Abstract
The reactions of nitrite with deoxygenated human erythrocytes were examined using membrane inlet mass spectrometry to detect the accumulation of NO in extracellular solution. In this method an inlet utilizing a silicon rubber membrane is submerged in cell suspensions and allows NO to pass from extracellular solution into the mass spectrometer. This provides a direct, continuous, and quantitative determination of nitric oxide concentrations over long periods without the necessity of purging the suspension with inert gas. We have not observed accumulation of NO when compared with controls on a physiologically relevant time scale and conclude that, within the limitations of the mass spectrometric method and our experimental conditions, erythrocytes do not generate a net efflux of NO following the addition of millimolar concentrations of nitrite. Moreover, there was no evidence at the mass spectrometer of the accumulation of a peak at mass 76 that would indicate N2O3, an intermediate that decays into NO and NO2. Inhibition of red cell membrane anion exchangers and aquaporins did not affect these processes.
Keywords: nitric oxide, nitrite, mass spectrometry, membrane inlet, erythrocytes, hemoglobin
Introduction
There has been great interest in the reactions of nitrite with erythrocytes following the initial reports of the reactions of nitrite with cell-free deoxyHb(FeII) [1]. These reactions are based on the well known one-electron reduction of nitrite by deoxyHb(FeII) resulting in the formation of metHb(FeIII). The fate of the resulting NO has been heavily studied, but it is certain that most of it binds to deoxyHb(FeII).
| (1) |
| (2) |
The reduction of nitrite by deoxyHb(FeII) is a complex reaction the properties of which depend on the ratio of nitrite to hemoglobin, showing autocatalytic behavior and ratios of NOHb(FeII) to metHb(FeIII) that deviate from unity under specific and well described conditions [2–4]. The ratio of products, NOHbFe(II) and metHbFe(III), depends on the concentration of nitrite. Doyle et al. [1] used excess nitrite and reported close to one equivalent each of NOHbFe(II) and metHbFe(III), confirmed in subsequent studies under specific conditions described therein [5, 6]. However, as concentrations of nitrite decreases below about 400 µM there is an excess of met-Fe(III) over NO-Fe(II), with the ratio Fe(III)/NO-Fe(II) as great as 1.8, which then approaches unity again at nitrite concentrations very low compared with heme [2]. We emphasize that the reactions of nitrite with hemoglobin in this report are determined at high nitrite concentrations, much higher than under physiological conditions. To further emphasize this, it was reported earlier that infusion of low concentrations of nitrite into the forearm caused increased blood flow [7]. Subsequent studies, however, show that the extent and mechanism of nitrite bioactivity is controversial [8–10].
Based on studies showing vasoactivity of nitrite under hypoxic conditions, increased consumption of nitrite during normoxic exercise, and positive arterial-to-venous gradients of nitrite, it has been suggested that the interactions of nitrite with erythrocytes could be a source of vasodilatory NO [4, 7, 11, 12]. Prominent among explanations of the vasodilatory effect of nitrite is the hypothesis that deoxyHb(FeII) in erythrocytes produces vasodilation through mechanisms related to the reactions of nitrite with deoxyHb(FeII) [6, 11, 13]. Furthermore, it has been proposed that the red cell membrane anion exchanger (AE-1) may help to mobilize extracellular nitrite for intracellular utlilization [3] and that aquaporin-1 may act as a membrane gas channel for NO permeation [14].
A significant problem with this scenario is that deoxyHb(FeII) avidly binds NO, and this autocapture is expected to prevent appreciable NO from exiting the erythrocyte. This effect may be mitigated by generation of an intermediate such as N2O3 which does not bind tightly to deoxyHb(FeII) and could pass across the red cell membrane [13]. However, mass spectrometric measurements showed no accumulation of N2O3 upon addition of nitrite to solutions of cell-free deoxyHb(FeII), and no NO above that of the uncatalyzed dismutation [15]. Additional data are accumulating that the vasodilatory effect of nitrite in vivo arises from nitrite reduction by the vascular endothelium and extravascular tissues. For example, studies detecting NO by EPR and chemiluminescence showed that nitrite caused appreciable increases in NO production in tissues such as liver and heart but only trace amounts in blood [16]. In addition, reactions of nitrite to generate NO under anerobic conditions (in vitro) have been demonstrated for myoglobin, xanthine oxidase, the bc1 complex of the mitochondrial electron transport chain, cytochrome P450, and endothelial NO synthase enzyme [17]. Other studies have suggested a role of S-nitrosothiols as a source of vasodilation, since potent S-nitrosating agents including N2O3 may be formed from nitrite [11]. Physiological concentrations of nitrite in humans are in a range up to 1 µM. The source of nitrite includes foods, the reactions of nitrate in bacteria in the oral cavity and GI tract, and eNOS-mediated NO production with subsequent oxidation which accounts for up to 70% of plasma nitrite [18].
In this report, we examine the reactions of nitrite with deoxygenated human erythrocytes using membrane inlet mass spectrometry (MIMS) to detect the accumulation of NO in extracellular solution [19]. This follows a study using the same methods applied to cell-free deoxyHb(FeII) [15]. In this method an inlet utilizing a silicon rubber membrane is submerged in cell suspensions and allows NO to pass from extracellular solution into the mass spectrometer. This provides a direct, continuous, and quantitative determination of nitric oxide concentrations over long periods without the necessity of purging the suspension with inert gas. We have not observed accumulation of NO when compared with controls on a physiologically relevant time scale and conclude that, within the limitations of the mass spectrometric method and our experimental conditions, erythrocytes do not generate a net efflux of NO following the addition of millimolar concentrations of nitrite. Moreover, inhibition of red cell membrane anion exchangers and aquaporins does not affect these processes.
Experimental Methods
Materials
Human blood was freshly collected and then diluted in isotonic buffer consisting of 50 mM sodium phosphate, 78 mM sodium chloride, 2.7 mM potassium chloride at pH 7.4. Cells were washed by repeated cycles of centrifugation and dilution in isotonic buffer prior to use. The washed packed red cells were then diluted into the same isotonic buffer described above at pH 6.7 supplemented with 2 mM of the chelator DTPA (diethylenetriamine-pentaacetic acid) within the reaction vessel. Experiments used 2.5 ml of red cells suspensions to which was added 0.5 µL of Antifoam A (Sigma). Degassed red cells were prepared from these samples by bubbling of helium for 5 minutes prior to the analysis.
The anion exchange inhibitors 4-acetamido-4’-isothiocyanstilbene-2,2’-disulfonic acid disodium salt (SITS), and 4,4'-diisothiocyano-stilbene-2,2'-disulfonic acid (DIDS) were purchased from AnaSpec (San Jose, CA). The inhibitor 4,4'-dinitrostilbene-2,2'-disulfonic acid, disodium salt (DNDS) was purchased from Invitrogen Corporation (Carlsbad, CA). The aquaporin channel blocker para-chloromercuribenzene sulfonic acid, sodium salt (PCMBS) was purchased from Toronto Research Chemicals, Inc. DIDS, SITS, and DNDS were incubated in red cell suspensions up to 30 minutes before experiments. Red cells were incubated with PCMBS for 10 minutes prior to experiments.
Inlet probe and kinetic measurements
The membrane inlet consisted of a length of silicon rubber (Silastic) tubing attached to a piece of glass tubing leading into a dry-ice acetone water trap and then into an Extrel EXM-200 mass spectrometer, as described elsewhere [19]. This membrane inlet was immersed in solution contained in a 3 ml glass reaction vessel modified for introduction of samples and inert gas, and sealable by injection septa and Teflon screw plugs [19]. When immersed in a red cell suspension, this inlet detects free, unbound, extracellular NO based on the magnitude of the m/z 30 peak (or N2O3 based on the m/z 76 peak if this species is present). The reaction vessel contained a small magnetic spinner to continuously mix the reactions. Mass spectra were obtained using electron impact ionization (70 eV) at an emission current of 1 mA. Source pressures were approximately 1 × 10−6 torr. The resulting mass scans were well resolved with a return of ion current (detector response) to the baseline separating each mass unit. Data collected using the membrane inlet were calibrated by injecting solutions containing known concentrations of NO into buffered solutions in the reaction vessel [19]. The sample preparation involved deoxygenating the red cell suspension with helium, as described above, followed by addition of nitrite through an injection septum.
Results
We have used membrane inlet mass spectrometry to observe the accumulation of free, unbound, extracellular NO caused by the addition of nitrite to deoxygenated suspensions of human red blood cells. In this method, the membrane inlet is submerged in the suspension and uncharged molecules of low molecular weight pass across a permeable membrane into the mass spectrometer. Inhibitors to block both anion exchange proteins and aquaporin were used to investigate mechanisms.
The addition of nitrite to a suspension of red cells
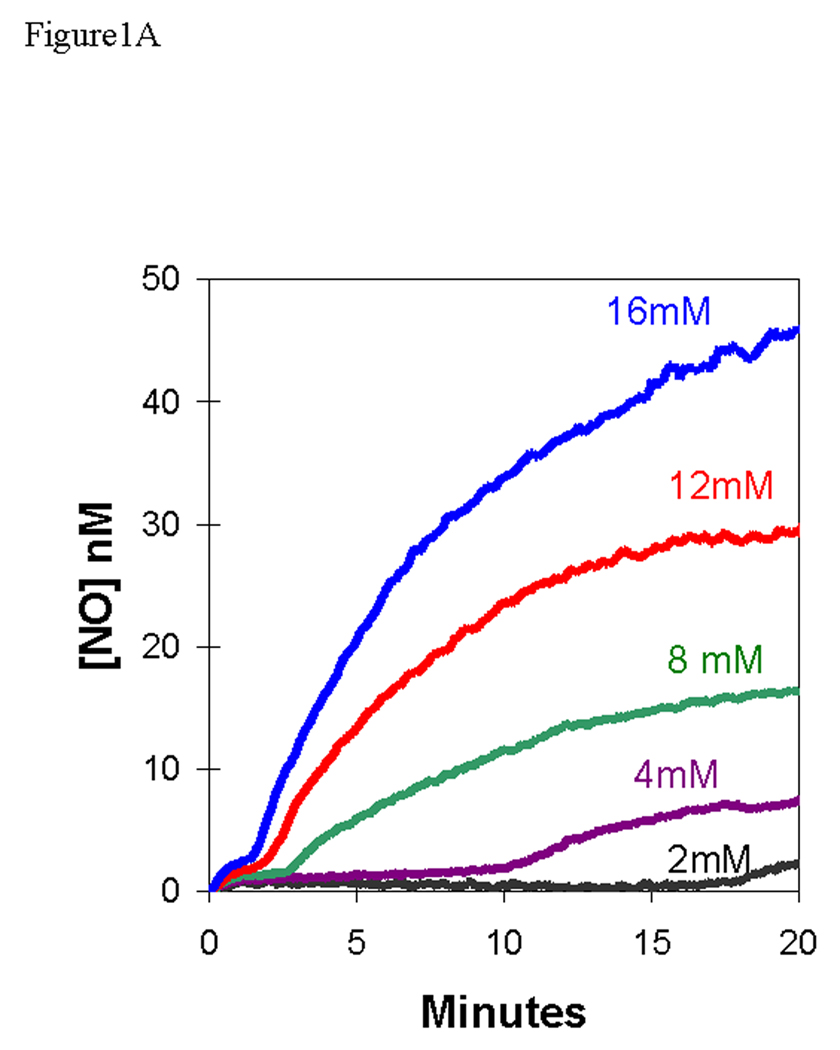
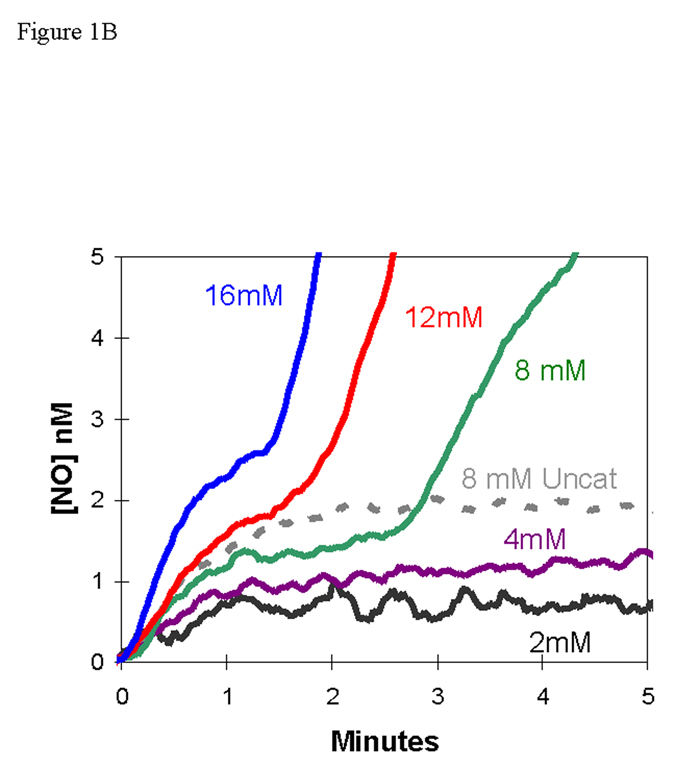
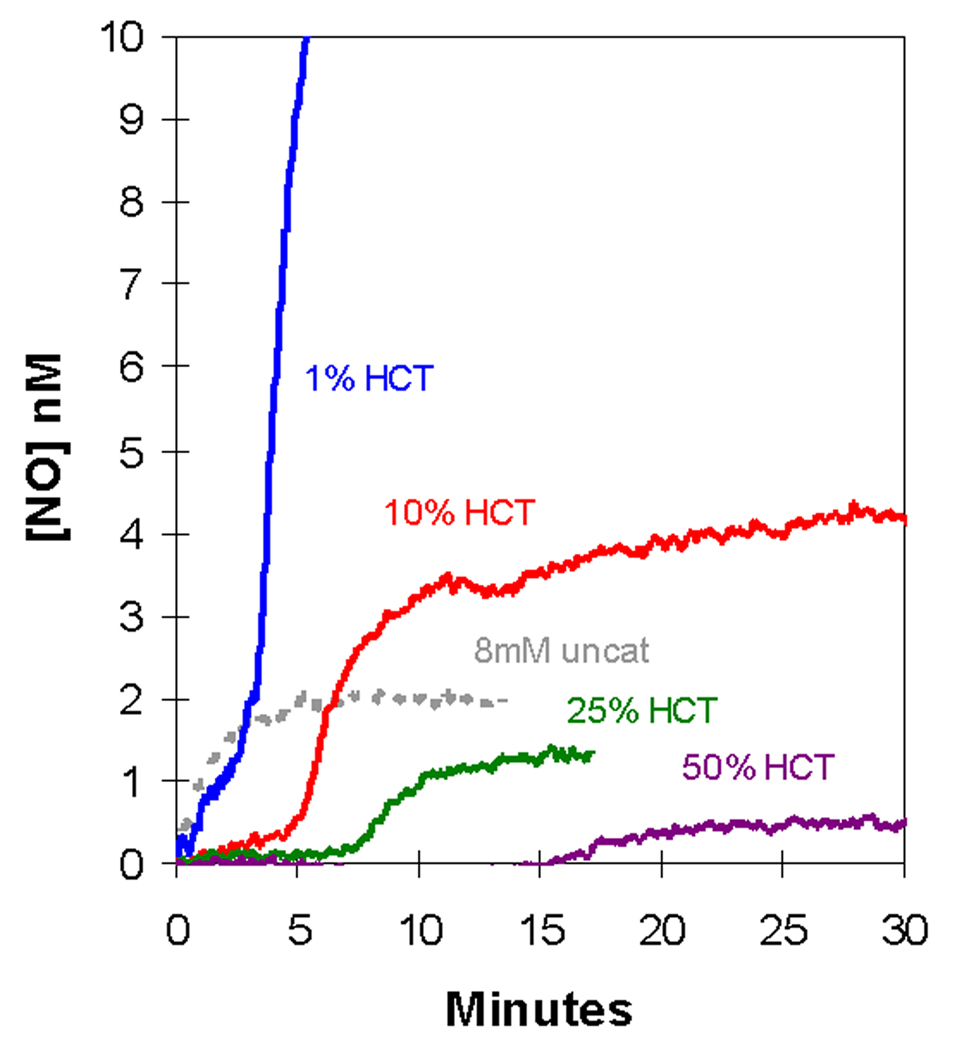
We added nitrite at initial concentrations of 2 – 16 mM to a suspension of deoxygenated, human red cells (0.8% hematocrit) and used the m/z 30 peak to detect extracellular NO. The appearance of NO after the addition of nitrite at time zero was characterized by two phases (Figures 1A,B). Each of the two phases shows an increase in NO approaching a plateau, and the second phase follows generally after the first phase has reached the plateau. For example, for the addition at time zero of 8 mM nitrite, the first phase extends from time zero to about 2.5 min, the second phase begins about 2.5 minutes (Figures 1A,B).
Figure 1.
(A) Time course of extracellular NO accumulation obtained from m/z 30 detected by membrane inlet mass spectrometry upon addition of sodium nitrite to a suspension degassed human red cells at 0.8% hematocrit. At time zero, NaNO2 was added at the concentrations identified on the plot. Solutions also contained 50 mM sodium phosphate, 78 mM sodium chloride, 2.7 mM potassium chloride, and 2 mM DTPA at pH 6.7 and 25 °C. (B) The same series of experiments showing an expansion of the early times. The dotted line represents the NO generated by addition of NaNO2 at a concentration of 8.0 mM to the identical solution but containing no red cells.
The first phase showed accumulation of extracellular NO forming a low-level plateau near 1.3 nM, for an initial nitrite concentration of 8.0 mM, with the plateau lasting over one minute (Figure 1B). This initial plateau was slightly less than about 2 nM formed in the uncatalyzed dismutation of an initial concentration of 8.0 mM (Figure 1B). The length of the initial plateau decreased with increasing nitrite concentration. These data can be compared with the accumulation of NO in solutions of cell-free hemoglobin (Figure 2) [15]. It is difficult to compare these results from MIMS with studies which purge red cell suspensions with inert gas and detect NO in the gas phase using chemiluminescence [7, 16]. These methods and conditions are different and so are results; for example, in measuring uncatalyzed dismutation of nitrite, MIMS allows equilibrium to establish and chemiluminescence removes NO with the inert gas as it is formed.
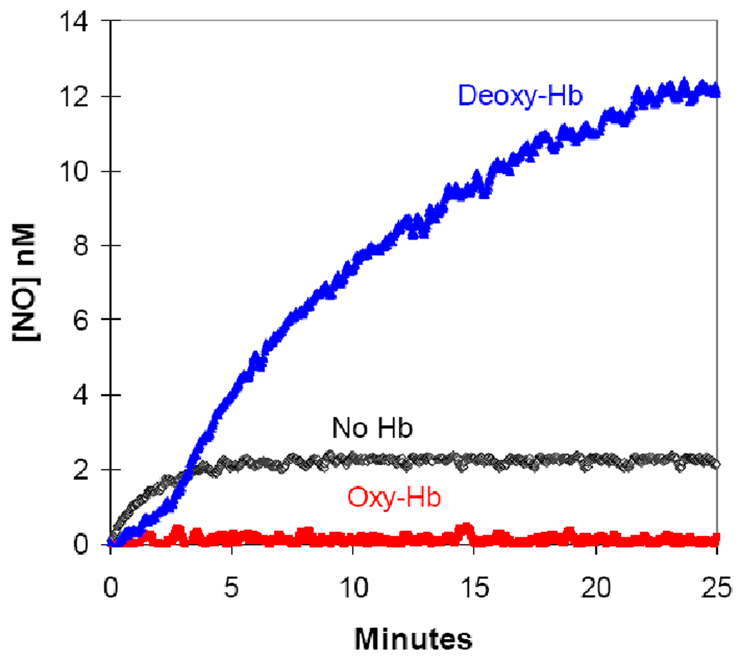
Figure 2.
The time course of dissolved NO concentrations (obtained from m/z 30) upon addition of nitrite to solutions containing (blue) 38 µM deoxy-Hb(FeII); or (red) 38 µM oxy-Hb(FeII); or (black) no hemoglobin. (These are heme concentrations.) At time zero, NaNO2 was added to attain a concentration of 8 mM. Solutions also contained 50 mM phosphate buffer at pH 6.8, 110 mM NaCl, 2 mM EDTA at 23 °C. These data from ref [15].
After this initial steady state plateau, there was a second phase in which extracellular NO concentrations increased and approached a higher plateau of NO concentration, one that exceeded the concentration of NO in the uncatalyzed reaction. In this second phase, the rate of increase of NO and level of the apparent second plateau increased with increasing concentrations of nitrite (Figure 1A). The data shown in Figure 1 are for human red cells that were washed three times in isotonic buffer; however, the results were identical when an equivalent amount of blood was used without prior washing of the red cells.
The following experiments were performed to determine whether the second phase was due to dissociation of NO from NOHb(FeII). MAHMA NONOate (Z-1-[N-methyl-N-[6-(N-methylammoniohexyl)amino]]-diazen-1-ium-1,2-diolate) at 25 µM was used to generate NO in a solution containing 40 µM deoxyHb(FeII) (heme concentration). Other conditions were as described in Figure 1 except no nitrite was added. The solution was monitored spectrophotometrically to observe the formation of the apparently fully nitrosylated hemoglobin. A stream of helium gas was used to remove unbound NO, monitored by observation of the m/z 30 peak. When this peak was near baseline, indicating the near complete removal of unbound NO, the helium stream was stopped. MIMS showed no increase in the m/z 30 peak over a period of 12 minutes. This is consistent with no dissociation of NO from NOHb(FeII) that could be detected.
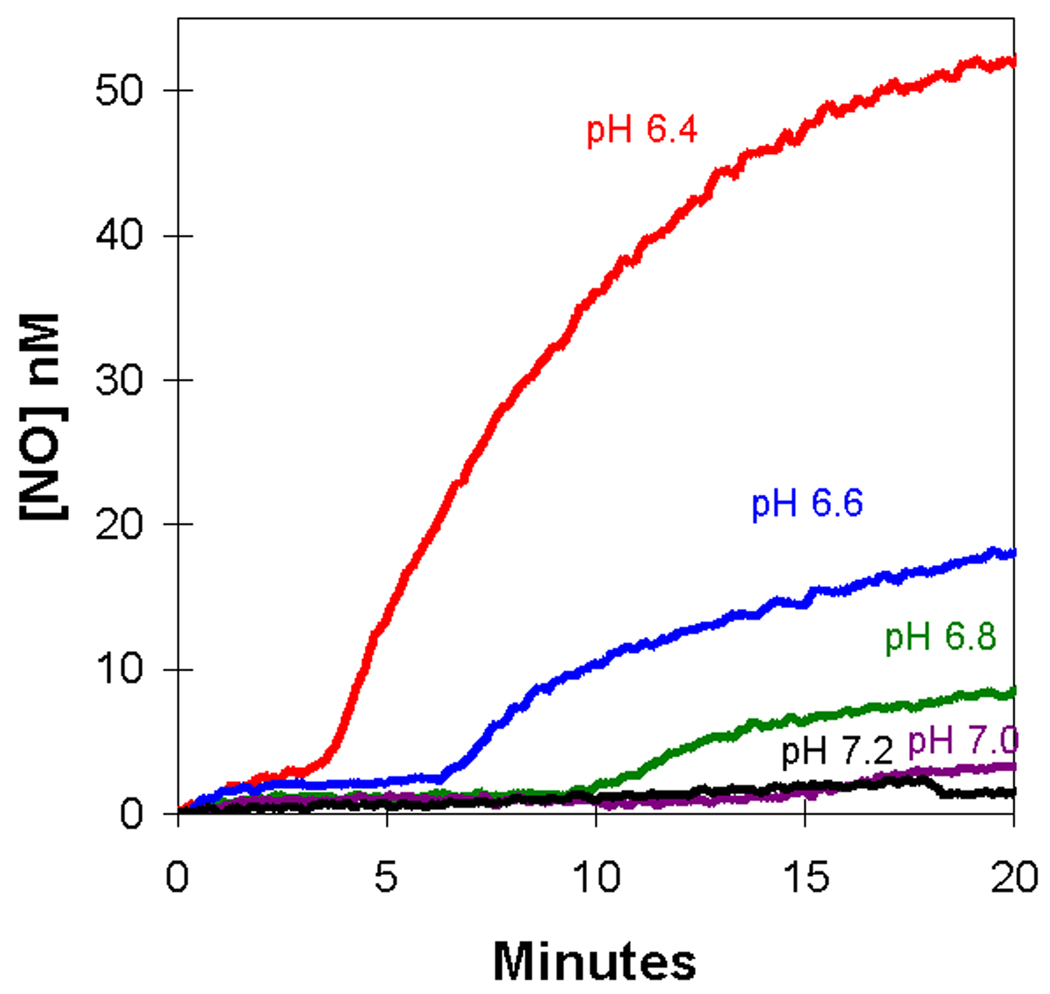
When the pH of the external solution was decreased, we observed a decrease in the length of the first plateau accompanied by an increase in the rate of appearance of extracellular NO and apparent plateau level for the second phase (Figure 3). A qualitatively similar effect was also observed in solutions of cell-free deoxyhemoglobin [15] and is related to the concentration of HNO2, the conjugate acid of nitrite with a pKa of 3.3. As with solutions of cell-free deoxyhemoglobin [15], the logarithm of the rates of NO accumulation in the second phase was a linear function of pH (R2 = 0.97) with rates increasing as pH decreases.
Figure 3.
The pH dependence of the accumulation of extracellular NO upon addition of sodium nitrite to a suspension of degassed human red cells at 0.8% hematocrit. At time zero, NaNO2 was added at a concentration of 8.0 mM at the pH identified on the plot. Other conditions were as described in the legend to Figure 1.
We extended these studies to suspensions of larger hematocrit using additions of 8 mM nitrite (Figure 4). Increasing the amount of deoxygenated erythrocytes increased the length of the initial plateau; at the larger hematocrits (25%, 50%) this initial phase showed no accumulation of NO. Also, increasing the hematocrit decreased the rate of extracellular NO accumulation and the level of the plateau in the second phase (Figure 4). We did not observe in these experiments an increase in the peak at m/z 76 that would correspond to the accumulation of N2O3. The limitations of MIMS to detect N2O3 was discussed in our earlier report on reactions of nitrite with hemoglobin [15]. This species is expected to have a lifetime in solution near 1 ms and to dissociate rapidly into NO and NO2 [20]. It is entirely possible that MIMS as used in this work would not detect an initial burst of N2O3. However, if N2O3 is escaping the red cell and rapidly decomposes to NO either in the red cell suspension or in the gas phase leading to the mass spectrometer, then we should be able to measure it by MIMS as NO. In this sense, it does not matter what species escapes the red cell as a molecule that decays to NO, we have not been able to detect it.
Figure 4.
The dependence of the accumulation of extracellular NO on the amount of human, degassed erythrocytes in suspensions. At time zero, NaNO2 was added at an initial concentration of 8.0 mM to the suspension containing 25 µL, 250 µL, 625 µL, or 1250 µL packed red cell volumes to the final hematocrits identified on the plot. Other conditions were as described in the legend to Figure 1.
The generation of NO in conditions closer to (but clearly not) physiological was then examined. We used human, deoxygenated erythrocyte suspensions at 50% hematocrit in isotonic buffer at pH 7.4 and 37 °C. The suspension was then injected with an initial 1.0 mM nitrite concentration and monitored for NO generation extracellularly. These conditions did not generate any detectable NO for up to 30 minutes following the addition of the nitrite.
Additional experiments were performed at varying levels of oxygenation under the conditions of Figure 1. Mixing oxygenated and deoxygenated packed red cells in different ratios was used to produce different levels of oxygenation. Completely oxygenated red cell suspensions showed no accumulation of NO upon addition of nitrite. Intermediate levels of oxygenation showed the two-stage accumulation of NO as in Figure 1 with NO concentrations which were intermediate between those observed for completely deoxygenated and oxygenated red cells. Further experiments were performed to force the transition between the relaxed state with high oxygen affinity and the tense state favoring deoxyhemoglobin in the red cell. The red cells were oxygenated and then injected into doxygenated buffer in the reaction vessel (conditions of Figure 1); at various times (up to 5 minutes) nitrite was added. The release of oxygen and accumulation of NO was measured in the mass spectrometer. There was no indication of NO released in a burst following nitrite addition, instead there was a slow increase of NO, much less than in the experiments using deoxygenated cells and on a slower time scale. When air or oxygen was bubbled into the reaction of completely deoxygenated red cells under conditions of Figure 1 it resulted in a similar decreased generation of NO.
Effect of DIDS on NO accumulation
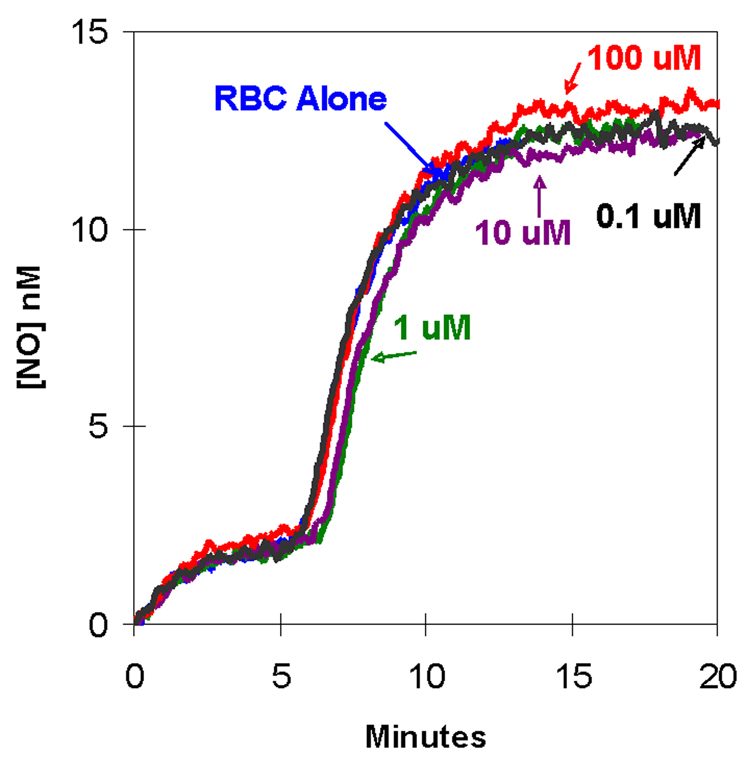
We tested several inhibitors of the band 3 anion exchanger (AE1) for their effect on the accumulation of extracellular NO after introduction of nitrite to deoxygenated red cells. Concentrations of DIDS from 0.1 µM to 1.0 mM had no effect on the accumulation of NO in extracellular fluid, shown for four concentrations of DIDS in Figure 5. An apparent IC50 of 0.1 µM DIDS was reported for the inhibition of the transport of sulfate by the anion exchangers in human red cells [21]. Results identical to Figure 5 were obtained when experiments were performed in the dark to prevent light induced isomerization of DIDS [22]. In additional experiments, we used solutions of DIDS containing potassium bicarbonate (0.1 mM) or DMSO (5% in final suspensions) to prevent the formation of polymeric DIDS [15]; results were identical to Figure 5.
Figure 5.
The effect of DIDS, an inhibitor of anion exchange, on accumulation of extracellular NO in suspensions of degassed human erythrocytes at 1.6% hematocrit. In each experiment 8.0 mM NaNO2 was added at time zero to a suspension containing the designated concentrations of DIDS: (black) 0.1 µM; (green) 1 µM; (purple) 10 µM; (red) 100 µM; and (blue) no DIDS. In each case, DIDS was incubated with erythrocytes 10 minutes prior to addition of nitrite. The other conditions were as described in the legend to Figure 1.
Experiments under the same conditions using the anion exchange inhibitors SITS and DNDS also showed no effect in the accumulation of NO (data not shown). SITS was examined in the concentration range from 10 µM to 100 µM. An IC 50 of 50 µM for SITS is reported for the inhibition of the transport of sulfate by the anion exchangers in human red cells [23]. DNDS was used at concentrations above and below its IC50 of 2 uM for inhibition of sulfate transport through the anion exchange protein of human red cells [21]. In control experiments, DIDS, SITS, DNDS in the concentration ranges used here caused no change in the rate of accumulation of NO in solutions containing deoxyhemoglobin but no erythrocytes.
Effect of PCMBS on NO accumulation
We added to our deoxygenated red cell suspensions PCMBS which is an inhibitor with complex effects on red cells including inhibition of the aquaporin-1 channel but also inhibition of anion exchange proteins to some extent [24]. The concentrations of PCMBS used in our studies are in the range of the inhibition of aquaporin demonstrated by the reduction of water diffusion by half at concentrations of 2 mM in adult human erythrocytes [25]. The reported IC50 for the inhibition of CO2 transport through the aquaporin-1 channel in human red cells is 0.5 mM [26] . A range of PCMBS from 25 µM to 2 mM was used in our studies; this range included both inhibition of the aquaporin channel as well as inhibition of the band 3 anion exchanger (IC50 near 2 mM) [24, 26].
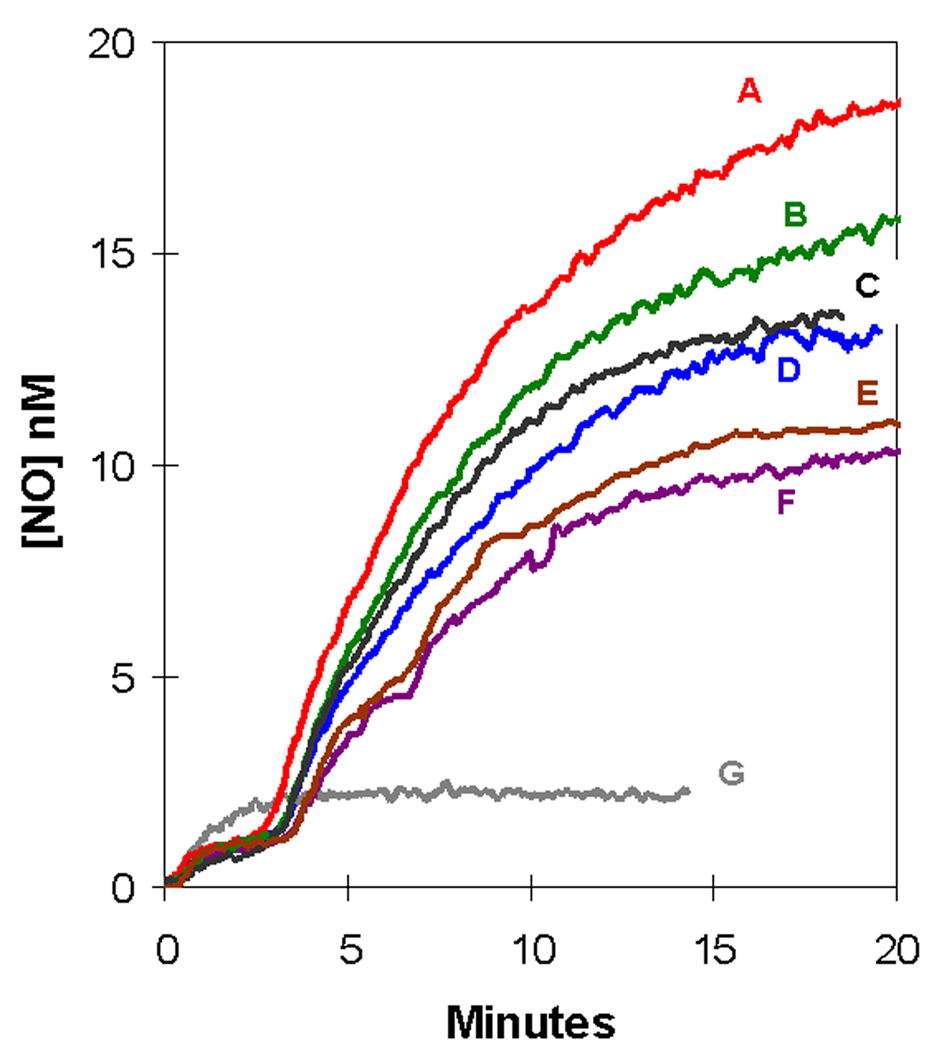
The presence of PCMBS showed no significant change in the level of NO for the initial plateau (up to 3 minutes) after addition of nitrite (Figure 6). For the second phase, when 25 µM or 50 µM PCMBS was incubated with the red cells, there was an increase in plateau level (Figure 5). At the higher PCMBS concentration of 1 mM and 2 mM the second plateau appeared lower than in the absence of PCMBS, which was observed in three repetitions of the experiment. PCMBS did not affect the rate of accumulation of NO in solutions containing deoxyhemoglobin but no erythrocytes at concentrations below 50 µM.
Figure 6.
Effect of PCMBS, an inhibitor of the aquaporin channel, on the accumulation of extracellular NO concentration (determined from m/z 30) in suspensions of human erythrocytes at 0.8% hematocrit following the addition of an initial concentration of 8.0 mM nitrite. Suspensions contained the following concentrations of PCMBS: (red) 25 µM; (green) 50 µM; (black) 100 µM; (blue) no PCMBS: (brown) 1 mM; and (purple) 2 mM labeled A–F respectively on the plot. The data in grey labeled G show the accumulation of NO after addition of 8.0 mM nitrite in solutions containing no erythrocytes. Other conditions were as described in the legend to Figure 1.
Discussion
In measuring the accumulation of NO in red cell suspensions by membrane inlet mass spectrometry, the inlet itself is immersed in the suspension. Hence, the free, unbound NO in the extracellular fluid is measured without purging the suspension with inert gas, giving a direct and real time measure of NO concentration and accumulation in the extracellular solution. We have observed a biphasic increase in NO measured in this manner after adding millimolar levels of nitrite to human, deoxygenated erythrocyte suspensions (Figure 1A,B). The uncatalyzed dismutation of nitrite observed by this method was reported earlier [19] and is shown in Figure 1B in comparison with data obtained using erythrocyte suspensions, and in Figure 2 in solutions containing cell-free hemoglobin. The topic of the uncatalyzed dismutation of nitrite is covered in a classic review from the 1950’s [27] which has been followed by numerous reports of the rate constants for this complex process [28, 29].
Accumulation of extracellular NO in red cell suspensions
The very low level of NO in the initial 1 – 2 minutes after addition of nitrite to deoxygenated erythrocyte suspensions is consistent with the tight binding of NO to deoxyHb(FeII) in the reaction schemes of Eqs. (1) and (2) in the interior of red blood cells. Even at the very large concentration of initial nitrite at 2 mM and 4 mM, the levels of NO detected in the extracellular fluid do not exceed 1 nM (Figure 1B) for at least five minutes, below the level of NO consistent with vasodilation (in a range up to 10 nM) [30]. Figure 1B shows that for the addition of 8 mM nitrite the concentrations of extracellular NO were somewhat lower than the control without red cells, consistent with the suggestion that NO generated is taken up by the red cells. This is particularly evident at the larger hematocrits shown in Figure 4 in which the concentration of NO outside of the cells decreases significantly when there are more erythrocytes present. This is understood as the efficient scavenging of extracellular NO by the increased number of red cells. Increased accumulation of extracellular NO in red cell suspensions was associated with a decrease in pH (Figure 3). The reduction in pH increases HNO2 concentrations and this may be a predominant species entering the red cell to be reduced to NO. Moreover, the uncatalyzed dismutation of nitrite is more rapid at lower pH [15].
In these red cell suspensions, the low-level plateau of the first phase (up to 2–3 minutes) represents a complex steady state in extracellular NO concentration involving several processes including the uncatalyzed dismutation of nitrite, the flux of nitrite and HNO2 into the cells where it reacts with hemoglobin according to Eqs. (1) and (2), and the diffusion of NO across the cell membrane. There are a number of other accompanying processes such as nitrosylation reactions, the possible role of other species of nitrogen oxides, other reactions of nitrite and NO in the cell, and the action of metHb(FeIII) reductase.
Nitrite concentrations used at 2 to 16 mM differ greatly from physiological concentrations of nitrite, which are near 1 µM; however, the much larger concentrations of nitrite needed to observe NO in red cell suspensions forms part of our conclusion. Addition of 1.0 mM nitrite under more physiological conditions did not show any accumulation of NO that we could measure. For the membrane inlet that we have used the half-time is near 5 seconds for response to step increases in concentration of NO. We would not be able to observe a small, rapid pulse of NO increase occurring within seconds after the addition of nitrite.
Other cautions apply when interpreting our measurements. Detection of NO in our reaction vessel and experimental conditions does not reproduce conditions in an arteriole, in which flowing red cells in plasma physiologically undergo a 25–75% reduction in hemoglobin-oxygen saturation with a simultaneous increase in the partial pressure of carbon dioxide (30 to 50 mmHg) and fall in pH (7.5 to 7.3)1 with only a minor reduction of extracellular nitrite at concentrations below 1 uM. Although our comparison of net NO accumulation with deoxygenated cells and uncatalyzed dismutation (Figure 1B and 4) is a direct observation, NO accumulation using deoxygenated compared with oxygenated red cells offers further interpretation. The results with oxygenated and deoxygenated red cells were very similar to those with cell free oxy- and deoxyHb(FeII) in Figure 2 where it is clear that initially NO accumulation is greater with deoxyHb(FeII). The reaction of NO and oxyHb(FeII) is near 9 × 107 M−1s−1 [31] and the value for the reaction of NO and deoxyHb(FeII) (Eq. (2)) is 2 × 107 M−1s−1 [32]. These values are viewed as rather similar; hence, a major difference between oxy and deoxy conditions appears to be the nitrite reductase activity of deoxyHb(FeII). In this interpretation, the greater accumulation of NO under deoxy compared with oxy conditions suggests efflux of NO from the cells. However, MIMS cannot detect the origin of the NO accumulated outside the cells; a fraction may be generated by the uncatalyzed dismutation of nitrite outside the cells and a fraction may be generated from the nitrite reductase activity of deoxyHb(FeII) with product NO that then exits the cell. However, direct observation shows no net accumulation of NO in the initial phase outside the cells compared with uncatalyzed levels of NO.
The second phase is an increase in the rate of extracellular NO accumulation that follows the initial plateau and occurs many minutes after addition of nitrite to degassed erythrocyte suspensions (Figure 1A,B). The cause of this second phase of increase and apparent second plateau is unclear; but it appears not due to the dissociation of NOHb(FeII). One possibility is the reaction of nitrite with forms of nitrosylated hemoglobin. The resulting progress curve in the second phase of the accumulation of NO in extracellular solution after addition of nitrite (Figure 1A) is very similar to that obtained by this method under similar conditions for solutions containing cell-free deoxyhemoglobin (Figure 2) [15]. Both experiments, using deoxygenated erythrocyte suspensions or solutions of cell-free deoxyHb(FeII), showed an initial phase of low levels of NO and a second phase of more rapid NO accumulation approaching a plateau. Moreover the time scales and magnitudes of NO observed are similar for the red cell and solution phase studies with purified hemoglobin (Figure 1, Figure 2).
Inhibition of band 3 anion exchanger of red cells
The exchange protein AE1 is a member of a family of anion exchangers that is located in the plasma membrane of erythrocytes as well as other tissues and is involved in the transport of CO2 and bicarbonate in respiration [33, 34]. Studies with intact red cells and ghosts show nitrite uptake blocked by DIDS, an inhibitor of anion exchange proteins including AE1 [35]. However, other studies show no effect of DIDS on nitrite induced formation of met-Hb(FeIII) in erythrocytes [36, 37]. There will be some diffusion of HNO2 (pKa 3.3) across the membrane, and studies indicate some uptake of nitrite by the sodium-dependent phosphate transporter [37].
We observed no significant change caused by the inhibitors of anion exchange DIDS, SITS, or DNDS in the rate of extracellular NO accumulation in this work (shown for DIDS in Figure 5). The disulfonic stilbene compounds including DIDS, SITS, and DNDS are a class of the most potent and commonly used inhibitors of the exchange protein AE1. These inhibitors in the concentration range of their respective IC50 values had no effect on NO accumulation under conditions of Figure 1. These results show that anion flux using the exchange protein AE1 is not a predominant factor in the accumulation of extracellular NO caused by addition of large quantities of extracellular nitrite. The insensitivity to DIDS in Figure 5 indicates that rate-limiting steps in the extracellular accumulation of NO do not involve AE1.
Inhibition of aquaporin-1 channel of red cells
Being a small uncharged molecule, NO is expected to pass readily across membranes [38], and there is evidence that some passes through the aquaporin channel [14]. Evidence that a large amount of CO2 fluxes out of red cells through the aquaporin channel suggests that it may be a conduit for NO and other dissolved gases to enter or exit the red cell, although the significance of this is uncertain [26].
PCMBS had no effect on the levels of extracellular NO in the initial plateau after addition of nitrite (Figure 6). In addition, PCMBS at the concentrations of Figure 6 had no effect on the accumulation of NO in solutions of cell-free deoxyhemoglobin (data not shown). These data show that the aquaporin channel does not play a predominant role in the complex steady-state processes that are involved in the initial response of red cells to large concentrations of nitrite. Rather, the steady state appears dominated by the flux of NO through other pathways and of HNO2 followed by the autocapture of NO in the red cells. The complex behavior of PCMBS toward the second plateau in Figure 6 is unexplained at present. We can conclude however that flux through the aquaporin channel is not a major factor for accumulation of extracellular NO in our experiments.
Summary
Application of membrane inlet mass spectrometry showed no initial burst of extracellular NO or N2O3 caused by addition of millimolar levels of nitrite to suspensions of deoxygenated red cells at pH 6.7. With a sensitivity as low at 0.5 nM, this method detected no accumulation of extracellular NO when 1.0 mM nitrite was added to red cells under more physiological conditions of hematocrit, pH, and temperature. These data support the hypothesis that, although the reactions of nitrite with deoxyHb(FeII) apply, the autocapture of NO by deoxyHb(FeII) precludes efflux of NO. Inhibition of the anion exchange protein and the aquaporin channel did not affect the accumulation of extracellular NO in these experiments; flux of nitrite and NO across the membrane by these processes are not rate contributing towards appearance of NO extracellularly.
Acknowledgments
We thank Dr. Bradley Fletcher for help with the experimental protocol. This work was supported in part by NIH grant GM25154 funds made available by the University of Florida.
Abbreviations
- MIMS
membrane inlet mass spectrometry
- deoxyHb(FeII)
the ferrous form of deoxyhemoglobin
- NOHb(FeII)
nitrosylated hemoglobin
- m/z
(m/e) is the mass-to-charge ratio detected with the mass spectrometer
- AE1
anion exchanger-1
- DTPA
diethylenetriaminepentaacetic acid
- SITS
4-acetamido-4’-isothiocyanstilbene-2,2’-disulfonic acid
- DIDS
4,4'-diisothiocyano-stilbene-2,2'-disulfonic acid
- DNDS
4,4'-dinitrostilbene-2,2'-disulfonic acid
- pCMBS
para-chloromercuribenzene sulfonate.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A limitation in the present experiments is the necessity for an absence of carbon dioxide and bicarbonate due to the generation in the mass spectrometer of C18O (m/z 30) from the natural abundance of 18O masking the NO peak. We are currently exploring the use of isotopic 15N-enriched nitrite to overcome this problem.
References
- 1.Doyle MP, Pickering RA, DeWeert TM, Hoekstra JW, Pater D. Kinetics and mechanism of the oxidation of human deoxyhemoglobin by nitrites. J Biol Chem. 1981;256:12393–12398. [PubMed] [Google Scholar]
- 2.Angelo M, Singel DJ, Stamler JS. An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8366–8371. doi: 10.1073/pnas.0600942103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang Z, Shiva S, Kim-Shapiro DB, Patel RP, Ringwood LA, Irby CE, Huang KT, Ho C, Hogg N, Schechter AN, Gladwin MT. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest. 2005;115:2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim-Shapiro DB, Gladwin MT, Patel RP, Hogg N. The reaction between nitrite and hemoglobin: the role of nitrite in hemoglobin-mediated hypoxic vasodilation. J Inorg Biochem. 2005;99:237–246. doi: 10.1016/j.jinorgbio.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 5.Luchsinger BP, Rich EN, Yan Y, Williams EM, Stamler JS, Singel DJ. Assessments of the chemistry and vasodilatory activity of nitrite with hemoglobin under physiologically relevant conditions. Journal of Inorganic Biochemistry. 2005;99:912–921. doi: 10.1016/j.jinorgbio.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Huang Z, Shiva S, Kim-Shapiro D, Patel R, Ringwood L, Irby C, Huang K, Ho C, Hogg N, Schechter A, Gladwin M. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. Journal of Clinical Investigation. 2005;115:2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu XL, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nature Medicine. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 8.Deem S, Min JH, Moulding JD, Eveland R, Swenson ER. Red blood cells prevent inhibition of hypoxic pulmonary vasoconstriction by nitrite in isolated, perfused rat lungs. American Journal of Physiology-Heart and Circulatory Physiology. 2007;292:H963–H970. doi: 10.1152/ajpheart.00812.2006. [DOI] [PubMed] [Google Scholar]
- 9.Dalsgaard T, Simonsen U, Fago A. Nitrite-dependent vasodilation is facilitated by hypoxia and is independent of known NO-generating nitrite reductase activities. American Journal of Physiology-Heart and Circulatory Physiology. 2007;292:H3072–H3078. doi: 10.1152/ajpheart.01298.2006. [DOI] [PubMed] [Google Scholar]
- 10.Hunter CJ, Dejam A, Blood AB, Shields H, Kim-Shapiro D, Machado RF, Tarekegn S, Mulla N, Hopper AO, Schechter AN, Power GG, Gladwin MT. Inhaled nebulized nitrite is a hypoxia-sensitive NO-dependent selective pulmonary vasodilator. Nature Medicine. 2004;10:1122–1127. doi: 10.1038/nm1109. [DOI] [PubMed] [Google Scholar]
- 11.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: The role of nitric oxide and S-nitrosohemoglobin. Annual Review of Physiology. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- 12.Schechter AN, Gladwin MT. Hemoglobin and the paracrine and endocrine functions of nitric oxide. New England Journal of Medicine. 2003;348:1483–1485. doi: 10.1056/NEJMcibr023045. [DOI] [PubMed] [Google Scholar]
- 13.Basu S, Grubina R, Huang J, Conradie J, Huang Z, Jeffers A, Jiang A, He X, Azarov I, Seibert R, Mehta A, Patel R, King SB, Hogg N, Ghosh A, Gladwin MT, Kim-Shapiro DB. Catalytic generation of N2O3 by the concerted nitrite reductase and anhydrase activity of hemoglobin. Nature Chemical Biology. 2007;3:785–794. doi: 10.1038/nchembio.2007.46. [DOI] [PubMed] [Google Scholar]
- 14.Herrera M, Garvin JL. Novel role of AQP-1 in NO-dependent vasorelaxation. American journal of physiology. Renal physiology. 2007;292:F1443. doi: 10.1152/ajprenal.00353.2006. [DOI] [PubMed] [Google Scholar]
- 15.Tu CK, Mikulski R, Swenson ER, Silverman DN. Reactions of nitrite with hemoglobin measured by membrane inlet mass spectrometry. Free Radical Biology & Medicine. 2009;46:14–19. doi: 10.1016/j.freeradbiomed.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li HT, Cui HM, Kundu TK, Alzawahra W, Zweier JL. Nitric oxide production from nitrite occurs primarily in tissues not in the blood - Critical role of xanthine oxidase and aldehyde oxidase. Journal of Biological Chemistry. 2008;283:17855–17863. doi: 10.1074/jbc.M801785200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lundberg J, Weitzberg E, Gladwin M. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008 doi: 10.1038/nrd2466. advanced online publication. [DOI] [PubMed] [Google Scholar]
- 18.Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, Scheeren T, Godecke A, Schrader J, Schulz R, Heusch G, Schaub GA, Bryan NS, Feelisch M, Kelm M. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radical Biology and Medicine. 2003;35:790–796. doi: 10.1016/s0891-5849(03)00406-4. [DOI] [PubMed] [Google Scholar]
- 19.Tu CK, Swenson ER, Silverman DN. Membrane inlet for mass spectrometric measurement of nitric oxide. Free Radical Biology and Medicine. 2007;43:1453–1457. doi: 10.1016/j.freeradbiomed.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 20.Shaw AW, Vosper AJ. Dinitrogen Trioxide.9. Stability of Dinitrogen Trioxide in Solution. Journal of the Chemical Society a -Inorganic Physical Theoretical. 1971 1592-&. [Google Scholar]
- 21.Barzilay M, Ship S, Cabantchik ZI. Anion Transport in Red Blood-Cells.1. Chemical Properties of Anion Recognition Sites as Revealed by Structure-Activity-Relationships of Aromatic Sulfonic-Acids. Membrane Biochemistry. 1979;2:227–254. doi: 10.3109/09687687909063866. [DOI] [PubMed] [Google Scholar]
- 22.Cabantchik ZI, Greger R. Chemical Probes for Anion Transporters of Mammalian-Cell Membranes. American Journal of Physiology. 1992;262:C803–C827. doi: 10.1152/ajpcell.1992.262.4.C803. [DOI] [PubMed] [Google Scholar]
- 23.Cabantch Zi, Rothstei A. Membrane Proteins Related to Anion Permeability of Human Red Blood-Cells.1. Localization of Disulfonic Stilbene Binding-Sites in Proteins Involved in Permeation. Journal of Membrane Biology. 1974;15:207–226. doi: 10.1007/BF01870088. [DOI] [PubMed] [Google Scholar]
- 24.Zhang ZH, Solomon AK. Effect of Pcmbs on Anion Transport in Human Red-Cell Membranes. Biochimica Et Biophysica Acta. 1992;1106:31–39. doi: 10.1016/0005-2736(92)90218-b. [DOI] [PubMed] [Google Scholar]
- 25.Conlon T, Outhred R. Temperature-Dependence of Erythrocyte Water Diffusion Permeability. Biochimica Et Biophysica Acta. 1978;511:408–418. doi: 10.1016/0005-2736(78)90277-8. [DOI] [PubMed] [Google Scholar]
- 26.Endeward V, Musa-Aziz R, Cooper GJ, Chen LM, Pelletier MF, Virkki LV, Supuran CT, King LS, Boron WF, Gros G. Evidence that aquaporin 1 is a major pathway for CO2 transport across the human erythrocyte membrane. Faseb Journal. 2006;20:1974–1981. doi: 10.1096/fj.04-3300com. [DOI] [PubMed] [Google Scholar]
- 27.Turney TA, Wright GA. Nitrous acid and nitrosation. Chem Rev. 1959;59:497–513. [Google Scholar]
- 28.Park J-Y, Lee Y-N. Solubility and decomposition kinetics of nitrous acid in aqueous solution. J Phys Chem. 1988;92:6294–6302. [Google Scholar]
- 29.Samouilov A, Kuppusamy P, Zweier JL. Evaluation of the magnitude and rate of nitric oxide production from nitrite in biological systems. Arch Biochem Biophys. 1998;357:1–7. doi: 10.1006/abbi.1998.0785. [DOI] [PubMed] [Google Scholar]
- 30.Carter TD, Bettache N, Ogden D. Potency and kinetics of nitric oxide-mediated vascular smooth muscle relaxation determined with flash photolysis of ruthenium nitrosyl chlorides. British Journal of Pharmacology. 1997;122:971–973. doi: 10.1038/sj.bjp.0701549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herold S, Exner M, Nauser T. Kinetic and mechanistic studies of the NO center dot-mediated oxidation of oxymyoglobin and oxyhemoglobin. Biochemistry. 2001;40:3385–3395. doi: 10.1021/bi002407m. [DOI] [PubMed] [Google Scholar]
- 32.Cooper CE. Nitric oxide and iron proteins. Biochimica Et Biophysica Acta-Bioenergetics. 1999;1411:290–309. doi: 10.1016/s0005-2728(99)00021-3. [DOI] [PubMed] [Google Scholar]
- 33.Casey JR. Why bicarbonate? Biochem. Cell Biol. 2006;84:930–939. doi: 10.1139/o06-184. [DOI] [PubMed] [Google Scholar]
- 34.Sterling D, Casey JR. Bicarbonate transport proteins. Biochemistry and Cell Biology-Biochimie Et Biologie Cellulaire. 2002;80:483–497. doi: 10.1139/o02-152. [DOI] [PubMed] [Google Scholar]
- 35.Shingles R, Roh MH, McCarty RE. Direct measurement of nitrite transport across erythrocyte membrane vesicles using the fluorescent probe, 6-methoxy-N-(3-sulfopropyl) quinolinium. Journal of Bioenergetics and Biomembranes. 1997;29:611–616. doi: 10.1023/a:1022491220299. [DOI] [PubMed] [Google Scholar]
- 36.Zavodnik IB, Lapshina EA, Rekawiecka K, Zavodnik LB, Bartosz G, Bryszewska M. Membrane effects of nitrite-induced oxidation of human red blood cells. Biochimica Et Biophysica Acta-Biomembranes. 1999;1421:306–316. doi: 10.1016/s0005-2736(99)00136-4. [DOI] [PubMed] [Google Scholar]
- 37.May JM, Qu ZC, Xia L, Cobb CE. Nitrite uptake and metabolism and oxidant stress in human erythrocytes. American Journal of Physiology-Cell Physiology. 2000;279:C1946–C1954. doi: 10.1152/ajpcell.2000.279.6.C1946. [DOI] [PubMed] [Google Scholar]
- 38.Liu X, Miller M, Joshi M, Sadowska-Krowicka H, Clark D, Lancaster JJ. Diffusion-limited Reaction of Free Nitric Oxide with Erythrocytes. Journal of Biological Chemistry. 1998;273:18709. doi: 10.1074/jbc.273.30.18709. [DOI] [PubMed] [Google Scholar]