Abstract
Task evoked functional MRI (fMRI) has been used successfully in the study of brain function and clinically for pre-surgical localization of eloquent brain regions prior to the performance of brain surgery. This method requires patient cooperation and is not useful in patients with cognitive dysfunction or physical impairment.
An alternative method that can overcome some of these disadvantages measures the intrinsic function of the brain using resting state fMRI. This method does not require any task performance and measures the spontaneous low frequency (<0.1 Hz) fluctuations of the fMRI signal over time. Resting state fMRI data can provide valuable pre-surgical information in many patients who cannot benefit from traditional task based fMRI. Our objective in the current review is to provide preliminary information, including advantages and remaining challenges, on the clinical utility of this technique for improving pre-surgical planning and individualized patient centered care.
Keywords: Functional MRI, Brain Mapping, CNS Tumors, Neurosurgery, Neuroradiology
Introduction
Blood oxygen level dependent (BOLD) functional magnetic resonance imaging (fMRI) measures neuronal activity using the ratio of oxyhemoglobin to deoxyhemoglobin as a contrast mechanism. In a typical application the patient alternates between a passive resting state and performing a task, such as finger tapping, that activates a region of interest in the cerebral cortex. During these periods the BOLD signal is measured in the MR scanner and the two images are subtracted from each other to reveal areas of the brain that were activated during the prescribed task (1–3). (Figure 1A). This technique has been an invaluable research tool in the laboratory and has helped increase our understanding of normal and abnormal brain function. Clinical applications of fMRI have focused on localizing areas of critical function for presurgical planning (4). Accurate localization of eloquent cortex (e.g. somatomotor, language) in relation to the tumor mass can help optimize resection and minimize morbidity and mortality. Functional foci identified using fMRI have been shown to correlate well with foci identified using more invasive techniques such as intra-operative electrophysiology (5) and Wada testing (6, 7). Further, the distance from an fMRI-identified functional region to the surgical margin has been shown to correlate with loss-of-function post-operatively (8). Although there is much accumulated evidence for the benefit of fMRI use in presurgical planning, large scale studies to demonstrate improved patient outcomes have not been performed.
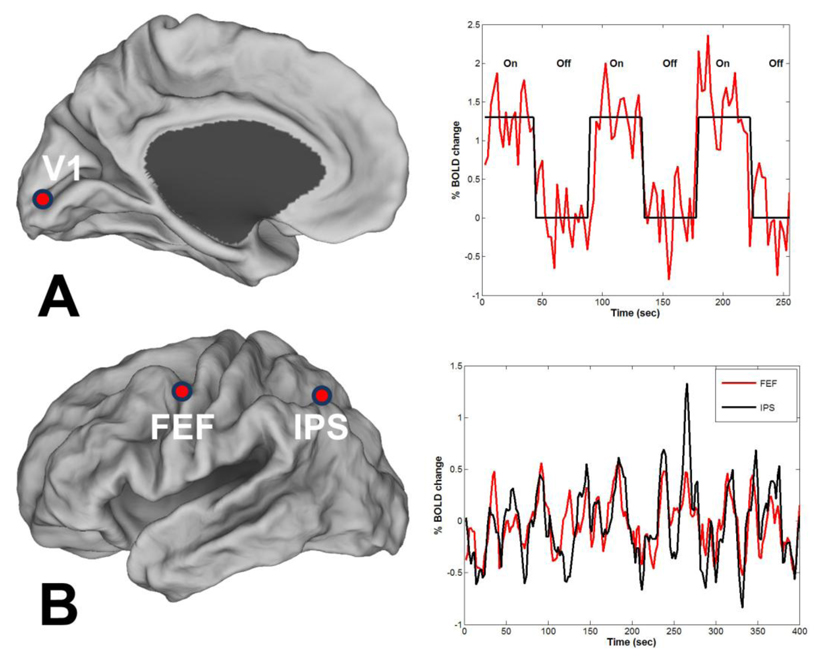
Figure 1.
The BOLD signal during task based fMRI (A) and during intrinsic resting state fMRI (B). In (A) the subject focused on a flickering checker board pattern that was periodically turned on and off. The measurement was made in the V1 region of the visual cortex (marked by red circle). Ongoing spontaneous fluctuations can be seen in this task paradigm. In (B) the spontaneous fluctuations from two regions of the dorsal attention system are presented. The regions are the intra- parietal sulcus (IPS) and the frontal eye fields (FEF) (marked by red circles). The two time curves demonstrate a high degree of correlation (r = 0.6).
Task based fMRI has several disadvantages that limit its application. The results are dependent on how well the patient can perform the prescribed task. Patients with brain tumors may be impaired and unable to cooperate (9). The patient must be awake during the procedure, so sedation cannot be used. Finally the task effects are small which may require long acquisition times to improve the signal to noise ratio of the measurement. We propose the method of localization of functional regions with spontaneous BOLD fluctuations as being able to overcome some of these problems (10).
Spontaneous BOLD fluctuation fMRI
Spontaneous BOLD fluctuations are fluctuations in neuronal activity that are correlated within distinct functional networks (10). This effect was first identified in a seminal paper by Biswal et al. (11). For example, strong coherence is reproducibly present between the left and right somatomotor cortices (11–17), between language areas (12, 18), and between numerous other functional regions (Figure 1B) even in the absence of overt task performance. Using spontaneous activity, one can generate resting state correlation maps that replicate the functional maps obtained from task activations.
The use of resting state fMRI for the evaluation of spontaneous fluctuations in the BOLD signal has several benefits over traditional task based fMRI. One important advantage of this method is that it can be performed even when the patient is unable to cooperate with the functional task. This will enable us to perform fMRI mapping on many populations previously excluded from traditional task based techniques such as young children, patients with cognitive impairments, and patients that are paralyzed, aphasic, or hard of hearing. Spontaneous fluctuations have been shown to persist under conditions of sleep (19, 20) and different levels of anesthesia (21–23), thus a second advantage of this technique is that is can be performed in agitated patients and in young children under sedation. A third advantage of this method is that one data acquisition can be used to study many different brain networks, thus possibly reducing the acquisition time when many systems are evaluated. This is in contrast to task activations which require dedicated data acquisitions for each function one is attempting to localize. Preliminary data (24) indicates that this technique compares favorably to task-based fMRI as illustrated in Fig. 2.
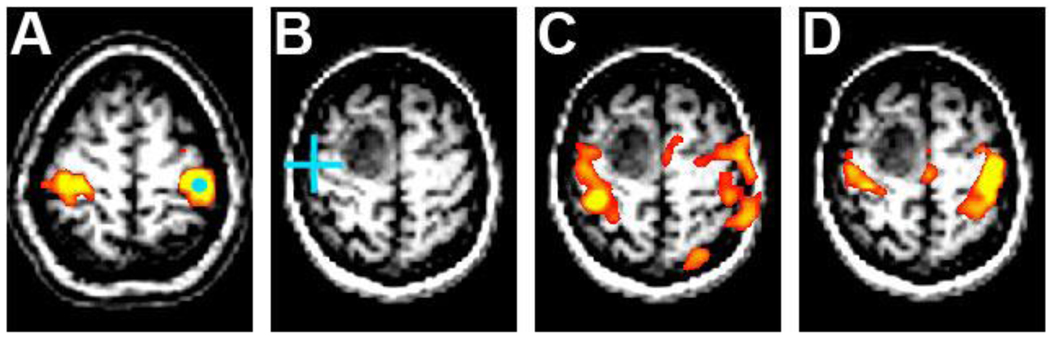
Figure 2.
Resting state correlation maps showing the distribution of the somatomotor network in a normal subject (A) after the selection of a seed region in the left somatomotor cortex (blue circle). The correlation with the right somatomotor cortex is evident. Images (B,C, and D) from a 58-year-old women with a diagnosis of glioblastoma multiforme (GBM). Intraoperative cortical mapping (B) shows evoked hand responses with stimulation posterolateral to the tumor (blue cross). Task-evoked activity with finger tapping in the tumor-affected motor cortex (C) showed a spatial distribution that approximates the resting state map, although several other foci of activity are seen that encompass part of motor cortex as well as extending posteriorly to parts of the parietal cortex. Resting state correlation map (D) after placement of a seed in the left somatomotor cortex (same coordinates as in (A)). The posteriorly displaced somatomotor cortex is seen, consistent with the cortical stimulation mapping and the task fMRI activity.
In addition to the somatomotor and language networks previously discussed, additional networks that have been characterized using resting state correlations from other seed regions include the visual system (12, 16), auditory (12), episodic memory (25, 26), default mode (27–30), and dorsal and ventral attention (30, 31). Additionally, abnormalities of these networks could potentially be defined. Most relevant from a surgical standpoint are aberrant connections created by epilepsy (32). Networks associated with a seizure focus may aid in guiding surgical resection and improving seizure free outcomes. When further automated, this method could potentially provide a comprehensive set of customized network localizations for each patient, thus substantially increasing the information available to the neurosurgeon and potentially improving patient centered care.
For the subject presented in Fig. 2, seed-based correlation maps were generated by extracting the BOLD fMRI time course from a spherical seed region of interest (6mm radius) and computing the Pearson correlation coefficient between this reference time course and the time course of every voxel in the brain. Resting state maps in tumor patients were obtained by placing seeds in morphologically normal cortical tissue when possible. For example when evaluating the somatomotor system the seed was placed in the hand motor area on the tumor-unaffected hemisphere based on known coordinates established by prior fMRI activation studies or on an anatomical basis as determined by structural MRI. In one case out of four tumor patients, the coordinate of the seed in right sensorimotor cortex was determined empirically by shifting placement of the seed from the standardized coordinate until the normal spatial pattern of the sensorimotor network was seen.
In a separate study (24) focusing on the somatomotor system we report on our initial experience with this technique. The method compared well with intra-operative cortical stimulation mapping in a small number of subjects. Additionally, resting state fMRI gave consistent results within individuals scanned multiple times. In comparison to task based fMRI, resting state analysis was more specific to the somatomotor cortex and showed less variability. These results are preliminary, based on a small number of patients and focused on only the somatomotor system. Future studies are required to confirm these findings in a larger cohort and in other functional networks.
Resting state fMRI has demonstrated great promise in this initial study. However, several technical challenges need to be addressed in the post processing phase of analysis. We rely on atlas coordinates for placement of our seed regions. Several networks, such as the language system are known to be more variable across subjects as compared to the somatomotor system (33). In addition, there are distortions in the brain configuration of patients with tumors that make the realignment task more difficult and hard to automate. We have partially solved these problems by masking out the tumor during this phase of the processing, but this was done by hand in the current study and automated algorithms for this masking are needed. We are testing other methods to improve the realignment, such as non-linear registration. Even with perfect alignment, a tumor may destroy functional tissue and lead to functional reorganization that makes seed based characterization of resting state networks difficult. This is less of a problem in bilateral networks, such as the somatomotor system, where we can use the healthy undistorted side for localization, but remains a challenge in unilateral systems, such as in the language and dorsal attention system. Independent component analysis, which does not require the placement of a priori seed regions stands out as a possible solution to this problem (21, 24, 34, 35).
Other challenges relate to the routine use of this method in the operating room. Of central concern will be the speed and efficiency of implementation. Whether it be in the extra-operative or in the intra-operative setting (i.e. intra-operative MRI) this method of signal acquisition and analysis will need to occur in a time frame that makes its use feasible. Currently, the time of acquisition is reasonable, but analysis for creation of functional images requires a dedicated researcher to perform the requisite processing to create useful image sets. This could not be practically done on a large scale. Further work will require a more automated version of analyses and image creation. This would allow for utilization to occur on a broader clinical scale (non-tertiary medical centers) or in real time as neurosurgeons begin to incorporate intraoperative MRIs more frequently in their surgical management.
Assesing a new fMRI Paradigm
When performing a technology assessment (36, 37) of presurgical planning with spontaneous BOLD fluctuations it is clear that it meets the criteria for technical performance, and preliminary data (24) indicate that it will at a minimum equal the diagnostic performance of current task based fMRI methods. Only a small number of reports address the issue of diagnostic and therapeutic impact of presurgical planning with fMRI. In one report (8), presurgical functional information from fMRI is mentioned in approximately 75% of patient’s neurosurgery notes. It is not clear if fMRI provides a benefit beyond that obtainable from anatomy or intra-operative functional localization, and there is no evidence that its use results in better patient outcomes (survival or morbidity), or in an impact on patient health.
To date, functional imaging has played a valuable but largely ancillary role in the localization of eloquent cortex for neurosurgical procedures. It has been additive to the current gold standard techniques, such as cortical stimulation and somatosensory evoked potentials. From a neurosurgical perspective, identification of zones essential for the implementation of a critical cognitive function such as speech and motor movements are what are sought with current electrophysiologic methods. The surgeon wishes to fundamentally know “if this region is resected, can the patient still perform the given task.” Task based functional imaging to date has largely identified cortical regions that are associated with a given cognitive function. Thus, activated sites may not only be those essential to the commission of the task, but also those that are secondarily associated with its performance (i.e. attention, memory, etc). These additional areas are essentially “localization noise” for finding cortex that is critical to preserve. Though it gives the surgeon improved awareness of where the critical zones are located, it does not provide more precise information on where the essential sites are. Beyond the current lack of precision, current imaging requires patient participation that further limits the number of patients that can benefit from these modalities due to a number of different clinical factors ranging from age to functional impairment. These same factors also adversely restrict a surgeon’s ability to perform classic neurophysiologic methods in the operating room. It is in this context of imaging imprecision and participation limitations that we have sought MRI methods that can enhance essential site localization and expand to populations not currently amenable to standard techniques.
Potential future developments of this technique could provide valuable benefits, but will require additional investigation and software development. In some complicated functional networks, such as the language network, intraoperative cortical stimulation is confined to the gyri. Resting state correlation mapping offers information throughout the gray matter and may provide information on previously inaccessible areas such as the intrasulcal language areas. Frameless stereotactic navigation is widely used by neurosurgeons to define brain anatomy in real time. An integration of this technique with our fMRI paradigm could allow the surgeon to navigate the anatomy simultaneously with several functional networks of their choosing. This would provide significant flexibility in intraoperative decision making. Combined anatomical and functional information could also be obtained from intraoperative MRI, providing a comprehensive picture of the shifts that occur in the brain during tumor resection surgery. Substantial progress will be needed in both computing hardware and software to realize this in real time for intraoperative evaluation. Finally, resting state fMRI could also be used to monitor post-operative patient progress, such as evaluating the reconstitution or lack thereof of functional networks that were disrupted during surgery (38).
Conclusions
In this report we present a new paradigm for the use of resting state fMRI data in presurgical planning. This technique analyzes spontaneous fluctuations in the BOLD signal across the brain and is substantially different from routine task based fMRI. BOLD fluctuations are correlated within neuroanatomically and functionally related areas of the brain and can help identify the location of critical functional networks, such as the somatomoter and language system. This information can help guide the surgical approach. This method has substantial advantages over the traditional task-based techniques, but also provides substantial challenges in post processing methods. One major advantage of the resting state method is that it does not require cooperation from the patient and can be conducted during sleep or under sedation, thus expanding the ability to obtain fMRI data to patients of all ages and all medical conditions. A second important advantage is that one data acquisition of clinically acceptable duration provides information on numerous different functional networks, thus saving time and increasing signal to noise ratio. Remaining post-processing challenges include automating the atlas registration and localization of functionally reorganized systems. Ultimately, the utility of preoperative functional mapping to neurosurgical planning will need to be evaluated in terms of benefit for post-operative functional outcome and survival.
Acknowledgements
This work was supported by Mallinkrodt Institue of Radiology Start up funds, by NIH K23 HD053212 (JSS), and by NIMH F30MH083483 (DZ). This publication was made possible by Grant Number UL1 RR024992 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. We wish to thank Dr. Raichle and Dr. Snyder for helpful comments and editing.
References
- 1.Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS. Time course EPI of human brain function during task activation. Magn Reson Med. 1992;25(2):390–397. doi: 10.1002/mrm.1910250220. [DOI] [PubMed] [Google Scholar]
- 2.Kwong KK, Belliveau JW, Chesler DA, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992;89(12):5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogawa S, Tank DW, Menon R, et al. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992;89(13):5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matthews PM, Honey GD, Bullmore ET. Applications of fMRI in translational medicine and clinical practice. Nat Rev Neurosci. 2006;7(9):732–744. doi: 10.1038/nrn1929. [DOI] [PubMed] [Google Scholar]
- 5.Vlieger EJ, Majoie CB, Leenstra S, Den Heeten GJ. Functional magnetic resonance imaging for neurosurgical planning in neurooncology. Eur Radiol. 2004;14(7):1143–1153. doi: 10.1007/s00330-004-2328-y. [DOI] [PubMed] [Google Scholar]
- 6.Adcock JE, Wise RG, Oxbury JM, Oxbury SM, Matthews PM. Quantitative fMRI assessment of the differences in lateralization of language-related brain activation in patients with temporal lobe epilepsy. Neuroimage. 2003;18(2):423–438. doi: 10.1016/s1053-8119(02)00013-7. [DOI] [PubMed] [Google Scholar]
- 7.Binder JR, Swanson SJ, Hammeke TA, et al. Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology. 1996;46(4):978–984. doi: 10.1212/wnl.46.4.978. [DOI] [PubMed] [Google Scholar]
- 8.Haberg A, Kvistad KA, Unsgard G, Haraldseth O. Preoperative blood oxygen level-dependent functional magnetic resonance imaging in patients with primary brain tumors: clinical application and outcome. Neurosurgery. 2004;54(4):902–914. doi: 10.1227/01.neu.0000114510.05922.f8. discussion 914-5. [DOI] [PubMed] [Google Scholar]
- 9.Pujol J, Conesa G, Deus J, Lopez-Obarrio L, Isamat F, Capdevila A. Clinical application of functional magnetic resonance imaging in presurgical identification of the central sulcus. J Neurosurg. 1998;88(5):863–869. doi: 10.3171/jns.1998.88.5.0863. [DOI] [PubMed] [Google Scholar]
- 10.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 11.Biswal B, Yetkin F, Haughton V, Hyde J. Functional connectivity in themotor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 12.Cordes D, Haughton VM, Arfanakis K, et al. Mapping functionally related regions of brain with functional connectivity MR imaging. American Journal of Neuroradiology. 2000;21:1636–1644. [PMC free article] [PubMed] [Google Scholar]
- 13.Cordes D, Haughton VM, Arfanakis K, et al. Frequencies contributing to functional connectivity in the cerebral cortex in 'resting-state' data. American Journal of Neuroradiology. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- 14.De Luca M, Smith SM, De Stefano N, Federico A, Matthews PM. Blood oxygenation level dependent contrast resting state networks are relevant to functional activity in the neocortical sensorimotor system. Experimental Brain Research. 2005;167:587–594. doi: 10.1007/s00221-005-0059-1. [DOI] [PubMed] [Google Scholar]
- 15.Fox MD, Snyder AZ, Zacks JM, Raichle ME. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat Neurosci. 2006;9(1):23–25. doi: 10.1038/nn1616. [DOI] [PubMed] [Google Scholar]
- 16.Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- 17.Xiong J, Parsons LM, Gao JH, Fox PT. Interregional connectivity to primary motor cortex revealed using MRI resting state images. Human Brain Mapping. 1999;8:151–156. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<151::AID-HBM13>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC. Detection of functional connectivity using temporal correlations in MR images. Human Brain Mapping. 2002;15:247–262. doi: 10.1002/hbm.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukunaga M, Horovitz SG, Van Gelderen P, et al. Large-amplitude, spatially correlated fluctuations in BOLD fMRI signals during extended rest and light sleep. Magnetic Resonance Imaging. 2006;24:979–992. doi: 10.1016/j.mri.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 20.Horovitz SG, Fukunaga M, de Zwart JA, et al. The default-mode network connectivity during awake and early sleep,: A simultaneous EEG-BOLD-fMRI study. Organization for Human Brain Mapping Annual Meeting, Florence, Italy. 2006:686. M-PM. [Google Scholar]
- 21.Kiviniemi V, Kantola JH, Jauhiainen J, Hyvarinen A, Tervonen O. Independent component analysis of nondeterministic fMRI signal sources. Neuroimage. 2003;19:253–260. doi: 10.1016/s1053-8119(03)00097-1. [DOI] [PubMed] [Google Scholar]
- 22.Peltier SJ, Kerssens C, Hamann SB, Sebel PS, Byas-Smith M, Hu X. Functional connectivity changes with concentration of sevoflurane anaesthesia. Neuroreport. 2005;16(3):285–288. doi: 10.1097/00001756-200502280-00017. [DOI] [PubMed] [Google Scholar]
- 23.Vincent JL, Patel GH, Fox MD, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447(7140):83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 24.Zhang D, Johnston JM, Fox MD, et al. Preoperative Sensorimotor Mapping in Brain Tumor Patients using Spontaneous Fluctuations in Neuronal Activity Imaged with fMRI: Initial Experience. Neurosurgery. 2008 doi: 10.1227/01.NEU.0000350868.95634.CA. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rombouts SA, Stam CJ, Kuijer JP, Scheltens P, Barkhof F. Identifying confounds to increase specificity during a "no task condition". Evidence for hippocampal connectivity using fMRI. Neuroimage. 2003;20(2):1236–1245. doi: 10.1016/S1053-8119(03)00386-0. [DOI] [PubMed] [Google Scholar]
- 26.Vincent JL, Snyder AZ, Fox MD, et al. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96(6):3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- 27.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26(1):15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laufs H, Krakow K, Sterzer P, et al. Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proc Natl Acad Sci U S A. 2003;100(19):11053–11058. doi: 10.1073/pnas.1831638100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103(26):10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bettus G, Guedj E, Joyeux F, et al. Decreased basal fMRI functional connectivity in epileptogenic networks and contralateral compensatory mechanisms. Hum Brain Mapp. 2008 doi: 10.1002/hbm.20625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanai N, Mirzadeh Z, Berger MS. Functional Outcome after Language Mapping for Glioma Resection. N Engl J Med. 2008;358:18–27. doi: 10.1056/NEJMoa067819. [DOI] [PubMed] [Google Scholar]
- 34.De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage. 2006;29(4):1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 35.van de Ven VG, Formisano E, Prvulovic D, Roeder CH, Linden DE. Functional connectivity as revealed by spatial independent component analysis of fMRI measurements during rest. Hum Brain Mapp. 2004;22(3):165–178. doi: 10.1002/hbm.20022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hollingworth W, Jarvik JG. Technology assessment in radiology: putting the evidence in evidence-based radiology. Radiology. 2007;244(1):31–38. doi: 10.1148/radiol.2441051790. [DOI] [PubMed] [Google Scholar]
- 37.Mackenzie R, Dixon AK. Measuring the effects of imaging: an evaluative framework. Clin Radiol. 1995;50(8):513–518. doi: 10.1016/s0009-9260(05)83184-8. [DOI] [PubMed] [Google Scholar]
- 38.Johnston JM, Vaishnavi SN, Smyth MD, et al. Loss of resting interhemispheric functional connectivity after complete section of the corpus callosum. J Neurosci. 2008;28(25):6453–6458. doi: 10.1523/JNEUROSCI.0573-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]