Abstract
Sex differences and gonadal hormone influences are well known for diverse aspects of forebrain amine and indolamine neurotransmitter systems, the cognitive and affective functions they govern and their malfunction in mental illness. This study explored whether hormone regulation/dysregulation of these systems could be related to gonadal steroid effects on catechol-O-methyltransferase and monoamine oxidase which are principal enzymatic controllers of forebrain dopamine, serotonin and norepinephrine levels. Driven by male over female differences in cortical enzyme activities, by male-specific associations between monoamine oxidase and catechol-O-methyltransferase gene polymorphisms and cognitive and dysfunction in disease and by male-specific consequences of gene knockouts in mice, the question of hormone sensitivity was addressed here using a male rat model where prefrontal dopamine levels and related behaviors are also known to be affected. Specifically, quantitative O-methylation and oxidative deamination assays were used to compare the activities of catechol-O-methyltransferase's soluble and membrane-bound isoforms and of monoamine oxidase's A and B isoforms in the pregenual medial prefrontal cortex and dorsal striatum of male rats that were sham operated, gonadectomized or gonadectomized and supplemented with testosterone propionate or with estradiol for 28 days. These studies revealed significant effects of hormone replacement but not gonadectomy on the soluble but not the membrane-bound isorfom of catechol-O-methyltransferase in both striatum and cortex. A significant, cortex-specific testosterone—but not estradiol—attenuated effect (increase) of gonadectomy on monoamine oxidase's A but not B isoform was also observed. Although none of these actions suggest potential roles in the reguation/dysregulation of prefrontal dopamine, the suppressive effects of testosterone on cortical monoamine oxidase-A that were observed could have bearing on the increased incidence of cognitive deficits and symptoms of depression and anxiety that are repeatedly observed in males in conditions of hypogonadalism related to aging, other biological factors or in prostate cancer where androgen deprivation is used as a neoadjuvant treatment.
Keywords: estrogen, androgen, schizophrenia, depression/anxiety, monoamines, indolamines
The prefrontal cortices are essential in mediating highest order executive functions such as planning, working memory and behavioral flexibility (Goldman-Rakic, 1990; Goldman-Rakic et al., 1990; Dalley et al., 2004). These functions are all exquisitely sensitive to prefrontal cortical dopamine (DA) signaling and information from disease processes and gene polymorphisms in humans (Davis et al., 1991; Egan et al., 2001; Goldberg et al., 2003; Goldberg and Weinberger, 2004) and from studies in experimental animal models (Tassin et al., 1978; Kessler and Markowitsch, 1981; Kalsbeek et al., 1989; Murphy et al., 1996; Verma and Moghaddam, 1996; Zahrt et al., 1997; Morrow et al., 2000) have shown that both too much and too little local DA tone is detrimental to performance on prefrontal cortical-dependant tasks. Gonadal hormones are also known to modulate prefrontal cortical cognitive information processing (Einon, 1980; Tees et al., 1981; van Haaren et al., 1990; Janowsky et al., 2000; Lacreuse, 2006; Luine, 2008) and recent studies in male rats suggest that this occurs in part via an induced exaggeration of prefrontal DA levels. Thus, gonadectomy in adult male rats has been shown to disrupt DA-dependent prefrontal cortical behaviors including open field activity, acquisition of T-maze delayed alternation, behavioral flexibility, and novel object recognition (Adler et al., 1999; Kritzer et al., 2001, 2007; Aubele et al., 2008a) in an androgen sensitive manner that is significantly correlated with gondectomy-induced increases in prefrontal cortical DA axon density (Kritzer et al., 1999; Kritzer, 2000) and that occurs in parallel with gonadectomy-induced increases in extracellular prefrontal DA levels (Aubele et al., 2008b). One objective of the work presented here was to explore whether this dysregulation might be a consequence of effects of gonadectomy and/or hormone replacement on the DA catabolic enzymes catechol-O-methyltransferase (COMT) and monoamine oxidase (MAO) which are principal controllers of the functionally critical parameter of DA tone in the prefrontal cortex (Westerink and Spaan, 1982; Karoum et al., 1994; Yavich et al., 2007). In addition, there are other, independent a priori reasons to suspect that both MAO and COMT may not only be sensitive to gonadal steroid stimulation but that they may be especially so in the cerebral cortex of males. These include evidence of male over female differences in human prefrontal cortical COMT activity (Chen et al., 2004), male-specific associations between MAO and COMT gene polymorphisms and specific higher order cognitive and/or affective disturbances, for example aggression, in mental illness (Volavka et al., 2004; Ducci et al., 2006; Biederman et al., 2008; Harrison and Tunbridge, 2008; Sjoberg et al., 2008) and male-specific physiological, pharmacological and/or behavioral effects that both COMT (Gogos et al., 1998; Huotari et al., 2002) and MAO-A (Cases et al., 1995) gene knockouts have on forebrain amine neurotransmitter systems in mice. Surprisingly, however, few studies have systematically assessed gonadal hormone impact on COMT or MAO activity in the CNS of either sex, fewer still have assessed hormone sensitivity of enzyme activity in forebrain structures such as the cerebral cortex, and few to none have explored hormone sensitivity of MAO or COMT in forebrain structures of males. To address this potentially important and as yet understudied area, the present studies paired functional enzyme assays with classical hormone manipulation paradigms to separately quantify and compare the activities of COMT's soluble and membrane-bound forms and of MAO's A and B isoforms in the medial prefrontal cortex and dorsal striatum of adult male rats that were sham operated, gonadectomized or gonadectomized and supplemented with testosterone propionate or with estradiol for 28 days.
Experimental Procedures
Animal subjects
Tissue from 72 adult male Sprague–Dawley rats (Taconic Farms, Germantown, NY, USA) was used. All animals were housed with food and water freely available under a 12-hour light/dark cycle and were gonadectomized, with and without replacement with 17-β-estradiol or testosterone propionate (below), or sham operated 28 days prior to euthanasia (below). All procedures involving animals are approved by the Institutional Animal Care and Use Committee of Stony Brook University, and minimize their use and discomfort.
Surgical procedures
Gonadectomy and sham surgeries were performed under aseptic conditions using ketamine/Ketaset HCL Injection (Fort Dodge Animal Health, Fort Dodge, IA, USA) (.09 ml/100 g) and xylazine/AnaSed Injection (Lloyd Laboratories, Shenandoah, IA, USA) (.05 ml/100 g) for anesthesia. For both operations, the sac of the scrotum and underlying tunica were incised; for gonadectomies, the vas deferens was also bilaterally ligated and the testis were removed. For gonadectomized, hormone-replaced rats, slow-release pellets (below) were implanted within the scrotal sac. Incisions were closed using sterile surgical staples that were removed after 10 days.
Hormone treatments
Subsets of gonadectomized animals were implanted with slow release pellets that contained either testosterone propionate or 17-β-estradiol (Innovative Research of America, Toledo, OH, USA). The testosterone propionate-containing pellets used have been used in previous studies in this laboratory and others and have been shown to release between 0.5–2ng of TP per milliliter of blood per day and yield plasma TP and E levels (ELISA) that are an average 70 and 90% of plasma TP and E levels, respectively, measured in gonadally intact controls (Turvin et al., 2007); likewise, the estradiol-containing pellets used were also identical to those used previously and have been shown to release between 10–40pg of E per milliliter of blood per day and yield plasma E levels that are roughly 90% of plasma E levels measured in gonadally intact controls (Turvin et al., 2007).
Euthanasia
Twenty-eight days after gonadectomy or sham surgery, rats were weighed and euthanized by rapid decapitation. Immediately afterward the brains were rapidly removed and regions of interest (pregenual medial prefrontal cortex, Fig. 1A, dorsal striatum Fig. 1B) were dissected out over ice. To maximize consistency, all brain dissections were performed by the same person (MFK). The bulbospongiosus muscles (BSM) were also dissected from all subjects and weighed.
Fig. 1.
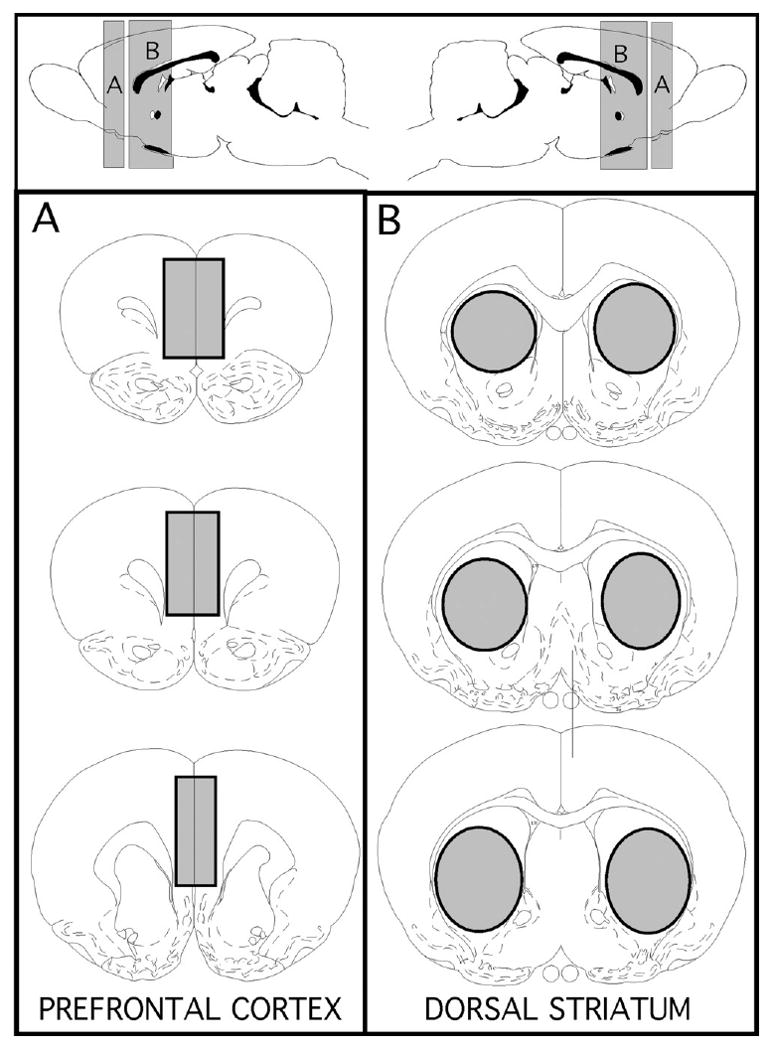
Schematic diagrams showing the anterior/poster (top panel) and coronal (lower left and right panels) locations of brain regions marked in grey that were micro-dissected out for the use in homogenate binding assays. The regions of interest that were collected were the pregenual medial prefrontal cortex (A) and dorsal striatum (B) taken at levels at and posterior to the anterior commisure. The templates showing these locations are modified from the atlas of Paxinos and Watson (1998).
Enzyme assays
Each individual assay was performed in triplicate using cortical or striatal tissue homogenates that were pooled from three animal subjects per hormone treatment group. Individual assay types (defined by enzyme- catechol-O-methyltransferase, membrane-bound isoform (COMT-mb), catechol-O-methyltransferase, soluble isoform (COMT-s), MAO-A, MAO-B; by brain region- striatum, cortex; and by animal group- control, gonadectomized (GDX), gonadectomised, testosterone propionate replaced (GDX-TP), gonadectomised, estradiol replaced, (GDX-E)) were also repeated three times using tissue homogenates prepared and pooled from three different sets of three animal subjects. Due to different requirements for preparation, the tissues used in MAO-A and MAO-B were obtained from a common set of animals while those for the COMT assays (s and mb isoforms) were pooled from a separate series of animal subjects.
COMT
COMT enzyme activity was determined using methods similar to those previously described (Zurcher and Da Prada, 1982).
Tissue preparation
Striatal and cortex tissue were dissected from three sets of three animal subjects per hormone treatment group. Tissue homogenates were pooled, weighed, re-supsended at 50 mg/ml in ice-cold homogenization buffer (1.2% Tris–HCl, 50% glycerol, pH 7.4, containing one EDTA-free Protease Inhibitor Cocktail tablet (Roche)/14 ml) and were homongenized using a glass dounce homogenizer. Tissues were then rapidly frozen in powdered dry ice and stored at −80 °C until assay.
On the day of assay, frozen homogenates were rapidly thawed and the soluble from membrane-bound forms of COMT were separated by centrifugation. Specifically, tissue homogenates were centrifuged at 14,000 g for 30 min. The supernatant was then collected and stored on ice prior to assay for COMT-s, and the pellet was re-suspended in 1 vol of homogenate buffer for COMT-mb assays. To assess the effectiveness of these centrifugation procedures in separating the two enzyme isoforms, six-point saturation assays were performed on control cortex and striatal (as per assay conditions, below) and Michaelis–Menten constants (Km) and maximal reaction velocities (Vmax.) were determined (GraphPad Prism, 4.0, Graph Pad Software, Inc., San Diego, CA, USA); these analyses revealed expected results of significantly higher catalytic activity and lower substrate affinity for COMT-s compared to COMT-mb samples.
Assays
Tissue homogenates were pre-incubated for 5 min in a water bath set to 37 °C in parallel with reaction buffer consisting of 10 mM Tris–HCl, pH 7.4, containing 1 mM MgCl2, 10 μM catechol, 1 μM DTT and 0.45 μCi [3H] adenosyl-S-methionine (PerkinElmer, Boston, MA, USA). Afterward, triplicate sets of reaction tubes (1.5 m; Ependorf) were prepared by adding 20 μl of tissue to 500 μl of reaction buffer. Total activity was assessed in parallel in additional triplicate sets of tubes that were prepared using the same reaction buffer but with the addition of 0.1 mg tropolone to assess non-specific activity. After 20 min, reactions were terminated by the addition of 500 μl of 1 M HCl; 1 ml samples were then taken from each tube, placed in 10 ml of scintillation fluid (Betafluor, National Diagnostics, Atlanta, GA, USA), vortexed and after settling were quantified by liquid scintillation counting using an LS-6500 Beckman-Coulter Scintillation Counter (Beckman Instruments, Inc, Fullerton, CA, USA). Time-course assays were performed on homogenates of cortical and striatal tissues collected from gonadally intact control animals using these methods but varying incubation times from 1 to 60 min to confirm that for each tissue and enzyme form evaluated, the twenty-minute incubation period selected for singe point assessments (below) was within an interval over which the rate of product formation was linear.
MAO
MAO-A and MAO-B activities were determined using methods similar to those described by Hallman et al. (1987).
Tissue preparation
For both MAO-A and MAO-B assays, tissue samples of medial prefrontal cortex and dorsal striatum were dissected from three sets of three animal subjects per hormone treatment group were combined, weighed, re-supsended at 100 mg/ml in ice-cold homogenization buffer (0.01 M phosphate buffer, pH 7.7, containing 1 mM EDTA and 0.25 M sucrose) and homogenized by hand using a small glass dounce homogenizer. Tissue homogenates were then rapidly frozen in powdered dry ice and stored at −80 °C until the time of assay.
Assays
Tissue samples were preincubated in a water bath set to 37 °C for 5 min in parallel with reaction buffer consisting of 0.05 mM phosphate buffer, pH 7.4. For MAO-A assays, reaction buffers additionally contained either 0.05 μCi/50 μM [14C] 5-hydroxytyrptamine (Perkin Elmer) as substrate or [14C] 5-hydroxytyrptamine plus 1 μM clorgyline to determine total and non-specific MAO-A activity, respectively. For MAO-B assays, reaction buffers also contained either 0.025 μCi/25 μM [14C] phenylethylamine (Perkin Elmer) as substrate or [14C] phenylethylamine plus 1μM pargyline to determine total and non-specific MAO-B activity, respectively.
Following preincubation, reaction tubes (1.5 ml Eppendorf) for both MAO-A and MAO-B assays were prepared by adding 10 μl of tissue to 700 μl of the reaction buffer or reaction buffer containing enzyme inhibitor. These tubes, prepared in triplicate for totals and non-specific blanks for each tissue and animal group, were vortexed and incubated for 20 min at 37 °C. The reactions were terminated by the addition of 50 μl 6 M HCl to each tube. The acid oxidation products formed were then extracted by adding 750 μl of a 1:1 solution of toluene-ethylacetate to each tube, vortexing, and then collecting 400 μl of the organic phase. This product was placed in glass scintillation vials containing 8 ml of scintillation cocktail (Betafluor, National Diagnostics, Atlanta, GA, USA), vortexed, and after settling and was counted by liquid scintillation spectrometry using an LS-6500 Beckman-Coulter Scintillation Counter. Time course assays were performed for MAO-A and MAO-B activity using homogenates of cortical and striatal tissues from control animals as per these methods but varying in incubation times from 1 to 60 min to confirm that the 20 min incubation period selected was within the time interval over which the rate of product formation was linear for MAO-A and MAO-B for cortical and subcortical tissue. MAO-A activity in prefrontal cortical tissue from control and GDX subjects was also evaluated in six-point saturation assays that were performed as above using substrate concentrations that ranges from 2 to 200 μM; Michaelis–Menten constants (Km) and maximal reaction velocities (Vmax) were determined using GraphPad Prism, 4.0 (Graph Pad Software, Inc., San Diego, CA, USA).
Protein quantification
Protein content was measured using a Bio Rad Protein Assay Kit (BioRad Life Sciences, Hercules, CA, USA). Triplicate samples of tissue homogenates used in each enzyme assay were diluted eightfold and measured at 595 nm using a Nanodrop 1000 Spectrophotometer (Nanodrop Technologies, Inc. Wilmington, DE, USA). Protein concentrations from these samples were calculated in relation to standard curves generated using 0.2–1.4 mg/ml of bovine γ globulin (provided with the kits). These values were used to normalize enzyme activity data prior to the within and across group analyses described below.
Data analysis
Protein-normalized, triplicate total and non-specific data points obtained from each of the three repeated enzyme activity assays performed per brain area and per hormone treatment group comprised the raw data that were used for all of the analyses described below. Because the experimental “n” for each was three, the data were first assessed first using descriptive statistics, and were then compared as allowed within and across animal groups using both parametric and non-parametric assessments to obtain the most reliable picture of the statistical power of resultant findings. Within group analyses utilized the Student's T-test and the Wilcoxon Signed Rank or Mann Whitney U-test; across animal group comparisons utilized analyses of variance (ANOVA) or the Kruskal–Wallis test. Individual bulbospongiosus muscle weights from each of the individual animal subjects used in the assays were also evaluated using descriptive statistics and were compared across hormone treatment groups using an ANOVA. For all statistical assessments, a P<0.05 level was accepted as significant, and allowed post-hoc testing utilized Fisher's PLSD (Stat View, 4.5).
Results
Effectiveness of hormone treatment
Separate analyses and comparisons of the weights of the androgen-sensitive BSM obtained from each animal subject used for COMT and MAO assays revealed expected differences in mass between the GDX and GDX-E groups on the one hand and the control and GDX-TP groups on the other (Fig. 2A, B). Thus, for both sets of animal groups, mean BSM weights of the GDX and GDX-E cohorts were only about 25% of the control whereas the weights of the GDX-TP groups were similar to or slightly higher than control. Separate analyses of variance (ANOVA) performed on the data collected from subjects used for the MAO and COMT assays further identified significant main effects of hormone treatment on BSM mass for both sets of subjects [for MAO-A assays: F(3,30)=14.67, P<0.0001; for COMT assays: F(3,30)=7.90, P<0.0001] and post hoc comparisons revealed significant differences in muscle mass between the GDX and GDX-E but not the GDX-TP groups compared to the sham-operated controls (Fig. 2A, B).
Fig. 2.
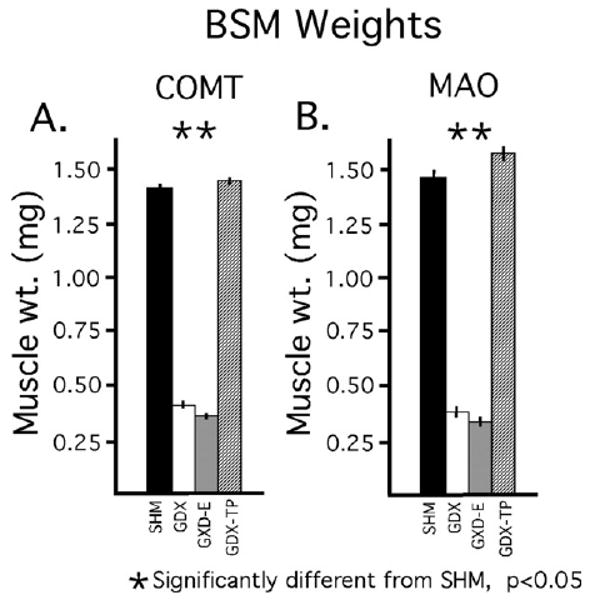
Bar graphs showing means weights of the bulbospongiousus muscles (BSM) in milligrams (±standard error of the mean), for animal subjects used for catechol-O-methylation (COMT) assays (A) and monoamine oxidase (MAO) assays (B). Data from animals that were sham-operated (SHM, black bars), gonadectomized (GDX, white bars) and gonadectomized and supplemented with estradiol (GDX-E, gray bars) or with testosterone propionate (GDX-TP, stippled bars) for 28 days are shown separately. As expected, there were significant effects of hormone treatment on the weights of these androgen-sensitive muscles, with the BSM weights in both of the 28 day GDX and GDX-E groups being significantly less (*) than those of the corresponding SHM and GDX-TP cohorts.
Catechol-O-Methyltransferse (COMT) activity
Assays of the COMT-mb revealed no obvious differences in protein-normalized enzyme activity in prefrontal compared to striatal tissues for any of the four animal groups (Fig. 3A). This was confirmed in within group parametric and non-parametric statistical comparisons (Student's t-test, Wilcoxon Signed Rank test) that found no significant or near significant differences in cortical compared to subcortical enzyme activity for any of the animal groups. There were also no obvious differences observed in normalized enzyme activity measures for either striatal or for cortical tissues in intact compared to GDX or GDX-hormone treated animals (Fig. 3A); this was further supported in both parametric and non-parametric statistical comparisons (ANOVA, Kruskal–Wallis test) that found no significant or near significant main effects of hormone treatment on COMT-mb activity for either prefrontal cortex or for dorsal striatum.
Fig. 3.
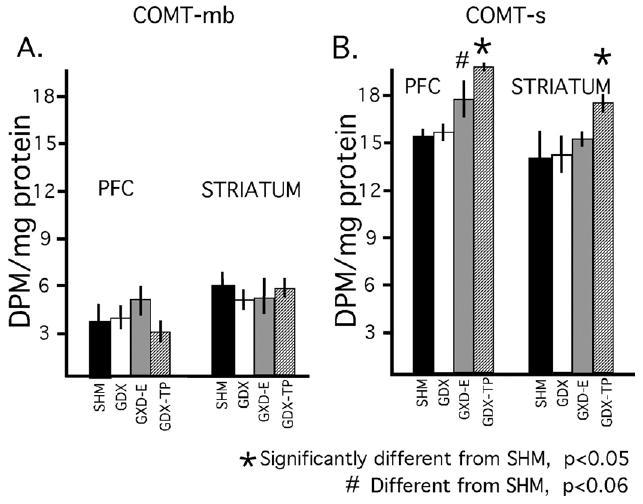
Bar graphs showing the mean (±standard error of the mean), protein normalized activities for the membrane-bound and soluble isoforms of catechol-O-methyltransferase (COMT-mb, COMT-s) in prefrontal cortex (PFC) and striatal tissue dissected from animals that were sham-operated (SHM, black bars), gonadectomized (GDX, white bars), gonadectomized and supplemented with estradiol (GDX-E, gray bars) or gonadectomized and supplemented with testosterone propionate (GDX-TP, stippled bars) for 28 days. There were no obvious effects of hormone deprivation or hormone replacement on the brain-abundant membrane bound isoform of COMT (A). There were also no obvious effects of gonadectomy on cortical or subcortical activity of COMT-s (B). There were, however, significant effects of hormone replacement of gonadectomized rats with testosterone propionate in cortical and striatal tissue, and near significant effects of hormone replacement of gonadectomized rats with estradiol in prefrontal cortex.
Protein-normalized enzyme activity levels for the soluble form of COMT (COMT-s) were similar and not significantly different for both cortical and subcortical tissue in all animal groups (Fig. 3B). Mean activity levels for cortex and for striatum were also similar and not significantly different from one another in GDX compared to control groups (Fig. 3B). However, normalized activity levels for both tissue types were about 15% higher than control in the GDX-E group and about 30% higher than control in the GDX-TP cohort (Fig. 3B). Subsequent statistical analyses confirmed that there were significant main effects of hormone treatment on COMT-s activity in prefrontal cortex (ANOVA, F3,18=8.66, P<0.0009; Kruskal–Wallis, H=13.63, P<0.0035) and near significant main effects of hormone treatment on COMT-s activity in striatum (ANOVA, F3,18=3.045, P<0.056; Kruskal–Wallis, H=5.60, P<0.133). Allowed post-hoc comparisons (Fisher's PLSD) further showed that these effects were driven by hormone replacement and not gonadectomy per se. Thus, there were significant differences between the GDX-TP and control groups for both striatum and cortex (P<0.011 and P<0.0003, respectively) and near significant differences between GDX-E and control in prefrontal cortex (P<0.057), but no significant or near significant differences in COMT-s in the GDX compared to control group for either tissue (Fig. 3B).
Monoamine oxidase (MAO) activity
Assays for MAO-B revealed protein-normalized enzyme activities that were similar and not significantly different in cortical compared to subcortical tissues in all four animal groups and that were also similar and not significantly different for both tissue types in the control compared to hormone treatment groups (Fig. 4A). Assays for MAO-A, on the other hand, revealed enzyme activity levels that in all animals groups were about 80% higher in striatum compared to prefrontal cortex (Fig. 4B); parametric (t-test) and non-parametric (Wilcoxon Signed Rank test) paired comparisons further identified these tissue differences as significant or near significant in all four animal groups (Student's T-test: corrected t-values from −3.129 to 6.061, P-values from <0.09 to 0.0005; Wilcoxon Signed Rank test: Z-values −1.540 to −2.521, P-values 0.123–0.012). For the dorsal striatum, there were no obvious trends or significant or near-significant differences in MAO-A activity in the control compared to hormone treatment groups (Fig. 4B). However, for the prefrontal cortex protein-normalized activity in the GDX cohort was about 30% higher than control whereas activities in the GDX-E and GDX-TP groups were both similar to each other and to control (Fig. 4B). Subsequent statistical analyses identified significant main effects of hormone treatment on prefrontal MAO-A activity (ANOVA, F3,24=4.505, P<0.012; Kruskal–Wallis, H=9.568, P<0.0226) and allowed post-hoc comparisons confirmed that these effects were a consequence of a significant difference between the GDX but not the GDX-TP or GDX-E groups and control.
Fig. 4.
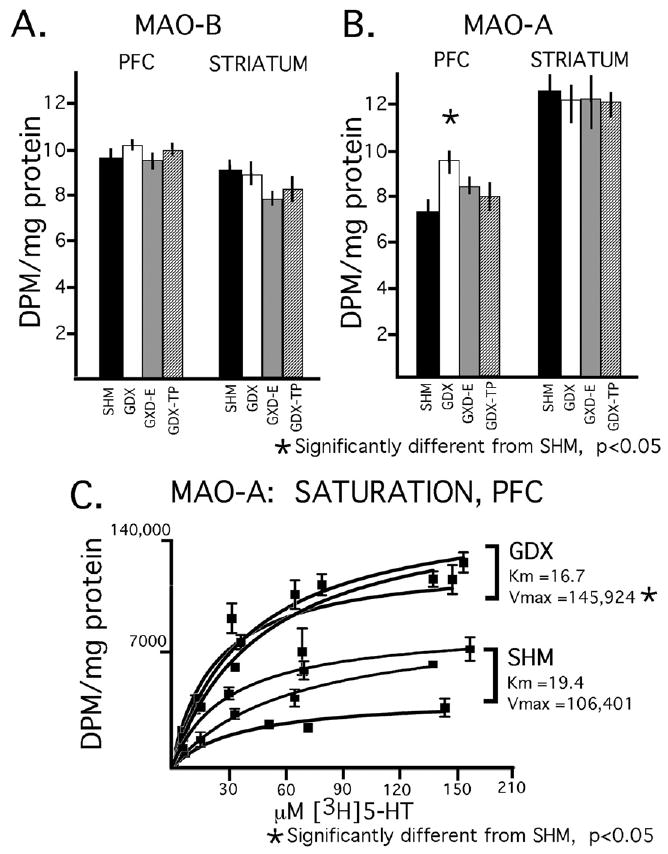
Bar graphs showing the mean (±standard error of the mean), protein normalized activities for the B- and A-isoforms of monoamine oxidase (panel A, MAO-B; panels B, C MAO-A) in prefrontal cortex (PFC) and dorsal striatum tissue dissected from animals that were sham-operated (SHM, black bars), gonadectomized (GDX, white bars), gonadectomized and supplemented with estradiol (GDX-E, gray bars) or gonadectomized and supplemented with testosterone propionate (GDX-TP, stippled bars) for 28 days. There were no obvious effects of hormone deprivation or hormone replacement on MAO-B in either cortical or subcortical tissue (A). There were, however, significant effects of gonadectomy on MAO-A activity in prefrontal cortical but not striatal tissue that were attenuated by both estradiol and testosterone replacement (B). The results from triplicate saturation assays (C) performed in prefrontal cortical tissue homogenates dissected from the gonadecotmized and sham-operated control groups revealed that GDX significantly elevated the maximal reaction velocity (Vmax) but had no significant effect on enzyme/substrate affinity (Km).
The stimulatory effects of GDX on prefrontal MAO-A activity were evaluated further in saturation assays that compared substrate affinity and reaction velocity between the GDX and control groups. These studies revealed Michaelis–Menten constants that were similar in GDX rats and controls (Km=16.70±3.11 and 19.40±3.46 μM, respectively) but Vmax values that were roughly 40% higher in the GDX compared to control group (145,924±5794 DPM/mg tissue/20 min versus 106,401±7679 DPM/mg tissue/20 min, respectively, Fig. 4C). Subsequent parametric (ANOVA) and non-parametric (Mann–Whitney U-test) comparisons confirmed that Km values in GDX and controls were similar to one another while the Vmax values of these two groups were significantly to nearly significantly different (ANOVA, F1,3=13.36, P<0.034; Mann–Whitney test, U=0, U′=6, P<0.083).
Discussion
Gonadal hormone influence over the monoamine and indolamine neurotransmitters in forebrain regions of the brain has been shown to include modulation of synthesis (Simerly, 1989; Fink et al., 1996, 1999; Pecins-Thompson et al., 1996; Thanky et al., 2002; Serova et al., 2004; Jeong et al., 2006; Guerra-Araiza et al., 2008), vesicular and/or synaptic release (Ji et al., 2007; Dluzen et al., 2008; Dluzen and McDermott, 2008), the number and/or affinity of the receptors and transporter proteins they interact with (Landry and Di Paolo, 2003; Bhatt and Dluzen, 2005; Le Saux and Di Paolo, 2006; Meyers and Kritzer, 2009), and the second messenger cascades these can activate (Le Saux and Di Paolo, 2005; Mani et al., 2009). It is likely that these actions—and very possibly others, help shape the sex differences and hormone malleability that has been observed for the complex cognitive, mnemonic and affective functions that these neurotransmitter systems support (Cosgrove et al., 2007), and could also have bearing on the sex differences that are also seen in the incidence, symptom severity and efficacy of drug treatment in the devastating forms of mental illness such as schizophrenia and major depression in which these forebrain systems fail (Goodman and Stevenson, 1989; Seeman and Lang, 1990). Accordingly, it is important to have as full a view as possible of the means by which gonadal steroid hormones affect these pivotal signaling systems. Here classical means of hormone deprivation and replacement were used to explore the hormone sensitivity of two catabolic enzymes, COMT and MAO, which are particularly active in regulating functional amine neurotransmitter levels in forebrain structures including the cerebral cortex (Westerink and Spaan, 1982; Karoum et al., 1994; Yavich et al., 2007). Stimulated by findings from this laboratory of a profound dysregulation of the prefrontal DA system in male rats, by evidence for male over female sex differences in and male-specific associations with polymorphisms of both enzymes in human health and mental illness (Volavka et al., 2004; Ducci et al., 2006; Sjoberg et al., 2007; Biederman et al., 2008; Harrison and Tunbridge, 2008) and by the striking male specific phenotypes seen in both MAO and COMT knockout mice (Cases et al., 1995; Gogos et al., 1998; Huotari et al., 2002), this approach was applied specifically to the adult male rat model. As discussed further below, these analyses revealed significant, regionally selective effects of hormone replacement on COMT-s but not COMT-mb, and of GDX on MAO-A but not MAO-B. Although none of the effects observed were consistent with roles in the regulation/dysregulation of DA in the prefrontal cortex (PFC) seen in the same animal model, they do provide new and more detailed information about the hormone sensitivity of identified enzyme isoforms in brain areas and in a sex where although there has been a wealth of data that predicts significant gonadal steroid impact on normal as well as pathological operations, systematic assessments have rarely to never been applied.
Effects of gonadectomy and hormone replacement on COMT: comparisons with previous studies
Given that DA disturbances in the prefrontal cortex are significant parts of the pathophysiology of schizophrenia, it is not surprising that the enzyme principally responsible for regulating prefrontal cortical DA levels, COMT has been identified as a susceptibility gene for this and other disorders including obsessive-compulsive disorder and attention deficit hyperactivity disorder (ADHD) where prefrontal DA systems are also at risk (Biederman et al., 2008; Harrison and Tunbridge, 2008). Further, as for the incidence, expression and/or outcome of these same disorders (Goodman and Stevenson, 1989; Seeman and Lang, 1990), there are also significant sexual dimorphisms associated with COMT. For example, the Met (A) allele of the COMT Val158 Met polymorphism has been associated with aggressive and impulsive behavior (Strous et al., 1997; Lachman et al., 1998; Kotler et al., 1999; Strous et al., 2003), ADHD (Biederman et al., 2008) and obsessive-compulsive disorder (Karayiorgou et al., 1997, 1999; Poyurovsky et al., 2005; Denys et al., 2006; Pooley et al., 2007) in males but not females, and with anxiety disorders in females but not males (Eley et al., 2003; Domschke et al., 2004; Woo et al., 2004; Stein et al., 2005; Rothe et al., 2006). In health, this allele has also been associated with better performance in behavioral measures of IQ, attention and working memory in boys but not girls (Barnett et al., 2007), and independent of the functional Val158 Met polymorphism, significant male over female differences have also been identified in COMT activity measured in prefrontal cortex (Chen et al., 2004), liver (Boudikova et al., 1990) and erythrocytes (Fahndrich et al., 1980; Floderus and Wetterberg, 1981; Philippu et al., 1981). Analyses in COMT −/−mice have also shown that the significant effects that this gene knockout has on forebrain DA levels and on certain DA drug responses are exclusive to males while behavioral consequences of this manipulation for measures of anxiety are female-specific (Gogos et al., 1998; Huotari et al., 2002).
Studies in this laboratory have shown that in adult male rats gonadal hormones exert a significant and selective stimulatory influence over basal prefrontal cortical DA levels and innervation densities (Kritzer et al., 1999; Kritzer, 2000). Given that the COMT enzyme is uniquely relied upon by the mesocortical DA system to regulate the key parameter of local DA tone, it seemed plausible that these findings and those noted above in humans and mice could be explained by testicular hormone regulation/gonadectomy-induced dysregulation of COMT. However, until now the question of hormone impact over this enzyme has been addressed mainly in studies that focused on ovarian hormones and female subjects. While these studies have clearly shown that COMT is estradiol-sensitive, they also suggest that hormone sensitivity may be largely limited to peripheral tissues and cell lines and to the soluble isoform of COMT that predominates therein. For example, estrogen response elements have been identified within the proximal COMT promoter region which specifically regulates transcription of COMT-s (Xie et al., 1999). Further, estradiol treatment has been shown to diminish COMT mRNA, activity and immunoreactivity in estrogen receptor expressing, COMT-s-enriched MCF-7 cells (Xie et al., 1999; Jiang et al., 2003). However, whereas estradiol administration in rats has also been shown to decrease COMT activity in liver, where the majority of COMT present is its soluble form, corresponding effects were not seen in the brain where COMT-mb predominates (Cohn and Axelrod, 1971; Tenhunen et al., 1993; Lundstrom et al., 1995; Matsumoto et al., 2003).
The present studies are to our knowledge a first to assess the effects of gonadectomy and hormone replacement on COMT in male subjects and a first to independently assess hormone actions on COMT-s versus COMT-mb in brain. The data that were obtained do, however, align with previous studies in indicating a gonadal hormone insensitivity of brain COMT and, more specifically, brain COMT-mb. Thus, no effects of either gonadectomy or hormone replacement were found on the activity of this enzyme isoform in either cortical or subcortical tissue. There were also no effects of gonadectomy itself on COMT-s. However, the present analyses did identify significant and near significant stimulatory effects on this enzyme isoform in GDX animals supplemented with testosterone and with estradiol, respectively. Given the paucity of COMT-s in brain and the low affinity that this isoform has for catecholamines (Lotta et al., 1995), it may be unlikely that this limited sphere of hormone sensitivity contributes significantly to the diverse sex differences that have come to be associated with brain COMT activity. Further, it is also unlikely that these effects are involved in the gonadectomy-induced upregulation of the prefrontal DA system that has been identified in the same animal model, where enzyme inhibition would be expected in the GDX cohort. The stimulation of COMT-s observed in both cortical and subcortical tissues of the testosterone- and the estradiol-supplemented groups is also at odds with the exclusively androgen-sensitive and prefrontal-selective effects that long-term gonadectomy in adult male rats exerts on mesocortical dopaminergic endpoints (Kritzer et al., 1999; Kritzer, 2000; Meyers and Kritzer, 2009).
In sum, all available data—including that added here, suggests that direct actions of gonadal steroids on enzyme activity may not be primary means by which sex differences are conferred on COMT in brain and the membrane-bound enzyme isoform that predominates there. However, there is a growing consensus for hormone regulation of COMT-s. Further, whereas previous studies had shown this isoform to be hormone malleable in non-neural tissues and cell cultures, the present study shows that the COMT-s present in brain is also hormone sensitive. Interestingly, however, both the testosterone- and the weaker estradiol-driven, stimulating effects on COMT-s that were identified here in the male brain are opposite to the inhibitory actions that have been previously identified for estradiol (above) and for the non-aromatizable androgen dihydrotestosterone in decreasing COMT transcription and activity in COMTs-enriched, cultured ovarian tissues (porcine and human granulose cell lines; Salih et al., 2008). Taken together, these findings not only suggest that gonadal hormones may be directly involved in regulating COMT-s, but that enzyme activity may be oppositely tuned by gonadal steroids in male versus female subjects. Understanding how this occurs may provide important clues as to how gonadal steroid stimulation contributes to the sex differences in the actions and activities of COMT/COMT-s that have been observed in non-neural tissues, that may have more to do with catabolism of catechol estrogens rather than the catecholamines, and that have been implicated in sex-specific patterns of protection from and vulnerability to certain estrogen-related cancers (Yin et al., 2004).
Effects of gonadectomy and hormone replacement on MAO: comparisons with previous studies
Studies in humans and animal models have identified male-specific links between MAO-A activity and MAO-A gene polymorphisms and/or gene knockouts with aggressive, impulsive and antisocial behaviors (Brunner et al., 1993; Cases et al., 1995; Kim et al., 1997; Caspi et al., 2002; Saito et al., 2002; Nilsson et al., 2006; Widom and Brzustowicz, 2006; Sjoberg et al., 2007), with related effects on forebrain levels of serotonin, norepnephrine and to a lesser degree DA (Cases et al., 1995; Chen et al., 2007), and with disorders including antisocial personality disorder, ADHD and schizophrenia where these functions and neurotransmitter systems are at risk (Sjoberg et al., 2008). That the promoter region of the human MAO-A gene has been shown to contain multiple androgen response elements (Ou et al., 2006) further suggests that this regulatory enzyme is influenced by gonadal, perhaps specifically testicular, hormones in males. Nonetheless, few studies have directly explored the question of gonadal hormone sensitivity of MAO in male subjects. Rather, most of the literature that exists to date has explored the potential hormone sensitivity of MAO enzyme activity in hindbrain and/or neuroendocrine brain centers in female rodent and non-human primate ovariectomy and hormone replacement models. While these analyses have identified effects of ovarian steroids on MAO that are in part brain region, hormone manipulation paradigm and MAO isoform specific, there are consensus findings of inhibitory effects of estrogen on MAO-A activity or mRNA (Luine et al., 1975; Chevillard et al., 1981; Luine and Rhodes, 1983; Ortega-Corona et al., 1994; Holschneider et al., 1998; Bethea et al., 2002; Gundlah et al., 2002) and more variable and generally more limited effects of estrogen on MAO-B (Holschneider et al., 1998; Gundlah et al., 2002).
In the two prior studies of MAO activity that were carried out in male rats (Luine et al., 1975; Birgner et al., 2008), the steroid hormone manipulations that were used were substantially different from those used in of this study. Perhaps as a consequence, both yielded results that do not readily match those identified here. Thus, whereas the current studies found that MAO-A activity in prefrontal cortex but not dorsal striatum was significantly increased by long-term gonadectomy, short-term testosterone treatment (3 days to 1 week) in gonadectomized rats was shown to increase MAO activity in the preoptic area and have no effects on MAO in cortex, amygdala, hippocampus or hypothalamus (Luine et al., 1975). A second study also showed that 14 days of i.m. administration of the anabolic steroid nandrolone in gonadally intact males dose-dependently decreased both MAO-A and MAO-B activity in striatum but not prefrontal cortex (Birgner et al., 2008). These findings are also essentially opposite to those identified here, where there were no effects of gonadectomy or hormone replacement on MAO-A or B in the striatum and no effects on MAO-B in prefrontal cortex. Thus, like other endpoints—and as presaged in studies of MAO in females (above), the effects of gonadal steroids on MAO activity in males may be sensitive to the specifics, for example, duration, dose, of the hormone deprivation, stimulation, supplementing and/or replacement used. This is supported in part by findings obtained in adult, male non-human primates where both the model and the findings obtained were more concordant with those of this study. Thus, in these previous analyses, MAO activity measured in plasma in gonadally intact animals at different times during the breeding season and in animals that had been gonadectomized for the long term revealed that MAO activity was inversely correlated with serum testosterone levels in the intact animals and significantly increased in a long-term gonadectomized cohort (Redmond et al., 1976). In the present study, testosterone suppressing effects over MAO were defined further in by isolating effects to MAO-A versus MAO-B, and by revealing an equal effectiveness of both TP and E in attenuating the effects of GDX on MAO-A; because both the TP and E replacement paradigms used in this study have been shown to result in similar plasma E levels (Turvin et al., 2007) this suggest that aromatized estrogenic derivatives of testosterone may be principally responsible for the hormone regulation observed. Finally, it was also shown that the influence of gonadectomy over the pivotal regulatory enzyme MAO-A relates to its abundance (Vmax.) rather than the efficiency of its interactions with substrate (Km).
Although DA is a known substrate for MAO-A (Shih, 1991; Shih et al., 1999), there are several reasons to reject the conclusion that the effects of GDX on MAO-A identified here are responsible for the effects that this same manipulation has on the prefrontal DA system. Specifically, although both endpoints show selectivity for the prefrontal cortex, the stimulatory actions of GDX identified here on the degradative enzyme MAO-A are counter to the stimulatory effects on prefrontal DA systems that this manipulation also brings. Further, whereas effects on prefrontal DA systems are typically androgen sensitive and estrogen insensitive, the effects observed here on MAO-A were attenuated in both the testosterone- and estradiol-supplemented, gonadectomized groups. However, whereas, the impact of GDX on MAO is more likely to be compensatory rather than causal for prefrontal DA systems, it is possible that the hormone influence identified here could have significant bearing on the preferred substrates of MAO-A, which for forebrain include 5-HT and NE (Shih, 1991; Shih et al., 1999). Further, the suppressive effects of testosterone on cortical MAO-A identified here could help explain the appearance of certain cognitive deficits, for example, in spatial memory, verbal fluency, and the increased incidence of symptoms of depression and anxiety that have been repeatedly identified in male subjects in clinical instances of hypogonadalism related to aging or other biological factors (Alexander et al., 1998; Cherrier et al., 2003b; Shores et al., 2004, 2005; Janowsky, 2006; Almeida et al., 2008; Amore et al., 2009), or in prostate cancer patients where androgen deprivation has been used as a neoadjuvant treatment (Cherrier et al., 2003b; Salminen et al., 2003, 2005; Janowsky, 2006). Given the present results from our surgical hypogonadalism rodent model and the previous data collected in gonadectomized, non-human primates (Redmond et al., 1976), it may well be predicted that in human conditions where circulating testosterone levels are decreased there would be increased levels of MAO-A and a related decrease in forebrain levels of 5-HT and NE, that in turn could produce a neurochemical milieu conducive to the production and/or exacerbation of exactly types of behavioral symptoms that are seen clinically (above) that have long been associated with deficits in catechol- and indolamine neural transmission. Finally, whereas testosterone replacement therapies in aging men and in certain male patient populations have had mixed or disappointing results in alleviating cognitive decline and/or affective symptoms (Janowsky et al., 2000; Kenny et al., 2002; Lu et al., 2006), the present findings that identified the MAO-A suppressing actions of testosterone as being androgen and/or estrogen-driven suggest that greater clinical benefit might be gained by the use of aromatizable rather than non-aromatizable androgens in applied hormone replacement therapies (Cherrier et al., 2003a, 2005).
Acknowledgments
This work supported by RO1-NS41966 to MFK.
Abbreviations
- ANOVA
analysis of variance
- BSM
bulbospongiosus muscles
- COMT
catechol-O-methyltransferase
- COMT-mb
catechol-O-methyltransferase, membrane-bound isoform
- COMT-s
catechol-O-methyltransferase, soluble isoform
- DA
dopamine
- GDX
gonadectomized
- GDX-E
gonadectomised, estradiol replaced
- GDX-TP
gonadectomised, testosterone propionate replaced
- MAO
monoamine oxidase
- MAO-A
monoamine oxidase, A, isoform
- MAO-B
monoamine oxidase, B isoform
- SHM
sham-operated control
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler A, Vescovo P, Robinson JK, Kritzer MF. Gonadectomy in adult life increases tyrosine hydroxylase immunoreactivity in the prefrontal cortex and decreases open field activity in male rats. Neuroscience. 1999;89:939–954. doi: 10.1016/s0306-4522(98)00341-8. [DOI] [PubMed] [Google Scholar]
- Alexander GM, Swerdloff RS, Wang C, Davidson T, McDonald V, Steiner B, Hines M. Androgen-behavior correlations in hypogonadal men and eugonadal men. II. Cognitive abilities. Horm Behav. 1998;33:85–94. doi: 10.1006/hbeh.1998.1439. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Yeap BB, Hankey GJ, Jamrozik K, Flicker L. Low free testosterone concentration as a potentially treatable cause of depressive symptoms in older men. Arch Gen Psychiatry. 2008;65:283–289. doi: 10.1001/archgenpsychiatry.2007.33. [DOI] [PubMed] [Google Scholar]
- Amore M, Scarlatti F, Quarta AL, Tagariello P. Partial androgen deficiency, depression and testosterone treatment in aging men. Aging Clin Exp Res. 2009;21:1–8. doi: 10.1007/BF03324891. [DOI] [PubMed] [Google Scholar]
- Aubele T, Kaufman R, Montalmant F, Kritzer MF. Effects of gonadectomy and hormone replacement on a spontaneous novel object recognition task in adult male rats. Horm Behav. 2008a;54:244–252. doi: 10.1016/j.yhbeh.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubele T, Meyers B, Liu A, Kritzer MF. Gonadectomy in adult male rats increases extracellular dopamine levels in the prefrontal cortex: Possible causative role for effects on the norepinephrine transporter, but not the dopamine transporter or the enzyme catechol-O-methyltransferase. Abstract, Society for Neuroscience annual meeting 2008b [Google Scholar]
- Barnett JH, Heron J, Ring SM, Golding J, Goldman D, Xu K, Jones PB. Gender-specific effects of the catechol-O-methyltransferase Val108/158Met polymorphism on cognitive function in children. Am J Psychiatry. 2007;164:142–149. doi: 10.1176/ajp.2007.164.1.142. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol. 2002;23:41–100. doi: 10.1006/frne.2001.0225. [DOI] [PubMed] [Google Scholar]
- Bhatt SD, Dluzen DE. Dopamine transporter function differences between male and female CD-1 mice. Brain Res. 2005;1035:188–195. doi: 10.1016/j.brainres.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Biederman J, Kim JW, Doyle AE, Mick E, Fagerness J, Smoller JW, Faraone SV. Sexually dimorphic effects of four genes (COMT, SLC6A2, MAOA, SLC6A4) in genetic associations of ADHD: a preliminary study. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1511–1518. doi: 10.1002/ajmg.b.30874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birgner C, Kindlundh-Hogberg AM, Oreland L, Alsio J, Lindblom J, Schioth HB, Bergstrom L. Reduced activity of monoamine oxidase in the rat brain following repeated nandrolone decanoate administration. Brain Res. 2008;1219:103–110. doi: 10.1016/j.brainres.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Boudikova B, Szumlanski C, Maidak B, Weinshilboum R. Human liver catechol-O-methyltransferase pharmacogenetics. Clin Pharmacol Ther. 1990;48:381–389. doi: 10.1038/clpt.1990.166. [DOI] [PubMed] [Google Scholar]
- Brunner HG, Nelen M, Breakefield XO, Ropers HH, van Oost BA. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 1993;262:578–580. doi: 10.1126/science.8211186. [DOI] [PubMed] [Google Scholar]
- Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, Muller U, Aguet M, Babinet C, Shih JC, et al. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995;268:1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Cases O, Rebrin I, Wu W, Gallaher TK, Seif I, Shih JC. Forebrain-specific expression of monoamine oxidase A reduces neurotransmitter levels, restores the brain structure, and rescues aggressive behavior in monoamine oxidase A-deficient mice. J Biol Chem. 2007;282:115–123. doi: 10.1074/jbc.M609830200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrier MM, Craft S, Matsumoto AH. Cognitive changes associated with supplementation of testosterone or dihydrotestosterone in mildly hypogonadal men: a preliminary report. J Androl. 2003a;24:568–576. doi: 10.1002/j.1939-4640.2003.tb02708.x. [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Matsumoto AM, Amory JK, Ahmed S, Bremner W, Peskind ER, Raskind MA, Johnson M, Craft S. The role of aromatization in testosterone supplementation: effects on cognition in older men. Neurology. 2005;64:290–296. doi: 10.1212/01.WNL.0000149639.25136.CA. [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Rose AL, Higano C. The effects of combined androgen blockade on cognitive function during the first cycle of intermittent androgen suppression in patients with prostate cancer. J Urol. 2003b;170:1808–1811. doi: 10.1097/01.ju.0000091640.59812.83. [DOI] [PubMed] [Google Scholar]
- Chevillard C, Barden N, Saavedra JM. Estradiol treatment decreases type A and increases type B monoamine oxidase in specific brain stem areas and cerebellum of ovariectomized rats. Brain Res. 1981;222:177–181. doi: 10.1016/0006-8993(81)90955-0. [DOI] [PubMed] [Google Scholar]
- Cohn CK, Axelrod J. The effect of estradiol on catechol-O-methyltransferase activity in rat liver. Life Sci I. 1971;10:1351–1354. doi: 10.1016/0024-3205(71)90335-3. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62:847–855. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- Denys D, Van Nieuwerburgh F, Deforce D, Westenberg H. Association between the dopamine D2 receptor TaqI A2 allele and low activity COMT allele with obsessive-compulsive disorder in males. Eur Neuropsychopharmacol. 2006;16:446–450. doi: 10.1016/j.euroneuro.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Bhatt S, McDermott JL. Differences in reserpine-induced striatal dopamine output and content between female and male mice: implications for sex differences in vesicular monoamine transporter 2 function. Neuroscience. 2008;154:1488–1496. doi: 10.1016/j.neuroscience.2008.04.051. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, McDermott JL. Sex differences in dopamine- and vesicular monoamine-transporter functions. Ann NY Acad Sci. 2008;1139:140–150. doi: 10.1196/annals.1432.010. [DOI] [PubMed] [Google Scholar]
- Domschke K, Freitag CM, Kuhlenbaumer G, Schirmacher A, Sand P, Nyhuis P, Jacob C, Fritze J, Franke P, Rietschel M, Garritsen HS, Fimmers R, Nothen MM, Lesch KP, Stogbauer F, Deckert J. Association of the functional V158M catechol-O-methyltransferase polymorphism with panic disorder in women. Int J Neuropsychopharmacol. 2004;7:183–188. doi: 10.1017/S146114570400416X. [DOI] [PubMed] [Google Scholar]
- Ducci F, Newman TK, Funt S, Brown GL, Virkkunen M, Goldman D. A functional polymorphism in the MAOA gene promoter (MAOA-LPR) predicts central dopamine function and body mass index. Mol Psychiatry. 2006;11:858–866. doi: 10.1038/sj.mp.4001856. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einon D. Spatial memory and response strategies in rats: age, sex and rearing differences in performance. Q J Exp Psychol. 1980;32:473–489. doi: 10.1080/14640748008401840. [DOI] [PubMed] [Google Scholar]
- Eley TC, Tahir E, Angleitner A, Harriss K, McClay J, Plomin R, Riemann R, Spinath F, Craig I. Association analysis of MAOA and COMT with neuroticism assessed by peers. Am J Med Genet B Neuropsychiatr Genet. 2003;120B:90–96. doi: 10.1002/ajmg.b.20046. [DOI] [PubMed] [Google Scholar]
- Fahndrich E, Coper H, Christ W, Helmchen H, Muller-Oerlinghausen B, Pietzcker A. Erythrocyte COMT-activity in patients with affective disorders. Acta Psychiatr Scand. 1980;61:427–437. doi: 10.1111/j.1600-0447.1980.tb00881.x. [DOI] [PubMed] [Google Scholar]
- Fink G, Sumner B, Rosie R, Wilson H, McQueen J. Androgen actions on central serotonin neurotransmission: relevance for mood, mental state and memory. Behav Brain Res. 1999;105:53–68. doi: 10.1016/s0166-4328(99)00082-0. [DOI] [PubMed] [Google Scholar]
- Fink G, Sumner BE, Rosie R, Grace O, Quinn JP. Estrogen control of central neurotransmission: effect on mood, mental state, and memory. Cell Mol Neurobiol. 1996;16:325–344. doi: 10.1007/BF02088099. [DOI] [PubMed] [Google Scholar]
- Floderus Y, Wetterberg L. The inheritance of human erythrocyte catechol-O-methyltransferase activity. Clin Genet. 1981;19:392–395. doi: 10.1111/j.1399-0004.1981.tb00732.x. [DOI] [PubMed] [Google Scholar]
- Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, Karayiorgou M. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci U S A. 1998;95:9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, Goldman D, Weinberger DR. Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psychiatry. 2003;60:889–896. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Weinberger DR. Genes and the parsing of cognitive processes. Trends Cogn Sci. 2004;8:325–335. doi: 10.1016/j.tics.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular and circuit basis of working memory in prefrontal cortex of nonhuman primates. Prog Brain Res. 1990;85:325–335. doi: 10.1016/s0079-6123(08)62688-6. discussion:326–335. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Funahashi S, Bruce CJ. Neocortical memory circuits. Cold Spring Harb Symp Quant Biol. 1990;55:1025–1038. doi: 10.1101/sqb.1990.055.01.097. [DOI] [PubMed] [Google Scholar]
- Goodman R, Stevenson J. A twin study of hyperactivity—I. An examination of hyperactivity scores and categories derived from Rutter teacher and parent questionnaires. J Child Psychol Psychiatry. 1989;30:671–689. doi: 10.1111/j.1469-7610.1989.tb00781.x. [DOI] [PubMed] [Google Scholar]
- Guerra-Araiza C, Miranda-Martinez A, Neri-Gomez T, Camacho-Arroyo I. Sex steroids effects on the content of GAD, TH, GABA(A), and glutamate receptors in the olfactory bulb of the male rat. Neurochem Res. 2008;33:1568–1573. doi: 10.1007/s11064-008-9665-1. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Lu NZ, Bethea CL. Ovarian steroid regulation of monoamine oxidase-A and -B mRNAs in the macaque dorsal raphe and hypothalamic nuclei. Psychopharmacology (Berl) 2002;160:271–282. doi: 10.1007/s00213-001-0959-0. [DOI] [PubMed] [Google Scholar]
- Hallman J, Oreland L, Edman G, Schalling D. Thrombocyte monoamine oxidase activity and personality traits in women with severe premenstrual syndrome. Acta Psychiatr Scand. 1987;76:225–234. doi: 10.1111/j.1600-0447.1987.tb02890.x. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Tunbridge EM. Catechol-O-methyltransferase (COMT): a gene contributing to sex differences in brain function, and to sexual dimorphism in the predisposition to psychiatric disorders. Neuropsychopharmacology. 2008;33:3037–3045. doi: 10.1038/sj.npp.1301543. [DOI] [PubMed] [Google Scholar]
- Holschneider DP, Kumazawa T, Chen K, Shih JC. Tissue-specific effects of estrogen on monoamine oxidase A and B in the rat. Life Sci. 1998;63:155–160. doi: 10.1016/s0024-3205(98)00255-0. [DOI] [PubMed] [Google Scholar]
- Huotari M, Santha M, Lucas LR, Karayiorgou M, Gogos JA, Mannisto PT. Effect of dopamine uptake inhibition on brain catecholamine levels and locomotion in catechol-O-methyltransferase-disrupted mice. J Pharmacol Exp Ther. 2002;303:1309–1316. doi: 10.1124/jpet.102.043042. [DOI] [PubMed] [Google Scholar]
- Janowsky JS. The role of androgens in cognition and brain aging in men. Neuroscience. 2006;138:1015–1020. doi: 10.1016/j.neuroscience.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Chavez B, Orwoll E. Sex steroids modify working memory. J Cogn Neurosci. 2000;12:407–414. doi: 10.1162/089892900562228. [DOI] [PubMed] [Google Scholar]
- Jeong H, Kim MS, Kim SW, Kim KS, Seol W. Regulation of tyrosine hydroxylase gene expression by retinoic acid receptor. J Neurochem. 2006;98:386–394. doi: 10.1111/j.1471-4159.2006.03866.x. [DOI] [PubMed] [Google Scholar]
- Ji J, McDermott JL, Dluzen DE. Sex differences in K+-evoked striatal dopamine output from superfused striatal tissue fragments of reserpine-treated CD-1 mice. J Neuroendocrinol. 2007;19:725–731. doi: 10.1111/j.1365-2826.2007.01581.x. [DOI] [PubMed] [Google Scholar]
- Jiang H, Xie T, Ramsden DB, Ho SL. Human catechol-O-methyltransferase down-regulation by estradiol. Neuropharmacology. 2003;45:1011–1018. doi: 10.1016/s0028-3908(03)00286-7. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, de Bruin JP, Matthijssen MA, Uylings HB. Ontogeny of open field activity in rats after neonatal lesioning of the mesocortical dopaminergic projection. Behav Brain Res. 1989;32:115–127. doi: 10.1016/s0166-4328(89)80079-8. [DOI] [PubMed] [Google Scholar]
- Karayiorgou M, Altemus M, Galke BL, Goldman D, Murphy DL, Ott J, Gogos JA. Genotype determining low catechol-O-methyl-transferase activity as a risk factor for obsessive-compulsive disorder. Proc Natl Acad Sci U S A. 1997;94:4572–4575. doi: 10.1073/pnas.94.9.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayiorgou M, Sobin C, Blundell ML, Galke BL, Malinova L, Goldberg P, Ott J, Gogos JA. Family-based association studies support a sexually dimorphic effect of COMT and MAOA on genetic susceptibility to obsessive-compulsive disorder. Biol Psychiatry. 1999;45:1178–1189. doi: 10.1016/s0006-3223(98)00319-9. [DOI] [PubMed] [Google Scholar]
- Karoum F, Chrapusta SJ, Egan MF. 3-Methoxytyramine is the major metabolite of released dopamine in the rat frontal cortex: reassessment of the effects of antipsychotics on the dynamics of dopamine release and metabolism in the frontal cortex, nucleus accumbens, and striatum by a simple two pool model. J Neurochem. 1994;63:972–979. doi: 10.1046/j.1471-4159.1994.63030972.x. [DOI] [PubMed] [Google Scholar]
- Kenny AM, Bellantonio S, Gruman CA, Acosta RD, Prestwood KM. Effects of transdermal testosterone on cognitive function and health perception in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci. 2002;57:M321–M325. doi: 10.1093/gerona/57.5.m321. [DOI] [PubMed] [Google Scholar]
- Kessler J, Markowitsch HJ. Delayed-alternation performance after kainic acid lesions of the thalamic mediodorsal nucleus and the ventral tegmental area in the rat. Behav Brain Res. 1981;3:125–130. doi: 10.1016/0166-4328(81)90033-4. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Shih JC, Chen K, Chen L, Bao S, Maren S, Anagnostaras SG, Fanselow MS, De Maeyer E, Seif I, Thompson RF. Selective enhancement of emotional, but not motor, learning in monoamine oxidase A-deficient mice. Proc Natl Acad Sci U S A. 1997;94:5929–5933. doi: 10.1073/pnas.94.11.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotler M, Barak P, Cohen H, Averbuch IE, Grinshpoon A, Gritsenko I, Nemanov L, Ebstein RP. Homicidal behavior in schizophrenia associated with a genetic polymorphism determining low catechol O-methyltransferase (COMT) activity. Am J Med Genet. 1999;88:628–633. [PubMed] [Google Scholar]
- Kritzer MF. Effects of acute and chronic gonadectomy on the catecholamine innervation of the cerebral cortex in adult male rats: insensitivity of axons immunoreactive for dopamine-beta-hydroxylase to gonadal steroids, and differential sensitivity of axons immunoreactive for tyrosine hydroxylase to ovarian and testicular hormones. J Comp Neurol. 2000;427:617–633. [PubMed] [Google Scholar]
- Kritzer MF, Adler A, Marotta J, Smirlis T. Regionally selective effects of gonadectomy on cortical catecholamine innervation in adult male rats are most disruptive to afferents in prefrontal cortex. Cereb Cortex. 1999;9:507–518. doi: 10.1093/cercor/9.5.507. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Brewer A, Montalmant F, Davenport M, Robinson JK. Effects of gonadectomy on performance in operant tasks measuring prefrontal cortical function in adult male rats. Horm Behav. 2007;51:183–194. doi: 10.1016/j.yhbeh.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, McLaughlin PJ, Smirlis T, Robinson JK. Gonadectomy impairs T-maze acquisition in adult male rats. Horm Behav. 2001;39:167–174. doi: 10.1006/hbeh.2001.1645. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Nolan KA, Mohr P, Saito T, Volavka J. Association between catechol O-methyltransferase genotype and violence in schizophrenia and schizoaffective disorder. Am J Psychiatry. 1998;155:835–837. doi: 10.1176/ajp.155.6.835. [DOI] [PubMed] [Google Scholar]
- Lacreuse A. Effects of ovarian hormones on cognitive function in nonhuman primates. Neuroscience. 2006;138:859–867. doi: 10.1016/j.neuroscience.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Landry M, Di Paolo T. Effect of chronic estradiol, tamoxifen or raloxifene treatment on serotonin 5-HT1A receptor. Brain Res Mol Brain Res. 2003;112:82–89. doi: 10.1016/s0169-328x(03)00049-4. [DOI] [PubMed] [Google Scholar]
- Le Saux M, Di Paolo T. Changes in 5-HT1A receptor binding and G-protein activation in the rat brain after estrogen treatment: comparison with tamoxifen and raloxifene. J Psychiatry Neurosci. 2005;30:110–117. [PMC free article] [PubMed] [Google Scholar]
- Le Saux M, Di Paolo T. Influence of oestrogenic compounds on monoamine transporters in rat striatum. J Neuroendocrinol. 2006;18:25–32. doi: 10.1111/j.1365-2826.2005.01380.x. [DOI] [PubMed] [Google Scholar]
- Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melen K, Julkunen I, Taskinen J. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34:4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- Lu PH, Masterman DA, Mulnard R, Cotman C, Miller B, Yaffe K, Reback E, Porter V, Swerdloff R, Cummings JL. Effects of testosterone on cognition and mood in male patients with mild Alzheimer disease and healthy elderly men. Arch Neurol. 2006;63:177–185. doi: 10.1001/archneur.63.2.nct50002. [DOI] [PubMed] [Google Scholar]
- Luine VN. Sex steroids and cognitive function. J Neuroendocrinol. 2008;20:866–872. doi: 10.1111/j.1365-2826.2008.01710.x. [DOI] [PubMed] [Google Scholar]
- Luine VN, Khylchevskaya RI, McEwen BS. Effect of gonadal steroids on activities of monoamine oxidase and choline acetylase in rat brain. Brain Res. 1975;86:293–306. doi: 10.1016/0006-8993(75)90704-0. [DOI] [PubMed] [Google Scholar]
- Luine VN, Rhodes JC. Gonadal hormone regulation of MAO and other enzymes in hypothalamic areas. Neuroendocrinology. 1983;36:235–241. doi: 10.1159/000123461. [DOI] [PubMed] [Google Scholar]
- Lundstrom K, Tenhunen J, Tilgmann C, Karhunen T, Panula P, Ulmanen I. Cloning, expression and structure of catechol-O-methyltransferase. Biochim Biophys Acta. 1995;1251:1–10. doi: 10.1016/0167-4838(95)00071-2. [DOI] [PubMed] [Google Scholar]
- Mani SK, Portillo W, Reyna A. Steroid hormone action in the brain: cross-talk between signalling pathways. J Neuroendocrinol. 2009;21:243–247. doi: 10.1111/j.1365-2826.2009.01844.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Weickert CS, Beltaifa S, Kolachana B, Chen J, Hyde TM, Herman MM, Weinberger DR, Kleinman JE. Catechol O-methyltransferase (COMT) mRNA expression in the dorsolateral prefrontal cortex of patients with schizophrenia. Neuropsychopharmacology. 2003;28:1521–1530. doi: 10.1038/sj.npp.1300218. [DOI] [PubMed] [Google Scholar]
- Meyers B, Kritzer MF. In vitro binding assays using (3)H nisoxetine and (3)H WIN 35,428 reveal selective effects of gonadectomy and hormone replacement in adult male rats on norepinephrine but not dopamine transporter sites in the cerebral cortex. Neuroscience. 2009;159:271–282. doi: 10.1016/j.neuroscience.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow BA, Roth RH, Elsworth JD. TMT, a predator odor, elevates mesoprefrontal dopamine metabolic activity and disrupts short-term working memory in the rat. Brain Res Bull. 2000;52:519–523. doi: 10.1016/s0361-9230(00)00290-2. [DOI] [PubMed] [Google Scholar]
- Murphy BL, Arnsten AF, Goldman-Rakic PS, Roth RH. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc Natl Acad Sci U S A. 1996;93:1325–1329. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson KW, Sjoberg RL, Damberg M, Leppert J, Ohrvik J, Alm PO, Lindstrom L, Oreland L. Role of monoamine oxidase A genotype and psychosocial factors in male adolescent criminal activity. Biol Psychiatry. 2006;59:121–127. doi: 10.1016/j.biopsych.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Ortega-Corona BG, Valencia-Sanchez A, Kubli-Garfias C, Anton-Tay F, Salazar LA, Villarreal JE, Ponce-Monter H. Hypothalamic monoamine oxidase activity in ovariectomized rats after sexual behavior restoration. Arch Med Res. 1994;25:337–340. [PubMed] [Google Scholar]
- Ou XM, Chen K, Shih JC. Glucocorticoid and androgen activation of monoamine oxidase A is regulated differently by R1 and Sp1. J Biol Chem. 2006;281:21512–21525. doi: 10.1074/jbc.M600250200. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in sterotaxic coordinates. 4th. New York: Academic Press; 1998. [Google Scholar]
- Pecins-Thompson M, Brown NA, Kohama SG, Bethea CL. Ovarian steroid regulation of tryptophan hydroxylase mRNA expression in rhesus macaques. J Neurosci. 1996;16:7021–7029. doi: 10.1523/JNEUROSCI.16-21-07021.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippu G, Hoo JJ, Milech U, Argarwall DP, Schrappe O, Goedde HW. Catechol-O-methyltransferase of erythrocytes in patients with endogenous psychoses. Psychiatry Res. 1981;4:139–146. doi: 10.1016/0165-1781(81)90017-2. [DOI] [PubMed] [Google Scholar]
- Pooley EC, Fineberg N, Harrison PJ. The met(158) allele of catechol-O-methyltransferase (COMT) is associated with obsessive-compulsive disorder in men: case-control study and meta-analysis. Mol Psychiatry. 2007;12:556–561. doi: 10.1038/sj.mp.4001951. [DOI] [PubMed] [Google Scholar]
- Poyurovsky M, Michaelovsky E, Frisch A, Knoll G, Amir I, Finkel B, Buniak F, Hermesh H, Weizman R. COMT Val158Met polymorphism in schizophrenia with obsessive-compulsive disorder: a case-control study. Neurosci Lett. 2005;389:21–24. doi: 10.1016/j.neulet.2005.06.064. [DOI] [PubMed] [Google Scholar]
- Redmond DE, Jr, Baulu J, Murphy DL, Loriaux DL, Zeigler MG, Lake CR. The effects of testosterone on plasma and platelet monoamine oxidase (MAO) an plasma dopamine-beta-hydroxylase (DBH) activities in the male rhesus monkey. Psychosom Med. 1976;38:315–326. doi: 10.1097/00006842-197609000-00004. [DOI] [PubMed] [Google Scholar]
- Rothe C, Koszycki D, Bradwejn J, King N, Deluca V, Tharmalingam S, Macciardi F, Deckert J, Kennedy JL. Association of the Val158Met catechol O-methyltransferase genetic polymorphism with panic disorder. Neuropsychopharmacology. 2006;31:2237–2242. doi: 10.1038/sj.npp.1301048. [DOI] [PubMed] [Google Scholar]
- Saito T, Lachman HM, Diaz L, Hallikainen T, Kauhanen J, Salonen JT, Ryynanen OP, Karvonen MK, Syvalahti E, Pohjalainen T, Hietala J, Tiihonen J. Analysis of monoamine oxidase A (MAOA) promoter polymorphism in Finnish male alcoholics. Psychiatry Res. 2002;109:113–119. doi: 10.1016/s0165-1781(02)00013-6. [DOI] [PubMed] [Google Scholar]
- Salih SM, Jamaluddin M, Salama SA, Fadl AA, Nagamani M, Al-Hendy A. Regulation of catechol O-methyltransferase expression in granulosa cells: a potential role for follicular arrest in polycystic ovary syndrome. Fertil Steril. 2008;89:1414–1421. doi: 10.1016/j.fertnstert.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Salminen E, Portin R, Korpela J, Backman H, Parvinen LM, Helenius H, Nurmi M. Androgen deprivation and cognition in prostate cancer. Br J Cancer. 2003;89:971–976. doi: 10.1038/sj.bjc.6601235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen EK, Portin RI, Koskinen AI, Helenius HY, Nurmi MJ. Estradiol and cognition during androgen deprivation in men with prostate carcinoma. Cancer. 2005;103:1381–1387. doi: 10.1002/cncr.20962. [DOI] [PubMed] [Google Scholar]
- Seeman MV, Lang M. The role of estrogens in schizophrenia gender differences. Schizophr Bull. 1990;16:185–194. doi: 10.1093/schbul/16.2.185. [DOI] [PubMed] [Google Scholar]
- Serova LI, Maharjan S, Huang A, Sun D, Kaley G, Sabban EL. Response of tyrosine hydroxylase and GTP cyclohydrolase I gene expression to estrogen in brain catecholaminergic regions varies with mode of administration. Brain Res. 2004;1015:1–8. doi: 10.1016/j.brainres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Shih JC. Molecular basis of human MAO A and B. Neuropsychopharmacology. 1991;4:1–7. [PubMed] [Google Scholar]
- Shih JC, Chen K, Ridd MJ. Monoamine oxidase: from genes to behavior. Annu Rev Neurosci. 1999;22:197–217. doi: 10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shores MM, Moceri VM, Sloan KL, Matsumoto AM, Kivlahan DR. Low testosterone levels predict incident depressive illness in older men: effects of age and medical morbidity. J Clin Psychiatry. 2005;66:7–14. doi: 10.4088/jcp.v66n0102. [DOI] [PubMed] [Google Scholar]
- Shores MM, Sloan KL, Matsumoto AM, Moceri VM, Felker B, Kivlahan DR. Increased incidence of diagnosed depressive illness in hypogonadal older men. Arch Gen Psychiatry. 2004;61:162–167. doi: 10.1001/archpsyc.61.2.162. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Hormonal control of the development and regulation of tyrosine hydroxylase expression within a sexually dimorphic population of dopaminergic cells in the hypothalamus. Brain Res Mol Brain Res. 1989;6:297–310. doi: 10.1016/0169-328x(89)90075-2. [DOI] [PubMed] [Google Scholar]
- Sjoberg RL, Ducci F, Barr CS, Newman TK, Dell'osso L, Virkkunen M, Goldman D. A non-additive interaction of a functional MAO-A VNTR and testosterone predicts antisocial behavior. Neuropsychopharmacology. 2008;33:425–430. doi: 10.1038/sj.npp.1301417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoberg RL, Nilsson KW, Wargelius HL, Leppert J, Lindstrom L, Oreland L. Adolescent girls and criminal activity: role of MAOA-LPR genotype and psychosocial factors. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:159–164. doi: 10.1002/ajmg.b.30360. [DOI] [PubMed] [Google Scholar]
- Stein MB, Fallin MD, Schork NJ, Gelernter J. COMT polymorphisms and anxiety-related personality traits. Neuropsychopharmacology. 2005;30:2092–2102. doi: 10.1038/sj.npp.1300787. [DOI] [PubMed] [Google Scholar]
- Strous RD, Bark N, Woerner M, Lachman HM. Lack of association of a functional catechol-O-methyltransferase gene polymorphism in schizophrenia. Biol Psychiatry. 1997;41:493–495. doi: 10.1016/s0006-3223(96)00474-x. [DOI] [PubMed] [Google Scholar]
- Strous RD, Nolan KA, Lapidus R, Diaz L, Saito T, Lachman HM. Aggressive behavior in schizophrenia is associated with the low enzyme activity COMT polymorphism: a replication study. Am J Med Genet B Neuropsychiatr Genet. 2003;120B:29–34. doi: 10.1002/ajmg.b.20021. [DOI] [PubMed] [Google Scholar]
- Tassin JP, Stinus L, Simon H, Blanc G, Thierry AM, Le Moal M, Cardo B, Glowinski J. Relationship between the locomotor hyperactivity induced by A10 lesions and the destruction of the frontocortical dopaminergic innervation in the rat. Brain Res. 1978;141:267–281. doi: 10.1016/0006-8993(78)90197-x. [DOI] [PubMed] [Google Scholar]
- Tees RC, Midgley G, Nesbit JC. The effect of early visual experience on spatial maze learning in rats. Dev Psychobiol. 1981;14:425–438. doi: 10.1002/dev.420140505. [DOI] [PubMed] [Google Scholar]
- Tenhunen J, Salminen M, Jalanko A, Ukkonen S, Ulmanen I. Structure of the rat catechol-O-methyltransferase gene: separate promoters are used to produce mRNAs for soluble and membrane-bound forms of the enzyme. DNA Cell Biol. 1993;12:253–263. doi: 10.1089/dna.1993.12.253. [DOI] [PubMed] [Google Scholar]
- Thanky NR, Son JH, Herbison AE. Sex differences in the regulation of tyrosine hydroxylase gene transcription by estrogen in the locus coeruleus of TH9-LacZ transgenic mice. Brain Res Mol Brain Res. 2002;104:220–226. doi: 10.1016/s0169-328x(02)00383-2. [DOI] [PubMed] [Google Scholar]
- Turvin JC, Messer WS, Jr, Kritzer MF. On again, off again effects of gonadectomy on the acoustic startle reflex in adult male rats. Physiol Behav. 2007;90:473–482. doi: 10.1016/j.physbeh.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haaren F, van Hest A, Heinsbroek RP. Behavioral differences between male and female rats: effects of gonadal hormones on learning and memory. Neurosci Biobehav Rev. 1990;14:23–33. doi: 10.1016/s0149-7634(05)80157-5. [DOI] [PubMed] [Google Scholar]
- Verma A, Moghaddam B. NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alternation performance in rats: modulation by dopamine. J Neurosci. 1996;16:373–379. doi: 10.1523/JNEUROSCI.16-01-00373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volavka J, Bilder R, Nolan K. Catecholamines and aggression: the role of COMT and MAO polymorphisms. Ann NY Acad Sci. 2004;1036:393–398. doi: 10.1196/annals.1330.023. [DOI] [PubMed] [Google Scholar]
- Westerink BH, Spaan SJ. Simultaneous determination of the formation rate of dopamine and its metabolite 3,4-dihydroxyphenylacetic acid (DOPAC) in various rat brain areas. Brain Res. 1982;252:239–245. doi: 10.1016/0006-8993(82)90391-2. [DOI] [PubMed] [Google Scholar]
- Widom CS, Brzustowicz LM. MAOA and the “cycle of violence”: childhood abuse and neglect, MAOA genotype, and risk for violent and antisocial behavior. Biol Psychiatry. 2006;60:684–689. doi: 10.1016/j.biopsych.2006.03.039. [DOI] [PubMed] [Google Scholar]
- Woo JM, Yoon KS, Choi YH, Oh KS, Lee YS, Yu BH. The association between panic disorder and the L/L genotype of catechol-O-methyltransferase. J Psychiatr Res. 2004;38:365–370. doi: 10.1016/j.jpsychires.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Xie T, Ho SL, Ramsden D. Characterization and implications of estrogenic down-regulation of human catechol-O-methyltransferase gene transcription. Mol Pharmacol. 1999;56:31–38. doi: 10.1124/mol.56.1.31. [DOI] [PubMed] [Google Scholar]
- Yavich L, Forsberg MM, Karayiorgou M, Gogos JA, Mannisto PT. Site-specific role of catechol-O-methyltransferase in dopamine overflow within prefrontal cortex and dorsal striatum. J Neurosci. 2007;27:10196–10209. doi: 10.1523/JNEUROSCI.0665-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin PH, Lee HC, Chau GY, Liu TY, Liu HC, Lui WY, Chi CW. Polymorphisms of estrogen-metabolizing genes and risk of hepatocellular carcinoma in Taiwan females. Cancer Lett. 2004;212:195–201. doi: 10.1016/j.canlet.2004.04.032. [DOI] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurcher G, Da Prada M. Rapid and sensitive single-step radio-chemical assay for catechol-O-methyltransferase. J Neurochem. 1982;38:191–195. doi: 10.1111/j.1471-4159.1982.tb10871.x. [DOI] [PubMed] [Google Scholar]


