Abstract
Deficiency of adrenal 4 binding protein/steroidogenic factor 1 (Ad4BP/SF-1; NR5A1) impairs adrenal development in a dose-dependent manner, whereas overexpression of Ad4BP/SF-1 is associated with adrenocortical tumorigenesis. Despite its essential roles in adrenal development, the mechanism(s) by which Ad4BP/SF-1 regulates this process remain incompletely understood. We previously identified a fetal adrenal enhancer (FAdE) that stimulates Ad4BP/SF-1 expression in the fetal adrenal gland by a two-step mechanism in which homeobox proteins initiate Ad4BP/SF-1 expression, which then maintains FAdE activity in an autoregulatory loop. In the present study, we examined the effect of transgenic expression of Ad4BP/SF-1 controlled by FAdE on adrenal development. When Ad4BP/SF-1 was overexpressed using a FAdE-Ad4BP/SF-1 transgene, FAdE activity expanded outside of its normal field, resulting in increased adrenal size and the formation of ectopic adrenal tissue in the thorax. The increased size of the adrenal gland did not result from a corresponding increase in cell proliferation, suggesting rather that the increased levels of Ad4BP/SF-1 may divert uncommitted precursors to the steroidogenic lineage. The effects of FAdE-controlled Ad4BP/SF-1 overexpression in mice provide a novel model of ectopic adrenal formation that further supports the critical role of Ad4BP/SF-1 in the determination of steroidogenic cell fate in vivo.
Overexpression of Ad4BP/SF-1 induces ectopic adrenal formation. This increased tissue size doesn’t result from cell proliferation, but rather increased commitment to the steroidogenic cell lineage.
The nuclear receptor adrenal 4 binding protein/steroidogenic factor 1 (Ad4BP/SF-1; officially designated NR5A1) is essential for gonadal and adrenal development. Studies of its expression identified a pool of Ad4BP/SF-1-positive precursor cells, designated the adrenogonadal primordium, which contribute to both the adrenal cortex and the gonads (1,2,3). Indeed, deletion of Ad4BP/SF-1 in mice results in adrenal and gonadal agenesis, with postnatal lethality due to adrenal insufficiency (4). The ventromedial hypothalamus, pituitary gonadotropes, and spleen also develop abnormally in mice lacking Ad4BP/SF-1 (2,5,6,7,8). These observations indicate that Ad4BP/SF-1 functions at multiple levels to regulate differentiation of the endocrine lineages. Moreover, Ad4BP/SF-1 gene dosage is an important factor in development of the steroidogenic tissues, because Ad4BP/SF-1 heterozygous (+/−) mice have hypoplastic adrenal glands (9) and lesser impairment in early gonadal development (10).
Forced expression of Ad4BP/SF-1 can induce embryonic stem cells and pluripotent bone marrow or adipose mesenchymal cells to differentiate into cells that express certain steroidogenic enzymes (11,12,13,14,15). In addition, overexpression of Ad4BP/SF-1 in cultured human adrenocortical tumor cells promoted cell proliferation (16). Thus, an improved understanding of how Ad4BP/SF-1 confers the steroidogenic phenotype on uncommitted precursors and stimulates their subsequent proliferation is an important goal for ongoing investigation.
We previously identified a fetal adrenal enhancer (FAdE) in the fourth intron of the Ad4BP/SF-1 gene that directs transgenic expression to the fetal adrenal gland; the FAdE contains binding sites for homeobox-containing proteins, which initiate Ad4BP/SF-1 expression, and for Ad4BP/SF-1 itself, which maintains Ad4BP/SF-1 expression in an autoregulatory loop (17). Although the FAdE is transiently activated in cells that contribute to the gonads, the adult (definitive) adrenal cortex, and poorly characterized cells in the thorax, FAdE-driven reporter gene expression persists beyond very early stages only in the cells that form the fetal adrenal zone (18). In the present study, we used the FAdE to target the overexpression of Ad4BP/SF-1 during mouse development. These transgenic studies, which may be relevant to the formation of ectopic adrenal tissue in human beings, demonstrate that Ad4BP/SF-1 can direct cell differentiation toward the steroidogenic pathway, leading to increased adrenal size and to the formation of ectopic adrenocortical cells in the thorax.
Results
Overexpression of Ad4BP/SF-1 expands the distribution of FAdE-active cells
Studies with heterozygous (+/−) and Ad4BP/SF-1 knockout mice strongly suggested that the level of Ad4BP/SF-1 expression determines the size of mouse adrenal glands (4,9). In the present work, we extended these studies by examining the effect of Ad4BP/SF-1 overexpression in transgenic mice. A construct that is expected to induce Ad4BP/SF-1 expression under the control of the FAdE and the 5.8-kb basal promoter of the Ad4BP/SF-1 gene [FAdE-Ad4BP-(Ad4BP/SF-1); see Fig. 1A] was used to generate transgenic mice with oocytes derived from mice carrying the Ad4BP-lacZ-FAdE transgene (17); this transgene permits facile detection of cells in which the FAdE is active because the lacZ-positive cells can be stained histochemically with X-gal.
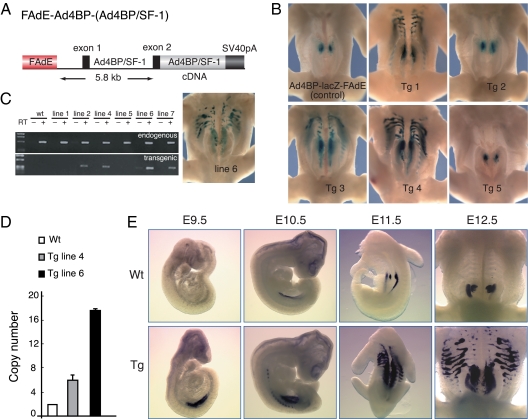
Figure 1.
Ad4BP/SF-1 overexpression driven by the FAdE results in an increased number of lacZ-positive adrenocortical cells. A, Schematic diagram of the FAdE-Ad4BP-(Ad4BP/SF-1) Tg construct. B, Transient Tg assays of the effect of overexpression of Ad4BP/SF-1 on lacZ expression driven by the Ad4BP-lacZ-FAdE reporter transgene (17). Using this assay, three of five Tg embryos at E12.5 revealed increased lacZ expression relative to the control embryo (upper left panel). C, Generation of Ad4BP/SF-1 overexpressing Tg mouse lines. Four Tg mouse lines (designated lines 2, 4, 6, and 7) expressed the Ad4BP/SF-1 transgene in the postnatal adrenal gland (P9) as assessed by RT-PCR. Line 6 mice had ectopic adrenal tissue and lacZ-positive cells in the thoracic region, matching the results in the transient assay. D, Determination of transgene copy number. Transgene copy number was determined by quantitative PCR using two pairs of primers as mentioned in Materials and Methods. E, Developmental expression of Ad4BP/SF-1 at the indicated times in Wt (upper panel) and FAdE-Ad4BP-(Ad4BP/SF-1) Tg embryos (bottom panel) analyzed by whole-mount in situ hybridization for Ad4BP/SF-1 transcripts. SV40, Simian virus 40.
We first used transient transgenesis in founder embryos at embryonic d 12.5 (E12.5) to examine the impact of the FAdE-Ad4BP-(Ad4BP/SF-1) transgene on adrenal development. Three of the five transgenic (Tg) embryos examined (Tg 1, Tg 3, and Tg 4) had larger adrenal glands than control embryos (Fig. 1B). This finding suggests that augmented expression of Ad4BP/SF-1 in cells in which the FAdE was active was sufficient either to recruit additional precursors into the adrenal pathway or to enhance the proliferation of cells committed to this fate. In addition, lacZ-positive cells in the same Tg embryos were distributed ectopically in the thorax in a manner highly reminiscent of our previous lineage tracing of FAdE-activated cells using a Cre-loxP strategy (18). Thus, FAdE activity, as revealed by persistent expression of lacZ driven by the Ad4BP-lacZ-FAdE transgene, was maintained abnormally in cells outside of the adrenal gland when levels of Ad4BP/SF-1 protein in FAdE-active cells were enhanced transgenically.
To explore the underlying mechanism(s) by which our Tg expression of Ad4BP/SF-1 expanded the field of persistent FAdE activity, we established six independent lines of FAdE-Ad4BP-(Ad4BP/SF-1) Tg mice. Based on RT-PCR analyses of adrenal RNA, the transgene was expressed in four of six lines (Fig. 1C). Of these, mice from line 6 recapitulated the ectopic lacZ expression and increased adrenal size seen in the transient transgenesis experiments described above (Fig. 1B); these mice were used for subsequent analyses comparing their development with that of mice lacking the transgene [termed “wild type” (Wt)].
To characterize Tg line 6 in more detail, we analyzed the transgene copy number by quantitative PCR. As shown in Fig. 1D, Tg line 6 and 4 carry 17–18 and six to seven copies of Ad4BP/SF-1 genes, respectively. Because they have two copies of endogenous genes as in the Wt case, the copy numbers of the transgene are estimated to be 15–16 for line 6 and four to five for line 4. Using Tg line 6, we examined the effect of Ad4BP/SF-1 overexpression at different developmental stages. At E9.5, Ad4BP/SF-1 was expressed at very low levels in the urogenital ridge of the non-Tg (Wt) embryo, whereas stronger and more widespread expression of Ad4BP/SF-1 was seen in the Tg embryo (Fig. 1E). This suggests that the number of Ad4BP/SF-1-positive cells, and not just the level of its expression within the same cells, was increased by the transgene. Thereafter, Ad4BP/SF-1 expression increased in Wt embryos, ultimately localizing to the developing adrenal glands and gonads at E11.5 and E12.5. Interestingly, in mice with Tg overexpression of Ad4BP/SF-1, the adrenal glands and gonads at E11.5 were not clearly resolved, and segmented signals extended anteriorly and laterally to the expanded urogenital ridge. This pattern is very similar to that seen in the transient transgenesis studies (Fig. 1B). By E12.5, the segmented ectopic expression expanded laterally, and the adrenal glands were obviously enlarged relative to Wt embryos.
The FAdE-Ad4BP-(Ad4BP/SF-1) transgene causes the formation of ectopic adrenal tissue in the thorax
To determine whether the ectopic lacZ-expressing cells in the thorax are authentic adrenocortical cells, we determined their expression of three steroidogenic enzymes: 3β-hydroxysteroid dehydrogenase (3β-Hsd), cholesterol side-chain cleavage enzyme (Cyp11a1), and 21-hydroxylase (Cyp21). Steroidogenic cells of both the adrenal gland and gonads express 3β-Hsd and Cyp11a1, whereas Cyp21 is expressed only in the adrenal cortex.
Consistent with previous studies, Ad4BP/SF-1 was expressed in both the adrenal glands and gonads in the Wt embryo at E13.5 (Fig. 2A), and all three steroidogenic markers were expressed in the Wt adrenal. In contrast, gonadal expression of 3β-Hsd and Cyp11a1 was limited to the interstitial region, and Cyp21 transcripts were not detected. In the FAdE-Ad4BP-(Ad4BP/SF-1) Tg embryo, the fields of expression of both Ad4BP/SF-1 and the steroidogenic genes were similarly expanded, and all three steroidogenic genes were expressed in the ectopic Ad4BP/SF-1-expressing cells. These results collectively suggest that the lacZ-positive cells observed in the thorax of Tg embryos express the steroidogenic markers, including Cyp21, and thus are bona fide adrenocortical cells. The ectopic cells expressing the steroidogenic enzymes were still present at 3 months of age (Fig. 2B). Thus, the overexpression of Ad4BP/SF-1 driven by the FAdE-Ad4BP-(Ad4BP/SF-1) transgene can evoke long-term changes in the gene expression profile of thoracic cells outside of the region where the adrenal cortex normally develops.
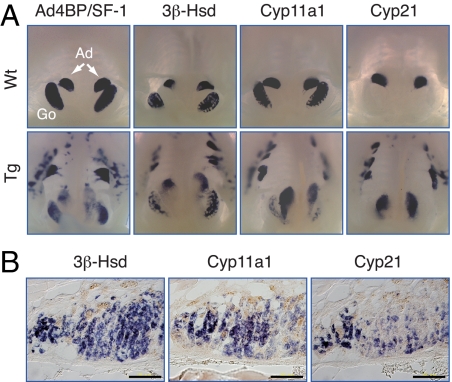
Figure 2.
The ectopic adrenal cells express steroidogenic enzymes. A, Expression of Ad4BP/SF-1 and its downstream target genes in FAdE-Ad4BP-(Ad4BP/SF-1) Tg mice. Shown are whole-mount in situ hybridization analyses of transcript for Ad4BP/SF-1 and three steroidogenic enzymes, 3β-Hsd (gonads and adrenal), Cyp11a1 (gonads and adrenal), and Cyp21 (adrenal specific) in Wt and FAdE-Ad4BP-(Ad4BP/SF-1) Tg embryos at E13. Ad, Adrenal; Go, gonad. B, Detection of three steroidogenic genes by section in situ hybridization. Transverse sections of the ectopic adrenal were subjected to in situ hybridization with adult (3 month old) Tg mice. Scale bars, 100 μm.
FAdE function is repressed in the ectopic adrenal tissue and adult adrenal cortex of Tg mice
Because FAdE function is maintained in an autoregulatory manner by Ad4BP/SF-1 during fetal age (17), we asked whether FAdE is activated until the adult age by Ad4BP/SF-1 overexpression. We performed X-gal staining combined with immunohistochemistry against Ad4BP/SF-1 on transverse sections of the ectopic adrenal tissue from young Tg mice [postnatal d 15(P15)]. Expression of lacZ varied greatly among the separate sites of the ectopic adrenal tissue. As shown in Fig. 3A, lacZ activity was limited to a few cells (left panel), whereas strong lacZ activity was observed in certain cell population (right panel). In the adrenal cortex of adult male mice, sparse lacZ activity was detected at the ages of 2 months and 7 months (Fig. 3B), although this activity was usually extinguished during puberty. These results demonstrate that many cells in the ectopic adrenal tissue and adult adrenal develop into adult (definitive) cells as the adult adrenal of the Wt.
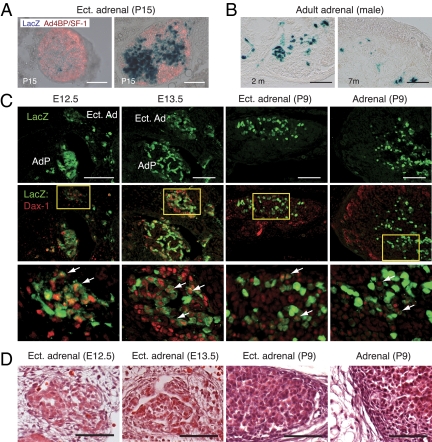
Figure 3.
FAdE function is repressed in the ectopic adrenal and adrenocortical cells of adult FAdE-Ad4BP-(Ad4BP/SF-1) Tg mice. A, Repression of lacZ expression in the ectopic adrenal of young Tg mouse (P15). Transverse sections of the ectopic adrenal tissue were prepared from the thoracic region of FAdE-Ad4BP-(Ad4BP/SF-1) Tg mice at P15 and subjected to X-gal staining (blue) combined with immunohistochemistry against Ad4BP/SF-1 (red). Merged images of lacZ and Ad4BP/SF-1 expression are shown. Scale bars, 100 μm. B, LacZ activity in the adult adrenals of the Tg. LacZ activity revealed by X-gal staining is detected in small numbers of the adrenocortical cells of 2- and 7-month old adult males. Scale bars, 100 μm. C, Expression of Dax-1 in the developing ectopic adrenal tissue. Expression of Dax-1 (red) and lacZ (green) was examined by immunohistochemistry in the transverse sections of the ectopic adrenal tissue of Tg mice at E12.5, E13.5, and P9 and adrenal cortex of P9. The areas enclosed by the rectangles in the middle panels are enlarged in the bottom panels. Arrows indicate cells expressing Dax-1, which show decreased expression of lacZ. Scale bars, 100 μm. D, Higher power views of hematoxylin-eosin (HE) staining of the ectopic adrenal tissue of Tg mice at E12.5, E13.5, and P9 and cortex of P9. AdP, Adrenal primordium; Ect. Ad, ectopic adrenal. Scale bars, 50 μm.
Recently, our studies have shown that the fetal adrenal enhancer (FAdE) is activated in all adrenocortical cells at E12.5 and is repressed gradually in cells of the outer adrenal layer. Moreover, we proposed that Dax-1 might play a crucial role for the eventual repression of FAdE function by suppressing the activity of Ad4BP/SF-1 in fetal adrenal cells (18). Therefore, we asked whether Dax-1 is expressed in the repression process in the ectopic adrenal tissue of the Tg mouse. The ectopic adrenal tissues at E12.5, E13.5, P9, and the adrenal at P9 were used. At E12.5, lacZ-positive, Dax-1-positive, and double-positive cells were distributed sporadically in the ectopic adrenal tissue (Fig. 3C). At E13.5, three types of cells were present, and lacZ-positive cells accumulated at the inner part of the ectopic adrenal tissue surrounded by Dax-1-positive and lacZ-negative cells. This distribution of the three types of the cells is basically similar in the ectopic adrenal tissue at P9 and adult adrenal. Interestingly, we noted that cells expressing Dax-1 show relatively decreased expression of lacZ (Fig. 3C, arrows). This inverse relationship suggests that Dax-1 suppresses FAdE function in cells transitioning from fetal to adult cells in the ectopic adrenal region as it occurs in the normal (Wt) adrenal (18). Furthermore, both ectopic adrenal tissue and enlarged adrenal glands of Tg mice were surrounded by fibrous capsular cells, comparable to those seen in Wt adrenal glands (Fig. 3D).
Ad4BP/SF-1 and its downstream gene expression levels at late developmental stages
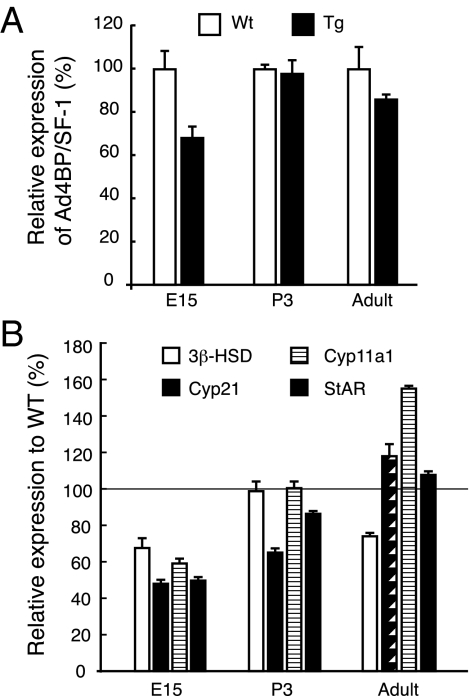
Next, we analyzed expression levels of Ad4BP/SF-1 and its downstream genes in the adrenal glands of the E15.5, P3, and adult Tg mice by quantitative RT-PCR. Unexpectedly, as shown in Fig. 4A, the mRNA level for Ad4BP/SF-1 in the Tg adrenal was decreased to 70% of Wt at E15.5, whereas it caught up to nearly 100% at neonatal stage. The mRNA level was slightly decreased to 85% of Wt at the adult stage. Moreover, we performed RT-PCR for downstream genes such as 3b-Hsd, Cyp11a1, Cyp21, and steroidogenic acute regulatory protein (StAR), all of which are necessary for steroidogenesis in the adrenal cortex. As shown in Fig. 4B, all genes examined were down-regulated to approximately 50–70% of Wt at E15.5. This decrease seems to be consistent with the decreased expression of Ad4BP/SF-1. Thereafter, their expressions became stronger at P3 and adult adrenals. These results suggest that the level of Ad4BP/SF-1 was finely controlled in vivo by an unknown mechanism, perhaps to normalize the effect of enlargement of the adrenal glands.
Figure 4.
Alteration of Ad4BP/SF-1 and its downstream gene expression levels. A, mRNA levels of Ad4BP/SF-1 in the adrenal glands of Wt and FAdE-Ad4BP-(Ad4BP/SF-1) Tg mice. Quantitative RT-PCR was performed using E15, P3, and adult adrenals of Wt and Tg mice. Total RNA was prepared from two mice for each sample as described in Materials and Methods. B, Downstream gene expression of Ad4BP/SF-1. The expression of downstream genes of Ad4BP/SF-1, 3b-Hsd, Cyp11a1, Cyp21 and steroidogenic acute regulatory protein (StAR), was determined by quantitative RT-PCR. The results were shown relative to Wt expression.
Developmental relationship of ectopic adrenocortical and sympathoadrenal progenitor cells in FAdE-Ad4BP-(Ad4BP/SF-1) Tg mice
The adrenal gland comprises two functionally distinct endocrine organs, the cortex and medulla. The cortex arises from the mesoderm-derived adrenogonadal primordium and normally forms anterior to the gonad. The medulla arises from neural crest-derived sympathoadrenal progenitors, which can differentiate into neurons of the sympathetic ganglia or can become chromaffin cells after migrating into the developing adrenal primordium.
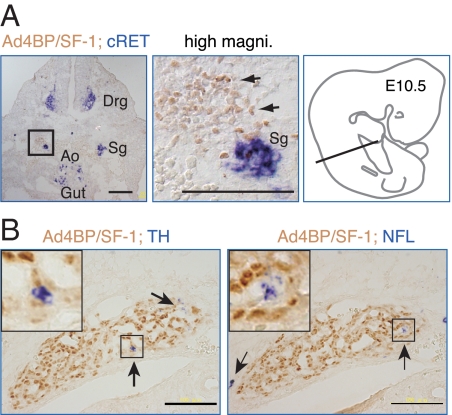
To determine whether the ectopic adrenal cells in the FAdE-Ad4BP-(Ad4BP/SF-1) Tg mice similarly maintained an intimate relationship with the sympathoadrenal precursors, we localized Ad4BP/SF-1 expression by immunohistochemistry and cRet expression by in situ hybridization. In cross-sections of Tg embryos at E10.5, as described previously (19), cRet was expressed in the sympathetic ganglia, dorsal root ganglia, and developing midgut (Fig. 5A). On higher power examination, the Ad4BP/SF-1-positive cells were localized immediately adjacent to the cRet-positive cells of the sympathetic ganglia. Thus, as normally seen in adrenal development, these adrenal precursor cells developed in close proximity to cells of the sympathoadrenal lineage.
Figure 5.
The ectopic adrenal tissue arises from cells adjacent to sympathoadrenal precursors. A, Transverse sections of E10.5 FAdE-Ad4BP-(Ad4BP/SF-1) Tg embryos at the level indicated in the schematic diagram of an E10.5 mouse embryo (right panel) were subjected to immunohistochemistry for Ad4BP/SF-1 and in situ hybridization for c-Ret, a marker of neurons of the sympathetic ganglia (sg). As shown by the arrows, Ad4BP/SF-1 (brown signal) is expressed in cells immediately adjacent to the sg (blue signal) in FAdE-Ad4BP-(Ad4BP/SF-1) Tg embryos. Drg, Dorsal root ganglia; Ao, aorta. Scale bars, 100 μm. B, The ectopic adrenal tissue contains scattered chromaffin cells. Sections containing the ectopic adrenal tissue of E13.5 Tg embryos were analyzed by immunohistochemistry for Ad4BP/SF-1 (brown signal) and either tyrosine hydroxylase (TH), a marker of catecholamine-synthesizing cells, or neurofilament 68 (NFL), a marker for neural crest derivatives. Arrows indicate blue cells expressing the sympathoadrenal cell markers, and the inserts show higher power views of the areas enclosed by the rectangles. Scale bar, 100 μm. Magni., Magnification.
Analyses of Ad4BP/SF-1 knockout mice have implicated the developing adrenocortical cells in the migration/differentiation of chromaffin cells (20). We therefore used in situ hybridization analyses of the catecholamine synthetic enzyme tyrosine hydroxylase and the neuroendocrine marker neurofilament 68 to determine whether the ectopic adrenal cells similarly recruited chromaffin cells. Intriguingly, at E13.5, a few cells expressing these neuroendocrine markers (arrows in Fig. 5B) were detected adjacent to the ectopic cells expressing Ad4BP/SF-1; this finding suggests that sympathoadrenal progenitor cells, possibly influenced by chemoattractant signals, can associate with the ectopic adrenal cells during development. However, based on the very limited number of blue cells, the recruitment process appears to be relatively inefficient.
Effects of Ad4BP/SF-1 overexpression on adrenal and urogenital development as revealed by adult FAdE-Ad4BP-(Ad4BP/SF-1) Tg mice
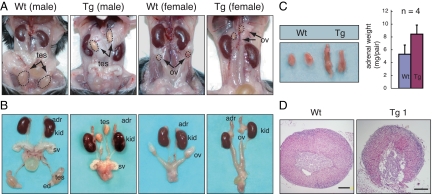
We next examined the effect of FAdE-directed Ad4BP/SF-1 overexpression on the adrenal glands and genitourinary tracts of 4-month-old adult Tg mice. In contrast to the Wt adrenal glands, those of the Tg mice were elongated in the rostral-caudal axis (Fig. 6) and weighed almost twice as much (Wt, 5.22 ± 1.41 mg/pair; Tg, 8.30 ± 1.39 mg/pair). Although the adrenal medulla normally is surrounded completely by the cortex, the medulla in Tg mice extended to the periphery of the gland, and there was a marked disorganization of the normal cortical architecture (Fig. 6D). Similar eccentric medullary displacement was reported in the adrenal glands of Ad4BP/SF-1 heterozygous (+/−) mice (21), although this has not been universally observed (Ref. 22 and M. Zubair, unpublished data).
Figure 6.
Abnormalities of the urogenital tracts and adrenals in adult FAdE-Ad4BP-(Ad4BP/SF-1) Tg mice. A, Adult male and female of Wt and FAdE-Ad4BP-(Ad4BP/SF-1) Tg mice are shown. Neither the testes (tes) nor the ovaries (ov) have descended normally in Tg mice. B, Dissected urogenital tracts and adrenal glands. Adrenal glands (adr) of the Tg mice were larger than those of Wt mice. Despite the abnormal location of the testes, the internal genitalia of the Tg mice were virilized normally. adr, Adrenal; tes, testis; sv, seminal vesicles; ed, epididymis; ov, ovary; kid, kidney. C, Dissected adrenal glands of Wt and FAdE-Ad4BP-(Ad4BP/SF-1) Tg mice, with corresponding weights (Wt, 5.22 ± 1.41 mg/pair; Tg, 8.30 ± 1.39 mg/pair). D, Sections of adrenal glands from Wt and Tg mice were stained with hematoxylin-eosin to reveal adrenal histology. The Tg adrenal glands were highly disorganized with eccentric location of the chromaffin cells. Scale bars, 200 μm.
In contrast to the normal position of the adrenal glands, both the testes and ovaries in the Tg mice remained adjacent to the kidneys. Despite their abnormal location, the testes in utero apparently produced both testosterone and anti-Müllerian hormone, as the internal genitalia (e.g. seminal vesicles and epididymis) were virilized and the Müllerian duct derivatives regressed (Fig. 6). However, the postnatal testes were smaller and had defective sperm maturation (supplemental Fig. 1 published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org), consistent with the known effects of cryptorchidism (23). Despite their abnormal location, the ovaries apparently were normal functionally; the females were fertile and the ovarian histology was normal (supplemental figure).
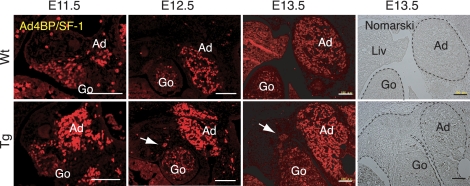
Because the gonads and adrenals arise from a common pool of Ad4BP/SF-1-expressing cells, the adrenogonadal primordium (1), it is plausible that incomplete separation of the two tissues might be associated with the abnormal gonadal location in the Tg mice. We therefore examined the distribution of Ad4BP/SF-1-expressing cells at E11.5–E13.5. In Wt embryos at E11.5, the Ad4BP/SF-1-expressing cells showed early evidence of separation into distinct gonadal and adrenal primordia separated by Ad4BP/SF-1-negative cells (Fig. 7). In the Tg embryo, in contrast, no such resolution of Ad4BP/SF-1-expressing cells into two pools was evident. By E12.5, the Ad4BP/SF-1-expressing cells in Wt embryos had largely resolved into two distinct populations that were separated by Ad4BP/SF-1-negative mesonephric cells. In Tg mice at the same stage, sporadic Ad4BP/SF-1-expressing cells remained distributed between the two primordia (arrows in Fig. 7). Complete separation becomes apparent in the Wt at E13.5, whereas it does not in the Tg with persistent presence of the connection formed by Ad4BP/SF-1 negative cells (Fig. 7, Nomarski view). Although not definitive, these data are consistent with the model that failure to achieve separation of the developing adrenals and gonads may contribute to the failure of the gonads to reach their normal location in the Tg mice.
Figure 7.
Aberrant distribution of Ad4BP/SF-1-expressing cells in the region of the developing steroidogenic organs. Immunohistochemical detection of Ad4BP/SF-1 (red) in the developing gonadal-adrenal region of Wt (upper panel) and FAdE-Ad4BP-(Ad4BP/SF-1) Tg embryos (lower panel) from E11.5 to E13.5 are shown. Sporadic Ad4BP/SF-1-positive cells are distributed between the two primordia (arrows) in the Tg sections. Nomarski views at E13.5 are shown in the right panel. The apparent signal in the liver results from autofluorescence of fetal erythrocytes. Scale bars, 100 μm. Ad, Adrenal; Go, gonad; Liv, liver.
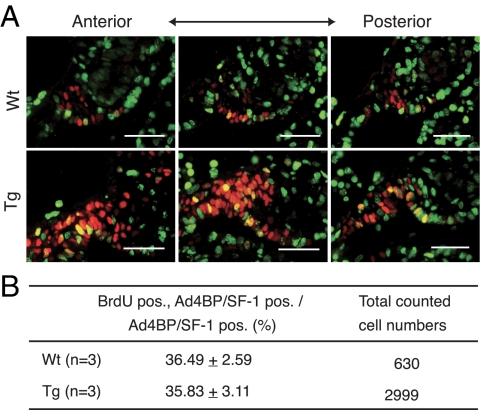
Cell proliferation is not altered in the fetal adrenal glands of FAdE-Ad4BP-(Ad4BP/SF-1) Tg mice
The observed increase in lacZ-expressing cells in Tg mice that overexpressed Ad4BP/SF-1 could reflect increased cell proliferation or decreased cell loss via programmed cell death. Consistent with the former model, forced overexpression of Ad4BP/SF-1 in adrenocortical cells in culture or in Tg mice promoted cell proliferation (16). We therefore examined proliferation in FAdE-Ad4BP-(Ad4BP/SF-1) Tg embryos at E9.5, a time when Ad4BP/SF-1 expression is already well established (see Fig. 1E). As shown in Fig. 8, the proliferation rate of Ad4BP/SF-1-positive cells at E9.5 did not differ significantly from that in Wt mice. Moreover, as assessed by terminal deoxynucleotide transferase-mediated dUTP nick end labeling assay, Tg and Wt mice did not differ significantly in apoptotic cell death in this region (data not shown). Thus, these studies argue that the increased number of Ad4BP/SF-1-positive cells caused by its Tg overexpression reflected neither increased proliferation nor decreased loss due to programmed cell death. Instead, the Tg overexpression of Ad4BP/SF-1 may direct uncommitted precursors toward the steroidogenic cell fate or may act to maintain the steroidogenic capacity in cells that normally lose this capacity.
Figure 8.
Cell proliferation is not increased in FAdE-Ad4BP-(Ad4BP/SF-1) Tg mice. A, Immunohistochemical detection of BrdU (green) and Ad4BP/SF-1 (red) in the adrenal cortex of Wt (upper panel) and FAdE-Ad4BP-(Ad4BP/SF-1) Tg (lower panel) embryos at E9.5. Superimposed images of BrdU and Ad4BP/SF-1 signals are shown. Scale bars, 50 μm. B, Summary of quantitative analyses of cell proliferation rate and cell number as described in Materials and Methods. pos, Postitive.
Discussion
Ad4BP/SF-1 as a master regulator of the steroidogenic lineage
The generation of ectopic adrenal cells in the FAdE-Ad4BP-(Ad4BP/SF-1) Tg mice demonstrates that Ad4BP/SF-1 can direct cell fate toward the steroidogenic lineage. Although such ectopic activity was seen in several embryos in transient transgenesis studies (Fig. 1), only one of four Tg lines supported the formation of ectopic adrenal tissue. Similarly, whereas Ad4BP/SF-1 overexpression driven by a YAC transgene produced adrenocortical hyperplasia and tumorigenesis, ectopic adrenal tissue was not described (16). Thus, we hypothesize that the diversion of uncommitted mesodermal precursors in thoracic regions to the steroidogenic lineage may require especially high levels of Ad4BP/SF-1. In fact, formation of ectopic adrenal becomes apparent in the Tg line 6 with high copy number (15–16 copies), whereas it does not in the Tg line 4 with low copy number (Fig. 1). We further propose that the enhanced expression of Ad4BP/SF-1 in a pool of cells that potentially serve as progenitors of the adrenal cortex likely increases the size of the adrenal gland by directing more of these cells toward an adrenocortical phenotype. A similar increase in ultimate organ size resulted from increasing the number of progenitor cells in the pancreas (24). Another possible reason to give rise to the different phenotypes between the YAC Tg and our Tg mice might be due to the difference in the constructs. YAC construct is expected to carry an adult adrenal enhancer, which has not yet been identified, as well as FAdE. Therefore, Ad4BP/SF-1 might be overexpressed in the adult adrenocortical cells, leading to hyperplastic adrenals.
In human beings, ectopic or accessory adrenal tissue usually is found in the upper abdomen or along the path of gonadal descent (25). Other reported sites of ectopic adrenal tissue include the gall bladder (26), liver (27), and the spinal cord (28). A recent report described thoracic adrenal tissue that expressed StAR, CYP11A1, 3β-HSD, CYP11B1, CYP17, CYP21, and the medullary marker tyrosine hydroxylase; based on this expression pattern, the ectopic tissue presumably was capable of steroid hormone and catecholamine biosynthesis (29). The authors speculated that more careful examination may reveal similar ectopic adrenal tissue in other human subjects. More commonly, ectopic adrenal tissue is described in boys with cryptorchidism or as testicular rests in patients with congenital adrenal hyperplasia; the ectopic tissue in these cases may reflect the common embryological origin of the adrenal cortex and gonads (25,30). We speculate that the ectopic adrenal tissue in human subjects also may have resulted from overexpression of Ad4BP/SF-1 during early development. Although the present model is difficult to examine in human beings, based on the case of our Tg mouse, the presence of the ectopic adrenocortical tissues in the inguinoscrotal region in boys may interrupt or delay the separation of the gonads from surrounding tissue and thus cause undescended testes, also known as cryptorchidism.
Several lines of evidence implicate Ad4BP/SF-1 in the determination of steroidogenic cell fate. Stable expression of Ad4BP/SF-1 in mouse embryonic stem cells induced the expression of the steroidogenic enzyme Cyp11a1, although it did not activate the entire steroidogenic pathway (11). More recently, Ad4BP/SF-1 overexpression in primary long-term cultured bone marrow or adipose tissue-derived stem cells stimulated the acquisition of steroidogenic competence (12,14,15). These studies, along with results described here, indicate that Ad4BP/SF-1 is a key factor in the determination of steroidogenic cell fate.
In fish, development of the interrenal gland, which serves as the adrenal equivalent, is tightly coupled to pronephric development, and both interrenal and pronephric primordial cells arise from the same region of the lateral intermediate mesoderm (31,32). Hence, the ectopic adrenal cells in the thoracic region described here may likewise originate from primitive pronephric cells that can be induced by Ad4BP/SF-1 to become steroidogenic cells.
The proper level of Ad4BP/SF-1 is necessary for normal adrenal formation
In mice, several lines of evidence suggest that Ad4BP/SF-1 gene dosage, presumably by affecting levels of Ad4BP/SF-1 protein, is critical for adrenal development. Embryos heterozygous for the Ad4BP/SF-1 knockout allele have hypoplastic adrenals (9); a comparable phenomenon was observed in M33 knockout mice, in which Ad4BP/SF-1 expression is diminished to approximately half that of Wt mice (33). Previous studies suggested that the adrenal impairment in the setting of Ad4BP/SF-1 haploinsufficiency reflects a decreased number of adrenal progenitor cells at E10.0, as detected by double labeling for GATA-4 and Ad4BP/SF-1 (9). Consistent with this critical effect of Ad4BP/SF-1 expression in adrenal development, bacterial artificial chromosome Tg expression of Ad4BP/SF-1 rescued the gonadal but not the adrenal defect of Ad4BP/SF-1 knockout mice (34).
Data presented here and elsewhere (18) suggest that ectopic adrenal formation from uncommitted precursors with steroidogenic potential normally is prevented in at least two ways. First, autoregulatory effects of Ad4BP/SF-1 maintain FAdE function via cognate binding sites within the FAdE itself (17). Thus, the level of Ad4BP/SF-1 expression in the uncommitted precursor cells normally falls below a threshold needed to maintain FAdE activity, a threshold that presumably was exceeded by our Tg overexpression of Ad4BP/SF-1. Second, the expression of the atypical nuclear receptor Dax-1 at early developmental stages may block Ad4BP/SF-1 from maintaining FAdE function in these precursor cells with steroidogenic potential. Consistent with its potential role in modulating the ultimate developmental fate of cells in which the FAdE is active, Dax-1 is expressed in the thoracic adrenocortical cells. Further studies that explore the effect of differing levels of Ad4BP/SF-1 overexpression or that modulate Dax-1 activity in these cells will be needed to provide definitive support for these models.
Materials and Methods
DNA construction and generation of Tg mice
The Ad4BP/SF-1 cDNA (SmaI-SacI fragment) from pCMX-Elp3 (35) was cloned upstream of the SV40 poly(A) sequence using fill-in reaction to blunt the SacI end. Thereafter, a SplI-NotI fragment from newly cloned vector was inserted downstream of an Ad4BP/SF-1 Tg cassette containing the FAdE and 5.8 kb of 5′-flanking region (17) to generate FAdE-Ad4BP-(Ad4BP/SF-1). This plasmid was digested with KpnI-NotI, and linearized DNA was used for microinjection. Tg mice were generated as described (36). Founder mice were identified by PCR with primers for Ad4BP/SF-1 (Table 1)that amplified a DNA fragment specific for the transgene. To determine transgene expression (Fig. 1C), total RNA was prepared from the adrenal glands at P9 using ISOGEN (Nippon Gene, Japan) and subjected to RT-PCR with primers that amplified either endogenous Ad4BP/SF-1 as a control or the transgene-derived transcript. Mice were maintained and experimental protocols were performed in accord with the guidelines of Institutional Animal Care and Use Committee of the National Institute for Basic Biology.
Table 1.
Primers used in this study
| Primer’s name | Sequences |
|---|---|
| Ad4BP/SF-1 (qRT-PCR) | CCCTTATCCGGCTGAGAATT and CCAGGTCCTCGTCGTACGA |
| 3b-Hsd (qRT-PCR) | GTTGTCATCCACACTGCTGC and AAGATGAAGGCTGGCACACT |
| Cyp21 (qRT-PCR) | TCTACCTGCTTGGCCTCACT and AAGGCCTCCTCAATGGTTCT |
| Cyp11a1 (qRT-PCR) | CGATACTCTTCTCATGCGAG and CTTTCTTCCAGGCATCTGAAC |
| StAR (qRT-PCR) | CTTGGCTGCTCAGTATTGAC and TGGTGGACAGTCCTTAACAC |
| Cyclophilin (qRT-PCR) | TGGAGAGCACCAAGACAGACA and TGCCGGAGTCGACAATGAT |
| FAdE (qPCR, copy number) | GGTTTATTGAGACACAGTGCG and GGCCAACAGGCTCCGTAATTC |
| Cyp11a1 (qPCR, copy number) | GGCAAACGATGGTGACAACA and GGGAGTCGGCAGGACACTT |
| Ad4BP/SF-1 (ex 4–7, founder Tg) | GACCAGATGACACTGCTGC and TCCTTGGCCTGCATGCTCA |
| Ad4BP/SF-1 (ex 7, Fig. 1C), | ATGCCACTGCCTCCAAAAGAC and GGGTTAGGGCAGGAATGTTGG |
| A4BP/SF-1 (ex 7-Sv40 PA, transgene exp. Fig. 1C) | CAGGAGTTCGTCTGTCTCAAG and ATGTGGTATGGCTGATTATGAT |
| cRet cDNA (in situ hyb. probe) | cctagaattCGGCTGTCCCTCTATGTGCTG and gccgtctagaTGGGGAGGGTGATTTGTTTCC |
| TH cDNA (in situ hyb. probe) | ggagggatccGGAAGCTGATTGCAGAGATTG and cacggaattcTGGGAGAACTGGGCAAATGTG |
| NFL cDNA (in situ hyb. probe) | cctagaattcACAGCCTGATGGACGAGATAG and CTGGTGAAACTGAGCCTGGTC |
Lowercase letters were artificially added for cloning. Ex, Exon; exp., experiment; hyb., hybridization; TH, tyrosine hydroxylase; NFL, neurofilament 68; qPCR, quantitative PCR; qRT-PCR, quantitative RT-PCR; Sv40, simian virus 40.
X-gal staining and immunohistochemistry
Whole-embryo staining for LacZ activity of Tg embryos at the indicated time points was examined as described elsewhere (36). For immunohistochemical and histological analyses, samples were fixed for 2–3 h in paraformaldehyde (PFA)/PBS at 4 C and then embedded in paraffin according to standard protocol and processed as described (18). A rabbit polyclonal antibody for Ad4BP/SF-1 (1:1500) (37) was detected with Cy3-conjugated goat antirabbit (Jackson ImmunoResearch Laboratories, West Grove, PA). For double-immunofluorescence analyses, the sections were deparaffinized, rehydrated, and boiled in a microwave oven for 10 min in 10 mm citrate (pH 2.0) for antigen retrieval. After washing with PBS for 5 min three times, the sections were blocked with 2% skim milk in PBS for 30 min and incubated with a guinea pig polyclonal antibody for Dax-1 (1:1000) (38), and rabbit anti-β-galactosidase (1:1500) [provided by Drs. K. Mihara and M. Sakaguchi (Kyushu University)] overnight at 4 C. After washing, the sections were incubated with Alexa 488-goat-antirabbit antibody (Invitrogen, Carlsbad, CA) together with Cy3-conjugated donkey antiguinea pig (Jackson ImmunoResearch Laboratories), washed with PBS, and mounted.
For combined analysis of lacZ activity and Ad4BP/SF-1 gene expression, samples were fixed in 0.5% PFA for 2 h at 4 C, washed with PBS, immersed with 20% sucrose for 3 h at 4 C, and then embedded in optimum cutting temperature compound. Transverse cryosections cut to 10 mm thickness were processed for lacZ staining for 2–3 h at 37 C as described elsewhere (36). The sections were fixed again in 4% PFA for 5 min at room temperature, followed by immunohistochemical analysis with the anti-Ad4BP/SF-1 antibody as described above.
Cell proliferation analysis using bromodeoxyuridine (BrdU)
To label proliferating cells, pregnant mice received a single ip injection of BrdU Sigma Chemical Co., St. Louis, MO; B-5002) (50 mg BrdU per kg of body weight) as described elsewhere (18). The pregnant females were killed 1 h after injection. The embryos were removed and fixed for 2 h in 4% PFA in PBS (PBS; PFA/PBS) at 4 C, and embedded in paraffin with standard procedures. BrdU incorporation was detected with a mouse monoclonal anti-BrdU antibody (Sigma, B8434; 1:500), whereas adrenocortical cells were detected using rabbit polyclonal antibody for Ad4BP/SF-1 (1:1500) (37). The adrenal glands were sectioned transversely at 5 μm thickness; immunohistochemical analyses were performed on every tenth of serial sections (a total of 16 or 25 sections, spanning roughly half of the adrenal gland from three embryos of Wt and Tg, respectively). For antigen retrieval, sections were deparaffinized, rehydrated, and boiled in a microwave oven for 10 min in 10 mm sodium citrate (pH 2.0). After washing with PBS for 5 min three times, the sections were blocked with 2% skim milk in PBS for 30 min and incubated overnight at 4 C with anti-BrdU antibody together with anti-Ad4BP/SF-1 antibody. After washing, the sections were incubated with Alexa 488-conjugated goat antimouse antibody (Invitrogen) for 1–2 h, and then with Cy3-conjugated goat antirabbit (Jackson ImmunoResearch Laboratories). After washing three times with PBS, they were mounted for fluorescence imaging. Three embryos at E9.5 (27–28 somites) from each genotype were analyzed.
In situ hybridization
Whole-mount and section in situ hybridizations were performed as described (18,39), respectively, with digoxigenin- labeled probes (Roche Molecular Biochemicals, Mannheim, Germany). The probes included: Ad4BP/SF-1 (accession no. AB000490, 192-2407), 3β-hydroxysteroid dehydrogenase (3β-Hsd, accession no. NM_008293, 512-1523), cholesterol side-chain cleavage enzyme (Cyp11a1, accession no. NM_019779, 132-691), and 21-hydroxylase (Cyp21, accession no. NM_ 009995, 469-1554) (18). Probes for cRet, tyrosine hydroxylase, and neurofilament 68 were synthesized by PCR and cloned into pBluescript II SK (Stratagene, La Jolla, CA) using primers shown in Table 1. To combine in situ hybridization analyses with immunohistochemical detection of Ad4BP/SF-1, the sections were first analyzed by in situ hybridization and then were fixed with 4% PFA for 15 min, washed with PBS, incubated in 3% H2O2 for 15 min, and processed for immunohistochemistry with antibody for Ad4BP/SF-1. Biotinylated antirabbit IgG (Nichirei Co., Tokyo, Japan) was used as the secondary antibody. Antibody-antigen complexes were detected by the streptavidin-biotin-peroxidase system using the Histofine kit (Nichirei Co.).
Quantitative PCR
RNA was isolated from adrenal using RNeasy mini (QIAGEN, Chatsworth, CA) according to the manufacturer’s protocol. The total RNA was treated with ribonuclease-free deoxyribonuclease (Roche), and then reverse transcribed with random hexamers using SuperScript II (Invitrogen). Quantitative PCR was performed using an Applied Biosystem 7900 HT sequence detection system (Applied Biosystems, Foster City, CA). Reactions were performed with a 2× SYBR Green PCR mastermix (Applied Biosystems), and gene-specific primers were designed located between two exons to avoid genomic amplification (Table 1). Each quantitative measurement was performed in triplicate. Transcript abundance was normalized in each sample to the average Ct value for mouse cyclophilin (40). For genomic quantitative PCR, FAdE primers (Table 1) were used, and DNAs were prepared from tails, and mixed with 2× SYBR Green PCR mastermix (Applied Biosystems) as described above. The results were normalized to Ct value for Cyp11a1 as a control (Table 1).
Supplementary Material
Acknowledgments
We thank Drs. K. Mihara and M. Sakaguchi (Kyushu University, Fukuoka, Japan) for providing the anti-β-galactosidase antibody, Professor O. Niwa (Hiroshima University, Hiroshima, Japan) for kindly providing pCMX-Elp3, Ms. Hiroe Kowa (National Institute for Basic Biology, Okazaki, Japan), for technical assistance, Etoh Tomoo (National Institute for Basic Biology, Okazaki, Japan) for helpful suggestions on generation of the Tg mice, and A. L. Reuter and N. R. Stallings (University of Texas Southwestern Medical Center, Dallas, TX) for reading and helpful discussions about the manuscript. K. Morohashi and M. Zubair are grateful to K. Parker for his generosity, friendship, and fairness. It is our great honor to live in the time when he lived, and to have studied the same transcription factor, SF-1 and Ad4BP.
Footnotes
This work was supported by Grants-in-Aid for Scientific Research on Priority Areas and Grant-in-Aid for Scientific Research (B) from the Ministry of Education, Culture, Sports Science, and Technology of Japan (to K.-i.M.) and National Institutes of Health Grants DK54480 and HD046743 (to K.L.P.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online July 23, 2009
Abbreviations: Ad4BP/SF-1, Adrenal 4 binding protein/steroidogenic factor 1; BrdU, bromodeoxyuridine; E12.5, embryonic d 12.5; FadE, fetal adrenal enhancer; 3β-Hsd, 3β-hydroxysteroid dehydrogenase; P15, postnatal d 15; PFA, paraformaldehyde; Tg, transgenic; Wt, wild type.
References
- Hatano O, Takakusu A, Nomura M, Morohashi K 1996 Identical origin of adrenal cortex and gonad revealed by expression profiles of Ad4BP/SF-1. Genes Cells 1:663–671 [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Shen WH, Ingraham HA, Parker KL 1994 Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol Endocrinol 8:654–662 [DOI] [PubMed] [Google Scholar]
- Keegan CE, Hammer GD 2002 Recent insights into organogenesis of the adrenal cortex. Trends Endocrinol Metab 13:200–208 [DOI] [PubMed] [Google Scholar]
- Luo X, Ikeda Y, Parker KL 1994 A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77:481–490 [DOI] [PubMed] [Google Scholar]
- Shinoda K, Lei H, Yoshii H, Nomura M, Nagano M, Shiba H, Sasaki H, Osawa Y, Ninomiya Y, Niwa O, Morohashi K, Li E 1995 Developmental defects of the ventromedial hypothalamic nucleus and pituitary gonadotroph in the Ftz-F1 disrupted mice. Dev Dyn 204:22–29 [DOI] [PubMed] [Google Scholar]
- Morohashi K, Tsuboi-Asai H, Matsushita S, Suda M, Nakashima M, Sasano H, Hataba Y, Li CL, Fukata J, Irie J, Watanabe T, Nagura H, Li E 1999 Structural and functional abnormalities in the spleen of an mFtz-F1 gene-disrupted mouse. Blood 93:1586–1594 [PubMed] [Google Scholar]
- Ingraham HA, Lala DS, Ikeda Y, Luo X, Shen WH, Nachtigal MW, Abbud R, Nilson JH, Parker KL 1994 The nuclear receptor steroidogenic factor 1 acts at multiple levels of the reproductive axis. Genes Dev 8:2302–2312 [DOI] [PubMed] [Google Scholar]
- Sadovsky Y, Crawford PA, Woodson KG, Polish JA, Clements MA, Tourtellotte LM, Simburger K, Milbrandt J 1995 Mice deficient in the orphan receptor steroidogenic factor 1 lack adrenal glands and gonads but express P450 side-chain-cleavage enzyme in the placenta and have normal embryonic serum levels of corticosteroids. Proc Natl Acad Sci USA 92:10939–10943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland ML, Fowkes RC, Ingraham HA 2004 Differential requirement for steroidogenic factor-1 gene dosage in adrenal development versus endocrine function. Mol Endocrinol 18:941–952 [DOI] [PubMed] [Google Scholar]
- Park SY, Meeks JJ, Raverot G, Pfaff LE, Weiss J, Hammer GD, Jameson JL 2005 Nuclear receptors Sf1 and Dax1 function cooperatively to mediate somatic cell differentiation during testis development. Development 132:2415–2423 [DOI] [PubMed] [Google Scholar]
- Crawford PA, Sadovsky Y, Milbrandt J 1997 Nuclear receptor steroidogenic factor 1 directs embryonic stem cells toward the steroidogenic lineage. Mol Cell Biol 17:3997–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondo S, Yanase T, Okabe T, Tanaka T, Morinaga H, Nomura M, Goto K, Nawata H 2004 SF-1/Ad4BP transforms primary long-term cultured bone marrow cells into ACTH-responsive steroidogenic cells. Genes Cells 9:1239–1247 [DOI] [PubMed] [Google Scholar]
- Tanaka T, Gondo S, Okabe T, Ohe K, Shirohzu H, Morinaga H, Nomura M, Tani K, Takayanagi R, Nawata H, Yanase T 2007 Steroidogenic factor 1/adrenal 4 binding protein transforms human bone marrow mesenchymal cells into steroidogenic cells. J Mol Endocrinol 39:343–350 [DOI] [PubMed] [Google Scholar]
- Gondo S, Okabe T, Tanaka T, Morinaga H, Nomura M, Takayanagi R, Nawata H, Yanase T 2008 Adipose tissue-derived and bone marrow-derived mesenchymal cells develop into different lineage of steroidogenic cells by forced expression of SF-1. Endocrinology 149:4717–4725 [DOI] [PubMed] [Google Scholar]
- Yazawa T, Mizutani T, Yamada K, Kawata H, Sekiguchi T, Yoshino M, Kajitani T, Shou Z, Umezawa A, Miyamoto K 2006 Differentiation of adult stem cells derived from bone marrow stroma into Leydig or adrenocortical cells. Endocrinology 147:4104–4111 [DOI] [PubMed] [Google Scholar]
- Doghman M, Karpova T, Rodrigues GA, Arhatte M, De Moura J, Cavalli LR, Virolle V, Barbry P, Zambetti GP, Figueiredo BC, Heckert LL, Lalli E 2007 Increased steroidogenic factor-1 dosage triggers adrenocortical cell proliferation and cancer. Mol Endocrinol 21:2968–2987 [DOI] [PubMed] [Google Scholar]
- Zubair M, Ishihara S, Oka S, Okumura K, Morohashi K 2006 Two-step regulation of Ad4BP/SF-1 gene transcription during fetal adrenal development: initiation by a Hox-Pbx1-Prep1 complex and maintenance via autoregulation by Ad4BP/SF-1. Mol Cell Biol 26:4111–4121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubair M, Parker KL, Morohashi K 2008 Developmental links between the fetal and adult zones of the adrenal cortex revealed by lineage tracing. Mol Cell Biol 28:7030–7040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachnis V, Mankoo B, Costantini F 1993 Expression of the c-ret proto-oncogene during mouse embryogenesis. Development 119:1005–1017 [DOI] [PubMed] [Google Scholar]
- Gut P, Huber K, Lohr J, Brühl B, Oberle S, Treier M, Ernsberger U, Kalcheim C, Unsicker K 2005 Lack of an adrenal cortex in Sf1 mutant mice is compatible with the generation and differentiation of chromaffin cells. Development 132:4611–4619 [DOI] [PubMed] [Google Scholar]
- Bland ML, Jamieson CA, Akana SF, Bornstein SR, Eisenhofer G, Dallman MF, Ingraham HA 2000 Haploinsufficiency of steroidogenic factor-1 in mice disrupts adrenal development leading to an impaired stress response. Proc Natl Acad Sci USA 97:14488–14493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu PS, Bavers DL, Beuschlein F, Shah S, Jeffs B, Jameson JL, Hammer GD 2002 Interaction between Dax-1 and steroidogenic factor-1 in vivo: increased adrenal responsiveness to ACTH in the absence of Dax-1. Endocrinology 143:665–673 [DOI] [PubMed] [Google Scholar]
- Hutson JM, Hasthorpe S, Heyns CF 1997 Anatomical and functional aspects of testicular descent and cryptorchidism. Endocr Rev 18:259–280 [DOI] [PubMed] [Google Scholar]
- Stanger BZ, Tanaka AJ, Melton DA 2007 Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature 445:886–891 [DOI] [PubMed] [Google Scholar]
- Ozel SK, Kazez A, Akpolat N 2007 Presence of ectopic adrenocortical tissues in inguinoscrotal region suggests an association with undescended testis. Pediatr Surg Int 23:171–175 [DOI] [PubMed] [Google Scholar]
- Busuttil A 1974 Ectopic adrenal within the gall-bladder wall. J Pathol 113:231–233 [DOI] [PubMed] [Google Scholar]
- Tajima T, Funakoshi A, Ikeda Y, Hachitanda Y, Yamaguchi M, Yokota M, Yabuuchi H, Satoh T, Koga M 2001 Nonfunctioning adrenal rest tumor of the liver: radiologic appearance. J Comput Assist Tomogr 25:98–101 [DOI] [PubMed] [Google Scholar]
- Cassarino DS, Santi M, Arruda A, Patrocinio R, Tsokos M, Ghatak N, Quezado M 2004 Spinal adrenal cortical adenoma with oncocytic features: report of the first intramedullary case and review of the literature. Int J Surg Pathol 12:259–264 [DOI] [PubMed] [Google Scholar]
- Shigematsu K, Toriyama K, Kawai K, Takahara O 2007 Ectopic adrenal tissue in the thorax: a case report with in situ hybridization and immunohistochemical studies. Pathol Res Pract 203:543–548 [DOI] [PubMed] [Google Scholar]
- Ketata S, Ketata H, Sahnoun A, FakhFakh H, Bahloul A, Mhiri MN 2008 Ectopic adrenal cortex tissue: an incidental finding during inguinoscrotal operations in pediatric patients. Urol Int 81:316–319 [DOI] [PubMed] [Google Scholar]
- Grassi Milano E, Basari F, Chimenti C 1997 Adrenocortical and adrenomedullary homologs in eight species of adult and developing teleosts: morphology, histology, and immunohistochemistry. Gen Comp Endocrinol 108:483–496 [DOI] [PubMed] [Google Scholar]
- Hsu HJ, Lin G, Chung BC 2003 Parallel early development of zebrafish interrenal glands and pronephros: differential control by wt1 and ff1b. Development 130:2107–2116 [DOI] [PubMed] [Google Scholar]
- Katoh-Fukui Y, Owaki A, Toyama Y, Kusaka M, Shinohara Y, Maekawa M, Toshimori K, Morohashi K 2005 Mouse Polycomb M33 is required for splenic vascular and adrenal gland formation through regulating Ad4BP/SF1 expression. Blood 106:1612–1620 [DOI] [PubMed] [Google Scholar]
- Fatchiyah, Zubair M, Shima Y, Oka S, Ishihara S, Fukui-Katoh Y, Morohashi K 2006 Differential gene dosage effects of Ad4BP/SF-1 on target tissue development. Biochem Biophys Res Commun 341:1036–1045 [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Okada M, Kotomura N, Suzuki K, Tsukiyama T, Niwa O 1995 Genomic organization and isoforms of the mouse ELP gene. J Biochem 118:380–389 [DOI] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Constantini F, Lacy E 1994 Manipulating the mouse embryo: a laboratory manual, 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- Morohashi K, Iida H, Nomura M, Hatano O, Honda S, Tsukiyama T, Niwa O, Hara T, Takakusu A, Shibata Y, Omura T 1994 Functional difference between Ad4BP and ELP, and their distributions in steroidogenic tissues. Mol Endocrinol 8:643–653 [DOI] [PubMed] [Google Scholar]
- Mukai T, Kusaka M, Kawabe K, Goto K, Nawata H, Fujieda K, Morohashi K 2002 Sexually dimorphic expression of Dax-1 in the adrenal cortex. Genes Cells 7:717–729 [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Nieto MA 1993 Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol 225:361–373 [DOI] [PubMed] [Google Scholar]
- Chuang JC, Cha JY, Garmey JC, Mirmira RG, Repa JJ 2008 Research resource: nuclear hormone receptor expression in the endocrine pancreas. Mol Endocrinol 22:2353–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.