Abstract
OBJECTIVE
To determine if sorafenib is associated with an improved 4-month probability of progression-free survival using radiographic and clinical criteria alone, in patients with metastatic castration-resistant prostate cancer (CRPC). Secondary endpoints included pharmacokinetics, toxicity analysis and overall survival.
PATIENTS AND METHODS
This was an open-label, phase II, 2 stage design, focusing on the results from the second stage since criteria for progression were modified after completion of the first stage. Sorafenib was given daily at a dose of 400 mg orally twice daily in 28-day cycles. Clinical and laboratory assessments were done every 4 weeks, radiographic scans were obtained every 8 weeks.
RESULTS
Twenty-four patients were accrued in the second stage. Patient characteristics included a median (range) age of 66 (49 – 85), on-study PSA of 68.45 ng/mL (5.8 – 995), Gleason of 8 (6 – 9), and ECOG of 1 (n=17). 21/24 had prior chemotherapy with docetaxel. All patients had bony metastases, either alone (n=11) or with soft tissue disease (n=13). One patient had a partial response. Ten patients had stable disease (median duration: 18 weeks, range: 15 – 48 weeks). At a median potential follow-up of 27.2 months, the median progression-free survival was 3.7 months and the median overall survival was 18.0 months. For the whole trial of 46 patients, median survival was 18.3 months. Most frequent toxicities included hand-foot skin reaction (Grade 2 in 9 patients, Grade 3 in 3 patients), rash, LFT abnormalities, and fatigue.
CONCLUSIONS
Sorafenib has moderate activity as 2nd line treatment in metastatic CRPC.
Keywords: castration resistant prostate cancer, angiogenesis, Raf-kinase inhibitor, sorafenib
INTRODUCTION
Sorafenib is an oral multi-kinase inhibitor that targets the Ras/Raf kinase pathway, vascular endothelial growth factor (VEGF), and platelet-derived growth factor receptor (PDGF) [1]. It has gained Food and Drug Administration approval for renal cell and hepatocellular cancer [2] and has shown promising activity in a variety of other cancers [3, 4]. Angiogenesis has been shown to have a role in the progression of prostate cancer [5]. As such, a phase II study using sorafenib in patients with metastatic castration resistant prostate cancer (mCRPC) was designed to determine if sorafenib was associated with a potentially improved 4-month probability of progression-free survival as determined by clinical, radiographic and prostate-specific antigen (PSA) criteria. The first stage of this two-stage design study was recently reported [6]. Of the 22 patients accrued to the first stage, 13 progressed only by prostate specific antigen (PSA) criteria in the absence of clinical or radiologic progression. Furthermore, two patients had reduction of metastatic bone lesions in bone scintigraphy while meeting PSA progression criteria. We determined that PSA was not a good surrogate marker for sorafenib activity as evidenced by in vitro cumulative increase in PSA with increasing drug concentrations. The observed discordance between the PSA and radiographic response led to the amendment of the protocol to define progression based only on clinical or radiographic criteria alone. This report describes the final analysis of the second stage of this clinical trial (n=24), and reports the overall survival for the whole cohort (n=46).
PATIENTS AND METHODS
Patient selection
All patients had histologically confirmed prostate adenocarcinoma and had progressive mCRPC as evidenced by any expanding measurable lesion, appearance of a new lesion, and/or an increasing PSA concentration on successive measurements. Patients were allowed to have no more than one prior cytotoxic chemotherapeutic regimen; Eastern Cooperative Oncology Group status of 0 to 2; life expectancy of ≥ 12 weeks; adequate organ function; and castrate levels of testosterone achieved either by surgical orchiectomy or administration of a gonadotropin releasing hormone agonist. Other eligibility criteria included being off prior chemotherapy for 4 weeks, absence of brain metastasis, bleeding diathesis or uncontrolled illnesses; well-controlled hypertension, if present.
Study design
This was an open-label, single-center, phase II clinical trial using an optimal two-stage design [7] with the first stage as previously published [6] and the second stage being presently reported. All patients gave written informed consent in accordance with federal, state, and institutional guidelines and the study was approved by the National Cancer Institute (NCI) Institutional Review Board. Patients received 400 mg of sorafenib orally twice daily each day of a 28-day cycle. Patients were evaluated in the clinic every 4 weeks, and radiographic assessments using computed tomography (CT) and bone scintigraphy were obtained every 2 months. Blood tests including complete blood count, chemistry, and PSA were obtained at each monthly visit. Response and progression was evaluated using Response Evaluation Criteria in Solid Tumors (RECIST).[8] For bone scans, progression was defined as appearance of a new lesion, and improvement was defined as the complete resolution of at least one lesion. PSA response was recorded but not used as a criterion for progression.
The primary endpoint was disease progression defined as either the appearance of new lesions on bone scan or progression by RECIST criteria. Secondary endpoints included measurement of overall response, pharmacokinetics, toxicity analysis, and pharmacodynamics.
Statistical considerations
The Simon two-stage design [7] was used in order to rule out a 30% probability of 4 month progression free survival while targeting a 50% probability of patients having 4 month progression free survival. Conventional error probabilities of alpha=0.10 and beta=0.10 were employed. Based on this design, during the first stage, 22 patients were enrolled and progression at four months was evaluated. Per the protocol, if 7 or fewer patients were found to be progression free at the 4 month evaluation, then no further patients were to be enrolled. However, the PSA and radiographic discordance noted during the first stage of the study allowed for the accrual to the full 46 patients following a change of endpoint evaluation felt to be desirable in view of the initial findings. The Kaplan-Meier method was used to calculate the progression-free survival for the second stage of accrued patients, as well as the overall survival.
Pharmacokinetics – Sample collection and analysis
Sorafenib doses and pharmacokinetic sample collection points were similar to stage 1 of this trial. Sorafenib was administered orally at 400 mg twice daily dose. The blood samples were collected at baseline and at 0.25, 0.50, 1, 2, 4, 6, 8, 12, and 24 hrs after the ingestion of initial doses. Immediately after collection samples were processed, plasma was separated and stored at −80 ° C. A validated LC-MS/MS method was used for determination of sorafenib concentration in plasma samples [9].
The samples were prepared by protein precipitation using acetonitrile and radiolabeled sorafenib was used as internal standard. The workable concentration range was 5–2000 ng/mL with mean accuracy and imprecision ranging from 92.86–99.88% and 1.19–4.53%. Pharmacokinetic parameters area under the curve (AUC0–12), maximum plasma concentration (Cmax) and time to maximum plasma concentration (tmax) were calculated by non-compartmental analysis using WinNonlin professional v5.0 (Pharsight Corporation, Mountain View, CA, USA).
Toxicity analysis and dose modifications
Adverse events were recorded using the NCI Common Terminology Criteria (NCI CTC) version 3 and dose adjustments made as previously described.[6] Briefly, no dose interruptions were required for grade 1 or 2 toxicities unless they were deemed intolerable by the patient and treatment was discontinued if ≥ grade 3 or grade 4 toxicities occurred and did not resolve to grade ≤ 1 or baseline within 3 weeks. Dose reductions by 200 mg/d were made but any subsequent dose reductions beyond 75% was not allowed.
RESULTS
Patient characteristics
Twenty-four patients were enrolled into the second stage of the trial between January 2006 and September 2007. The baseline characteristics are presented in Table 1. Twenty-one of 24 (87.5%) patients in the second stage had received prior chemotherapy with docetaxel compared to only 55% (12 of 22 patients) in stage 1. Majority had ECOG status of 1 (n=17).
Table 1.
Patients Demographics and Characteristics
| Characteristics | |
|---|---|
| Total number of patients | 24 |
| Age, yrs | |
| Median | 66 |
| Range | 49 – 87 |
| Race | |
| Caucasian | 18 |
| African American | 5 |
| Hispanic | 1 |
| Gleason score | |
| Median | 8 |
| Range | 6 – 9 |
| ECOG performance status | |
| 0 | 7 |
| 1 | 17 |
| Median | 1 |
| PSA on-study ng/ml | |
| Median | 68.45 |
| Range | 5.8 – 995 |
| Hemoglobin g/dL | |
| Median | 12.35 |
| Range | 10.4 – 14.2 |
| Alkaline phosphatase | |
| Median | 83 |
| Range | 45 – 414 |
| Sites of metastasis | |
| Bone only | 11 |
| Bone and soft tissue | 13 |
| Prior chemotherapy | |
| Docetaxel, n (%) | 21 (87.5%) |
Pharmacokinetics
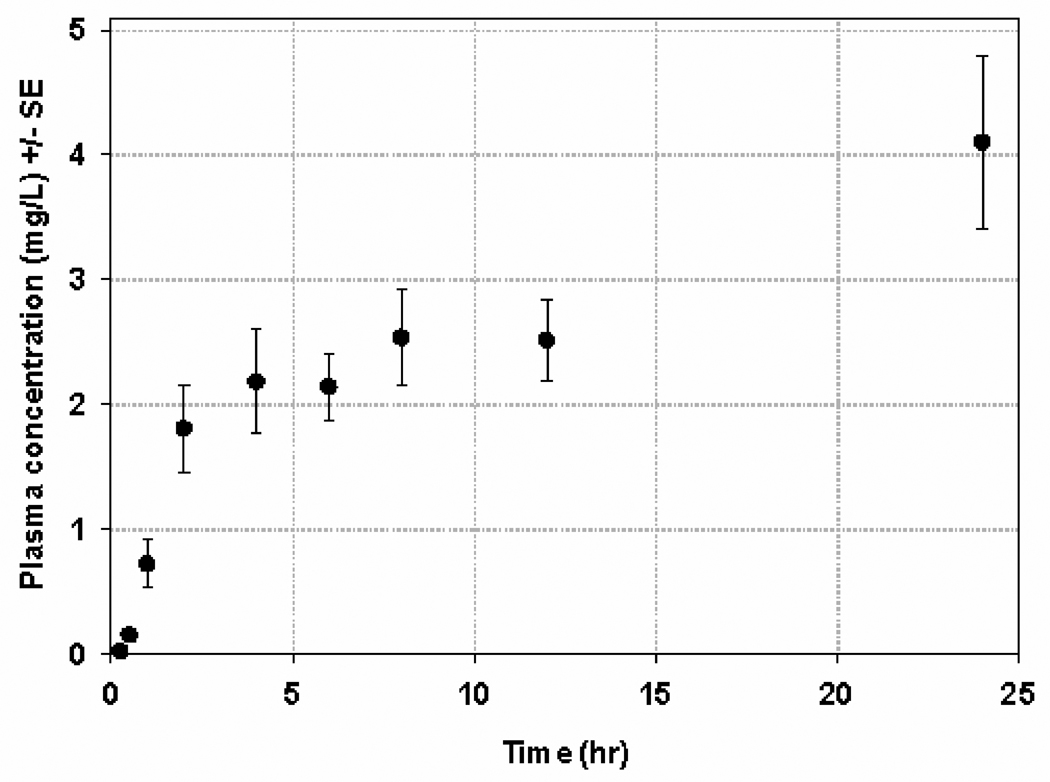
Plasma-concentration time profile for patients on the second stage of this trial is shown in Figure 1. Following administration of first dose, the geometric mean for exposure (AUC0–12) was 18.63 mg/L*hr (95% CI, 13.1–26.4; %CV, 69 %) and for Cmax was 2.57 mg/L (95% CI, 1.9–3.5; %CV, 71 %). The tmax ranged from 2–12.2 hr with a median value of 8 hr. The geometric mean AUC0–12 and Cmax for the second stage were found to be significantly higher than those reported for the first stage, perhaps due to three patients who had significantly higher AUCs (> 2 times mean AUC) than the rest of patients in stage 2. Of these 3 patients with the highest AUC, one patient had UGT1A9*3/*3 homozygous variant polymorphism, the second one was the oldest with the lowest body surface area, and the third one had high alkaline phosphatase levels. However, comparison between the two stages between patient demographics (weight, age, BSA), liver function markers (albumin, total protein, SGOT, SGPT) and serum creatinine were not found to be significantly different. The median accumulation (concentration at 24th hr/ concentration at 12th hr) after second dose was 1.46 and ranged from 0.54 – 4.41.
Figure 1.
Plasma concentration time profile for patients in stage 2
Toxicities
All patients who received treatment were analyzed for toxicity. Patients received a median of 2.5 cycles (range <1 – 12). However, of the 24 patients, 5 discontinued drug treatment prior to the radiographic evaluation at 8 weeks secondary to refusal (n=3), adverse event (n=1), and death (n=1). The patient who died on-study was an 85 year-old patient with pre-existing cerebrovascular accident within the past 5 years, and was on-study for only 20 days when he suffered from a recurrent hemorrhagic cerebrovascular accident. Of note, this patient was one of the three who had the highest sorafenib AUC. Table 2 lists the most common treatment-related adverse events occurring in > 10% of patients enrolled in the second stage and all grade 3 or 4 events. The incidence of hand-foot skin reaction (HFSR) was notably higher in patients in the second stage (3 patients with Grade 3 and 9 patients with Grade 2) as compared with the first stage where only one patient each had Grade 2 and 3 HFSR. The second stage patients also experienced a higher incidence of LFT abnormalities and more severe fatigue but less hypertension compared to those in the first stage. Dose reductions occurred in 54% (13 of 24) patients in the second stage.
Table 2.
Treatment-related adverse events (n = 24 patients)
| Adverse Events | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Blood/Bone Marrow | ||||
| Anemia | 2 | 1 | 1 | |
| Thrombocytopenia | 2 | 1 | ||
| Cardiovascular | ||||
| CNS cerebrovascular ischemia | 1 | |||
| Hypertension | 3 | |||
| Thrombosis/embolism (vascular access-related) | 1 | 1 | ||
| Constitutional symptoms | ||||
| Fatigue | 10 | 2 | 2 | |
| Weight loss | 3 | 2 | ||
| Dermatology/skin | ||||
| Hand-foot skin reaction | 1 | 9 | 3 | |
| Rash/desquamation | 11 | 3 | 1 | |
| Gastrointestinal | ||||
| Diarrhea | 6 | 2 | ||
| Nausea | 1 | 1 | 1 | |
| Infection | 1 | |||
| Metabolic/Laboratory | ||||
| ALT,SGPT | 6 | 2 | ||
| AST,SGOT | 8 | 1 | ||
| Alkaline phosphatase | 1 | 4 | 1 | |
| Hyperkalemia | 1 | |||
| Hyponatremia | 1 | 1 | ||
| Hypophosphatemia | 3 | 5 | ||
| Pain | ||||
| Musculoskeletal | 3 | 7 | ||
| Throat/larynx | 1 | 1 | ||
| Pulmonary/Upper respiratory | ||||
| Voice changes | 3 |
Response, progression free survival, and overall survival
Of the 13 patients enrolled in the second stage who had measurable disease, one patient had a partial response (PR) by RECIST criteria. Of the 24 patients in the second stage, 10 patients had stable disease. The median duration of stable disease is 18 weeks, currently with a range of 15 to 48 weeks, including two patients still stable at 35 and 37 weeks. No PSA responses were noted, the one patient who had PR on CT scan had a 48% reduction in PSA after 2 cycles of sorafenib. This patient received a total of 6 cycles before disease progression was noted. At a median potential follow-up of 27.2 months, the median progression-free survival (PFS) for patients in the second stage was 3.7 months (Figure 2A). The median overall survival (OS) for patients in the second stage was 18 months while the median OS for the whole cohort of 46 patients was 18.3 months (Figure 2B).
Figure 2.
Figure 2A. Kaplan-Meier curve for the progression-free survival of 24 patients enrolled in stage 2
Figure 2B. Kaplan-Meier curve for the Overall Survival of the whole cohort of 46 patients
DISCUSSION
We have previously reported the results of the first stage of this phase II trial of sorafenib [6] in metastatic castration resistant prostate cancer. Of the 22 patients in the first stage, 13 patients progressed by PSA alone and of all patients with bony lesions, only 4 had progressive disease. In vitro experiments showed that sorafenib treatment in LNCaP prostate cancer cell lines showed growth inhibition but increased cumulative PSA secretion over time. The observed discordance between the PSA increase and improvement in bone scans brought about a protocol amendment that resulted in further accrual of the trial to investigate the effect of targeting the Ras/Raf/Extracellular signal regulated kinase (ERK)/mitogen activated protein kinase (MAPK) signaling pathway and vascular endothelial growth factor (VEGF) in metastatic CRPC.
Indeed, the interpretation of post-therapy PSA changes as a measure of response in the era of targeted agents is of unclear clinical significance, especially since noncytotoxic agents may modulate PSA secretion independent of its activity on tumor suppression [10]. Two other clinical studies using sorafenib for prostate cancer has shown similar results with sorafenib exhibiting limited activity using PSA-defined criteria for progression [11, 12]. In the first study, the primary endpoint of progression-free survival of ≥ 12 weeks using sorafenib was achieved with 4 of 55 evaluable patients achieving SD by RECIST criteria, 2 patients with PSA response, and 11 patients with stable PSA [11], while a 3.8% PSA response was seen in the study by Chi et. al. [12], thereby not meeting the primary end point of > 20% possibility of a PSA response as defined by 50% decline in ≥ 4 weeks. The conclusion for both of these trials, including our previous published first stage, was that while sorafenib did exhibit some activity in prostate cancer, PSA was not a reliable marker for disease progression. However, no reliable surrogate marker has yet been established. Analysis of phospho-ERK levels did not show a correlative reduction in the obtained samples of patients treated with sorafenib [6]. Therefore, subsequent bone marrow biopsies were not performed. Of note, the two previous clinical studies using sorafenib enrolled patients who were chemotherapy-naïve [11, 12]. In comparison, the majority of patients in the second stage of this trial had prior docetaxel (21 of 24 patients) since the accrual began in January 2006, long after docetaxel and prednisone had become standard of care [13].
Second line treatment after docetaxel failure has been studied using several agents including mitoxantrone [14], ixabepilone [15], carboplatin [16], and satraplatin [17], with reported median overall survival using these agents in the range of 9.8 months to 17 months. The median overall survival for sorafenib is 18.3 months in this study, comparable to other 2nd line cytotoxic regimens. In addition, there was one PR and 10 patients with SD. The modest activity seen warrants further study of sorafenib, perhaps in the docetaxel-failure population.
Sorafenib is fairly well tolerated, although an increase in patients who had to discontinue treatment in the second stage compared to the first stage was noted. More patients experienced hand-foot skin reaction (HFSR) with Grade 3 toxicity occurring in 3 patients and Grade 2 toxicity in 9 patients in contrast to the first stage in which only one patient each developed Grade 2 and 3 HFSR. Further explorations of risk factors associated with the dermatologic toxicities are reported elsewhere (personal communication). Although high variability was observed in rate and extent of sorafenib absorption for the second stage of this trial, this was consistent with the first stage and other reported pharmacokinetics trials [18–20] where geometric mean on exposure and Cmax ranges from 9.76–71.7 hr*mg/L and 1.28–9.35 mg/L, respectively and the corresponding % CV ranges from 43–90% and 44–106%. The variability in exposure and Cmax does not account for the higher frequency of HFSR observed in the second stage. Exploration of covariate factors that might explain variability in individual response or toxicity to sorafenib is ongoing. One possible example of sources of variability is polymorphism in UGT1A9 enzyme which may influence the sorafenib blood levels by altering its elimination. In the current analysis, the patient with UGT1A9*3/*3 polymorphism (only 1) had significantly higher exposure and was the only patient who had grade 3 skin rash/desquamation toxicity.
CONCLUSIONS
While it is difficult to compare the two stages of this phase II trial since the PSA-defined progression endpoint was no longer considered in the second stage as a progression criterion, sorafenib in prostate cancer seems to benefit a select population of patients. Also, the assessment used in the second stage of this trial is in line with the evolving concept that in the absence of a clinically compelling indicator of progression, early changes in the PSA should not be heavily weighed upon in the decision to withhold or discontinue treatment [10]. However, ongoing challenges remain as we attempt to identify the appropriate early outcome measures that may be used in the assessment of response using these newly available targeted agents.
Acknowledgements
We would like to thank our research nurse Ms. Lea Latham, data manager Ms. Cynthia Graves, and Dr. Howard Parnes, in the conduct of this trial; Erin Gardner for assistance in PK analysis; Cancer Therapy and Evaluation Program (CTEP) for providing sorafenib; the NCI nurses and fellows in their care of our patients, and most of all, our patients who participated in this trial. This work was supported by the Intramural Research Program of the National Cancer Institute. This is a US Government work. There are no restrictions on its use. The views expressed within this paper do not necessarily reflect those of the US Government.
REFERENCES
- 1.Wilhelm S, Carter C, Lynch M, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5:835–844. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- 2.Pazdur R, Keegan P. [cited February 28, 2008];National Cancer Institute: FDA Approval for Sorafenib Tosylate, 11/19/07 update. Available from: http://www.cancer.gov/cancertopics/druginfo/fda-sorafenib-tosylate.
- 3.McDermott DF, Sosman JA, Gonzalez R, et al. Double-blind randomized phase II study of the combination of sorafenib and dacarbazine in patients with advanced melanoma: a report from the 11715 Study Group. J Clin Oncol. 2008;26:2178–2185. doi: 10.1200/JCO.2007.14.8288. [DOI] [PubMed] [Google Scholar]
- 4.Siu LL, Awada A, Takimoto CH, et al. Phase I trial of sorafenib and gemcitabine in advanced solid tumors with an expanded cohort in advanced pancreatic cancer. Clin Cancer Res. 2006;12:144–151. doi: 10.1158/1078-0432.CCR-05-1571. [DOI] [PubMed] [Google Scholar]
- 5.Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993;143:401–409. [PMC free article] [PubMed] [Google Scholar]
- 6.Dahut WL, Scripture C, Posadas E, et al. A phase II clinical trial of sorafenib in androgen-independent prostate cancer. Clin Cancer Res. 2008;14:209–214. doi: 10.1158/1078-0432.CCR-07-1355. [DOI] [PubMed] [Google Scholar]
- 7.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 8.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 9.Jain L, Gardner ER, Venitz J, Dahut W, Figg WD. Development of a rapid and sensitive LC-MS/MS assay for the determination of sorafenib in human plasma. J Pharm Biomed Anal. 2008;46:362–367. doi: 10.1016/j.jpba.2007.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinbild S, Mross K, Frost A, et al. A clinical phase II study with sorafenib in patients with progressive hormone-refractory prostate cancer: a study of the CESAR Central European Society for Anticancer Drug Research-EWIV. Br J Cancer. 2007;97:1480–1485. doi: 10.1038/sj.bjc.6604064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chi KN, Ellard SL, Hotte SJ, et al. A phase II study of sorafenib in patients with chemo-naive castration-resistant prostate cancer. Ann Oncol. 2008;19:746–751. doi: 10.1093/annonc/mdm554. [DOI] [PubMed] [Google Scholar]
- 13.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 14.Oh WK, Manola J, Babcic V, Harnam N, Kantoff PW. Response to second-line chemotherapy in patients with hormone refractory prostate cancer receiving two sequences of mitoxantrone and taxanes. Urology. 2006;67:1235–1240. doi: 10.1016/j.urology.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg JE, Weinberg VK, Kelly WK, et al. Activity of second-line chemotherapy in docetaxel-refractory hormone-refractory prostate cancer patients : randomized phase 2 study of ixabepilone or mitoxantrone and prednisone. Cancer. 2007;110:556–563. doi: 10.1002/cncr.22811. [DOI] [PubMed] [Google Scholar]
- 16.Ross RW, Beer TM, Jacobus S, et al. A phase 2 study of carboplatin plus docetaxel in men with metastatic hormone-refractory prostate cancer who are refractory to docetaxel. Cancer. 2008;112:521–526. doi: 10.1002/cncr.23195. [DOI] [PubMed] [Google Scholar]
- 17.Sternberg CN, Petrylak D, Witjes F, et al. Satraplatin (S) demonstrates significant clinical benefits for the treatment of patients with HRPC: Results of a randomized phase III trial. J Clin Oncol (Meeting Abstracts) 2007;25:5019. [Google Scholar]
- 18.Strumberg D, Clark JW, Awada A, et al. Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. Oncologist. 2007;12:426–437. doi: 10.1634/theoncologist.12-4-426. [DOI] [PubMed] [Google Scholar]
- 19.Clark JW, Eder JP, Ryan D, Lathia C, Lenz HJ. Safety and pharmacokinetics of the dual action Raf kinase and vascular endothelial growth factor receptor inhibitor, BAY 43-9006, in patients with advanced, refractory solid tumors. Clin Cancer Res. 2005;11:5472–5480. doi: 10.1158/1078-0432.CCR-04-2658. [DOI] [PubMed] [Google Scholar]
- 20.Moore M, Hirte HW, Siu L, et al. Phase I study to determine the safety and pharmacokinetics of the novel Raf kinase and VEGFR inhibitor BAY 43-9006, administered for 28 days on/7 days off in patients with advanced, refractory solid tumors. Ann Oncol. 2005;16:1688–1694. doi: 10.1093/annonc/mdi310. [DOI] [PubMed] [Google Scholar]