Abstract
Recent studies have shown that estrogens promote the growth of lung cancer cells and may potentially be responsible for increased susceptibility to lung cancer in women. These observations raise the possibility of using antiestrogens in treating and preventing lung cancer. However, it is not clear how estrogen receptors (ERs) modulate the growth of non-small cell lung cancer (NSCLC) cells. Our Western blotting and real-time PCR analysis showed that NSCLC cells expressed ERβ, but not ERα. In addition, ERβ-specific ligands, but not ERα-specific ligands, promoted the growth of lung cancer cells. Furthermore, knockdown of ERβ by short hairpin RNA constructs resulted in loss of estrogen-dependent growth of lung cancer cells. Interestingly, endogenous ERβ failed to transcriptionally activate estrogen response element (ERE)-luciferase constructs in NSCLC cells, suggesting a lack of genomic function. Upon further investigation, ERβ was found to be in the cytoplasm in all lung cancer cells and failed to translocate to the nucleus in the presence of estrogen, as observed by biochemical, ArrayScan, and confocal microscopy experiments. Nonetheless, estrogen caused rapid activation of cAMP, Akt, and MAPK signaling pathways in lung cancer cells. Immunohistochemical analysis of lung tumor biopsies showed strong ERβ staining in the cytoplasm, whereas no staining was observed for ERα. In conclusion, our results suggest that that proliferative effects of estrogen in lung cancer cells is mediated primarily, if not exclusively, by the nongenomic action of ERβ.
Estrogenic signaling in lung cancer cells is mediated through ERβ, but not ERα, and functions through non-genomic mechanisms.
There is increasing evidence to show that estrogens may contribute to lung cancer risk in women. Lung adenocarcinoma, which shows weaker association with tobacco smoke than other types of lung cancer, is found predominantly in women, suggesting a possible role for estrogens in the development of the disease (1) .
Estrogens are key signaling molecules that regulate various physiological processes, such as cell growth, development, and differentiation, and also play a major role in many pathological processes of hormone-dependent diseases (for review, see Ref. 2). Estrogens exert their biological effect through two estrogen receptor (ER) subtypes, ERα and ERβ. In the classical model of estrogen action, referred to as genomic function, ERs are in an inactive conformation and are sequestered in a multiprotein complex involving heat shock proteins in the absence of the ligand. Binding of the ligand induces a conformational change in the receptor, resulting in dissociation of heat shock proteins and corepressors and recruitment of coactivators with histone modification activities. Thus, by the binding of ERs to estrogen response elements (EREs) in the promoter regions of target genes, transcriptional regulation occurs in a ligand-dependent manner (3). In many cases, ERs can also modulate non-ERE-containing genes by interacting with the DNA-bound transcription factors such as AP-1 (4), Sp1 (5), and nuclear factor-κB (NF-κB) (6).
In addition to transcriptional activation in the nucleus, estrogen action occurs at the cell surface within minutes after administration of 17β-estradiol (E2). This nongenomic function of ER involves rapid activation of many signaling molecules, such as IGF-I and epidermal growth factor (EGF) receptors, MAPK, Akt, protein kinase C, and release of calcium and nitric oxide. It is believed that the liganded receptor assembles as a part of a large signalsome complex that includes G proteins, receptor tyrosine kinases such as IGF-IR and EGF receptor, and Src family adaptor proteins (for review, see Refs. 7 and 8). An ERα deletion mutant that lacks the N terminus can still activate signaling at the membrane as efficiently as wild-type protein, suggesting that the membrane-localizing function of ER is facilitated by the ligand-binding domain (LBD) of the receptor (9). Further, Ser 522 and Cys 447 residues in the LBD play an important role in interaction of ERα with caveolin-1 and subsequent membrane translocation (10,11). Cys 447 residue is also the site of posttranslational modification such as palmitoylation (11), characteristic of membrane-associated proteins. Recent work has identified the motifs in the LBD region of ERα and ERβ that are required for membrane localization and function (12).
A number of recent studies have provided strong evidence for the role of ERs in lung cancer. Estrogens promote growth of non-small cell lung cancer (NSCLC) cells, whereas antiestrogens inhibit them (13,14,15), suggesting that the ER pathway can be a potential target for lung cancer treatment and prevention. Transgenic mice expressing a luciferase reporter construct under the control of estrogen response element display an increase in luciferase activity in their lungs upon E2 treatment, indicating that the lung is an estrogen-responsive tissue (16). Treatment of rats and mice with combined carcinogenic and estrogenic compounds led to an increase in lung tumors when compared with carcinogen treatment alone, suggesting the role of estrogens in tumor progression (17,18). Furthermore, ERβ also plays an important role in normal lung biology. Targeted disruption of ERβ in mice results in abnormal lung structure and systemic hypoxia (19).
Although these studies reveal the importance of estrogenic signaling in lung cancer cells, they do not examine the molecular mechanisms by which ERs promote the growth of lung cancer cells. For example, it is not clear which ER isotype is involved in mediating these estrogenic effects in the lung (14,20), and whether ER signals through either genomic or nongenomic pathways. Furthermore, there have been conflicting reports of the presence of ERs in lung tumors. Some studies have reported the expression of both ERα and ERβ in lung tumors (20,21), whereas others have reported only ERβ expression (22,23).
In this study, we have systematically examined the function of ERα and ERβ in non-small lung cancer (NSCLC) cells. We show by real-time PCR and Western blotting analysis that ERβ is the predominant isotype expressed in lung cancer cells. We show that endogenous ERβ is localized in cytoplasm of NSCLC cells and is unable to translocate to nucleus after estrogen addition. Treatment of NSCLC cells with estrogen caused rapid activation of cAMP, MAPK, and Akt signaling pathways, suggesting that estrogen-dependent growth of lung cancer cells is through nongenomic actions of ERβ. Immunohistochemical analysis of primary lung tumor specimens reveals predominant ERβ staining in the cytoplasm.
Results
ERβ mediates estrogen-dependent growth of lung cancer cells
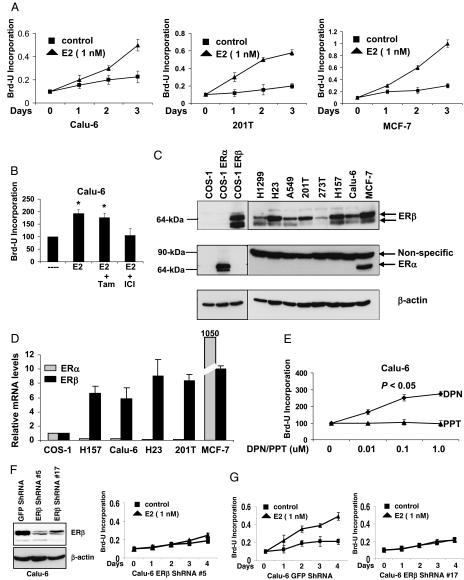
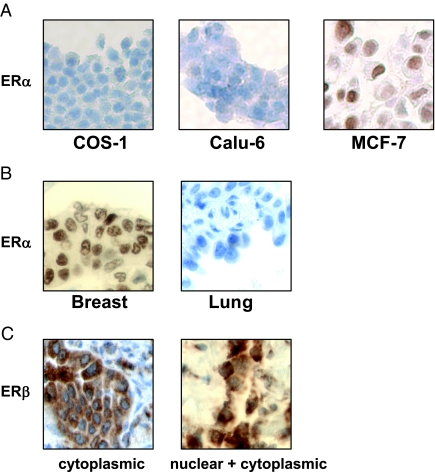
We examined the growth of Calu-6 and 201T lung adenocarcinoma cells in response to estrogen treatment. As expected, estrogen stimulated the growth of Calu-6 and 201T cells and was comparable to the effects seen on MCF-7 breast cancer cells (Fig. 1A). This estrogen-dependent growth in Calu-6 cells was blocked by antiestrogen ICI 182,780 but not by tamoxifen (Fig. 1B), in agreement with previous reports (13). Next, we examined the levels of ER in a panel of NSCLC cells. Western blotting analysis revealed that NSCLC cells predominantly expressed ERβ protein but not ERα (Fig. 1C), similar to earlier reports (14). ERα and ERβ constructs overexpressed in ER negative COS-1 cells were used to determine antibody specificity (Fig. 1C). ERβ levels varied among NSCLC cells and migrated as a doublet around 64-kDa in most cell lines. In agreement with this, real-time PCR analysis revealed very low ERα message when compared with ERβ in NSCLC cells. ERβ mRNA levels were comparable to those seen in MCF-7 cells (Fig. 1D). Similar results were obtained by Bookout et al. (24), who showed undetectable levels of ERα mRNA when compared with ERβ in mouse lung tissue.
Figure 1.
ERβ mediates estrogen-dependent growth of lung cancer cells. A, NSCLC cells and MCF-7 cells were treated with E2 for 3 d and cell proliferation was measured by Brd-U incorporation. Values are represented as mean (±sd) from four identical wells. Statistical analysis was performed by two-tailed t test (P < 0.05). B, Calu-6 cells were treated with E2 (1 nm), tamoxifen (100 nm), or ICI 182,780 (100 nm) for 4 d, and cell proliferation was measured by Brd-U incorporation. Values are represented as mean (±sd) from five identical wells. Statistical analysis was performed by one-way ANOVA (*, P < 0.05; control vs. different treatments). C, Western blotting analysis of NSCLC cells, MCF-7 cells, and COS-1 cells transfected with ERα and ERβ constructs. A nonspecific band at 90 kDa was seen with ERα antibody in all the cells. D, Quantitative PCR analysis of ERα and ERβ1 mRNA levels in NSCLC cells and MCF-7 cells. Values are expressed relative to levels seen in COS-1 cells. E, Calu-6 cells were treated with DPN or PPT for 6 d, and cell proliferation was measured by Brd-U incorporation. Values are represented as mean (±sd) from five identical wells. Statistical analysis was performed by one-way ANOVA (*, P < 0.05; control vs. different treatments). F, Western blotting analysis of GFP ShRNA (control) and ERβ ShRNA expressing Calu-6 cells. G, Calu-6 cells stably expressing ShRNA constructs were treated with E2 for 4 d, and cell proliferation was measured by Brd-U incorporation. Values are represented as mean (±sds) from five identical wells. Statistical analysis was performed by two-tailed t test (P < 0.05). ICI, ICI 182,780; Tam, tamoxifen.
To further show that estrogenic signals in NSCLC cells are mediated through ERβ, we performed cell proliferation assays with ERβ- and ERα-selective ligands, diarylpropionitrile (DPN) and propyl-pyrazole-triol (PPT), respectively (25). DPN, but not PPT, stimulated the growth of Calu-6 cells (Fig. 1E). Further, ERβ levels in Calu-6 cells were down-regulated by stably expressing ShRNA constructs (Fig. 1F), and the down-regulation of ERβ mRNA was also confirmed by real-time PCR (data not shown). ERβ down-regulated cells responded poorly to estrogen when compared with control cells (Fig. 1G), indicating that ERβ is required for estrogen-dependent growth of lung cancer cells.
Estrogen treatment activates kinase pathways in lung cancer cells
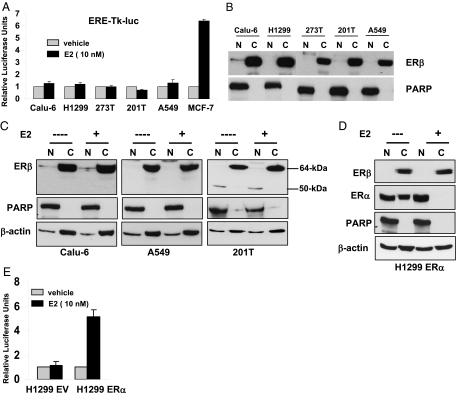
We examined whether ERβ is involved in rapid activation of MAPK, Akt, and cAMP signaling pathways. Exposure of 201T NSCLC cells to estrogen caused phosphorylation of MAPK, Akt, and cAMP response element-binding protein (CREB) proteins (Fig. 2A), with maximum activation seen at the 5-min time point. Similar results were obtained with E2-BSA, a membrane-impermeable estrogen conjugate (26) (Fig. 2A). The rapid activation of kinase pathways by estrogen, but not by EGF-I, was abolished in ERβ knockdown cells (Fig. 2B). To further confirm that estrogen activates only nongenomic signaling in lung cancer cells, we used estrogen response element-driven luciferase construct (ERE-TK-luc) (27) and a luciferase construct driven by serum response element (SRE) (28). The former construct requires ER to bind to DNA whereas the latter is activated by MAPK and cAMP signaling pathways. As shown in Fig. 2C, estrogen activated SRE-luc construct, but not ERE-TK-luc, in 201T cells. We also obtained similar results with E2-BSA and DPN, but not with PPT (Fig. 2C). Further, we examined whether inhibition of kinase pathways prevented estrogen-dependent proliferation of lung cancer cells. Estrogen-dependent cell growth was inhibited by protein kinase A inhibitor H89, phosphatidylinositol 3-kinase inhibitor LY294002, and MAPK kinase 1 inhibitor U0126 in Calu-6 cells (Fig. 2D). Together, these results suggest that estrogen-dependent growth of lung cancer cells requires nongenomic functions of ERβ.
Figure 2.
Nongenomic signaling by ERβ. A, 201T cells grown in phenol red free media were treated with E2 (10 nm) or E2-BSA (10 nm) for indicated time points, and lysates were subjected to Western blotting with phospho-specific antibodies. B, 201T cells were transfected with GFP SiRNA or ERβ SiRNA oligos, and cells were treated with E2 (10 nm) or EGF-I (20 ng/ ml) for 5 min. Lysates were subjected to Western blotting with phospho-specific antibodies. C, 201T cells were transiently transfected with ERE-TK-luc or pSRE-luc plasmids. After 24 h, cells were treated with various ligands and subjected to luciferase assays. Results are expressed as relative luciferase units, the ratio of firefly to Renilla luciferase. Values are shown as means (±sd) from three identical wells. D, Calu-6 cells were treated with E2 (1 nm), H89 (4 mm), LY 294002 (4 mm), or U 0126 (1 mm) for 3 d, and cell-proliferation was measured by Brd-U incorporation. Values are represented as mean (±sd) from five identical wells. Statistical analysis was performed by one-way ANOVA (*, P < 0.05; control vs. different treatments).
ERβ does not translocate to the nucleus
We further examined the genomic functions of endogenous ERβ in lung cancer cells. To measure the transcriptional activity of endogenous ERβ in NSCLC cells, we performed luciferase assays by transfecting ERE-TK-luc reporter construct. There was no significant increase in estrogen-dependent reporter activity in any of the NSCLC cells, when compared with MCF-7 cells (Fig. 3A). Our experiments with another reporter construct (ERE-TATA-luc) (29) also yielded the same results (data not shown), suggesting that it is not due to reporter construct-specific effects. Fractionation of the cell lysates revealed that ERβ was predominantly localized in the cytosol in all NSCLC cells (Fig. 3B).
Figure 3.
ERβ does not translocate to nucleus. A, NSCLC cells and MCF-7 cells were transiently transfected with ERE-TK-luc and pRLTK-luc plasmids. After 24 h, cells were treated with or without E2 overnight and subjected to luciferase assays. Results are expressed as relative luciferase units, the ratio of firefly to Renilla luciferase. Values are shown as means (±sd) from three identical wells. B, Nuclear and cytosolic fractions were prepared from NSCLC cell lysates and subjected to Western blotting with ERβ antibodies (Upstate Biotechnology). PARP was used as loading control for nuclear fractions. C, NSCLC cells grown in phenol red free media were treated with E2 (100 nm) for 1 h. Nuclear and cytosolic fractions were prepared, and ERβ was analyzed by Western blotting with antibodies from Upstate Biotechnology. PARP and β-actin were used as loading controls for nuclear and cytosolic fractions, respectively. D, Western blotting analysis in H1299 cells stably expressing ERα. E, H1299 cells stably expressing empty vector (EV) or ERα constructs were transfected with ERE-TK-luc and pRLTK-luc plasmids, and luciferase assays were performed as described above. Values are shown as means (±sd) from three identical wells. C, Cytoplasmic; N, nuclear.
It is well documented in the literature that many nuclear receptors, including ERs, rapidly translocate into the nucleus upon binding to their ligands. To examine this possibility, we treated Calu-6, A549, and 201T NSCLC cells with E2 for 1 h and fractionated the cell lysates. To our surprise, estrogen treatment did not induce ERβ translocation into the nucleus (Fig. 3C). We failed to see ERβ translocation even when cells were exposed to estrogen overnight (data not shown). Studies with another ERβ antibody that recognizes a different epitope (Affinity BioReagents, Inc., Golden, CO) also gave us similar results (data not shown).
One possible explanation for lack of ERβ translocation is NSCLC cells do not support nuclear import of ERs. To investigate this, we generated H1299 NSCLC cells stably expressing ERα, which otherwise do not express detectable levels of ERα. Estrogen treatment led to translocation of ERα, but not ERβ, into the nucleus (Fig. 3D). These studies infer that lack of endogenous ERβ transcriptional function in NSCLC cells is due to inability to translocate to the nucleus in the presence of the ligand. Supporting this, H1299 ERα cells showed increased ERE-TK-luc activity in the presence of estrogen, when compared with empty vector (H1299 EV) control (Fig. 3E).
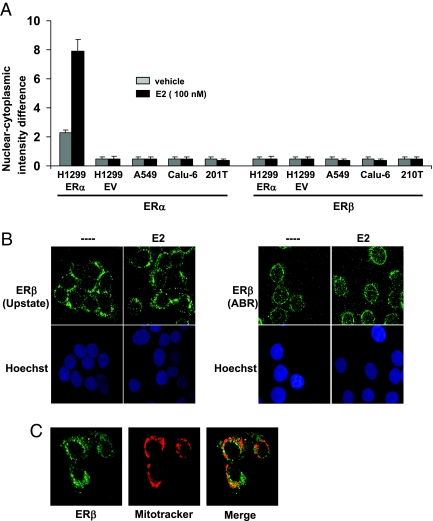
To further track ERβ translocation, we used an immunofluorescence localization technique called ArrayScan (30). The ArrayScan instrument measures the fluorescence intensity in the nucleus and in the small circular boundary around the nucleus, which represents cytosol. The instrument uses an algorithm to calculate the fluorescence intensity difference between nucleus and cytosol, and a higher nuclear-cytoplasmic intensity difference denotes increased protein localization in the nucleus. A number of studies have used ArrayScan to monitor nuclear localization of various proteins (31,32,33). To measure ERα and ERβ translocation, NSCLC cells were treated with E2 for 1 h, and cells were processed as described in Materials and Methods. As shown in Fig. 4A, in the absence of estrogen, we observed a background nuclear-cytoplasmic intensity difference in H1299 ERα cells because a small fraction of ERα is present in the nucleus (Fig. 3D). Estrogen treatment further increased nuclear-cytoplasmic intensity difference in these cells, suggesting ERα translocation into the nucleus. As expected, H1299 EV (empty vector transfected cells), Calu-6, 201T, and A549 NSCLC cells did not show any fluorescent signal because they do not express ERα (Fig. 4A). Under similar conditions, none of the cells displayed ERβ translocation after estrogen treatment (Fig. 4A), thus confirming our biochemical observations. Our experiments with ERβ antibody from a different source (Affinity BioReagents) also gave us similar results (data not shown). To determine whether the assay system was working, we assessed nuclear translocation of NF-κB in all lung cancer cells and observed that NF-κB readily translocated into the nucleus in all lung cancer cells upon exposure to TNFα (data not shown).
Figure 4.
ERβ translocation analysis. A, NSCLC cells grown in phenol red free media were treated with E2 for 1 h, and ER nuclear localization was measured by ArrayScan instrument as described in Materials and Methods. ERβ antibodies were from Upstate Biotechnology. Values are shown as means (±sd) from three identical wells. B, 201T cells grown in phenol red free media were treated with E2 for 1 h, and confocal microscopy studies were performed with ERβ antibodies from Upstate Biotechnology (left) and Affinity BioReagents (right). ERβ and nucleus staining are represented by green and blue, respectively. C, 201T cells were treated with Mitotracker Red, and ERβ was visualized as described above with antibodies from Upstate Biotechnology. Overlap is seen in yellow. ABR, Affinity BioReagents; EV, empty vector.
We further assessed ERβ localization by confocal microscopy using the two ERβ antibodies mentioned above. ERβ protein was localized in the cytoplasm, irrespective of E2 treatment, in 201T cells (Fig. 4B). Z-stack images revealed barely detectable levels of ERβ in the nucleus by both the antibodies (data not shown). These results are in agreement with a previous report that showed lack of ERβ translocation in a murine hippocampus cell line (34). Further, ERβ colocalized with mitochondria-specific dye Mitotracker Red in 201T cells (Fig. 4C), suggesting that a part of cytoplasmic ERβ localizes to mitochondria.
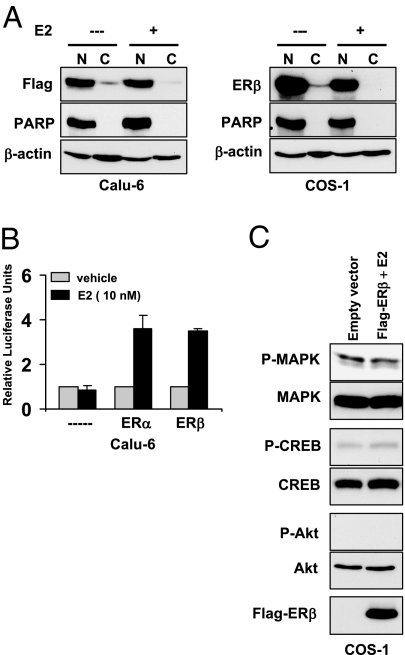
Our studies so far suggest that endogenous ERβ is unable to translocate to the nucleus in the presence of estrogen. This is in contrast to numerous studies that show transcriptional function of ERβ in various cell lines (4,29,35,36,37). Because many of these studies were performed with exogenously expressed ERβ construct, we examined whether exogenously expressed protein behaves differently when compared with endogenous protein. We transiently transfected Calu-6 and COS-1 cells with Flag-tagged ERβ construct and performed Western blotting analysis. To our surprise, almost all of exogenously expressed Flag-ERβ was localized in the nucleus in Calu-6 and COS-1 cells, irrespective of ligand treatment (Fig. 5A). In agreement, both Flag-ERα and Flag-ERβ could efficiently activate ERE-TK-luc construct to similar levels (Fig. 5B), suggesting that ERβ can perform genomic functions in lung cancer cells if present in the nucleus. Further, exogenously expressed nuclear ERβ failed to activate kinase pathways in response to estrogen in COS-1 cells (Fig. 5C). Thus, our studies suggest that forced expression causes mislocalization of ERβ in lung cancer cells.
Figure 5.
Overexpressed ERβ localizes to nucleus. A, Calu-6 and COS-1 cells transfected with Flag-ERβ were treated with E2 (100 nm) for 1 h. Nuclear and cytosolic fractions were prepared, and ERβ localization was analyzed with Flag or ERβ (Upstate Biotechnology) antibodies. B, Calu-6 cells were transfected with ERE-TK-luc along with Flag-ERα or Flag-ERβ plasmids, and luciferase assays were performed as described earlier. Values are shown as means (±sd) from three identical wells. C, COS-1 cells transfected with Flag-ERβ were treated with E2 (10 nm) for 5 min, and lysates were subjected to Western blotting with phospho-specific antibodies. C, Cytoplasmic; N, nuclear.
Immunohistochemical analysis of primary lung tissue specimens
To investigate ERα and ERβ localization, we performed immunohistochemical analysis on 29 NSCLC biopsy samples. The patients’ clinical variables (gender, smoking status) and tumor histological features are summarized in Table 1. ERβ staining was observed in all 29 tumor samples, with cytoplasmic-only staining in 69% of the samples (20 of 29), and both nuclear and cytoplasmic staining in 31% of the samples (nine of 29). In contrast, ERα staining was not detected in any of the tumor specimens. Specificity for ERα staining was evaluated by using paraffin-embedded COS-1 and Calu-6 cell pellets (ERα negative), and MCF-7 cell pellet (ERα positive) (Fig. 6A). Figure 6B shows a representative slide stained for ERα in lung tumor samples. A breast adenocarcinoma specimen was used as a positive control. Figure 6C shows a representative staining of ERβ in lung tumor samples. Thus, ERβ was detected in all biopsy samples, predominantly in the cytoplasm. However, we found no significant correlation between ERβ expression and clinicopathological variables due to small sample size.
Table 1.
Cinical variables (gender, smoking status) and tumor histological features
| Variables | |
|---|---|
| Gender | |
| Male | 14 (48.2%) |
| Female | 15 (51.7%) |
| Race | |
| Caucasian | 26 (89.6%) |
| African American | 2 (6.8%) |
| Undetermined | 1 (3.4%) |
| Age | |
| Median | 65.7 yr |
| Range | 42–81 yr |
| Smoking history | |
| Active | 10 (34.4%) |
| Former | 16 (55.1%) |
| Never | 2 (6.8%) |
| Undetermined | 1 (3.4%) |
| Histology | |
| Adenocarcinoma | 15 (51.7%) |
| Squamous | 11 (37.9%) |
| Large cell | 1 (3.4%) |
| Undetermined | 2 (6.8%) |
| Tumor stage | |
| IA/IB | 15 (51.7%) |
| IIA/IIB | 6 (20.6%) |
| IIIA/IIIB | 7 (24.1%) |
| Undetermined | 1 (3.4%) |
Figure 6.
Immunohistochemical analysis in NSCLC biopsy samples. A, Staining of paraffin-embedded COS-1, Calu-6, and MCF-7 cell pellets with ERα antibodies. B, Examples of breast and NSCLC tissue stained with ERα antibodies. C, A representative of lung tissue showing ERβ staining in cytoplasm only (left), and staining in both nucleus and cytoplasm (right). ERβ antibodies were from Upstate Biotechnology.
Discussion
It is well established that ERs play a major role in pathological processes of many hormone-dependent diseases such as ovarian, breast, and endometrial cancers. Hence, antiestrogens and selective ER modulators are routinely used in the treatment and prevention of these cancers (38). There is an emerging interest in the role of ERs in lung cancer development (13,14,20,39) and the possible use of antiestrogens and aromatase inhibitors in lung cancer treatment (39,40). However, very little is known about the mechanism of ER function in lung cancer cells. The key observations from our study are: 1) ERβ, but not ERα, promotes estrogen-dependent growth of lung cancer cells; 2) endogenous ERβ is mainly localized in the cytoplasm and mitochondria, and does not translocate to the nucleus in the presence of ligand; and 3) estrogens rapidly activate multiple kinase pathways in lung cancer cells, suggesting a nongenomic action of ERβ in lung cancer cells.
To date, this is the first report regarding nuclear translocation studies of ERs in lung cancer cells. Our data suggest that in NSCLC cells, a large fraction of ERβ is present in the cytosol and is unable to translocate to the nucleus in the presence of estrogen. Our findings are confirmed by biochemical, confocal, and ArrayScan experiments in various cell lines and by using two antibodies that recognize different epitopes on ERβ. Our findings are in agreement with a previous report that showed a lack of endogenous ERβ translocation in murine neuronal cells (34).
We have initiated studies to identify the mechanism responsible for localization of ERβ in the cytoplasm of lung cancer cells. Treatment of lung cancer cells with leptomycin B, an inhibitor of chromosome region maintenance 1 nuclear exporter, did not alter ERβ localization in our studies (data not shown). This suggests that nuclear export signals do not play a role in nuclear exclusion of ERβ in lung cancer cells. One potential explanation for cytosolic localization of endogenous ERβ is posttranslational modifications. For example, phosphorylation of a conserved serine residue in the DNA-binding domain by protein kinase C confers cytoplasmic localization of many nuclear receptors such as hepatocyte nuclear factor-4α, retinoic acid receptor-α, retinoic X receptor-α, and thyroid hormone receptor (41). Alternatively, fatty acylation of ERβ can target it to plasma membrane. ERβ undergoes palmitoylation, which is required for efficient interaction with caveolin-1 protein at the plasma membrane (12). Our preliminary studies did not show evidence of phosphorylation of endogenous (cytoplasmic) and exogenously expressed (nuclear) ERβ. Further, treatment of Calu-6 cells with staurosporine, a global kinase inhibitor or trichostatin A, a histone deacetylase inhibitor, did not cause ERβ to translocate to the nucleus in the presence of estrogen, suggesting that posttranslational modifications such as phosphorylation and acetylation may not play a role in ERβ localization (data not shown). Further, sequence analysis of ERβ mRNA in lung cancer cells showed no change, thus ruling out mutations in nuclear localizing signal (data not shown).
It is possible that ERβ is retained by a cytoplasmic protein complex in lung cancer cells. Kumar et al. (42) have shown that ERα is sequestered in the cytoplasm by a splice variant of metastatic tumor antigen-1 (MTA1s) in some breast cancer cells. To examine this, we performed RT-PCR analysis and found the expression of MTA1s splice variant in a panel of lung cancer cells (data not shown). Thus, it is possible that endogenous ERβ is sequestered in the cytoplasm by MTA1s in lung cancer cells, and further studies are warranted to support this hypothesis.
It is also possible that ERβ is targeted to other organelles such as mitochondria, rather than nucleus, in lung cancer cells. In addition to our observations, others have reported ERβ to be localized in the mitochondria (34,43). A putative mitochondria localization signal has been mapped to the hinge region of ERβ. It is implicated that ERβ binds to EREs in the mitochondrial DNA and up-regulates respiratory chain proteins (43). Further, mitochondrial ER can inhibit apoptosis by preventing reactive oxygen species formation through up-regulating manganese superoxide dismutase (44). Thus, it remains unclear why endogenous ERβ does not translocate to the nucleus in lung cancer cells and requires further investigation.
Our studies show that ERβ is necessary for estrogen-dependent growth of lung cancer cells and potentially may play a role in the development of lung adenocarcinomas. However, the role of ERβ in tumor progression is controversial. Earlier studies suggested that ERβ inhibits tumorigenesis due to lack of its expression in ovarian, breast, and cervical cancers, when compared with normal tissue (45). These reports were further supported by ERβ overexpression studies in breast cancer cells, which demonstrated growth inhibition (46,47). Care should be taken when interpreting these overexpression studies because ERα overexpression also leads to growth inhibition in breast cancer cells (48,49). Recent reports have shown that ERβ can function as a tumor promoter in certain conditions. ERβ expression is associated with tamoxifen response in ERα-negative breast tumors, suggesting its role in growth and proliferation of breast cancer cells (50,51). ERβ causes estrogen-dependent proliferation of stromal cells in rodent mammary glands (52). In metastatic prostate cancer and stomach adenocarcinomas, ERβ is present whereas ERα is lost (53,54). Furthermore, ERβ contributes to cell proliferation of LNCaP prostate cancer cells (55). Clearly, these studies suggest an intriguing hypothesis that ERβ functions as a tumor promoter in a situation when ERα is absent, but this requires additional documentation. In agreement with this, our studies show loss of ERα expression in lung cancer cells and primary lung tumor specimens. Our studies are in strong agreement with two reports that showed ERβ, but not ERα, expression in NSCLC tumor specimens (22,23).
In addition to cytoplasmic staining, we observed ERβ nuclear staining in 31% of the tumor samples. Similarly, we also observed a signal at 50 kDa with ERβ antibodies in nuclear fractions in some Western blots (Fig. 3C). We are uncertain whether these signals are nonspecific proteins or truncated isoforms of ERβ. Involvement of these truncated ERβ isoforms in transcriptional functions is questionable because they are unable to bind to the ligand (56).
The current study provides compelling evidence to show that ERβ functions through nongenomic mechanisms in lung cancer cells. Our report is supported by the recent findings in human small airway epithelial cells that show cooperation between nongenomic mechanisms of ERβ and β-adrenergic receptors in response to tobacco carcinogen (methylnitrosamine)-1-(3-pyridyl)-1-butanone (NNK). The genomic functions of ERβ did not play a role in mediating NNK effects in these cells (57). Further, an interesting parallel exists between NSCLC cells and LNCaP prostate cancer cells wherein both cell types lack ERα expression, and ERβ promotes estrogen-dependent cell growth through nongenomic mechanisms. ERβ promotes estrogen-dependent growth of LNCaP cells by up-regulating IGF-IR and androgen-responsive genes through activation of kinase pathways (55). In some instances, nongenomic actions of ERβ are stronger than those of ERα. ERβ is more efficient than ERα in protecting cells from apoptosis through nongenomic actions in breast cancer cells (44). Thus, there is ample evidence to support nongenomic functions of ERβ in various cell types. Physical interactions between ERβ and membrane-associated proteins, caveolin-1, Src, and modulator of nongenomic activity of ER (MNAR), have been reported in mediating nongenomic signaling (58). It remains to be tested whether these interactions are functionally significant in lung cancer cells.
In summary, our data indicate that ERβ is not a nuclear protein in lung cancer cells and provide a unique model system to study the extranuclear functions of ERβ. The studies reported here are different from previously reported studies in breast cancer wherein ERβ is localized in the nucleus and antagonizes growth-promoting functions of ERα. It is possible that the outcomes of genomic functions of ERβ are distinct from those of nongenomic functions, which promote estrogen-dependent growth of lung and prostate cancer cells. Although further investigation is required to understand the lack of ERβ nuclear translocation, our studies highlight the role of ERβ in promoting growth of lung cancer cells and provide a rationale for use of antiestrogens in lung cancer treatment.
Materials and Methods
Cell lines
H157, Calu-6, and 201T cells are lung adenocarcinomas. 273T is a squamous-cell carcinoma cell line. H1299 is a large-cell carcinoma cell line. A549 is a bronchioalveolar cell line. A549, H157, H1299, and COS-1 cells were from Dr. Jonathan Kurie (M.D. Anderson Cancer Center). 201T and 273T NSCLC cells were established in Dr. Siegfried’s laboratory (39). Calu-6 cells were purchased from American Type Culture Collection (Manassas, VA). MCF-7 breast cancer cells were from Dr. Pamela Hershberger (University of Pittsburgh Cancer Institute). NSCLC cells were maintained in RPMI containing 10% fetal bovine serum. COS-1 and MCF-7 cells were maintained in DMEM containing 10% fetal bovine serum. Cells were incubated at 37 C in a humidified atmosphere containing 5% CO2.
Reagents and antibodies
ERα and ERβ (530 amino acids) cDNA constructs were from Dr. Mark Nichols (University of Pittsburgh Cancer Institute). PPT and DPN were from Tocris (Ellisville, MO). We purchased E2, E2-BSA [E26-(O-carboxymethyl)oxime:BSA)] and monoclonal antibodies against β-actin from Sigma-Aldrich (St. Louis, MO); tamoxifen and ICI 182,780 were purchased from Tocris (Ellisville, MO); polyclonal antibodies against ERβ were purchased from Upstate Biotechnology (Lake Placid, NY) and Affinity BioReagents (Golden, CO); polyclonal antibodies against phospho-MAPK (Thr202/Tyr204), MAPK, phospho-Akt (Ser473), Akt, phospho-CREB (Ser133), and CREB were purchased from Cell Signaling Technology (Beverly, MA); Hoechst, Mitotracker Red CMXRos, and secondary antibody conjugated to Alexa-488 were purchased from Invitrogen Life Technologies (Carlsbad, CA); and polyclonal antibodies against ERα and poly(ADP-ribose) polymerase (PARP) were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Real-time PCR
RNA was isolated from NSCLC cells using an RNA isolation kit. (QIAGEN, Valencia, CA). Reverse-transcriptase reactions were performed with 1 μg of total cellular RNA using random hexamers. The reverse-transcriptase reaction (1 μl) was added to 20 μl of PCR mix containing primers and TaqMan probes specific for ERα (Applied Biosystems, Foster City, CA). ERα transcript levels were normalized to β-glucuronidase mRNA, which was used as a control. For ERβ, primers specific for ERβ1 (56) were used, and ERβ transcripts were normalized to β-actin mRNA, which was used as control.
Cell proliferation assays
NSCLC cells and MCF-7 cells grown in phenol red free media containing charcoal-stripped serum were seeded into 96-well plates (4 × 10 cells per well). Cells were serum starved for 2 d and treated with the indicated compounds for 4–6 d under serum-free (NSCLC cells) or low-serum (MCF-7) conditions. Cells were incubated, 3 h before harvest, with bromodeoxyuridine (Brd-U) followed by fixing and lysis of cells. Brd-U incorporation was measured colorimetrically using Cell Proliferation ELISA kit from Roche (Indianapolis, IN). Each experiment was performed three times, and data from a representative experiment are shown as the means and sds (indicated by error bars) from five identical wells.
Stable cell lines
ERβ ShRNA cells were generated by cloning small interfering RNA (SiRNA) oligos specific for ERβ (59) into pSUPER vector (Oligoengine, Seattle, WA). The construct was transfected into Calu-6 cells, and transfectants were selected under puromycin. Individual clones were isolated and analyzed for ERβ expression. H1299 cells stably expressing ERα were generated by transfecting the cells with pcDNA-ERα construct and selecting the cells under G418. Individual clones were isolated and analyzed for ERα expression.
Luciferase assays
NSCLC cells and MCF-7 cells (8 × 104 cells per well) grown in phenol red-free media containing charcoal-stripped serum were transfected in 24-well plates with the pERE-TK-luc or pSRE-luc (Stratagene, La Jolla, CA). pRLTK -luc was transfected as an internal control. The total amount of plasmid was kept to 1 μg/well. Cells were treated 24 h later with E2 (10−8 m) for 16 h, and cell lysates were assayed for luciferase activity using a dual-luciferase reporter assay system (Promega Corp., Madison, WI). Data were expressed as relative luciferase activity, the ratio of firefly to Renilla luciferase activity. Each experiment was performed three times, and data from a representative experiment are shown as the means and sds (indicated by error bars) from three identical wells.
Nuclear and cytosolic extracts
NSCLC cells grown in phenol red-free medium were treated with E2 (100 nm) for 1 h. Cells were harvested, and nuclear and cytosolic fractions were prepared as previously described (60). ERα and ERβ were analyzed by Western blotting, and PARP and β-actin were used as loading controls for nuclear and cytosolic fractions, respectively.
Kinase signaling
201T NSCLC cells grown in phenol red-free media were serum starved for 2 d. Cells were treated with vehicle, E2 (10 nm) or E2-BSA (10 nm) for various time points. Cells were harvested and lysed in radioimmune precipitation assay buffer containing phosphatase and protease inhibitors, and phosphoproteins were analyzed by Western blotting. For down-regulation of ERβ, 201T cells were transfected with green fluorescent protein (GFP) SiRNA or ERβ SiRNA oligos, and Western blotting was performed as mentioned above.
ArrayScan experiments
NSCLC cells grown in phenol red-free media were seeded onto collagen-coated black-walled 96-well plates (10,000 cells per well). The cells were treated 72 h later with E2 (100 nm) for 1 h. The media were removed and washed, and the cells were fixed with 4% paraformaldehyde for 15 min at room temperature. Cells were permeabilized with a buffer containing 300 mm sucrose and 0.1% Triton X-100 in PBS. Cells were blocked with Tris-buffered saline-Tween 20 containing 10% goat serum and 1% BSA followed by incubation with anti-ERα or anti-ERβ antibodies overnight. Cells were washed and stained with blocking buffer containing Hoechst and secondary antibody conjugated to Alexa-488. The cells were maintained in PBS until image analysis.
The wells were scanned in the ArrayScan instrument (Cellomics, Inc., Pittsburgh, PA), which is an automated fluorescent imaging microscope, and the fluorescently labeled components in the nucleus and cytoplasm are quantified using an algorithm. The system was used to scan multiple fields from each well until a preselected number of cells was imaged and analyzed (500 cells per well). Each experiment was performed three times, and data from a representative experiment are shown as the means and sds (indicated by error bars) from three identical wells.
Confocal microscopy
NSCLC cells were grown on coverslips in phenol red-free media and treated with E2 (100 nm) for 1 h. Cells were fixed with methanol/acetone and stained with ERβ antibodies as mentioned in ArrayScan experiments. Fluorescent images were collected with a confocal scanning laser system (Olympus Fluoview 1000; Olympus Corp., Lake Success, NY) attached to an inverted microscope (IX81; Olympus Corp., Tokyo, Japan). Where mentioned, cells were pretreated with Mitotracker Red CMXRos (100 nm) for 45 min and analyzed as mentioned above.
Immunohistochemistry
Paraffin-embedded lung tissue biopsies were obtained from Lung SPORE tissue bank. After paraffin removal, slides were hydrated and antigen retrieval was done using a high pH buffer (Biocare Medical, Concord, CA). Endogenous peroxidase was quenched using 3% hydrogen peroxide, and slides were washed with Tris-buffered saline/Tween 20 and treated with a blocking buffer (for ERα from Biocare Medical) or 10% normal goat serum (for ERβ). Slides were incubated with anti-ERα antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA; 1:2000 dilution, 30 min at room temperature) or anti-ERβ antibody (Upstate Biotechnology, Lake Placid, NY; 1:1000 dilution, overnight at 4 C) and then incubated with DakoEnvision Dual Link System Plus (DAKO Corp., Denmark) for 30 min, rinsed, and incubated in the substrate peroxide and the chromogen diaminobenzidine according to manufacturer’s instructions (DAKO). The slides were then rinsed and counterstained with hematoxylin. Staining of ER-negative COS-1 cells and an absence of primary antibody were used as negative controls for the antibodies. ERα and ERβ staining was quantified based on the percentages of cells staining positively in cytoplasmic, nuclear, or both compartments. A tumor was considered positive if at least 10% of the cells demonstrated staining.
Statistical analysis
Statistical analysis was done using GraphPad Prism software (GraphPad Software, Inc., San Diego, CA). Multiple group data were analyzed using one-way ANOVA, and data between two groups were analyzed using unpaired Student’s t test. Values were considered significant when P < 0.05.
Supplementary Material
Footnotes
This work was supported by the career development award from the University of Pittsburgh SPORE in Lung Cancer (to H.S.) a grant from the Hillman Foundation (to H.S), and by the Core Grant for Vision Research EY08098.
Disclosure Statement: The authors have nothing to disclose.
First Published Online December 23, 2008
Abbreviations: Brd-U, Bromodeoxyuridine; CREB, cAMP response element-binding protein; DPN, diarylpropionitrile; E2, 17β-estradiol; EGF, epidermal growth factor; ER, estrogen receptor; ERE, estrogen response element; GFP, green fluorescent protein; LBD, ligand-binding domain; MTA1, metastatic tumor antigen-1; NF-κB, nuclear factor-κB; NSCLC, non-small-cell lung cancer; PARP, poly(ADP-ribose) polymerase; PPT, propyl-pyrazole-triol; ShRNA, short hairpin RNA; SiRNA, small interfering RNA.
References
- Siegfried JM 2001 Women and lung cancer: does oestrogen play a role? Lancet Oncol 2:506–513 [DOI] [PubMed] [Google Scholar]
- Ascenzi P, Bocedi A, Marino M 2006 Structure-function relationship of estrogen receptor α and β: impact on human health. Mol Aspects Med 27:299–402 [DOI] [PubMed] [Google Scholar]
- Matthews J, Gustafsson JA 2003 Estrogen signaling: a subtle balance between ER α and ER β. Mol Interv 3:281–292 [DOI] [PubMed] [Google Scholar]
- Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS 1997 Differential ligand activation of estrogen receptors ERα and ERβ at AP1 sites. Science 277:1508–1510 [DOI] [PubMed] [Google Scholar]
- Saville B, Wormke M, Wang F, Nguyen T, Enmark E, Kuiper G, Gustafsson JA, Safe S 2000 Ligand-, cell-, and estrogen receptor subtype (α/β)-dependent activation at GC-rich (Sp1) promoter elements. J Biol Chem 275:5379–5387 [DOI] [PubMed] [Google Scholar]
- Galien R, Garcia T 1997 Estrogen receptor impairs interleukin-6 expression by preventing protein binding on the NF-κB site. Nucleic Acids Res 25:2424–2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song RX, Santen RJ 2006 Membrane initiated estrogen signaling in breast cancer. Biol Reprod 75:9–16 [DOI] [PubMed] [Google Scholar]
- Levin ER 2005 Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol 19:1951–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razandi M, Oh P, Pedram A, Schnitzer J, Levin ER 2002 Ers associate with and regulate the production of caveolin: implications for signaling and cellular actions. Mol Endocrinol 16:100–115 [DOI] [PubMed] [Google Scholar]
- Razandi M, Alton G, Pedram A, Ghonshani S, Webb P, Levin ER 2003 Identification of a structural determinant necessary for the localization and function of estrogen receptor α at the plasma membrane. Mol Cell Biol 23:1633–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, Visca P, Marino M 2005 Palmitoylation-dependent estrogen receptor α membrane localization: regulation by 17β-estradiol. Mol Biol Cell 16:231–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER 2007 A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem 282:22278–22288 [DOI] [PubMed] [Google Scholar]
- Stabile LP, Davis AL, Gubish CT, Hopkins TM, Luketich JD, Christie N, Finkelstein S, Siegfried JM 2002 Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor α and β and show biological responses to estrogen. Cancer Res 62:2141–2150 [PubMed] [Google Scholar]
- Hershberger PA, Vasquez AC, Kanterewicz B, Land S, Siegfried JM, Nichols M 2005 Regulation of endogenous gene expression in human non-small cell lung cancer cells by estrogen receptor ligands. Cancer Res 65:1598–1605 [DOI] [PubMed] [Google Scholar]
- Pietras RJ, Marquez DC, Chen HW, Tsai E, Weinberg O, Fishbein M 2005 Estrogen and growth factor receptor interactions in human breast and non-small cell lung cancer cells. Steroids 70:372–381 [DOI] [PubMed] [Google Scholar]
- Ciana P, Di Luccio G, Belcredito S, Pollio G, Vegeto E, Tatangelo L, Tiveron C, Maggi A 2001 Engineering of a mouse for the in vivo profiling of estrogen receptor activity. Mol Endocrinol 15:1104–1113 [DOI] [PubMed] [Google Scholar]
- Jiang YG, Chen JK, Wu ZL 2000 Promotive effect of diethylstilbestrol on urethan-induced mouse lung tumorigenesis. Chemosphere 41:187–190 [DOI] [PubMed] [Google Scholar]
- Seike N, Wanibuchi H, Morimura K, Wei M, Nishikawa T, Hirata K, Yoshikawa J, Fukushima S 2003 Enhancement of lung carcinogenesis by nonylphenol and genistein in a F344 rat multiorgan carcinogenesis model. Cancer Lett 192:25–36 [DOI] [PubMed] [Google Scholar]
- Morani A, Barros RP, Imamov O, Hultenby K, Arner A, Warner M, Gustafsson JA 2006 Lung dysfunction causes systemic hypoxia in estrogen receptor β knockout (ERβ−/−) mice. Proc Natl Acad Sci USA 103:7165–7169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez-Garban DC, Chen HW, Fishbein MC, Goodglick L, Pietras RJ 2007 Estrogen receptor signaling pathways in human non-small cell lung cancer. Steroids 72:135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai H, Ishii A, Washiya K, Konno T, Kon H, Yamaya C, Ono I, Minamiya Y, Ogawa J 2005 Estrogen receptor α and β are prognostic factors in non-small cell lung cancer. Clin Cancer Res 11:5084–5089 [DOI] [PubMed] [Google Scholar]
- Wu CT, Chang YL, Shih JY, Lee YC 2005 The significance of estrogen receptor β in 301 surgically treated non-small cell lung cancers. J Thorac Cardiovasc Surg 130:979–986 [DOI] [PubMed] [Google Scholar]
- Omoto Y, Kobayashi Y, Nishida K, Tsuchiya E, Eguchi H, Nakagawa K, Ishikawa Y, Yamori T, Iwase H, Fujii Y, Warner M, Gustafsson JA, Hayashi SI 2001 Expression, function, and clinical implications of the estrogen receptor β in human lung cancers. Biochem Biophys Res Commun 285:340–347 [DOI] [PubMed] [Google Scholar]
- Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ 2006 Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell 126:789–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington WR, Sheng S, Barnett DH, Petz LN, Katzenellenbogen JA, Katzenellenbogen BS 2003 Activities of estrogen receptor α- and β-selective ligands at diverse estrogen responsive gene sites mediating transactivation or transrepression. Mol Cell Endocrinol 206:13–22 [DOI] [PubMed] [Google Scholar]
- Taguchi Y, Koslowski M, Bodenner DL 2004 Binding of estrogen receptor with estrogen conjugated to bovine serum albumin (BSA). Nuclear Receptor 2:5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P, Kanterewicz B, Hershberger PA, McCarty Jr KS, Day BW, Nichols M 2004 Inhibition of estrogen receptor α-mediated transcription by antiestrogenic 1,1-dichloro-2,2,3-triarylcyclopropanes. Mol Pharmacol 66:970–977 [DOI] [PubMed] [Google Scholar]
- Marais R, Wynne J, Treisman R 1993 The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell 73:381–393 [DOI] [PubMed] [Google Scholar]
- Hall JM, McDonnell DP 1999 The estrogen receptor β-isoform (ERβ) of the human estrogen receptor modulates ERα transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology 140:5566–5578 [DOI] [PubMed] [Google Scholar]
- Ding GJ, Fischer PA, Boltz RC, Schmidt JA, Colaianne JJ, Gough A, Rubin RA, Miller DK 1998 Characterization and quantitation of NF-κB nuclear translocation induced by interleukin-1 and tumor necrosis factor-α. Development and use of a high capacity fluorescence cytometric system. J Biol Chem 273:28897–28905 [DOI] [PubMed] [Google Scholar]
- Agler M, Prack M, Zhu Y, Kolb J, Nowak K, Ryseck R, Shen D, Cvijic ME, Somerville J, Nadler S, Chen T 2007 A high-content glucocorticoid receptor translocation assay for compound mechanism-of-action evaluation. J Biomol Screen 12:1029–1041 [DOI] [PubMed] [Google Scholar]
- Vogt A, Cooley KA, Brisson M, Tarpley MG, Wipf P, Lazo JS 2003 Cell-active dual specificity phosphatase inhibitors identified by high-content screening. Chem Biol 10:733–742 [DOI] [PubMed] [Google Scholar]
- Vakkila J, DeMarco RA, Lotze MT 2004 Imaging analysis of STAT1 and NF-κB translocation in dendritic cells at the single cell level. J Immunol Methods 294:123–134 [DOI] [PubMed] [Google Scholar]
- Yang SH, Liu R, Perez EJ, Wen Y, Stevens Jr SM, Valencia T, Brun-Zinkernagel AM, Prokai L, Will Y, Dykens J, Koulen P, Simpkins JW 2004 Mitochondrial localization of estrogen receptor β. Proc Natl Acad Sci USA 101:4130–4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley SM, Parker MG 1999 A comparison of transcriptional activation by ER α and ER β. J Steroid Biochem Mol Biol 69:165–175 [DOI] [PubMed] [Google Scholar]
- Duong BN, Elliott S, Frigo DE, Melnik LI, Vanhoy L, Tomchuck S, Lebeau HP, David O, Beckman BS, Alam J, Bratton MR, McLachlan JA, Burow ME 2006 AKT regulation of estrogen receptor β transcriptional activity in breast cancer. Cancer Res 66:8373–8381 [DOI] [PubMed] [Google Scholar]
- Tremblay A, Tremblay GB, Labrie F, Giguere V 1999 Ligand-independent recruitment of SRC-1 to estrogen receptor β through phosphorylation of activation function AF-1. Mol Cell 3:513–519 [DOI] [PubMed] [Google Scholar]
- Jordan VC 2004 Selective estrogen receptor modulation: concept and consequences in cancer. Cancer Cell 5:207–213 [DOI] [PubMed] [Google Scholar]
- Stabile LP, Lyker JS, Gubish CT, Zhang W, Grandis JR, Siegfried JM 2005 Combined targeting of the estrogen receptor and the epidermal growth factor receptor in non-small cell lung cancer shows enhanced antiproliferative effects. Cancer Res 65:1459–1470 [DOI] [PubMed] [Google Scholar]
- Weinberg OK, Marquez-Garban DC, Fishbein MC, Goodglick L, Garban HJ, Dubinett SM, Pietras RJ 2005 Aromatase inhibitors in human lung cancer therapy. Cancer Res 65:11287–11291 [DOI] [PubMed] [Google Scholar]
- Sun K, Montana V, Chellappa K, Brelivet Y, Moras D, Maeda Y, Parpura V, Paschal BM, Sladek FM 2007 Phosphorylation of a conserved serine in the deoxyribonucleic acid binding domain of nuclear receptors alters intracellular localization. Mol Endocrinol 21:1297–1311 [DOI] [PubMed] [Google Scholar]
- Kumar R, Wang RA, Mazumdar A, Talukder AH, Mandal M, Yang Z, Bagheri-Yarmand R, Sahin A, Hortobagyi G, Adam L, Barnes CJ, Vadlamudi RK 2002 A naturally occurring MTA1 variant sequesters oestrogen receptor-α in the cytoplasm. Nature 418:654–657 [DOI] [PubMed] [Google Scholar]
- Chen JQ, Delannoy M, Cooke C, Yager JD 2004 Mitochondrial localization of ERα and ERβ in human MCF7 cells. Am J Physiol 286:E1011–E1022 [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Wallace DC, Levin ER 2006 Functional estrogen receptors in the mitochondria of breast cancer cells. Mol Biol Cell 17:2125–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin A, Boulle N, Lazennec G, Vignon F, Pujol P 2004 Loss of ERβ expression as a common step in estrogen-dependent tumor progression. Endocr Relat Cancer 11:537–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazennec G, Bresson D, Lucas A, Chauveau C, Vignon F 2001 ERβ inhibits proliferation and invasion of breast cancer cells. Endocrinology 142:4120–4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom A, Hartman J, Foster JS, Kietz S, Wimalasena J, Gustafsson JA 2004 Estrogen receptor β inhibits 17β-estradiol-stimulated proliferation of the breast cancer cell line T47D. Proc Natl Acad Sci USA 101:1566–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazennec G, Katzenellenbogen BS 1999 Expression of human estrogen receptor using an efficient adenoviral gene delivery system is able to restore hormone-dependent features to estrogen receptor-negative breast carcinoma cells. Mol Cell Endocrinol 149:93–105 [DOI] [PubMed] [Google Scholar]
- Garcia M, Derocq D, Freiss G, Rochefort H 1992 Activation of estrogen receptor transfected into a receptor-negative breast cancer cell line decreases the metastatic and invasive potential of the cells. Proc Natl Acad Sci USA 89:11538–11542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skliris GP, Leygue E, Watson PH, Murphy LC 2008 Estrogen receptor α negative breast cancer patients: estrogen receptor β as a therapeutic target. J Steroid Biochem Mol Biol 109:1–10 [DOI] [PubMed] [Google Scholar]
- Gruvberger-Saal SK, Bendahl PO, Saal LH, Laakso M, Hegardt C, Eden P, Peterson C, Malmstrom P, Isola J, Borg A, Ferno M 2007 Estrogen receptor β expression is associated with tamoxifen response in ERα-negative breast carcinoma. Clin Cancer Res 13:1987–1994 [DOI] [PubMed] [Google Scholar]
- Cheng G, Weihua Z, Warner M, Gustafsson JA 2004 Estrogen receptors ER α and ER β in proliferation in the rodent mammary gland. Proc Natl Acad Sci USA 101:3739–3746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leav I, Lau KM, Adams JY, McNeal JE, Taplin ME, Wang J, Singh H, Ho SM 2001 Comparative studies of the estrogen receptors β and α and the androgen receptor in normal human prostate glands, dysplasia, and in primary and metastatic carcinoma. Am J Pathol 159:79–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S, Ohkura Y, Eguchi H, Kobayashi Y, Akagi K, Uchida K, Nakachi K, Gustafsson JA, Hayashi S 2002 Estrogen receptor β is expressed in human stomach adenocarcinoma. J Cancer Res Clin Oncol 128:319–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandini G, Genua M, Frasca F, Squatrito S, Vigneri R, Belfiore A 2007 17β-Estradiol up-regulates the insulin-like growth factor receptor through a nongenotropic pathway in prostate cancer cells. Cancer Res 67:8932–8941 [DOI] [PubMed] [Google Scholar]
- Leung YK, Mak P, Hassan S, Ho SM 2006 Estrogen receptor (ER)-β isoforms: a key to understanding ER-β signaling. Proc Natl Acad Sci USA 103:13162–13167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majidi M, Al-Wadei HA, Takahashi T, Schuller HM 2007 Nongenomic β estrogen receptors enhance β1 adrenergic signaling induced by the nicotine-derived carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in human small airway epithelial cells. Cancer Res 67:6863–6871 [DOI] [PubMed] [Google Scholar]
- Wong CW, McNally C, Nickbarg E, Komm BS, Cheskis BJ 2002 Estrogen receptor-interacting protein that modulates its nongenomic activity-crosstalk with Src/Erk phosphorylation cascade. Proc Natl Acad Sci USA 99:14783–14788 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pedram A, Razandi M, Levin ER 2006 Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol 20:1996–2009 [DOI] [PubMed] [Google Scholar]
- Schreiber E, Matthias P, Muller MM, Schaffner W 1989 Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res 17:6419 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.