Abstract
Herpes Simplex Virus-2 (HSV-2) is a sexually transmitted infection that is the leading cause of genital ulcers worldwide. Infection is life long and is characterized by repeated asymptomatic and symptomatic shedding episodes of virus that are initiated when virus is released from neurons into the genital tract. The pattern of HSV-2 release from neurons into the genital tract is poorly understood. We fit a mathematical model of HSV-2 pathogenesis to curves generated from daily quantification of HSV in mucosal swabs performed from patients with herpetic genital ulcers. We used virologic parameters derived from model fitting for stochastic model simulations. These simulations reproduced previously documented estimates for shedding frequency, and herpetic lesion diameter and frequency. The most realistic model output occurred when we assumed minimal amounts of daily neuronal virus introduction. In our simulations, small changes in average total quantity of HSV-2 released from neurons influenced detectable shedding frequency, while changes in frequency of neuronal HSV-2 release had little effect. Frequent HSV-2 shedding episodes in humans are explained by nearly constant release of small numbers of viruses from neurons that terminate in the genital tract.
Herpes Simplex Virus-2 (HSV-2) is a widely prevalent sexually transmitted infection that is the leading cause of genital ulcer disease (1), and is a risk factor for human immunodeficiency virus-1 (HIV-1) acquisition and transmission (2, 3). Initial infection results in viral replication in epithelial cells of the genital tract and spread into innervating peripheral neurons. The virus is not cytopathic to neurons and exists indefinitely in the nuclei of neurons within the dorsal root ganglia in a protected state termed “latency” (4). Virus is released from the neurons back into the genital tract during “reactivations”.
Until recently, a common view of HSV-2 pathogenesis was that reactivations occur one to two times per month, and almost always result in a genital ulcer (5). Recent work with intensive sampling methods and sensitive assays for HSV DNA by polymerase chain reaction (PCR) indicates that most people who exhibit antibodies to HSV-2 in their blood shed virus frequently in the genital tract (6). “Viral shedding”, or detection of virus in the genital tract by culture or PCR, is characterized by distinct “shedding episodes” that are preceded and followed by periods in which HSV is not detected. Eight-five percent of shedding episodes are “subclinical” or “asymptomatic” and are not perceived by the infected host (7), despite the presence of virus from genital swabs. Sixty percent of shedding episodes last less than 12 hours, implying rapid production and clearance of virus in the genital mucosa (7).
There is substantial heterogeneity among patients regarding frequency of subclinical episodes (6, 8) and “recurrences” which are shedding episodes associated with a genital lesion (9). While individuals who have recurrences also experience subclinical episodes (7), many infected persons who exhibit antibodies to HSV-2 only shed virus asymptomatically (8). Factors that influence shedding frequency in an individual or among individuals are poorly understood. Studies in animals indicate that quantity of virus in ganglia, as well as host responses can influence reactivation frequency (10, 11). However, the relevance of animal models to human HSV infection is unclear. Because asymptomatic shedding can lead to HSV-2 transmission (12), determinants of shedding frequency are important to understand.
Mathematical models are useful tools to evaluate the interplay between host and viral pathogens and model findings can be informative to the practicing clinician. The original HIV-1 models estimated extremely rapid production and clearance of HIV-1 during plasma virus steady state, which necessitates the need for multiple antiviral medications to avoid drug resistance; other models predicted the existence of a latently infected pool of cells with slower turnover and viral production, making cure of HIV a daunting prospect (13–15).
In this article, we describe a stochastic mathematical model of mucosal HSV-2 infection. The model utilizes experimentally-derived virologic data pertaining to mucosal HSV-2 reactivation, and a wide array of clinical data on the frequency, pattern and duration of human HSV-2 infection in immunocompetent persons. Our results indicate that release of only a few HSV viruses per day is sufficient to initiate subclinical and clinical shedding episodes, and that overall quantity of neuronal virus release per unit time, rather than frequency of neuronal reactivation, is a dominant factor in determining the frequency of HSV-2 shedding.
Results
Model of the mucosal reactivation pattern in patients with recurrent HSV-2 infection
We achieved good fit between curves generated from the deterministic form of our mathematical model, which included parameters of viral replication and infection as well as parameters of CD8+ T-cell response (Fig. 1), and empirical measures of viral expansion and clearance during herpetic genital lesions. We achieved good fit to all 89 patient curves derived from swabs of the entire anogenital tract, and all 15 patient curves derived from lesion-only swabs (Fig. 2, Fig. S2). We were unable to fit a deterministic form of the model, which included parameters of viral replication and infection but no parameters of CD8+ T-cell response, to 20 patient-derived curves in which this was attempted (Fig. S3), confirming that host immune response is an essential component of the empirically observed pattern of genital shedding.
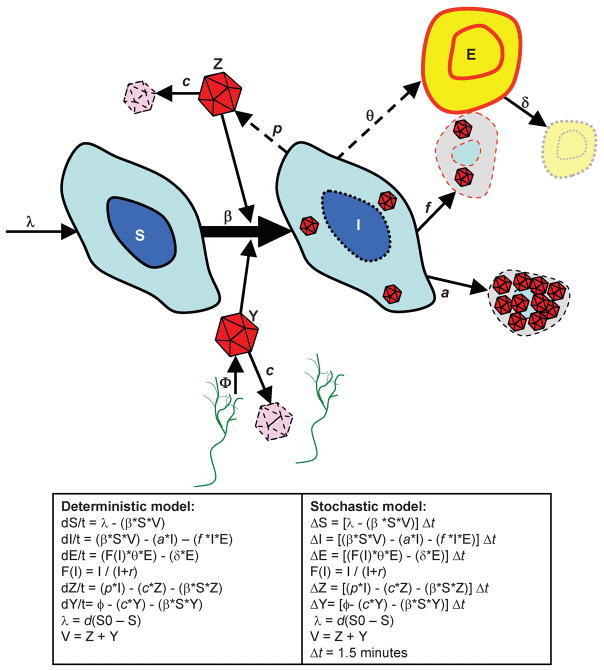
Figure 1.
Cartoon diagram of model. S = Susceptible epithelial cells; I = Infected epithelial cells; E = CD8+ lymphocyte cells; Z = Viral particles from infected epithelial cells; Y = Viral particles from the neuronal bed. Green = sensory neurons.
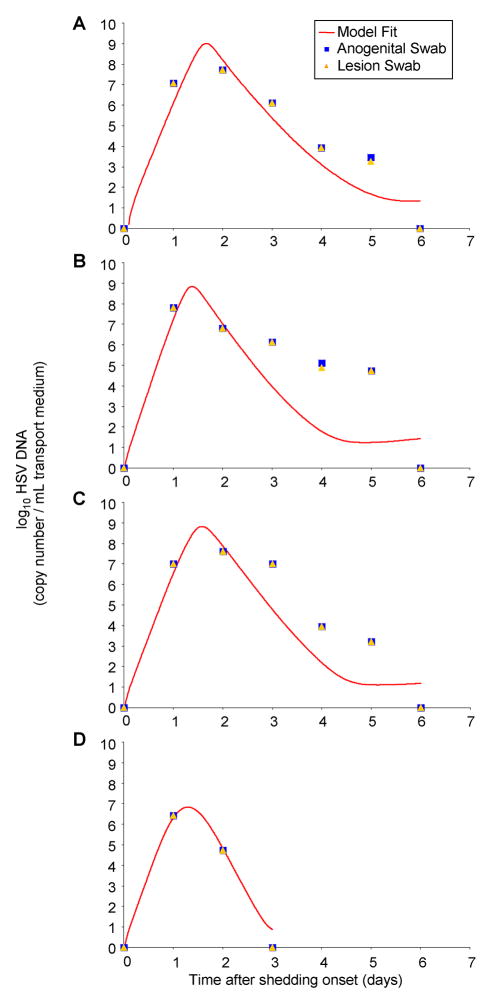
Figure 2. The deterministic form of the model achieves good fit with empiric data from lesional swabs.
Four examples of deterministic model curve fitting to mucosal HSV-2 shedding curves with daily serial quantitative PCRs performed on anogenital lesions. Actual patient quantitative HSV PCR data from anogenital swabs (blue circles) and lesional swabs (orange triangles) performed during herpetic lesions, and deterministic model fits (red lines).
The measured HSV copy number was identical in concurrently performed anogenital and lesional swabs in 11 out of 15 patients on each day of sampling, and differed by less than one log in no more than 2 data points in the other 4 patients. Parameter values derived from curve fitting to anogenital swab data and lesion-only swab data, from concurrent swabs on the same patient during a genital HSV lesion, were the same in all 15 cases.
Parameter estimates from the deterministic model-fitting highlight key features of pathogenesis (Table 1). First, considerable variability was noted among patients. For example, model-derived estimates of viral infectivity differed among patients by as much as a factor of eight. Second, viral lifespan was brief (1.5 – 3 hours) implying rapid viral turn over in the genital tract. Finally, the model predicted that epithelial cells burst after a median of 8807 HSV DNA copies are produced within the cell, provided the cell avoids being killed by CD8+ lymphocytes. Thus, a single epithelial cell can release 170 times more viruses than was released by all of the sensory neurons in the area during a single day [(50 HSV DNA copies per day (see below)]. Therefore, amplification of sufficient virus to produce a detectable shedding episode occurs mainly by means of viral replication within and release from epithelial cells.
Table 1.
Model parameter range estimates from the literature, and model-derived parameter estimates from 89 curve fitting exercises.
| Parameter | Units | Range in literature (ref) | Model estimates: Mean, [% range] |
|---|---|---|---|
| Viral infectivity: (1/(β*S0)) | Virion-days per infected cell | 3 – 69(34) | 19.3 [6.4, 53.3] |
| Infected cell DNA burst size: (p/a) |
p = Per cell per day 1/a = Days |
p=1000–26700 1/a =0.75–0.83 (35–37) |
8807 [4241, 15098] |
| Viral life span: (24/c) | Hours | 0.25 – 3 (38) | 2.4 [1.5, 3] |
| Peak CD8+ lymphocyte production rate: (θ) | CD8+ cells per day | 0.98 – 3.1 (39) | 1.68 [0.98, 2.62] |
| CD8+ antigen recognition: (r) | Infected cells | 5–200 | 63 [5,157] |
| CD8+ killing efficiency: (f) | Infected cell per CD8+ cell per day | 0.001 – 0.2(40–43) | 0.0081 [0.0011,0.016] |
| Initial CD8 level (E0) | CD8 cells | 1000 – 15000(16, 44) | 2053 [1000, 3092] |
| Neuronal viral production (φ) | Virions per day | 1 – 2000 | -- |
| CD8+ mucosal lifespan: (1/δ) | Days | 12–28 (16) | -- |
Stochastic model simulation output
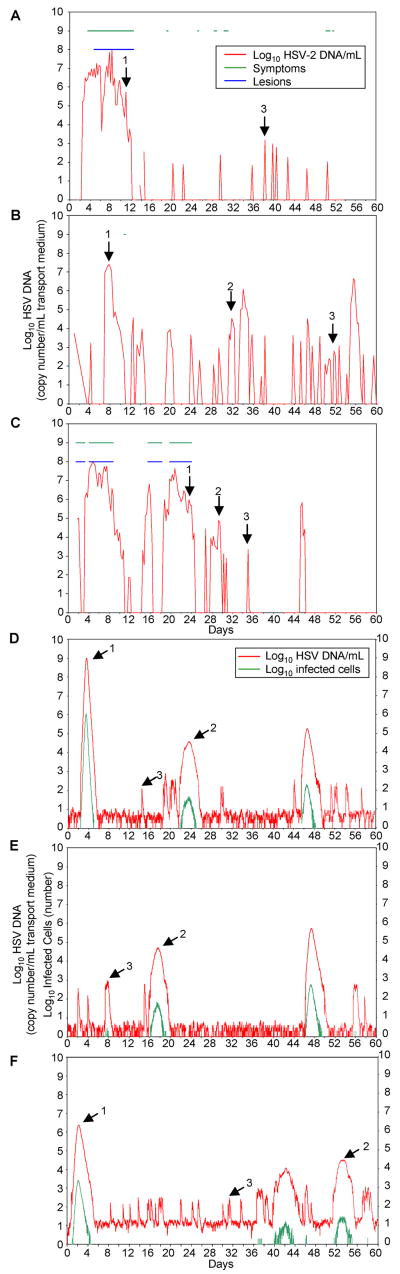
We evaluated how closely the model with parameters of viral replication and CD8+ lymphocyte dynamics, predicted variability that we observed in subclinical and clinical reactivation patterns from prospectively studied patients. Empiric patterns of viral shedding were reproduced when we simulated the stochastic model over a year. Each simulation demonstrated episodes that varied in duration, frequency and severity (Fig. 3D–F). HSV-2 shedding patterns resembled those gathered prospectively from patients who underwent anogenital swabs every six hours for sixty-days (Fig. 3A–C). Stochastic simulations using the model that lacked terms for CD8+ lymphocyte response, resulted in persistent rather than episodic viral production from epithelial cells (Fig. S4A). This simulated shedding pattern is not consistent with the typical pattern of HSV-2 shedding in an immunocompetent host.
Figure 3. In a stochastic form, the model generates shedding patterns that resemble those from actual patients.
(A, B, C) Actual patient HSV-2 shedding patterns over 60 days (x axis) with anogenital swabs performed every 6 hours. Red line, log-10 HSV-2 DNA/mL transport medium. Green line, symptoms present. Blue line, lesion present. Arrow 1, high-copy episode. Arrow 2, medium copy episode. Arrow 3, low-copy episode. (A) There is 1 high-copy episode, no medium-copy episodes, 11 low-copy episodes episode, and 22.0% detectable shedding. (B) There are 3 high-copy episodes, 3 medium-copy episodes, 21 low-copy episodes, and 34.8% detectable shedding. (C) There are 3 high-copy episodes, 4 medium-copy episodes, 4 low-copy episodes and 39.3% detectable shedding. (D, E, F) Variations in the number of HSV particles introduced by neurons per day (φ) directly affect frequency of shedding and number of reactivations. Sixty-day stochastic model simulations using mean model-derived parameters from Table 1. Red line, log-10 HSV-2 DNA/mL transport medium. Green line, log-10 infected cells. Arrow 1, high-copy episode. Arrow 2, medium copy episode. Arrow 3, low-copy episode. (D) Neuronal release rate is 50 HSV-2 copies per day per 2-centimeter model area. There is 1 high-copy episode, 2 medium-copy episodes, 13 low-copy episodes, and 22.9% detectable shedding. (E) Neuronal release rate is 25 copies per day per 2-centimeter model area. There is 1 high-copy episode, 1 medium-copy episodes, 6 low-copy episodes and 16.9% detectable shedding. (F) Neuronal release rate is 100 copies per day per 2-centimeter model area. There is 1 high-copy episode, 2 medium-copy episodes, 22 low-copy episodes and 36.9% detectable shedding.
Reflecting observed variability in reactivation patterns of HSV-2 infection, the ranges of outcomes generated from 445 model stochastic runs of the CD8+ lymphocyte inclusive model, were broad and consistent with previously defined outcomes in patient cohorts (Table 2). The median values for percent of time shedding greater than 50 HSV-2 copies (21%), and peak estimated lesion diameter (7.2 millimeters) per year were virtually equivalent to those in the literature (20.5% and 8.7 millimeters, respectively).
Table 2. Model-predicted and empiric phenotypic variables for genital herpes.
Predicted values are from 445 stochastic simulations of the model using 89 patient parameters.
| Phenotypic variable | Model predicted values | Empiric values (ref) | ||||
|---|---|---|---|---|---|---|
| Mean | Median | Range | Mean | Median | Range | |
| Percent of time > 50 copies HSV-2 DNA/mL | 22.5 | 21 | 5.5–78.8 | 20.5(7) 28.0(6) |
-- | 0–77.3(6) |
| Percent of time > 103 copies HSV-2 DNA/mL | 12.7 | 11.2 | 0–66.2 | -- | -- | -- |
| Percent of time >104 copies HSV-2 DNA/mL | 5.8 | 5.3 | 0–47.5 | -- | -- | -- |
| Total shedding episodes/year | 70 | 72 | 11–173 | -- | 18 | 0–128(7) |
| Clinical recurrences/year | 1.4 | 2 | 0–10 | -- | 2.1(17) 8.5(8) 4(9) |
0–25(17) 0–30(8) |
| Peak diameter of an ulcer (mm) | 8.4 | 7.2 | 2–18.6 | -- | 8.7(20) | 1.6 – 18.5(20) |
| Percent of shedding episodes that are clinical recurrences | 3.6 | 1.2 | 0–29.4 | 15(7) | -- | -- |
| Maximum duration of shedding episode/year (days) | 7.5 | 6.3 | 3.1–27.4 | -- | 4(6) | -- |
Failure to detect the briefest shedding episodes
The model predicted a higher number of shedding episodes per year and lower percent of episodes that result in clinical recurrences, than has been previously reported from studies in patients (Table 2) (6, 7). Thus, the model suggested that subclinical reactivation might be even more frequent than current empirical data suggest. We previously demonstrated that sampling frequency influences number of detected subclinical episodes (7). We therefore performed an additional run of the model using mean values of each parameter with continuous simulated sampling (1000 samples per day) versus simulated sampling every six-hours. Although frequency of shedding greater than 50 copies was equal under both modeled conditions (19.69% versus 19.66%), continuous sampling predicted more episodes lasting six hours or less (43 versus 21), more total episodes (56 versus 34), and a lower proportion of clinical episodes (5.4%[3/56] versus 8.8%[3/34]). Episodes that were missed with simulated sampling every six hours were 1.5 to 4 hours long. Thus, the model suggests that greater numbers of subclinical episodes of HSV-2 reactivation would be detected if we sampled patients every hour.
If a limited number of epithelial cells become infected, then viruses may not reach the skin surface because not enough cells die to create a micro-ulceration. In this circumstance, model simulations would again describe brief episodes that are not documented clinically with PCR swabs. We have described the existence of such episodes in PCR sampling from biopsy specimens (16). We re-examined the above 365-day stochastic simulation to measure the impact of this phenomenon on model output: if episodes with no more than one infected cell at any point in time (31/43) were not counted in our analysis, the model predicted that 25% (3/12) of detectable episodes were lesional compared to 15% in the clinical literature (7).
Distinctions between subclinical shedding episodes and recurrences based on number of cells infected
Detailed analysis of a 365-day stochastic simulation suggested three types of shedding episodes that we define as low-copy (<104 copies HSV/mL), medium-copy (104–106 copies HSV/mL) and high-copy or lesional (>106 copies HSV/mL). These types of episodes are observed in patients (Fig 3A–C). However, our model simulations allow for a more detailed analysis of their structure: during the many low-copy episodes, very few cells were infected, duration of shedding was brief, and HSV copy number was low; medium-copy episodes were less frequent and characterized by higher number of infected cells, longer shedding duration and higher copy numbers of HSV. Medium-copy episodes accounted for the largest proportion of time with detectable shedding; during high-copy episodes, enough cells were killed to generate a lesion diameter of greater than two millimeters (Table 3).
Table 3. Distinguishing features of low, medium and high-copy HSV-2 shedding episodes.
During a 365-day stochastic model simulation mean patient-derived parameter values were derived from 89 HSV PCR curves.
| Low copy | Medium copy | High copy | ||||
|---|---|---|---|---|---|---|
| Number of episodes | 37 | 8 | 3 | |||
| Quantitative episode characteristics | Median | Range | Median | Range | Median | Range |
| Log peak instantaneous HSV copy number/mL | 2.2 | 1.6–3.2 | 5.3 | 4.5–5.6 | 6.6 | 6.5–8.4 |
| Log number of HSV copies during the entire episode | -- | 1.7–4.2 | -- | 5.6–6.5 | -- | 7.4–9.0 |
| Peak instantaneous infected cells | 1 | 1–4 | 232 | 44–446 | 4103 | 3719–256000 |
| Number of infected cells during the entire episode | 1 | 1–115 | -- | 3158–23009 | -- | 165250–4300814 |
| Peak lesion diameter (mm): | 0 | -- | 0.9 | 0.6–1.2 | 3.5 | 3.1–16.2 |
| Shedding duration (days): | 0.4 | 0.1–2.1 | 3.2 | 2.8–4.4 | 3.1 | 3.0–3.3 |
| Other episode characteristics | ||||||
| Number of episodes missed with every six hour shedding protocol | 18 | 0 | 0 | |||
| Number of episodes not detectable with PCR swabbing due to insufficient cell death for micro-ulceration | 26 | 0 | 0 | |||
| Clinical/histologic correlate | None or micro-ulceration | Micro-ulceration | Vesicles, ulcers | |||
| Contribution to time spent with > 50 copies HSV DNA/mL | 30% | 52% | 18% | |||
In our simulations, the total number of infected cells and viruses generated over the course of an entire medium or high copy episode were higher than the peak instantaneous number of infected cells and peak number of viruses during the episode because turnover of infected cells and viruses was extremely rapid. The average lifespan of infected cells was considerably shorter than the several days during which a lesion forms and heals. Even during large lesions, only a small percentage of the total number of infected cells over the course of the episode was infected at any point in time. Similarly, average lifespan of a virus during this simulation was only 2.7 hours (Table 3). The model predicted that viral production and clearance were rapid during the first two days of a high-copy episode. HSV DNA copy number was approximately 1.4e7 and number of infected cells was approximately 21,000 at the time of lesion detection (> 2 mm diameter) during a typical genital lesion simulation, while total number of HSV DNA copies and infected cells produced before lesion detection was 3.3e7 and 93,500 respectively (Fig S5A, B).
High variability in shedding frequency due to small variations in neuronal introduction rate of virus
Model simulations with rate of neuronal virus introduction of 50 copies per day per 2-centimeter diameter surface area generated numerous shedding episodes and realistic ranges of outcomes. The average ratio of viruses produced by epithelial cells versus neurons in 445 simulations was 103.5 (range for individual simulation: 101.3, 106.1). Higher ratios occurred during simulations with large lesions. An average of one out of every 241 (range: 65, 1088) viruses infected an adjacent epithelial cell.
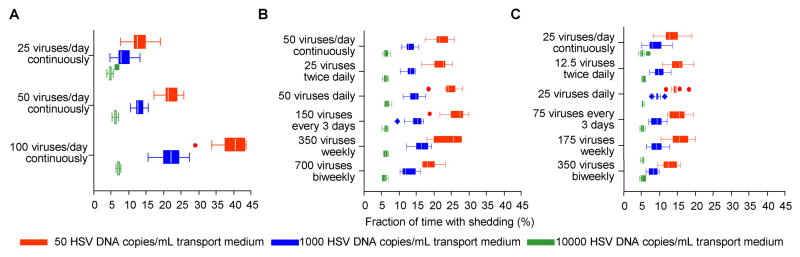
Reduction in HSV-2 release rate from neurons to 25 copies per day in our simulations led to a decrease in shedding frequency (Fig. 3E, Fig. 4A), while an increase to 100 copies per day had the opposite effect (Fig. 3F, Fig. 4A). Subclinical and clinical shedding episodes occurred when we ran simulations with as few as two HSV copies per day in the model area. There was only limited alteration in shedding frequency when we altered pattern of viral introduction from the neuronal tissue (even when virus was introduced as infrequently as every 2 weeks), provided that average amount of virus added per day remained the same (Fig. 4B and C).
Figure 4. Determination of the detectable shedding of HSV-2 in the genital tract by average amount of HSV-2 introduced from sensory neurons into the genital tract per day.
The pattern of reactivation from the sensory neurons has minimal effect on detectable HSV-2 shedding frequency provided that the average amount of HSV-2 per day is unchanged. (A) Simulations of constant HSV-2 introduction at rates of 25, 50 and 100 copies per day per 2-centimeter diameter area (~ 1, 2 and 4 copies HSV per hour) with percent of time with detectable shedding > 50 copies, > 1000 copies and > 10000 copies HSV/mL medium as outcomes. (B) Simulations of introduction of HSV-2 into the genital tract at an average rate of 50 copies per day per 2-centimeter diameter area with different patterns of viral introduction (constant versus bolus introductions twice daily, daily, every three days, weekly or bi-weekly). (C) Simulations of introduction of HSV-2 into the genital tract at an average rate of 25 copies per day per 2-centimeter diameter area with different patterns of viral introduction (constant versus bolus introductions twice daily, daily, every three days, weekly or bi-weekly). For each example, we performed ten simulations for each set of parameters to provide a range of shedding frequency. Boxes represent intra-quartile range (IQR). Whiskers represent the furthest data point, or 1.5*IQR below or above the quartile, whichever is closer. Dots represent data points more than 1.5*IQR above or 1.5*IQR below the quartile.
Discussion
We present a stochastic model of mucosal HSV-2 pathogenesis that closely reproduces the pattern of actual HSV-2 genital shedding and measurement-based estimates of shedding frequency (6, 7, 17), annual clinical recurrences (8, 18), peak copy number during episodes (6, 7), duration of shedding episodes (8, 19), and lesion diameter (20). The output from our stochastic model mirrors the substantial variability in HSV-2 shedding frequency and clinical recurrence among patients and within the same patient over time (7, 8, 21). Although HSV-2 is detectable in the genital tract on a median of 20% of days, the range within the 95% of seropositive patients who shed HSV-2 during 6–12 weeks of observation is 0–78% of days (6). Our model generates similar heterogeneity within patients by virtue of its stochastic nature, and among patients as a result of patient-to-patient differences in parameters that determine viral replication, viral spread and cytolytic immune response.
Our simulations suggest that genital HSV-2 infection represents a highly dynamic system in which viral particles are frequently but slowly introduced into the genital area in very low numbers by sensory neurons, and periodically released in an explosive fashion by infected epithelial cells. Once an epithelial cell produces a burst of viral particles, these particles have a few hours to infect contiguous cells before losing infectivity. Whether any of the hundreds of particles released from the first infected cells are able to infect surrounding cells and initiate a chain reaction of infection determines which of three distinct shedding episodes will ensue: (i) a brief low-copy episode, (ii) a more prolonged subclinical, medium-copy episode, or (iii) a high-copy episode associated with a clinically apparent genital lesion.
In our model, no more than a single epithelial cell is infected at any point in time during many low-copy shedding episodes. These smallest reactivations are the most likely to be missed with current methods of detecting shedding. First, if a tiny number of nucleated epithelial cells are infected during an episode, then only cells at the dermal epidermal junction will produce HSV: viruses may never reach the skin surface, and will not be detected, even with well-timed sampling. Second, because most low-copy shedding episodes are brief, many are missed with every six-hour swabbing protocols. Our model’s output reinforces our observation that current estimates of shedding frequency with every 6-hour sampling are accurate (7, 17, 18), but implies that measures of number of detectable shedding episodes per year are likely to be underestimates.
Medium-copy episodes may be responsible for person-to-person transmission because, although not enough epithelial cells die to generate a visible lesion, shedding is prolonged. In addition, there is a much higher inoculum of HSV-2 during a medium than a low-copy episode. High-copy episodes create enough infected cells to generate a detectable lesion. Lesions used for our curve fitting all were associated peak copy numbers greater than 106. Fitting results were equivalent whether we used anogenital or lesion only swabs. This does not rule out concurrent HSV shedding at other anogenital sites during a genital lesion. Rather, the amount of virus produced at the lesion site so far exceeds that from other subclinical shedding sites that curve fitting is not affected, and swabs of the entire anogenital region are adequate for this study.
In order to initiate shedding episodes in epithelial cells of the genital mucosa, our model suggests that miniscule numbers of viruses must be introduced from the neuronal tissue per day. Moreover, the vast majority of detectable viral DNA in the genital tract appears to be of epithelial cell origin, rather than from neurons. Several clinical phenomena are potentially explained by a slow but steady release of HSV from genital tract neurons. The low amount of neuronal virus required for disease and transmission might explain why neurons survive HSV-2 infection, and why peripheral neuropathy is not a disease manifestation (22). The prodromes that often precede ulcer formation are conventionally thought to result from travel of viral particles down the neuron (20). However, asymptomatic episodes, which lack prodromes, also occur after neuronal reactivation. Our model suggests that prodromes could possibly be from high levels of viral replication among epithelial cells early during recurrence and prior to lesion detection. The frequent pattern of neuronal viral release might also explain why currently available DNA nucleoside analogues prevent recurrences more than sub-clinical shedding episodes and transmissions (6, 23). Recurrences occur over several days. Therefore, high levels of an antiviral medication that is dosed on a daily basis are certain to coincide with early lesion formation. However, because viral release from neurons is so frequent, even brief periods with sub-therapeutic drug levels might be sufficient to allow for shedding episode initiation.
Even small, modeled variations in neuronal virus introduction rate have a dramatic impact on detectable shedding frequency. Determinants of neuronal viral production remain unclear but may include ganglionic lymphocyte infiltration, cytokine production, and immune response to primary HSV-2 infection (10, 11, 24, 25). These mechanisms likely determine the rate of virus release into the genital tract, which in turn predicts shedding frequency in our model. If the number of viruses produced from the neurons is below a certain threshold, then shedding episodes are rare. This scenario may explain findings in six patients with HSV-2 directed antibodies who appear to never shed virus (26). If over 200 copies of HSV are shed from neurons per day in our simulations, then detectable shedding from infected epithelial cells is present over 50% of the time. This result suggests a mechanism for persistent shedding described in immunocompromised patients, as well as a subset of immunocompetent patients (6, 27).
It was once common wisdom that infectious HSV-2 particles are only periodically introduced into the mucosa from neuronal tissue, and that these reactivations almost always caused genital lesions (5). Because initiation of an HSV-2 shedding episode implies that ganglionic reactivation recently occurred, reactivation must be at least as common as episode frequency. Recent work documenting sub-clinical detection of HSV-2 in the genital mucosa on 20% of days suggests that reactivation occurs at least this often (7). Our model is consistent with another study that concluded that HSV-2 release from sacral ganglia is likely to occur much more frequently than actual HSV-2 detection (17). Our findings also suggest that a constant, extremely slow release of infectious viruses from sensory neurons can explain localized HSV-2 shedding episodes in the genital mucosa. The same net amount of virus, whether introduced on a continuous, daily, weekly or bi-weekly basis in our model, causes the same frequency of detectable virus. The key periodic event in HSV-2 shedding may not be release of HSV-2 from the neurons, which is possibly a constant, low-grade process, but rather infection of a first epithelial cell that denotes episode initiation.
Our model has important limitations. First, the mucosal immune compartment involves innate and humoral processes not included in our current model for lack of adequate in vivo data. Since our model accurately reproduced true clinical outcomes, these immunologic phenomena are at least partially captured within included model parameters such as viral lifespan, infectivity, and burst phase. Second, our model oversimplifies kinetics of the infected cell in its progress towards death. There is more viral production over time as transcriptional activity is up-regulated (28), and HSV-2 may inactivate the cytolytic immune response against the infected cell as infection progresses (29). With accrual of more precise laboratory and clinical data, we hope to incorporate these relevant concepts of viral replication into the model.
Despite the model’s oversimplification of viral pathogenesis, we include a high number of parameters and we may have arrived at local optima for estimates of certain parameter values. Nevertheless, we incorporated the minimum possible number of parameters to describe HSV-2 shedding in the genital tract: exclusion of basic CD8+ T-cell parameters resulted in poor model fit to empirical data. In addition, our parameter estimates are from several sources and are not all disease or host specific. Increasing the complexity of the model and detail of empirical data for model fitting will inevitably change certain parameter estimates, although the fundamental concepts described here will likely remain intact. Our conclusions are model-derived and represent hypothesis generation. Future studies should focus on validating our findings experimentally and clinically, and will inform more detailed permutations of our model.
The findings of our model suggest a formidable challenge in controlling HSV shedding and person-to-person transmission. In persons infected with HSV, successful antiviral medication or immunotherapy will need to completely eliminate the frequent trickle of HSV from the neurons into the genital tract. A vaccine that is effective in promoting mucosal immunity may limit recurrences only, while elimination of both asymptomatic shedding and recurrences may require immune activation in both neurons and genital mucosa.
Materials and Methods
Model format
We developed a model to evaluate the pathway of mucosal HSV reactivation from release of viral particles at the neuro-epithelial junction to development of mucosal ulceration (Fig. 1). The model’s ordinary differential equations describe infection of susceptible epithelial cells (S), release of virus (Y) from infected neurons, release of virus (Z) from infected epithelial cells (I), and clearance of infected cells by CD8+ lymphocytes (E). Each model parameter has a specific influence on these variables at a certain stage of pathogenesis. Estimates and sources for parameter value ranges that determine the rate of change for the variables S, I, Y, Z and E are listed in Table 1 and described in the SM.
Model fitting to patient data
To estimate model parameters during an HSV-2 shedding episode associated with a genital lesion, we employed a deterministic form of our model that we fit to empirically derived data from patients with clinically, serologically and virologically documented recurrent HSV-2 infection. The data were obtained from natural history studies of genital HSV-2 in which quantitative HSV PCR was collected on a daily basis from the entire anogenital tract of 81 patients with a genital ulcer (Fig. 2) (8, 30, 31). We solved our ordinary differential equations for unknown parameter values using nonlinear least-squares regression (SM). Based on inclusion criterion, we used 89 of 115 curves from 64 patients for our analysis (35 male/54 female, 18 penile/45 vulvar/26 peri-anal) (SM). Fifteen of 89 curves were derived separately from swabs of the entire anogenital tract and lesion-only swabs: we calculated parameter estimates for each of these curves.
Stochastic model simulations
Once parameters were estimated for the 89 curves, we converted the model to a stochastic stage-structured form with unchanged compartmental structure (SM) (32). The advantage of the discrete stochastic system is that by allowing for only integer values of variables and run-to-run heterogeneity, it highlights variability of infection over time, and functions well when small numbers of cells are initially infected. We ran the model for 365 days on five occasions for each set of 89 patient parameters to allow for average measurements of outcomes (445 simulations).
For the 445 simulations, we selected outcomes for clinical and epidemiologic relevance. Possible transmission surrogates included a) percentage of time with > 50, and >1000 HSV-2 copies/mL, and b) shedding episodes (>50 copies) per year (33); surrogates for disease severity included a) recurrences (>2 millimeter diameter lesions) per year, and b) highest estimated lesion diameter during a year (millimeters). For each simulation, we measured proportion of viruses produced from epithelial cells versus neurons, and proportion of viruses that died before infecting another cell. We also ran the stochastic model with mean values of the 89 sets of derived parameters to obtain detailed measures of total numbers of cells, and viruses produced during individual subclinical and clinical (> 2 millimeter diameter) shedding episodes.
Neuronal patterns of introduction
To assess effect of neuronal introduction rate on shedding frequency, we adjusted rate of neuronal virus introduction to 25, 50 and 100 viruses per day. For these simulations, we used mean values of 89 patient-derived parameters from curve fitting (Table 1). For each introduction rate, we ran the model ten times accounting for 30 simulations.
We also introduced HSV into the genital mucosa at different frequencies to simulate different rates of ganglionic reactivation (continually, every twelve hours, daily, every three days, weekly or bi-weekly). To isolate the effect of frequency of introduction, we held total amount of HSV-2 per day constant at 50 or 25 copies per day for each change in frequency.
Supplementary Material
Acknowledgments
We thank Dr. David Koelle for conceptual assistance with this project, and Andy Blair for assistance with figure production.
Funding: Supported by NIH grants PO1 AI030731, R37 AI042528, T32 CA80416, T32 AI07140, and the NIH supported HIV Vaccine Trials Network.
Footnotes
Author contributions: JTS conceived the project, designed the model, performed the analyses and wrote the manuscript; LAR designed the model and edited the manuscript; KM performed shedding studies and edited the manuscript; JZ performed staining for CD8+ lymphocytes; SS arranged the data for analysis; AA assisted with data analysis; AW performed shedding studies and edited the manuscript; LC conceived the project, analyzed the data and edited the mansucript.
Competing interests: The authors declare that they have no competing financial interests.
Methods (15 pages), Supplementary Figures 1–5, References, and Four files [a graphical display of 115 curves of combined anogenital swabs from patients with genital lesions (SM1), the data needed to generate these curves (SM2), code for the mathematical model in Berkeley Madonna Version 8.3.18 (SM3) and in Word format (SM4).]
References
- 1.Weiss H. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes. 2004;11(Suppl 1):24A. [PubMed] [Google Scholar]
- 2.Freeman E, et al. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 3.Gray R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357:1149. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 4.Stanberry LR, Kern ER, Richards JT, Abbott TM, Overall JC., Jr Genital herpes in guinea pigs: pathogenesis of the primary infection and description of recurrent disease. J Infect Dis. 1982;146:397. doi: 10.1093/infdis/146.3.397. [DOI] [PubMed] [Google Scholar]
- 5.Rattray MC, Corey L, Reeves WC, Vontver LA, Holmes KK. Recurrent genital herpes among women: symptomatic v. asymptomatic viral shedding. Br J Vener Dis. 1978;54:262. doi: 10.1136/sti.54.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wald A, et al. Frequent genital herpes simplex virus 2 shedding in immunocompetent women. Effect of acyclovir treatment. J Clin Invest. 1997;99:1092. doi: 10.1172/JCI119237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mark KE, et al. Rapidly cleared episodes of herpes simplex virus reactivation in immunocompetent adults. J Infect Dis. 2008;198:1141. doi: 10.1086/591913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wald A, et al. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med. 2000;342:844. doi: 10.1056/NEJM200003233421203. [DOI] [PubMed] [Google Scholar]
- 9.Benedetti J, Corey L, Ashley R. Recurrence rates in genital herpes after symptomatic first-episode infection. Ann Intern Med. 1994;121:847. doi: 10.7326/0003-4819-121-11-199412010-00004. [DOI] [PubMed] [Google Scholar]
- 10.Hoshino Y, Pesnicak L, Cohen J, Straus S. Rates of reactivation of latent herpes simplex virus from mouse trigeminal ganglia ex vivo correlate directly with viral load and inversely with number of infiltrating CD8+ T cells. J Virol. 2007;81:8157. doi: 10.1128/JVI.00474-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu T, Khanna KM, Chen X, Fink DJ, Hendricks RL. CD8(+) T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J Exp Med. 2000;191:1459. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mertz GJ, et al. Frequency of acquisition of first-episode genital infection with herpes simplex virus from symptomatic and asymptomatic source contacts. Sex Transm Dis. 1985;12:33. doi: 10.1097/00007435-198501000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Ho D, et al. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 14.Perelson A, et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 15.Wei X, et al. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 16.Zhu J, et al. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med. 2007;204:595. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crespi CM, Cumberland WG, Wald A, Corey L, Blower S. Longitudinal study of herpes simplex virus type 2 infection using viral dynamic modelling. Sex Transm Infect. 2007;83:359. doi: 10.1136/sti.2006.022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mark K, et al. Topical resiquimod 0.01% gel decreases herpes simplex virus type 2 genital shedding: a randomized, controlled trial. J Infect Dis. 2007;195:1324. doi: 10.1086/513276. [DOI] [PubMed] [Google Scholar]
- 19.Wald A, et al. Two-day regimen of acyclovir for treatment of recurrent genital herpes simplex virus type 2 infection. Clin Infect Dis. 2002;34:944. doi: 10.1086/339325. [DOI] [PubMed] [Google Scholar]
- 20.Corey L, Adams HG, Brown ZA, Holmes KK. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Intern Med. 1983;98:958. doi: 10.7326/0003-4819-98-6-958. [DOI] [PubMed] [Google Scholar]
- 21.Wald A, Zeh J, Barnum G, Davis L, Corey L. Suppression of subclinical shedding of herpes simplex virus type 2 with acyclovir. Ann Intern Med. 1996;124:8. doi: 10.7326/0003-4819-124-1_part_1-199601010-00002. [DOI] [PubMed] [Google Scholar]
- 22.Sawtell N, Thompson R. Rapid in vivo reactivation of herpes simplex virus in latently infected murine ganglionic neurons after transient hyperthermia. J Virol. 1992;66:2150. doi: 10.1128/jvi.66.4.2150-2156.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corey L, et al. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N Engl J Med. 2004;350:11. doi: 10.1056/NEJMoa035144. [DOI] [PubMed] [Google Scholar]
- 24.Chen S, Garber D, Schaffer P, Knipe D, Coen D. Persistent elevated expression of cytokine transcripts in ganglia latently infected with herpes simplex virus in the absence of ganglionic replication or reactivation. Virology. 2000;278:207. doi: 10.1006/viro.2000.0643. [DOI] [PubMed] [Google Scholar]
- 25.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity. 2003;18:593. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Posavad C, et al. T cell immunity to herpes simplex viruses in seronegative subjects: silent infection or acquired immunity? J Immunol. 2003;170:4380. doi: 10.4049/jimmunol.170.8.4380. [DOI] [PubMed] [Google Scholar]
- 27.Posavad C, et al. Frequent reactivation of herpes simplex virus among HIV-1-infected patients treated with highly active antiretroviral therapy. J Infect Dis. 2004;190:693. doi: 10.1086/422755. [DOI] [PubMed] [Google Scholar]
- 28.Aguilar J, et al. Quantitative comparison of the HSV-1 and HSV-2 transcriptomes using DNA microarray analysis. Virology. 2006;348:233. doi: 10.1016/j.virol.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 29.Sloan D, et al. CTL are inactivated by herpes simplex virus-infected cells expressing a viral protein kinase. J Immunol. 2003;171:6733. doi: 10.4049/jimmunol.171.12.6733. [DOI] [PubMed] [Google Scholar]
- 30.Wald A, Zeh J, Selke S, Ashley R, Corey L. Virologic characteristics of subclinical and symptomatic genital herpes infections. N Engl J Med. 1995;333:770. doi: 10.1056/NEJM199509213331205. [DOI] [PubMed] [Google Scholar]
- 31.Brock B, Selke S, Benedetti J, Douglas JJ, Corey L. Frequency of asymptomatic shedding of herpes simplex virus in women with genital herpes. JAMA. 1990;263:418. [PubMed] [Google Scholar]
- 32.Chao DL, Davenport MP, Forrest S, Perelson AS. A stochastic model of cytotoxic T cell responses. J Theor Biol. 2004;228:227. doi: 10.1016/j.jtbi.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Magaret A, Wald A, Huang M, Selke S, Corey L. Optimizing PCR positivity criterion for detection of herpes simplex virus DNA on skin and mucosa. J Clin Microbiol. 2007;45:1618. doi: 10.1128/JCM.01405-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strand SS, Vanheyningen TK, Leib DA. The virion host shutoff protein of herpes simplex virus type 1 has RNA degradation activity in primary neurons. J Virol. 2004;78:8400. doi: 10.1128/JVI.78.15.8400-8403.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roizman KDB. In: Fields Virology. Knipe HPDM, Griffin DE, et al., editors. Lippincott Williams & Wilkins; Philadelphia: 2007. [Google Scholar]
- 36.Jiang C, et al. Herpes simplex virus mutants with multiple substitutions affecting DNA binding of UL42 are impaired for viral replication and DNA synthesis. J Virol. 2007;81:12077. doi: 10.1128/JVI.01133-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang C, Hwang YT, Randell JC, Coen DM, Hwang CB. Mutations that decrease DNA binding of the processivity factor of the herpes simplex virus DNA polymerase reduce viral yield, alter the kinetics of viral DNA replication, and decrease the fidelity of DNA replication. J Virol. 2007;81:3495. doi: 10.1128/JVI.02359-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turner R, Shehab Z, Osborne K, Hendley JO. Shedding and survival of herpes simplex virus from ‘fever blisters’. Pediatrics. 1982;70:547. [PubMed] [Google Scholar]
- 39.De Boer RJ, Homann D, Perelson AS. Different dynamics of CD4+ and CD8+ T cell responses during and after acute lymphocytic choriomeningitis virus infection. J Immunol. 2003;171:3928. doi: 10.4049/jimmunol.171.8.3928. [DOI] [PubMed] [Google Scholar]
- 40.Regoes RR, Yates A, Antia R. Mathematical models of cytotoxic T-lymphocyte killing. Immunol Cell Biol. 2007;85:274. doi: 10.1038/sj.icb.7100053. [DOI] [PubMed] [Google Scholar]
- 41.Regoes RR, Barber DL, Ahmed R, Antia R. Estimation of the rate of killing by cytotoxic T lymphocytes in vivo. Proc Natl Acad Sci U S A. 2007;104:1599. doi: 10.1073/pnas.0508830104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barchet W, et al. Direct quantitation of rapid elimination of viral antigen-positive lymphocytes by antiviral CD8(+) T cells in vivo. Eur J Immunol. 2000;30:1356. doi: 10.1002/(SICI)1521-4141(200005)30:5<1356::AID-IMMU1356>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 43.Ganusov VV, De Boer RJ. Estimating in vivo death rates of targets due to CD8 T-cell-mediated killing. J Virol. 2008;82:11749. doi: 10.1128/JVI.01128-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu J, et al. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat Med. 2009;15:886. doi: 10.1038/nm.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.