonadal hormones was determined using expression of the transcription factor c-fos (Fos) as a marker of neuronal activity. We observed sex- and estrous cycle- dependent differences in HPA activation by EtOH as measured by both these parameters. ACTH secretion was highest in females in proestrus or estrus, just prior to and after the endogenous peak of E2, as was Fos expression in the paraventricular nucleus of the hypothalamus (PVN) and the locus coreuleus (LC) of the brainstem. In OVX animals, E2 replacement caused an increase in PVN and LC Fos expression in response to icv EtOH as compared to OVX controls, but a decrease in ACTH secretion. Taken together these results indicate that at the level of the CNS, EtOH stimulates HPA activity more robustly at times when the effects of E2 are high, but that E2 alone is not responsible for the effect. The data further suggest that the LC plays an important role in the circuitry, which appears to be different from that activated following the systemic administration of EtOH.
Keywords: ethanol, hypothalamic-pituitary-adrenal, adrenocorticotropin, paraventricular nucleus of the hypothalamus, locus coeruleus, estrous cycle
Introduction
The hypothalamic-pituitary-adrenal (HPA) axis is a key component of the circuit that regulates the way in which mammalian organisms perceive and respond to stressors. Over the years, evidence has accumulated that the activation of this axis is gender-dependent, with females generally exhibiting greater HPA response to stressors than males [1–2]. Since some components of the HPA axis have been linked to the development of alcohol (ethanol, EtOH) addiction [3], and because women who abuse EtOH exhibit increased anxiety and health problems associated with the drug compared to males [4–5], there may be a link between gender, HPA axis activity, and the development of EtOH dependence. This link may be due to the actions of reproductive hormones, which play an important role in modulating HPA axis responses to stressors such as EtOH. One reason for this is that receptors for gonadal hormones, including oestradiol (E2), progesterone (P4) and testosterone (T) have been localized to brain areas that regulate HPA function. These areas include the paraventricular nucleus of the hypothalamus (PVN) and the locus coeruleus (LC) of the brainstem [6–9]. In addition to expressing receptors for gonadal hormones, E2, P4, and T have been shown to alter neuronal activity in these brain nuclei and others [10–11]. In view of these findings, the studies presented here investigated the role of reproductive hormones in HPA responses to EtOH administration in the rat.
It was previously shown that systemic administration of EtOH activates the HPA axis in a sex- and estrous cycle- dependent manner [12], with females exhibiting a more robust HPA response to the drug than males. This phenomenon was linked to the effects of circulating E2 on HPA function. However, a variety of factors make determining the specific endocrine organ(s) responsible for this effect difficult. For instance, systemic EtOH can cause the production of liver cytokines [13], plus alter blood pressure and body temperature [14–15], all of which stimulate HPA activity irrespective of the direct CNS actions of the drug. Additionally, each of these physiological parameters has been shown to be regulated in a sexually dimorphic manner [16–20], further complicating the interpretation of results from experiments investigating the role of the specific organs in mediating sexually dimorphic HPA responses to systemic EtOH. The studies presented here investigated interactions between circulating gonadal hormones and HPA responses to EtOH that are activated specifically at the level of the brain.
In order to resolve whether the drug works directly at the level of the CNS to generate sexually dimorphic HPA responses, we utilized an EtOH administration technique whereby a small (5 μL of 100%, 86 μMol), non-toxic amount of the drug was slowly microinjected into a lateral ventricle (intracerebroventricular injection, icv). EtOH injected icv rapidly diffuses throughout the brain [21], and does not leak to the periphery in measurable amounts or cause neuronal damage. It is only briefly measurable in the CSF (with a peak of 0.08%), and is able to stimulate the secretion of the pituitary stress hormone adrenocorticotropin (ACTH) in a dose dependent manner [22]. Experimental groups included intact males, intact cycling females, and ovariectomized (OVX) animals with or without E2 replacement. Following icv EtOH or vehicle administration, HPA activation was measured both by determining plasma ACTH levels and by assessing neuronal activation levels in brain areas that regulate HPA function using Fos expression as a marker of neuronal activity. Brain areas analyzed have all been shown to be responsive to systemic EtOH administration and express receptors for gonadal hormones. These included the PVN and the LC [23–24], plus the cingulate cortex, which is responsive to EtOH [25], expresses receptors for estradiol [26], and sends inhibitory feedback to the PVN following HPA activation [27]. We also investigated the ability of icv EtOH to activate Fos expression in the central amygdala (CeA), which in addition to being responsive to EtOH and expressing estrogen receptors [28–29], is involved in the formation and regulation of emotional memory/output [30]. Overall, the results presented below indicate that high levels of E2 alone are not sufficient for increasing CNS-triggered HPA activity in females.
Materials and Methods
Animals
Adult male and female Sprague Dawley rats (200–220 g) were housed in an AAALAC approved facility. Animals were kept under standard lighting (12L/12D) and temperature (70°F) conditions, and had access to food and water ad libitum. All animals were singly housed after any surgical procedure.
Surgeries
Rats were anesthetized using a cocktail consisting of ketamine, xylazine, and acepromazine. The anesthetic was injected subcutaneously (s.c.), and icv cannulae (Plastics One, Roanoke Va) were inserted using the following coordinates from bregma: anterior-posterior: −0.4mm, lateral: −1.4mm, and dorsal-ventral: −3.8mm. Cannulae were held in place using small stainless steel screws and cranioplastic cement, also purchased from Plastics One. When relevant, gonadectomies were performed bilaterally at the same time as icv surgery. For studies investigating ACTH secretion in response to icv EtOH, intravenous (i.v.) catheters were inserted 72 hours prior to experimentation. Briefly, rats were given an intraperitoneal injection of the above-mentioned anesthetic cocktail, the jugular vein was exposed, and an i.v. catheter made of polyethelyene tubing with a silastic tip was inserted into the vein and externalized through the skin on the back of the neck.
Estrous cycle synchronization
In order to ensure that animals were in the appropriate stage of the estrous cycle on the days of experimentation, cycle synchronization was performed by giving two same-day subcutaneous injections (at 0900 at 1400) of the gonadotropin releasing hormone (GnRH) agonist ([DTRP6, PRO9-Net] GnRH). This method synchronizes estrous cycles with approximately 80% accuracy. The day of the estrous cycle that each rat was in on the day of experimentation was confirmed via microscopic examination of post-mortem vaginal smears.
Estrogen replacement protocols
E2 (17β estradiol, Sigma Corp) replacement injections were given using two different paradigms. For our initial ACTH experiments, rats were injected s.c. with E2 (10 μg/animal in olive oil, Sigma-Aldrich, St. Louis MO) daily for 7 days prior to experimentation. This E2 injection paradigm results in plasma E2 levels of approximately 160 pg/ml [31]. A second group of animals received cyclic E2 (50 μg/animal in olive oil) injections every 4th day, including early on the day of experimentation. Since the ACTH response to icv EtOH was similar in both groups of animals, the lower dose was used in subsequent Fos studies.
Experimental protocol
All studies took place in a sound-proof room, with animals housed in opaque experimental chambers. Experimentation began at 6 a.m. (lights on), when endogenous HPA activity is near its nadir in the rat [32], and were completed before 1 PM in order to assure consistency between studies in terms of endogenous ACTH and ovarian hormone secretion levels. Briefly, i.v. catheters and icv cannulae were connected to lengths of polyethylene tubing that extended to peripheral sites, allowing for the non-invasive icv injection of EtOH, and for blood withdrawal. The conscious, freely moving animals were then allowed a 3 hour re-acclimation period following connection of the icv cannulae and i.v. catheters prior to any blood draws or icv injections. This period is necessary for basal hormone levels to return to basal values following the initial set-up period of for the experiments. Pure EtOH (Aaper alcohol, Shelbyville, KY) or vehicle was injected icv at a rate of 1 μl/10 seconds to a total volume of 5 μl.
Tissue harvesting for Fos Analysis
Forty-five minutes after icv EtOH administration, rats used in experiments examining Fos expression were deeply anesthetized using chloral hydrate, and transcardially perfused with saline followed by 4% paraformaldehyde (PFA) in PBS (pH 7.0). Following perfusion brains were removed and postfixed in 4% PFA for 4 hours then cryoprotected overnight in a 30% sucrose/PBS solution. They were then flash-frozen in cold methylbutane, and stored at −80C until used. The 45 minute time point was chosen using results from an icv EtOH/Fos expression time course study that indicated that average levels of Fos expression were highest after icv EtOH administration at this time point (data not shown).
Immunocytochemistry
Coronal brain sections were cut through the cingulate cortex, PVN, CeA, and LC at a thickness of 40μM, using a Leica cryostat (Leica Microsystems, Bannockburn IL). Every 3rd section throughout the brain areas of interest was used for Fos analysis. Tissue was processed for Fos immnoreactivity using Vectastain ABC pereoxidase kits and standard ICC techniques (Vector Laboratories, Burlingame, CA). Fos antibody was purchased from Sigma Corp (St. Louis, MO) and used at a concentration of 1:5000. Fos immunoreactivity was developed using cobalt enhanced DAB tablets, also purchased from Sigma Corp.
Image analysis
Brain sections were examined using a 10X objective attached to a Leica light microscope equipped with a digital microscope camera connected to a PC running Moticam software (Motic, Vancouver BC). Fos-positive nuclei in matched sections of the cingulate cortex, PVN, LC, and CeA were counted using NIH Image J software. All Fos expression analysis was done by two individuals who were blind to the experimental treatment groups. Fos counts for each brain area were averaged and are presented as Fos positive nuclei/section for each brain area.
ACTH level quantification
Plasma ACTH levels were determined using a commercially available ACTH ELISA kit (MDBiosciences, St. Paul, MN), which has been validated for the measurement of this hormone in the rat [33]. The kit has a detection limit of 0.46 pg/ml. The intra- and inter- assay coefficients of variation are less than 4.2 and 6.2%, respectively.
Statistical Analysis
Statistics were determined using GraphPad Prism 4.0 software (La Jolla, CA). For ACTH studies, one way ANOVA with repeated measures plus a Neuman-Keuls post-test were used. Fos data were analyzed using one way ANOVAs and/or Student’s T tests, where applicable.
Results
Sex- and estrous cycle- dependent ACTH secretion following the icv administration of EtOH
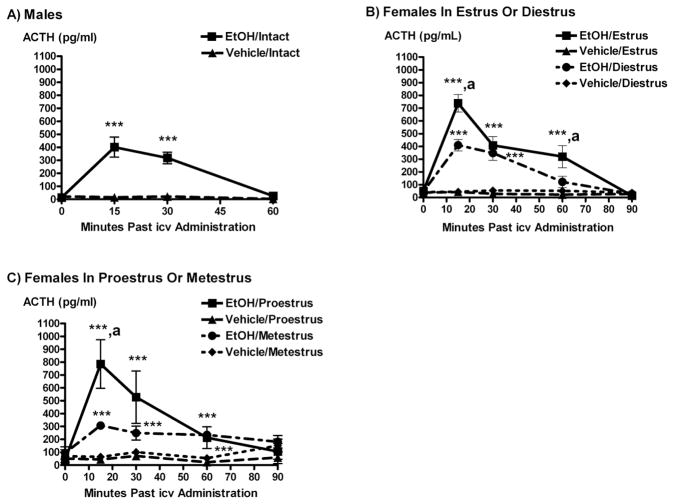
EtOH administered icv significantly stimulated ACTH secretion in all experimental groups as compared to controls, with the release of this hormone peaking 15–30 minutes after the icv EtOH injection (P < 0.05 to 0.001, Figure 1). This effect was most profound in females in proestrus or estrus. Animals receiving icv EtOH on proestrus, estrus and metestrus also showed a prolonged release of ACTH as compared to males or females in diestrus, with plasma levels being significantly higher than basal values up to 60 minutes after administration of EtOH (P < 0.05 to 0.01).
Figure 1.
The icv administration of EtOH (5 μL of 100%) significantly stimulated ACTH secretion compared to vehicle in: A) intact males, B) intact females in estrus or diestrus, and C) intact females in proestrus or metestrus. Each time point represents mean ACTH ± SEM. ***, P < 0.001 versus vehicle; **, P < 0.01 versus vehicle; *, P < 0.05 versus vehicle; a, P < 0.05 versus the same time point in diestrus (Panel B), or metestrus (Panel C); N = 6–9 animals/group.
Effect of icv EtOH administration on Fos expression in the PVN in intact animals
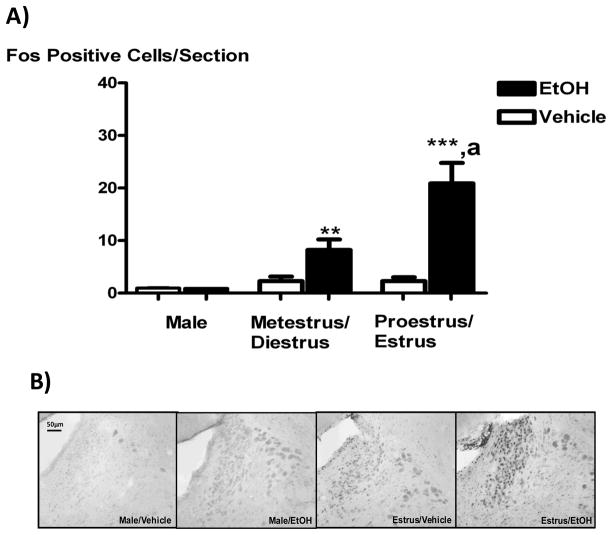
The expression of the transcription factor and marker of neuronal activity Fos was significantly increased in the PVN of animals receiving icv EtOH in all experimental groups, as compared to controls (P < 0.01, Figure 2A). PVN Fos expression induced by icv EtOH was sex- and estrous cycle- dependent, with the immediate early gene expressed more significantly on proestrus and estrus than in all other treatment groups. Since these animals exhibited a similar ACTH and Fos responses to icv EtOH, the data for proestrus/estrus or metestrus/diestrus were pooled for all our studies examining Fos expression. As can be seen in the representative pictures in Figure 2B, icv EtOH- induced Fos expression occurred most abundantly in the medial parvocellular PVN, where most CRF-positive neurons that project to the median eminence reside [34].
Figure 2.
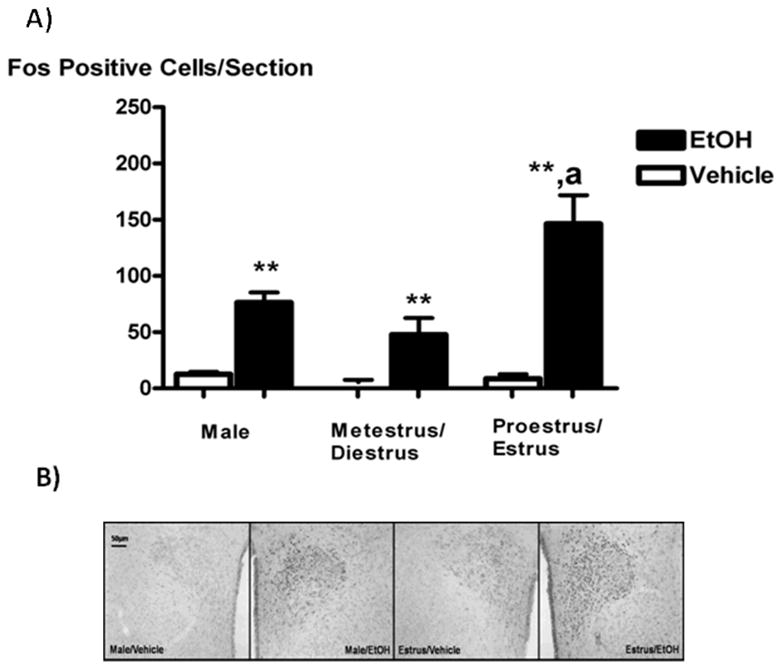
The icv administration of EtOH (5 μL of 100%) significantly increased Fos expression in the PVN compared to vehicles in all experimental animals females, with this effect being highest on proestrus (Panel A). Panel B shows representative 10X pictures of PVN Fos expression (black nuclei) in response to icv EtOH (5μL of 100%) or vehicle in intact males (two panels on left) and females in estrus (two panels on right). Bar height represents the mean ± SEM. **, P < 0.01 versus vehicle; ***, P < 0.001 versus vehicle; a, P < 0.05 versus metestrus/diestrus animals receiving EtOH. N = 8 (male) or 11–12 (female) animals per group and 4–6 PVN sections per animal.
Effect of icv EtOH administration on Fos expression in the locus coeruleus in intact animals
The icv injection of EtOH did not stimulate Fos expression in the LC of males (Figure 3). Conversely, in females in all stages of the estrous cycle, icv EtOH caused a modest but significant increase in Fos expression in the LC, as compared to controls (P < 0.01). This occurred in an estrous cycle- dependent manner, with Fos expression levels being highest on proestrus/estrus as compared to metestrus/diestrus.
Figure 3.
The icv administration of EtOH (5 μL of 100%) stimulated Fos expression in the LC compared to vehicle in intact females but not in males. Panel A shows average icv EtOH vs vehicle Fos counts for males and females in metestrus/diestrus or proestrus/estrus. Panel B shows representative 10X pictures of Fos expression (black nuclei) in the LC in response to icv EtOH (5 μL of 100%) or vehicle in males (left two panels) and estrous females (right two panels). Bar height represents the mean ± SEM. **, P < 0.01 versus vehicle; a, P < 0.05 versus metestrus and diestrus/EtOH; N = 11–12 animals per group and 4–6 LC sections per animal.
Effect of icv EtOH on Fos expression in the cingulate cortex and the CeA
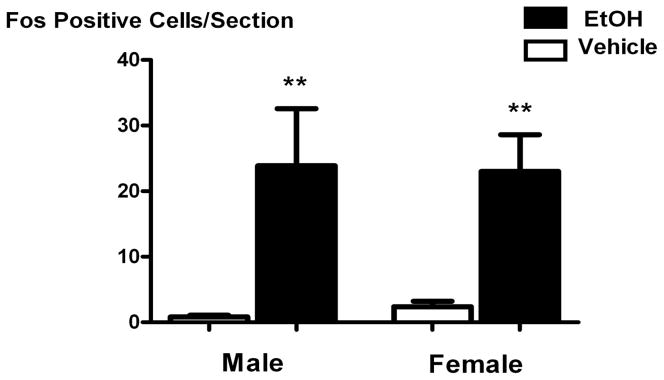
The icv administration of EtOH did not activate Fos expression in the CeA in a series of studies where tissue was harvested 30, 45, 60, or 90 minutes after icv injection (data not shown). Conversely, icv EtOH significantly stimulated Fos expression in the cingulate cortex compared to vehicle 45 minutes after injection (P < 0.01), but did not do so in a sexually dimorphic manner. Because of this, Fos counts from 3 representative females in each day of the estrous cycle were combined, and the data for these animals versus males are presented as a function of sex in Figure 4.
Figure 4.
The icv administration of EtOH (5 μL of 100%) stimulated Fos expression as compared to vehicle in the cingulate cortex of males and intact, cycling females. Bar height represents the mean ± SEM. **, P < 0.01 versus estrus/vehicle. N = 12 animals per group and 5–6 cingulate cortex sections per animal.
Effect of icv EtOH administration on ACTH secretion in OVX animals receiving replacement E2
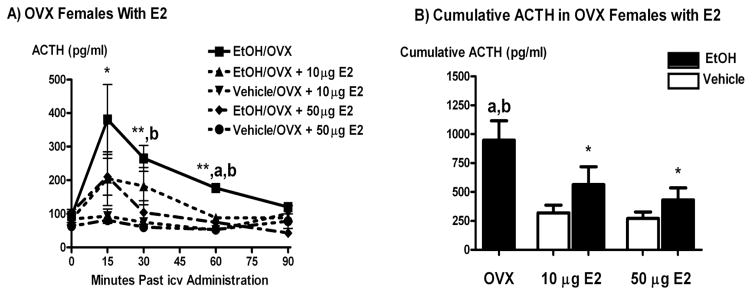
The icv administration of EtOH caused a decrease in ACTH secretion in OVX animals receiving E2 replacement as compared to those receiving sham hormone injections (P < 0.05 to 0.01, Figure 5). This occurred when E2 was administered s.c. daily at a dose of 10 μg, and when it was administered every 4th day at a dose of 50 μg/kg in order to mimic fluctuating E2 secretion that occurs throughout the estrous cycle.
Figure 5.
The icv administration of EtOH (5 μl of 100%) significantly stimulated ACTH secretion as compared to vehicle in OVX shams and OVX animals receiving replacement E2 injections (10 μg/day for 7 days or 50 μg every fourth day). Panel A shows a time course for ACTH secretion following icv EtOH or vehicle and Panel B shows cumulative ACTH secretion over 60 minutes. *, P < 0.05 versus vehicle; a, P < 0.01 versus EtOH/OVX + 10 μg and 50 μg E2; b, P < 0.05 versus EtOH/50 μg E2; N = 8 animals/group.
Effect of icv EtOH administration on Fos expression in the PVN and LC of OVX animals receiving replacement E2
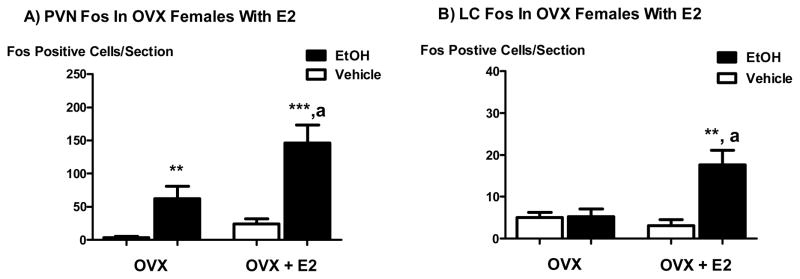
OVX animals receiving replacement E2 (10 μg) injected s.c. daily for 7 days prior to experimentation exhibited a higher level of PVN Fos expression in response to icv EtOH as compared to OVX animals receiving sham E2 injections and icv EtOH (P < 0.01, Figure 6a). These results indicate that E2 directly or indirectly influences PVN neurons in females to be more responsive to icv EtOH as determined by the activation of Fos expression. OVX animals also exhibited slightly elevated basal LC Fos expression levels as compared to intact females. However, icv EtOH did not significantly stimulate Fos expression in these animals compared to vehicle controls. Conversely, OVX animals receiving E2 replacement (10 μg daily for 7 days) exhibited LC Fos expression levels in response to icv EtOH that were significantly greater than controls (P < 0.01) and similar to intact females in proestrus (Figure 6b), once again indicating that E2 replacement in females increases the responsiveness of some neurons to EtOH.
Figure 6.
The icv administration of EtOH (5 μl of 100%) significantly stimulated Fos expression in the PVN of OVX animals with or without E2 (10 μg/day for 7 days) replacement (panel A) as compared to controls, and in the LC of OVX animals with E2 replacement (panel B). **, P < 0.01 versus vehicle; ***, P < 0.001 versus vehicle; a, P < 0.01 versus EtOH/OVX with or without E2 replacement. N = 6–8 animals per group and 4–6 PVN or LC sections per animal.
Discussion
The results presented here indicate a complicated role for gonadal hormones in altering CNS- mediated HPA responses to EtOH. Briefly, we found that the icv administration of a small amount of EtOH stimulated both ACTH secretion as well as PVN and LC Fos expression in a sexually dimorphic manner. ACTH and Fos responses to icv EtOH were similar in intact males, intact females in metestrus and diestrus (when endogenous E2 and P4 levels are relatively low), and in OVX animals. However, as compared to other experimental groups both ACTH secretion and Fos expression in response to icv EtOH were significantly increased in proestrous and estrous females (just prior to and after the endogenous peaks of E2 and P4). These results in intact animals indicate that sex differences in HPA responses to EtOH may be mediated directly at the level of the brain and be driven by changing levels of gonadal hormones. Further, the small but statistically significant sexually dimorphic Fos response in the LC we observed indicates that a proposed feed-forward loop between this structure and the PVN [35] may be involved in producing more robust HPA responses to stressors in females as compared to males.
In studies using E2 replacement paradigms, a dose of 10 μG/rat/day given s.c. in OVX animals increased the ability of icv EtOH to induce Fos expression in the PVN and LC. Conversely, the icv EtOH- induced ACTH response in animals receiving replacement E2 injections at both this and a higher dose (50 μg/ml every 4th day) was smaller in animals receiving olive oil injections with no E2. This disassociation between increased Fos expression levels in the PVN and ACTH secretion following icv EtOH in E2 replacement animals was not found in experiments using a systemic EtOH delivery paradigm [7], indicating that the drug’s actions as a noxious stimulus in the periphery stimulate HPA responses on their own.
Sexually dimorphic HPA responses to icv EtOH in intact animals
Our studies indicate that at least some aspects of sexually dimorphic HPA responses to EtOH arise strictly from the drug’s central effects. This was predicted based on results of studies by Ogilvie and Rivier [12]. These authors reported findings similar to those here in intact animals, but using a systemic EtOH delivery paradigm. However, a major difference between our study and the one by Ogilive and Rivier is that we used a central delivery paradigm in order to test the CNS- specific actions of the drug. Doing so controlled for the potentially confounding effects of EtOH in the periphery that feedback to the brain and activate HPA responses independently of the explicit actions of the drug at the level of the brain. Potentially confounding effects that need to be accounted for in determining the role of systemic EtOH on CNS- mediated HPA activation include that the drug alters cytokine production [36], blood pressure [37], and irritates the stomach lining [38]. Interestingly, changes in liver and immune responses to noxious stimuli [16–18], blood pressure [19], and gastrointestinal function [20] have all been shown to be differentially regulated in males and females.
Of note in the results presented here is that there was a comparatively large amount of variation in the ACTH response to icv EtOH in animals in proestrus as opposed to other days of the cycle. This is likely due to the slight variability of the timing of the onset of this stage of the estrous cycle. This variability results in different plasma E2/P4 ratios between animals. Since there is a great deal of interaction between these two hormones in a variety of instances, including the onset of the GnRH surge [39], it is a possibility that P4 may positively influence HPA responsiveness to icv EtOH given a high background of E2. For instance, higher levels of P4 have been shown to increase estrogen receptor beta (ERβ) synthesis in a hypothalamic cell line [40], and activation of this receptor has been linked to increased CRF synthesis [40]. However, it should be noted that others have found that the addition of P4 in OVX animals receiving replacement E2 inhibited HPA responses to restraint stress [41]. Unlike that used for our studies, though, the restraint stress paradigm entails the activation of peripheral as well as centrally mediated pathways that stimulate HPA function, thereby potentially stimulating neuronal circuits not activated by icv EtOH.
Since we observed concordant increases in PVN and LC Fos expression following icv EtOH on proestrus and estrus, our initial hypothesis was that gonadal hormone feedback to the brain either directly or indirectly confers a greater responsiveness to EtOH in CRF neurons involved in regulating HPA function. The small, but estrous cycle-dependent ability of icv EtOH to stimulate Fos expression in the LC of females, plus the ability of this treatment to deplete noradrenergic stores here [42] indicates that the positive feedback loop between the PVN and LC [43] may be more active in females at times when circulating gonadal hormone levels are high. This in turn would result in greater HPA responsiveness to stressors that activate the LC, such as EtOH [42]. In further support of the idea that the LC is potentially more responsive to stressors during proestrus and estrus, estrogen receptor alpha (ERα) is expressed more robustly in the LC during these time [44]. In addition to our finding of increased Fos expression in response to icv EtOH, Curtis et al [10] reported sexually dimorphic noradrenergic responses to stressors that were mediated at the level of the LC. Taken together, these findings support the notion of the LC as an E2-sensitive brain area that may play a major role in generating sexually dimorphic HPA responses to stressors. However, our results in studies that investigated icv EtOH- induced ACTH secretion in OVX rats receiving E2 replacement (below) call into question this conclusion.
Effect of E2 replacement on HPA responsiveness to icv EtOH in females
As can be seen in Figures 5 and 6, E2 replacement in females caused increased PVN and LC Fos expression in response to icv EtOH as compared to OVX animals not receiving replacement hormone. However, OVX rats with replacement E2 injections exhibited a blunted ACTH response to icv EtOH as compared to OVX control rats. In our laboratory, this occurred in OVX animals receiving several different E2 injection paradigms, including the two shown in the results section (Figure 7; other data not shown). While a disassociation between stressor- induced PVN Fos expression and ACTH secretion have been shown before [45], to our knowledge this is the first time it has been reported when the stressor was EtOH, and with apparent involvement of the LC in responding to the stressor.
There are several potential explanations for the seemingly discrepant findings between intact animals in proestrus and estrus versus OVX animals receiving replacement E2. One possibility is that the results were due to E2 levels that are higher than those found in a normally cycling female. For instance, Ree et al [46] found that relatively high levels of E2 can decrease estrogen receptor mRNA in some tissues. This effect was reversed when E2 levels were lower [46]. It has also been shown that under conditions of prolonged high E2 levels, HPA axis responsiveness to a stressor can be decreased, compared to conditions that include a lower dose of E2 [47]. However, this does not appear to be the case when the stressor is mild [48]. As the magnitude of HPA axis activation by icv alcohol is smaller than that observed with other alcohol administration paradigms, this explanation is also unlikely to be valid. Indeed, in other studies carried out in our laboratory using a different (pellet) E2 replacement paradigm, we also observed a blunting effect of E2 replacement on the ACTH response to icv EtOH when circulating E2 levels were in the 60–80 pg/ml range (Larkin and Selvage, unpublished data). Taken together, our interpretation of the data presented here is that while circulating E2 levels were higher than normally found in our E2 replacement studies, the dose was not so high as to cause an inverse (inhibitory) effect on HPA activation on its own.
Yet another feasible explanation for our findings is that the neurons expressing Fos in response to icv EtOH in E2 replacement rats are involved in sending inhibitory signals to CRF neurons that project to the median eminence. Alternatively, it may be that E2 chronically increased HPA activity in our experiments, such that our experimental conditions caused a large secretion of corticosterone that in turn provided feedback signals to the hypothalamus and pituitary and inhibited the ACTH response to the subsequent mild stressor of icv EtOH. Indeed, increased blood corticosterone levels have been shown to decrease the ACTH response to a subsequent stressor [49–50] and experiments using similar E2 dosing paradigms as ours showed decreased HPA responses to stressors [47]. However, as mentioned above, others have shown the opposite, i.e. that E2 replacement caused increased HPA responses to stressors [51]. For instance, a recent report by Weiser and Handa [52] suggests that E2 decreases the efficacy of glucocorticoid negative feedback loops via an ERα-mediated mechanism. Thus, the role of E2 in generating sexually dimorphic HPA responses is still very much in question.
Summary and conclusions
The research presented here reveals CNS- mediated, sexually dimorphic HPA responses to EtOH. Results for studies done in intact animals show an increase in both PVN and LC responsiveness to icv EtOH at times when the effects of gonadal hormones are high (proestrus and estrus). These data suggests that in females in these stages of the estrous cycle, the hypothesized PVN-LC positive feedback loop [35] may be more active in response to EtOH. However, despite E2 replacement causing increases in neuronal activity in both the PVN and LC in our studies, the hormone replacement regimens we used also caused decreased ACTH secretion in animals receiving icv EtOH as compared to controls. While there is literature indicating a stimulatory role for E2 in ACTH responses to stressors [6, 31], others show decreases in the ACTH response [47, 53]. Because of this, it appears that the role of E2 in modulating stress responses depends largely on the specific stressor involved. Indeed, the importance of the type of stressor used to try to determine the role of E2 in HPA activation is illustrated by the conflict between our findings and those of Ogilvie and Rivier [12], who used a systemic EtOH administration paradigm to test the sex differences in HPA activation by the drug. Thus, while the results presented here agree with some of the literature in the field, they contradict findings of others; as such, more research obviously needs to be done to determine how E2 affects HPA responses to various stressors, including EtOH.
Acknowledgments
This work was in part supported by funding from NIH grant number 1R01AA016947-01A2
References
- 1.Iwasaki-Sekino A, et al. Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinology. 2009;34(2):226–37. doi: 10.1016/j.psyneuen.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Jezova D, et al. Neuroendocrine response during stress with relation to gender differences. Acta Neurobiol Exp (Wars) 1996;56(3):779–85. doi: 10.55782/ane-1996-1183. [DOI] [PubMed] [Google Scholar]
- 3.Clarke TK, Schumann G. Gene-environment interactions resulting in risk alcohol drinking behaviour are mediated by CRF and CRF1. Pharmacol Biochem Behav. 2009;93(3):230–6. doi: 10.1016/j.pbb.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Roman PM. Biological features of women’s alcohol use: a review. Public Health Rep. 1988;103(6):628–37. [PMC free article] [PubMed] [Google Scholar]
- 5.Zilberman ML, et al. Substance use disorders: sex differences and psychiatric comorbidities. Can J Psychiatry. 2003;48(1):5–13. doi: 10.1177/070674370304800103. [DOI] [PubMed] [Google Scholar]
- 6.Handa RJ, et al. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28(4):464–76. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- 7.Handa RJ, Weiser MJ, Zuloaga DG. A role for the androgen metabolite, 5alpha-androstane-3beta,17beta-diol, in modulating oestrogen receptor beta-mediated regulation of hormonal stress reactivity. J Neuroendocrinol. 2009;21(4):351–8. doi: 10.1111/j.1365-2826.2009.01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choleris E, et al. Steroidal/neuropeptide interactions in hypothalamus and amygdala related to social anxiety. Prog Brain Res. 2008;170:291–303. doi: 10.1016/S0079-6123(08)00424-X. [DOI] [PubMed] [Google Scholar]
- 9.Hamson DK, Jones BA, Watson NV. Distribution of androgen receptor immunoreactivity in the brainstem of male rats. Neuroscience. 2004;127(4):797–803. doi: 10.1016/j.neuroscience.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Curtis AL, Bethea T, Valentino RJ. Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology. 2006;31(3):544–54. doi: 10.1038/sj.npp.1300875. [DOI] [PubMed] [Google Scholar]
- 11.Kow LM, Pfaff DW. Responses of hypothalamic paraventricular neurons in vitro to norepinephrine and other feeding-relevant agents. Physiol Behav. 1989;46(2):265–71. doi: 10.1016/0031-9384(89)90266-7. [DOI] [PubMed] [Google Scholar]
- 12.Ogilvie KM, Rivier C. Gender difference in hypothalamic-pituitary-adrenal axis response to alcohol in the rat: activational role of gonadal steroids. Brain Res. 1997;766(1–2):19–28. doi: 10.1016/s0006-8993(97)00525-8. [DOI] [PubMed] [Google Scholar]
- 13.Schaffert CS, et al. Alcohol metabolites and lipopolysaccharide: roles in the development and/or progression of alcoholic liver disease. World J Gastroenterol. 2009;15(10):1209–18. doi: 10.3748/wjg.15.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li G, Abdel-Rahman AA. Estrogen-dependent enhancement of NO production in the nucleus tractus solitarius contributes to ethanol-induced hypotension in conscious female rats. Alcohol Clin Exp Res. 2009;33(2):366–74. doi: 10.1111/j.1530-0277.2008.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ristuccia RC, Spear LP. Adolescent ethanol sensitivity: hypothermia and acute tolerance. Ann N Y Acad Sci. 2004;1021:445–7. doi: 10.1196/annals.1308.061. [DOI] [PubMed] [Google Scholar]
- 16.Kovacs EJ, Messingham KA. Influence of alcohol and gender on immune response. Alcohol Res Health. 2002;26(4):257–63. [PMC free article] [PubMed] [Google Scholar]
- 17.Lieber CS. Susceptibility to alcohol-related liver injury. Alcohol Alcohol Suppl. 1994;2:315–26. [PubMed] [Google Scholar]
- 18.Crabb DW. Pathogenesis of alcoholic liver disease: newer mechanisms of injury. Keio J Med. 1999;48(4):184–8. doi: 10.2302/kjm.48.184. [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Ruiz A, et al. Sex differences in control of blood pressure: role of oxidative stress in hypertension in females. Am J Physiol Heart Circ Physiol. 2008;295(2):H466–74. doi: 10.1152/ajpheart.01232.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sim SM, Back DJ. Intestinal absorption of oestrone, oestrone glucuronide and oestrone sulphate in the rat in situ--I. Importance of hydrolytic enzymes on conjugate absorption. J Steroid Biochem. 1985;22(6):781–8. doi: 10.1016/0022-4731(85)90286-9. [DOI] [PubMed] [Google Scholar]
- 21.Friedman HJ, Lester D. Intraventricular ethanol and ethanol intake: a behavioral and radiographic study. Pharmacol Biochem Behav. 1975;3(3):393–401. doi: 10.1016/0091-3057(75)90047-7. [DOI] [PubMed] [Google Scholar]
- 22.Lee S, et al. Site of action of acute alcohol administration in stimulating the rat hypothalamic-pituitary-adrenal axis: comparison between the effect of systemic and intracerebroventricular injection of this drug on pituitary and hypothalamic responses. Endocrinology. 2004;145(10):4470–9. doi: 10.1210/en.2004-0110. [DOI] [PubMed] [Google Scholar]
- 23.Makino S, Hashimoto K, Gold PW. Multiple feedback mechanisms activating corticotropin-releasing hormone system in the brain during stress. Pharmacol Biochem Behav. 2002;73(1):147–58. doi: 10.1016/s0091-3057(02)00791-8. [DOI] [PubMed] [Google Scholar]
- 24.Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848(1–2):141–52. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- 25.Vongvatcharanon U, et al. Alcohol administration during adulthood induces alterations of parvalbumin and glial fibrillary acidic protein immunoreactivity in rat hippocampus and cingulate cortex. Acta Histochem. 2009 doi: 10.1016/j.acthis.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 26.O’Keefe JA, Handa RJ. Transient elevation of estrogen receptors in the neonatal rat hippocampus. Brain Res Dev Brain Res. 1990;57(1):119–27. doi: 10.1016/0165-3806(90)90191-z. [DOI] [PubMed] [Google Scholar]
- 27.Saphier D, Feldman S. Electrophysiology of limbic forebrain and paraventricular nucleus connections. Brain Res Bull. 1986;17(6):743–50. doi: 10.1016/0361-9230(86)90085-7. [DOI] [PubMed] [Google Scholar]
- 28.McBride WJ. Central nucleus of the amygdala and the effects of alcohol and alcohol-drinking behavior in rodents. Pharmacol Biochem Behav. 2002;71(3):509–15. doi: 10.1016/s0091-3057(01)00680-3. [DOI] [PubMed] [Google Scholar]
- 29.Mitra SW, et al. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144(5):2055–67. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- 30.Vilpoux C, et al. Ethanol-sensitive brain regions in rat and mouse: a cartographic review, using immediate early gene expression. Alcohol Clin Exp Res. 2009;33(6):945–69. doi: 10.1111/j.1530-0277.2009.00916.x. [DOI] [PubMed] [Google Scholar]
- 31.Figueiredo HF, et al. Estrogen potentiates adrenocortical responses to stress in female rats. Am J Physiol Endocrinol Metab. 2007;292(4):E1173–82. doi: 10.1152/ajpendo.00102.2006. [DOI] [PubMed] [Google Scholar]
- 32.Brodish A. Control of ACTH secretion by corticotropin-releasing factor(s) Vitam Horm. 1979;37:111–52. doi: 10.1016/s0083-6729(08)61069-9. [DOI] [PubMed] [Google Scholar]
- 33.Qiao S, Okret S, Jondal M. Thymocyte-synthesized glucocorticoids play a role in thymocyte homeostasis and are downregulated by adrenocorticotropic hormone. Endocrinology. 2009 doi: 10.1210/en.2009-0195. [DOI] [PubMed] [Google Scholar]
- 34.Kiss JZ, Mezey E, Skirboll L. Corticotropin-releasing factor-immunoreactive neurons of the paraventricular nucleus become vasopressin positive after adrenalectomy. Proc Natl Acad Sci U S A. 1984;81(6):1854–8. doi: 10.1073/pnas.81.6.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunn AJ, Swiergiel AH, Palamarchouk V. Brain circuits involved in corticotropin-releasing factor-norepinephrine interactions during stress. Ann N Y Acad Sci. 2004;1018:25–34. doi: 10.1196/annals.1296.003. [DOI] [PubMed] [Google Scholar]
- 36.Bode C, Bode JC. Activation of the innate immune system and alcoholic liver disease: effects of ethanol per se or enhanced intestinal translocation of bacterial toxins induced by ethanol? Alcohol Clin Exp Res. 2005;29(11 Suppl):166S–71S. doi: 10.1097/01.alc.0000189280.19073.28. [DOI] [PubMed] [Google Scholar]
- 37.Altura BM, Altura BT. Microvascular and vascular smooth muscle actions of ethanol, acetaldehyde, and acetate. Fed Proc. 1982;41(8):2447–51. [PubMed] [Google Scholar]
- 38.Olson CE. A chronobiologic approach to ethanol and acidified aspirin injury of the gastric mucosa in the rat. Chronobiol Int. 1987;4(1):19–29. doi: 10.1080/07420528709078505. [DOI] [PubMed] [Google Scholar]
- 39.Zalanyi S. Progesterone and ovulation. Eur J Obstet Gynecol Reprod Biol. 2001;98(2):152–9. doi: 10.1016/s0301-2115(01)00361-x. [DOI] [PubMed] [Google Scholar]
- 40.Ogura E, et al. Effects of estradiol on regulation of corticotropin-releasing factor gene and interleukin-6 production via estrogen receptor type beta in hypothalamic 4B cells. Peptides. 2008;29(3):456–64. doi: 10.1016/j.peptides.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129(5):2503–11. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- 42.Selvage DJ, Parsons L, Rivier C. Role played by brainstem neurons in regulating testosterone secretion via a direct neural pathway between the hypothalamus and the testes. Endocrinology. 2006;147(6):3070–5. doi: 10.1210/en.2005-1358. [DOI] [PubMed] [Google Scholar]
- 43.Reyes BA, et al. Hypothalamic projections to locus coeruleus neurons in rat brain. Eur J Neurosci. 2005;22(1):93–106. doi: 10.1111/j.1460-9568.2005.04197.x. [DOI] [PubMed] [Google Scholar]
- 44.Helena CV, et al. Changes in alpha-estradiol receptor and progesterone receptor expression in the locus coeruleus and preoptic area throughout the rat estrous cycle. J Endocrinol. 2006;188(2):155–65. doi: 10.1677/joe.1.06268. [DOI] [PubMed] [Google Scholar]
- 45.Kasckow JW, et al. Stability of neuroendocrine and behavioral responsiveness in aging Fischer 344/Brown-Norway hybrid rats. Endocrinology. 2005;146(7):3105–12. doi: 10.1210/en.2004-1648. [DOI] [PubMed] [Google Scholar]
- 46.Ree AH, et al. Autologous down-regulation of messenger ribonucleic acid and protein levels for estrogen receptors in MCF-7 cells: an inverse correlation to progesterone receptor levels. Endocrinology. 1989;124(5):2577–83. doi: 10.1210/endo-124-5-2577. [DOI] [PubMed] [Google Scholar]
- 47.Ochedalski T, et al. Interaction between oestrogen and oxytocin on hypothalamic-pituitary-adrenal axis activity. J Neuroendocrinol. 2007;19(3):189–97. doi: 10.1111/j.1365-2826.2006.01525.x. [DOI] [PubMed] [Google Scholar]
- 48.Burgess LH, Handa RJ. Chronic estrogen-induced alterations in adrenocorticotropin and corticosterone secretion, and glucocorticoid receptor-mediated functions in female rats. Endocrinology. 1992;131(3):1261–9. doi: 10.1210/endo.131.3.1324155. [DOI] [PubMed] [Google Scholar]
- 49.O’Connor KA, et al. Stress-induced sensitization of the hypothalamic-pituitary adrenal axis is associated with alterations of hypothalamic and pituitary gene expression. Neuroendocrinology. 2004;80(4):252–63. doi: 10.1159/000082876. [DOI] [PubMed] [Google Scholar]
- 50.Ginsberg AB, et al. Acute glucocorticoid pretreatment suppresses stress-induced hypothalamic-pituitary-adrenal axis hormone secretion and expression of corticotropin-releasing hormone hnRNA but does not affect c-fos mRNA or fos protein expression in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 2003;15(11):1075–83. doi: 10.1046/j.1365-2826.2003.01100.x. [DOI] [PubMed] [Google Scholar]
- 51.Young EA, et al. Effects of estrogen antagonists and agonists on the ACTH response to restraint stress in female rats. Neuropsychopharmacology. 2001;25(6):881–91. doi: 10.1016/S0893-133X(01)00301-3. [DOI] [PubMed] [Google Scholar]
- 52.Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience. 2009;159(2):883–95. doi: 10.1016/j.neuroscience.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Serova LI, Maharjan S, Sabban EL. Estrogen modifies stress response of catecholamine biosynthetic enzyme genes and cardiovascular system in ovariectomized female rats. Neuroscience. 2005;132(2):249–59. doi: 10.1016/j.neuroscience.2004.12.040. [DOI] [PubMed] [Google Scholar]