Abstract
Splenic toxicity of aniline is characterized by vascular congestion, hyperplasia, fibrosis and development of a variety of sarcomas in rats. However, underlying mechanisms by which aniline elicits splenotoxic response are not well understood. Previously we have shown that aniline exposure causes oxidative damage to the spleen. To further explore the oxidative mechanism of aniline toxicity, we evaluated the potential contribution of heme oxygenase-1 (HO-1), which catalyzes heme degradation and releases free iron. Male SD rats were given 1 mmol/kg/day aniline in water by gavage for 1, 4 or 7 days, while respective controls received water only. Aniline exposure led to significant increases in HO-1 mRNA expression in the spleen (2- and 2.4-fold at days 4 and 7, respectively) with corresponding increases in protein expression, as confirmed by ELISA and Western blot analyses. Furthermore, immunohistochemical assessment of spleen showed stronger immunostaining for HO-1 in the spleens of rats treated for 7 days, confined mainly to the red pulp areas. No changes were observed in mRNA and protein levels of HO-1 following 1 day exposure. The increase in HO-1 expression was associated with increases in total iron (2.4- and 2.7- fold), free iron (1.9- and 3.5-fold), and ferritin levels (1.9- and 2.1-fold) at 4 and 7 days of aniline exposure. Our data suggest that HO-1 up-regulation in aniline-induced splenic toxicity could be a contributing pro-oxidant mechanism, mediated through iron release, and leading to oxidative damage.
Keywords: Heme oxygenase-1, oxidative stress, iron, ferritin, aniline, spleen
Introduction
Aniline, a toxic aromatic amine, is an extensively used industrial chemical. Exposure to aniline is known to cause toxicity to the hematopoietic system [1-5]. Aniline toxicity is generally characterized by methemoglobinemia, hemolysis and hemolytic anemia [6-9], and by the development of splenic hyperplasia, fibrosis, and a variety of primary sarcomas after chronic exposure in rats [2,10-13]. While mechanisms of erythrocyte damage have been the focus of many studies, little attention has been given to the delineation of molecular mechanisms in aniline-induced toxicity to the spleen.
Heme oxygenase-1 (HO-1) is a rate-limiting microsomal enzyme that catalyzes the oxidative degradation of heme moiety of hemoglobin to biliverdin, carbon monoxide and free iron [14,15]. HO-1 transcription can be induced by a whole array of stressors, including transition-metals [16,17], heme, hemoglobin and other heme proteins [18,19], and oxidative/nitrosative stress [20-22]. HO-1 can exert cytoprotective and cytotoxic effects through several mechanisms, including serving as a molecular chaperone, degrading pro-oxidant heme to produce antioxidants (bilirubin and carbon monoxide) and releasing iron [23-25]. Even though an antioxidant role of HO-1 has been extensively studied [15,24], several studies also support a pro-oxidant role for HO-1 [14,26-28]. However, a specific role for HO-1 in aniline-induced splenic oxidative damage is not known.
Earlier studies have demonstrated that severity of toxic responses in the spleen is closely associated with erythrocyte damage [2,3,12]. The deposition and subsequent breakdown of damaged erythocytes during aniline insult will result in release and accumulation of iron/iron-storage proteins in the spleen. Indeed, studies have shown that aniline exposure in rats leads to iron release/overload [3,4,8,12,29] and oxidative stress in the spleen [4,8,30-32]. We hypothesize that up-regulation of HO-1 contributes to oxidative damaging reactions in the spleen by catalyzing the oxidative degradation of the heme moiety of hemoglobin and releasing free iron. This study was, therefore, focused on evaluating the regulation of HO-1, release of free iron and status of iron storage protein, ferritin, in the spleens of rats exposed to aniline.
Materials and methods
Animals and treatments
Male Sprague-Dawley rats (∼200 g), obtained from Harlan (Indianapolis, IN), were housed in wire-bottom cages over absorbent paper with free access to tap water and Purina rat chow. The animals were acclimatized in a controlled-environment animal room (temperature, 22 °C; relative humidity, 50%; photoperiod, 12-h light/dark cycle) for 7 days prior to treatment. The experiments were performed in accordance with the guidelines of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of University of Texas Medical Branch. The animals, in groups of 6 each, were given 1mmol/kg/day aniline in drinking water by gavage for 1, 4 or 7 days; respective control animals received water only. The choice of aniline dose was based on our earlier studies that showed significant increases in lipid peroxidation, protein oxidation and DNA damage (oxidative stress) in the spleen [4,29,30]. The rats were euthanized 24 h after the last dose under nembutal (sodium pentoparbital) anesthesia and the spleens were removed immediately, blotted, weighted and stored at -80 °C until further analysis. A portion of spleen was snap-frozen in liquid nitrogen and stored at -80 °C for RNA isolation. Also, portions of the spleen from control and aniline-treated rats were fixed in 10% neutral buffered formalin for immunohistological processing.
Real-time RT-PCR for HO-1 mRNA
RNA isolation
Total RNA was isolated from spleen tissues using RiboPure Kit (Ambion, Austin, TX) as per manufacturer's instructions. To eliminate genomic DNA contamination, isolated RNA was treated with RNase free DNase I (DNA-free kit, Ambion, Austin, TX). The total RNA concentration was determined by measuring the absorbance at 260nm. RNA integrity was verified electrophoretically by ethidium bromide staining and by measuring A260/A280 ratio.
Real-time RT-PCR
Real-time RT-PCR was performed essentially as described earlier [32-34]. First-strand cDNA was prepared from total RNA by using SuperScript First-Strand Synthesis Kit (Invitrogen, Carlsbad, CA) as per manufacturer's instructions. Real-time PCR employing a two-step cycling protocol (denaturation and annealing/extension) was carried out using Mastercycler realplex (Eppendorf, Westbury, N.Y.) and using primer sense 5′-TGGAAGAGGAGATAGAGCGA and antisense, 5′-TGTTGAGCAGGAAGGCGGTC. For each cDNA sample, parallel reactions were performed in triplicate for the detection of 18S and rat HO-1. The reaction samples in a final volume of 25 μl contained 2 μl of cDNA templates, 2 μl primer pair, 12.5 μl iQ SYBR Green Supermix and 8.5 μl water. Amplification conditions were identical for all reactions: 95 °C for 2 min for template denaturation and hot start prior to PCR cycling. A typical cycling protocol consisted of three stages: 15s at 95 °C for denaturation, 30s at 65 °C for annealing, 30s at 72 °C for extension, and an additional 20s hold for fluorescent signal acquisition [33]. To avoid non-specific signal from primer-dimers, the fluorescence signal was detected 2 °C below the melting temperature (Tm) of individual amplicon and above the Tm of the primer-dimers [35,36]. A total of 40 cycles were performed.
Quantitation of PCR was done using the comparative CT method as described in User Bulletin No. 2 of Applied Biosystems (Foster City, CA), and reported as fold difference relative to the calibrator cDNA (QuantumRNA Universal 18S Standards, Ambion). The fold change in HO-1 cDNA (target gene) relative to the 18S endogenous control was determined by:
Fold change = 2-ΔΔCT, where ΔΔCT = (CT Aniline - CT 18S) – (CT Control – CT 18S).
HO-1 ELISA
HO-1 protein levels in the spleen tissue lysates (prepared by using the lysis buffer provided with ELISA kit) were quantitated by using rat HO-1 ELISA Kit (Assay Designs, Ann Arbor, MI). OD was read at 450 nm on a Bio-Rad Benchmark Plus microplate spectrophotometer.
Western blot for HO-1
Protein samples from control and aniline-treated rats were subjected to Western blot analysis as described earlier [31]. Briefly, spleen tissue lysates were prepared using the lysis buffer essentially as described by the manufacturer (Cell Signaling, Beverly, MA). The lysate proteins (50 μg) were subjected to 10% SDS-PAGE and transferred to a PVDF membrane (Amersham, Arlington Heights, IL). After blocking with non-fat dry milk (5%, w/v), HO-1 protein band was detected using anti-HO-1 antibody (1:3000; Santa Cruz Biotech, Santa Cruz, CA) and goat anti-rabbit IgG-HRP (1:5000; Santa Cruz Biotech) as secondary antibody. ECL Plus (Amersham, Arlington Heights, IL) was used to detect the signals. HO-1 bands were quantitated by densitometry and normalized using the actin signal to correct for differences in loading of proteins from control and experimental groups. For densitometric analysis, the protein bands on the blot were measured by Eagle Eye II software. Protein concentration in the lysates was determined by Bio-Rad Protein Assay kit (Bio-Rad Laboratories).
Immunohistochemistry of HO-1 and CD68
Paraffin sections were cut and deparaffinized in an oven at 55 °C for 1 h and treated with xylene and various concentrations of ethanol, and finally rehydrated with water [32,37]. The slides were incubated with sodium citrate buffer at 95 °C for 20 min for antigen retrieval and subsequently incubated with various reagents, which included quenching of endogenous peroxidase activity with 0.3% H2O2 in PBS, blocking the non-specific binding sites with 5% non-fat dry milk, 1% BSA, 5% goat serum (horse serum in case of CD68) in PBS, respectively, and avidin and biotin block solutions (Vector Laboratories, Burlingame, CA). The sections were then incubated with antibodies to HO-1 (monoclonal, 1:50 dilution, Stressgen Bioreagents, Ann Arbor, MI,) or CD68 (a macrophage marker; 1:100 dilution, Abcam Inc., Cambridge, MA) overnight at 4 °C. Sections were washed with PBS and incubated with secondary antibodies. Immunoreactivity was detected by the ABC Method (Vectastain Elite ABC kit, Vector Laboratories, Burlingame, CA) with color development using 3,3′-diaminobenzidine (DAB). Mayer's Hematoxylin was then added as a counterstain for 1 min. The negative controls were immunostained as above, but with goat or horse serum instead of the anti-HO-1 or anti-CD68 antibody. Histological evaluation for staining was done with an OLYMPUS BX51 Microscope (Leads Instruments, Inc., Irving, TX).
Total iron and free iron analysis
Total iron in the spleens was analyzed by atomic absorption spectrophotometer as described earlier [4,38]. Analysis of low molecular weight chelatable iron (LMWC-Fe, free iron) was done as described earlier [13,29].
Ferritin Determination
Spleen tissues from control and aniline-treated rats were cut into small pieces and homogenized in a buffer containing 30 mM Tris-Cl, pH 8.0, 15 mM EDTA, 1.0 mM DTT, 0.5 mM spermine and spermidine, 15% glycerol and a complete protease inhibitor cocktail (Roche, Germany). After homogenization, 0.2% Triton X-100 and 5.0 M NaCl (to achieve a final concentration of 150 mM) were added. After incubating 30 minutes on ice, the extracts were centrifuged at 14,000 g for 30 min. The supernatant was saved and used for ferritin assay.
Ferritin was quantitated using an ELISA kit, essentially as described by the manufacturer (Innovative Research Inc., Novi, MI). Briefly, 100 μl of the standard or samples from control and aniline-treated rats (in duplicate) were added to ELISA plate wells precoated with anti-ferritin antibodies, and incubated at room temperature for 1 h with gentle mixing. After incubation, the plate was washed thoroughly with the wash buffer and 100 μl of the diluted (1:100) enzyme-antibody conjugate was added and incubated for 10 min at room temperature. After washing with wash buffer, 100 μl TMB substrate (3′,3′,5′,5′-tetramethylbenzidine) solution was added to each well. The plate was then incubated at room temperature for 10 min with continuous shaking followed by addition of 100 μl stop solution (0.3 M H2SO4) to terminate the reaction. The OD was read at 450 nm on a Bio-Rad Benchmark plus microplate spectrophotometer (Bio-Rad Laboratories, Hercules, CA).
Statistical analysis
Data are expressed as means ± SD. One-way ANOVA followed by Tukey-Kramer multiple comparison test (GraphPad InStat 3 software, La Jolla, CA) was performed for the statistical analysis. A p value of <0.05 was considered to be statistically significant.
Results
HO-1 mRNA expression in response to aniline exposure
To evaluate the impact of aniline exposure on the expression of HO-1 in the spleen, HO-1 mRNA levels were analyzed by real-time PCR and their levels are shown in Fig. 1. Aniline exposure for 4 and 7 days resulted in 2.0- and 2.4-fold increases, respectively, in HO-1 mRNA expression as compared to their respective controls. No change in HO-1 mRNA was observed at day 1 following aniline exposure.
Fig. 1.
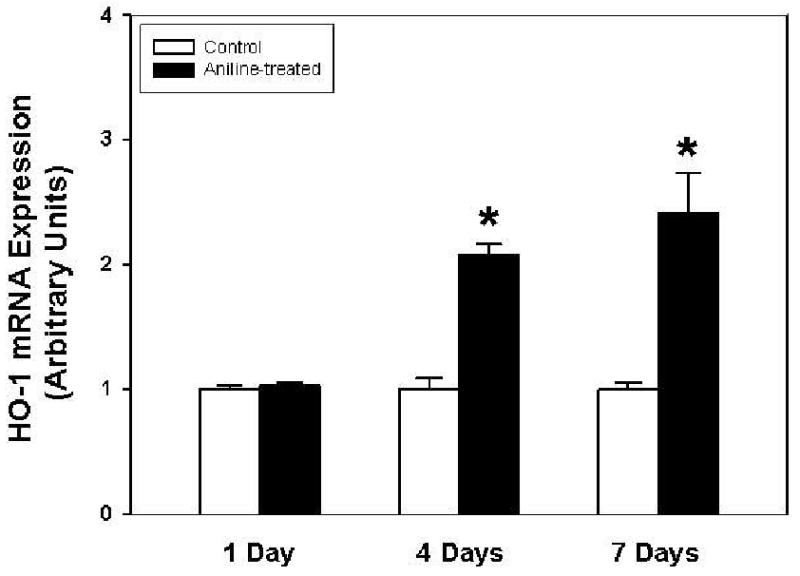
Real-time PCR analysis of HO-1 gene expression in the spleens of control and aniline-treated rats. Total RNA was extracted from spleens, real-time PCR was performed, and the fold change in mRNA expression (2-ΔΔCT) was determined. Values are means ± SD (n=3). *p<0.05 vs. respective controls.
Effect of aniline exposure on HO-1 protein expression in the spleen
To investigate whether increases in HO-1 gene expression were also associated with increases in protein levels, HO-1 protein levels were quantitated by a rat specific HO-1 ELISA kit in the spleen tissue lysates and results are shown in Fig. 2. Aniline exposure led to significant increases of 1.9- and 2.6-fold in HO-1 levels at 4 and 7 days, respectively, whereas no change was observed at day 1.
Fig. 2.
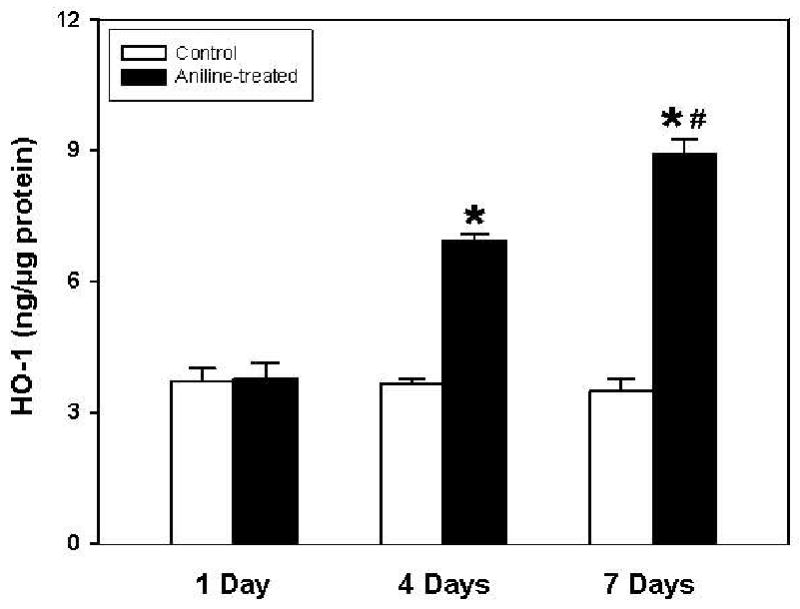
HO-1 protein levels in the spleens of rats treated with aniline. HO-1 was quantitated using a rat-specific ELISA kit. Values are means ± SD (n=6). *p < 0.05 vs. respective controls; # p < 0.05 vs. 4 days aniline group.
To further validate and confirm our quantitative ELISA results, HO-1 protein expression in the spleen lysates was also determined by Western blotting. As evident from Fig. 3, the HO-1 protein levels in the tissue lysates of aniline-treated rats increased by 2 and 2.5 fold at 4 and 7 days, respectively. However, similar to mRNA and ELISA results, no changes in HO-1 protein levels were observed 24 h following a single dose of aniline.
Fig. 3.
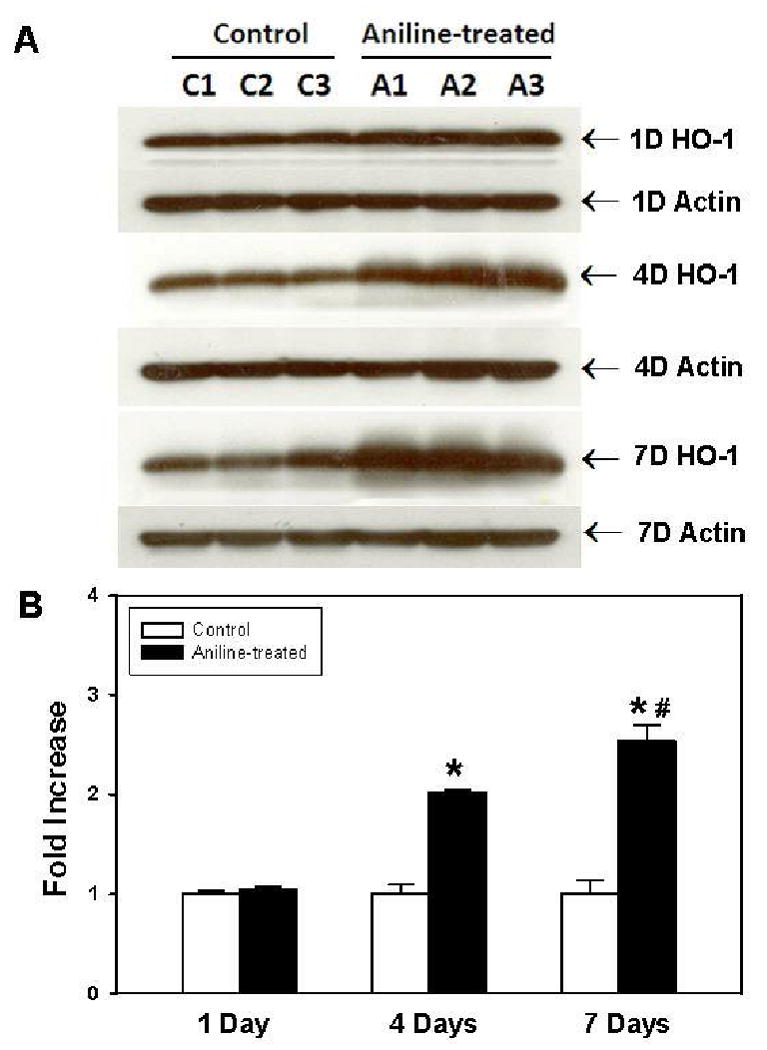
HO-1 protein expression in rat spleens following aniline exposure. (A) Western blot detection of HO-1 in the spleens of control and aniline-treated [1, 4 or 7 days (D)] rats. (B) Densitometric analysis of HO-1 bands. Values are means ± SD (n=3). *p < 0.05 vs. respective controls; # p < 0.05 vs. 4 days aniline group.
Immunohistochemical assessment of HO-1 and CD68 in the spleen of rats exposed to aniline
To demonstrate HO-1 expression and localization in the spleens of experimental animals, immunohistochemical studies were conducted. Since mRNA and protein expression of HO-1 showed no change at 1 day and maximum increases at 7 days, HO-1 immunohistochemical assessment was done at 1 and 7 days only. While spleens from control rats showed sparse immunostaining for HO-1 (Fig. 4A), increased immunostaining was evident in rats treated with aniline for 7 days (Fig. 4B). The immunoreactivity for HO-1 appeared predominantly in the red pulp areas of the spleen (Fig. 4B). Consistent with HO-1 mRNA and protein results, no changes in HO-1 immunoreactivity were observed 24 h following a single dose of aniline (data not included). To evaluate the contribution of macrophages in the increased expression of HO-1 in the spleen, immunoreactivity for CD68+ cells was also investigated. As evident from Fig. 4, aniline treatment for 7 days resulted in an increased immunoreactivity for CD68+ cells in the red pulp areas of the spleen (D) in comparison to controls (C), suggesting that macrophages are one of the major contributors to the increased HO-1 expression in the spleen in response to aniline exposure.
Fig. 4.
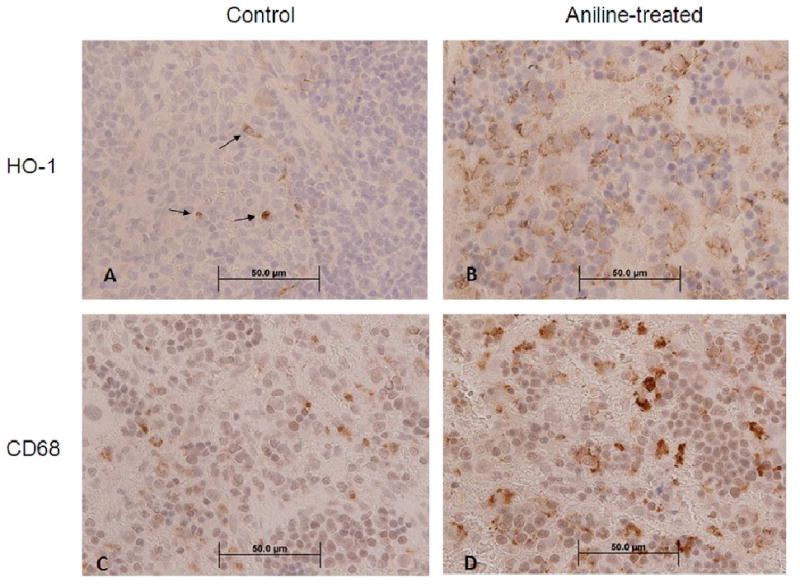
Immunohistochemistry of HO-1 and CD68 in rat spleen. Spleen from control rats (A) showed comparatively mild staining for HO-1 with occasional heavily stained cells (arrows), whereas spleen from rats treated with aniline for 7 days showed consistently strong immunoreactivity for HO-1 (B), with many heavily stained cells confined to the red pulp areas of the spleen. Similarly, there was an increased presence of CD68+ (macrophage marker) cells in the red pulp areas of spleen from rats treated with aniline for 7 days (D) compared to the controls (C).
Effect of aniline exposure on total and free iron
Aniline exposure is associated with erythrocyte damage [2,3]. Excessive deposition and degradation of erythrocytes in the spleen could potentially lead to iron release/overload. Therefore, changes in both total and free iron were quantitated by atomic absorption spectrophotometry. As evident from Fig. 5, exposure to a single dose of aniline did not result in any change in the total iron levels. However, total iron content increased significantly at 4 days (2.4 fold) and 7 days (2.7 fold) in comparison to their respective controls. LMWC-Fe (free iron) followed a similar pattern, showing increases of 1.9- and 3.5-fold at 4 and 7 days, respectively (Fig. 6), while no change after day 1.
Fig. 5.
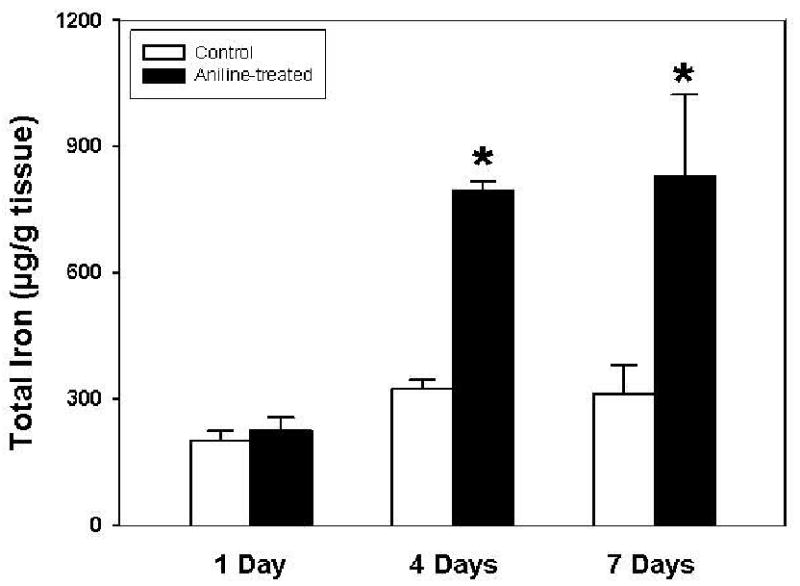
Splenic total iron content in the control and aniline-treated rats. Total iron was analyzed by atomic absorption spectrophotometry. Values are means ± SD (n=6). *p < 0.05 vs. respective controls.
Fig. 6.
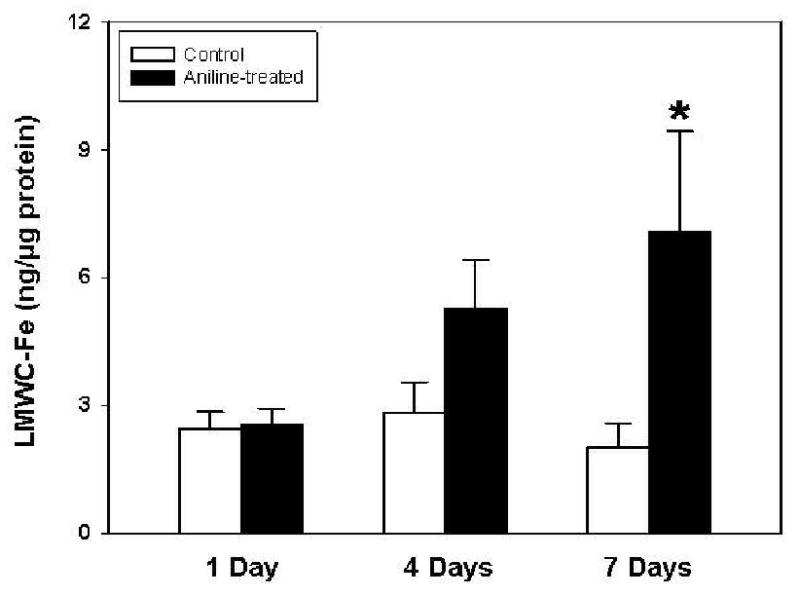
LMWC-Fe (free iron) in the spleens of control and aniline-treated rats. Values are means ± SD (n=4-5). *p < 0.05 vs. controls.
Effect of aniline exposure on ferritin levels in spleen
Ferritin is an iron-storage protein which could exert antioxidant properties by mediating the sequestration and storage of reactive iron, thus preventing ferryl or hydroxyl radical formation [39]. On the other hand, ferritin could also be a potential source of catalytic iron during oxidative stress [40,41]. Therefore, in view of the anti- and pro-oxidant potential of ferritin, its status was evaluated in the spleens of rats exposed to aniline. ELISA determination of total ferritin levels in the spleens of aniline-treated rats showed significant increases of 1.9- and 2.1-fold at days 4 and 7, respectively, in comparison to respective controls (Fig. 7). Exposure to a single dose of aniline, however, did not result in any change in ferritin levels in the spleen.
Fig. 7.
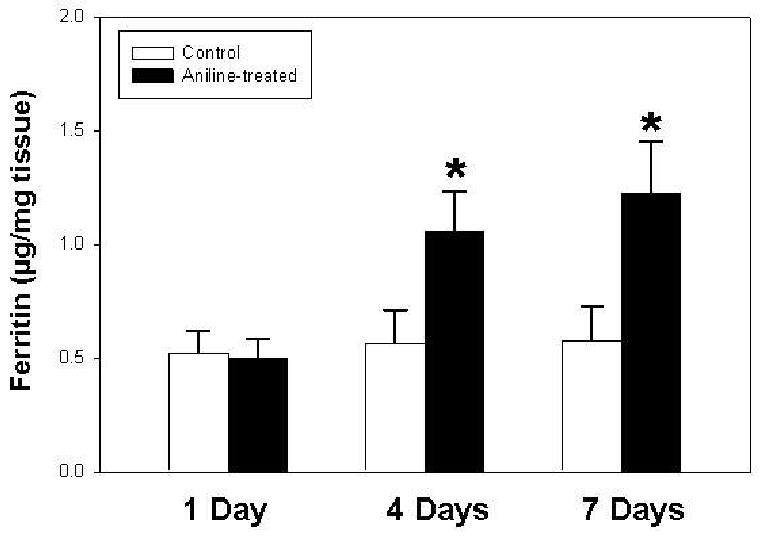
Ferritin levels in the spleens of control and aniline-treated rats. Total ferritin was quantitated using an ELISA. Values are means ± SD (n=5-6). *p < 0.05 vs. respective controls.
Discussion
Aniline and substituted anilines are known to cause splenic toxicity [2,12,13,31]. Among the most significant splenotoxic changes, preceding the formation of splenic sarcomas that result from exposure to aniline and/or structurally-related compounds, are splenomegaly, sinusoidal congestion, capsular hyperplasia and diffuse fibrosis [10-13]. Splenotoxicity could also lead to reduced ability of the spleen to participate in the immune response and/or its phagocytic function of clearing damaged erythrocytes and infectious organisms from the blood. This necessitates a clear understanding of early molecular events in the spleen that could contribute to such toxic responses. It has been established previously that aniline exposure leads to erythrocyte damage [2,3,7] and oxidative stress in the spleen [4,13,30]. Recognizing the role of HO-1 in catalyzing the oxidative degradation of heme moiety of hemoglobin to release free iron, it was of particular interest to evaluate its contribution to the splenic toxicity of aniline. It is evident from our data that HO-1 expression indeed increases in the spleen following aniline exposure, and its overexpression could be a pro-oxidant action in the splenic toxicity.
A major finding of this study is the significant increase in the expression of HO-1 (both at mRNA and protein levels) in the spleen following aniline exposure. The increases were highly significant in both repeated dose groups (4 and 7 days). Interestingly, the HO-1 was localized mostly in the red pulp of the spleen – areas which also showed intense iron staining (Prussian blue) [4]. To our knowledge, this is the first study to show an up-regulation of HO-1 in spleen following aniline exposure. Interestingly, the increases in HO-1 expression in this study also corresponded with previous findings of increased oxidative stress observed under similar experimental conditions [4]. While oxidative/nitrosative stress is known to induce HO-1 [20-22], up-regulation of HO-1, as observed in this study, could contribute to increased oxidative stress [14,26-28]. Other hemolytic agents such as phenylhydrazine and phenacetin also cause upregulation of HO-1 gene expression in rat spleens [42], and, thus, compliment our findings.
Aniline exposure in this study also led to significant increases in the total iron content – a finding consistent with earlier observations [4,13]. We also observed a substantial increase in LMWC-Fe (free iron) after repeated dose administration of aniline. Iron overload is associated with carcinogenesis in animal models and also in human diseases such as genetic hemochromatosis and asbestosis [43,44]. Free iron is redox active and can promote, through the Fenton reaction, the formation of harmful oxygen species such as hydroxyl radicals [45,46]. Thus, the iron-catalyzed oxyradicals can damage cellular lipids, proteins and nucleic acids, resulting in wide-ranging impairment of cellular function and integrity. Our earlier studies did show that aniline exposure was indeed associated with significant increases in lipid peroxidation [4,13], protein oxidation [13], DNA damage [29,32], activation of redox-sensitive transcription factors NF-κB and AP-1 [31,33] and up-regulation of fibrogenic cytokines [30,31,33,34] in the spleen.
There has been considerable debate concerning the pro-oxidant or antioxidant nature of HO-1 [14,24]. Heme has pro-oxidant properties, so its removal by HO-1 can be considered an antioxidant effect [15,47]. However, heme breakdown by HO-1 leads to the formation of LMWC-Fe (free iron), which is a more versatile catalyst of oxidative damage than heme [48]. Interestingly, exogenously added bilirubin at an equimolar concentration to low-molecular-mass iron present in both microsomal and liposomal systems was unable to prevent the pro-oxidant effect of the iron [14], further supporting a pro-oxidant role for HO-1.
Normally, iron is transported and stored in specific proteins (ferritin, transferrin and lactoferritin), which prevent or minimize its reaction with reduced oxygen derivatives [49,50]. Iron released from HO-mediated reactions stimulates ferritin synthesis, which ultimately provides an iron detoxification mechanism that may offer long-term cytoprotection [24]. However, if iron transport and storage proteins do not rapidly and effectively remove this redox-active iron, HO-1 induction could be a pro-oxidant effect. The aniline exposure protocol used in this study represents an experimental condition known to cause oxidative stress [4,30,32]. This protocol led to increases in ferritin levels, along with increases in free iron, suggesting that despite greater presence of ferritin, increased cytoprotection was not evident and tissue damaging oxidative reactions still prevailed [4,30]. Our findings are in general agreement with others, showing increased ferritin levels along with increased lipid peroxidation following ozone exposure [51], L-methionine treatment [52] and during non-alcoholic steatohepatitis [53]. Further support to this is evident from the finding that patients with iron overload exhibit increased iron and oxidative stress, despite up-regulated ferritin levels [54].
It is possible that ferritin can also be a potential source of catalytic iron during oxidative stress. Superoxide generating systems such as xanthine/xanthine oxidase or compounds/drugs which undergo redox cycling to produce superoxide radicals, may promote the release of ferritin iron [40,55]. Thus, ferritin may promote oxidative damage under conditions where excessive superoxide formation occurs [40]. Indeed, studies suggest that aniline and its metabolites have the potential to generate superoxide radicals [56]. Despite increased presence of ferritin, it is also possible that intracellular iron may overwhelm the ability of ferritin to sequester redox-active iron and thus result in oxidative stress.
In conclusion, our studies clearly show that aniline exposure results in HO-1 up-regulation, increased ferritin levels, and excessive release and availability of iron (free iron) in the spleen. Greater release and presence of redox-active iron as a result of exaggerated expression of HO-1 would result in increased oxidative stress, as observed previously [4,30,32,33]. Our data thus suggest that HO-1 up-regulation could be a significant pro-oxidant mechanism in the splenic toxicity of aniline, leading to oxidative damage through iron release in the spleen.
Acknowledgments
This work was supported by Grant ES06476 from the National Institute of Environmental Health Sciences (NIEHS), NIH, and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kim YC, Carlson GP. The effect of an unusual work shift on chemical toxicity II. Studies on theexposure of rats to aniline. Fundam Appl Toxicol. 1986;7:144–152. doi: 10.1016/0272-0590(86)90208-3. [DOI] [PubMed] [Google Scholar]
- 2.Bus JS, Popp JA. Perspectives on the mechanism of action of the splenic toxicity of aniline and structurally related compounds. Food Chem Toxicol. 1987;25:619–626. doi: 10.1016/0278-6915(87)90024-x. [DOI] [PubMed] [Google Scholar]
- 3.Khan MF, Boor PJ, Kaphalia BS, Alcock NW, Ansari GAS. Hematopoietic toxicity of linoleic acid anilide: importance of aniline. Fundam Appl Toxicol. 1995;25:224–232. doi: 10.1006/faat.1995.1058. [DOI] [PubMed] [Google Scholar]
- 4.Khan MF, Gu Y, Alcock NW, Boor PJ, Ansari GAS. Oxidative stress in splenotoxicity of aniline. Fundam Appl Toxicol. 1997;35:22–30. doi: 10.1006/faat.1996.2259. [DOI] [PubMed] [Google Scholar]
- 5.Pauluhn J. Subacute inhalation toxicity of aniline in rats: Analysis of time-dependence and concentration dependence of hematotoxic and splenic effects. Toxicol Sci. 2004;81:198–215. doi: 10.1093/toxsci/kfh187. [DOI] [PubMed] [Google Scholar]
- 6.Harrison JH, Jollow DJ. Role of aniline metabolites in aniline-induced hemolytic anemia. J Pharmacol Exp Ther. 1986;238:1045–1054. [PubMed] [Google Scholar]
- 7.Khan MF, Wu X, Kaphalia BS, Boor PJ, Ansari GAS. Acute hematopoietic toxicity of aniline in rats. Toxicol Lett. 1997;92:31–37. doi: 10.1016/s0378-4274(97)00032-5. [DOI] [PubMed] [Google Scholar]
- 8.Ciccoli L, Ferrali M, Rossi V, Signorini C, Alessandrini C, Comporti M. Hemolytic drugs aniline and dapsone induce iron release in erythrocytes and increase the free iron pool in spleen and liver. Toxicol Lett. 1999;110:57–66. doi: 10.1016/s0378-4274(99)00138-1. [DOI] [PubMed] [Google Scholar]
- 9.Mellert W, Deckardt K, Gembardt C, Zwirner-Baier I, Jackh R, Ravenzwaay BV. Aniline: early indiators of toxicity in male rats and their relevance to spleen carcinogenicity. Human Exptl Toxicol. 2004;23:379–389. doi: 10.1191/0960327104ht466oa. [DOI] [PubMed] [Google Scholar]
- 10.Goodman DG, Ward JM, Reichardt WD. Splenic fibrosis and sarcomas in F344 rats fed diets containing aniline hydrochloride, p-chloroaniline, azobenzene, o-toluidine hydrochloride, 4,4'sulfonyldianiline, or DRC Red No. 9. J Natl Cancer Inst. 1984;73:265–273. [PubMed] [Google Scholar]
- 11.Weinberger MA, Albert RH, Montgomery SB. Splenotoxicity associated with splenic sarcomas in rats fed high doses of DRC Red No. 9 or aniline hydrochloride. J Natl Cancer Inst. 1985;75:681–690. [PubMed] [Google Scholar]
- 12.Khan MF, Kaphalia BS, Boor PJ, Ansari GAS. Subchronic toxicity of aniline hydrochloride in rats. Arch Environ Contant Toxicol. 1993;24:368–374. doi: 10.1007/BF01128736. [DOI] [PubMed] [Google Scholar]
- 13.Khan MF, Wu X, Boor PJ, Ansari GAS. Oxidative modification of proteins and lipids in aniline-induced splenic toxicity. Toxicol Sci. 1999;48:134–140. doi: 10.1093/toxsci/48.1.134. [DOI] [PubMed] [Google Scholar]
- 14.Lamb NJ, Quinlan GJ, Mumby S, Evans TW, Gutteridge JMC. Haem oxygenase shows pro-oxidant activity in microsomal and cellular systems: implications for the release of low-molecular-mass iron. Biochem J. 1999;344:153–158. [PMC free article] [PubMed] [Google Scholar]
- 15.Prawan A, Kundu JK, Surh YJ. Molecular basis of heme oxygenase-1 induction: Implications for chemoprevention and chemoprotection. Antioxid Redox Signal. 2005;7:1688–1703. doi: 10.1089/ars.2005.7.1688. [DOI] [PubMed] [Google Scholar]
- 16.Maines MD, Kappas A. Metals as regulators of heme metabolism. Science. 1977;198:1215–1221. doi: 10.1126/science.337492. [DOI] [PubMed] [Google Scholar]
- 17.Brown KE, Dennery PA, Ridnour LA, Fimmel CJ, Kladney ED, Brunt RM, Spitz DR. Effect of iron overload and dietary fat on indices of oxidative stress and hepatic fibrogeneis in rats. Liver Intl. 2003;23:232–242. doi: 10.1034/j.1600-0676.2003.00832.x. [DOI] [PubMed] [Google Scholar]
- 18.Alam J, Shibahara S, Smith A. Transcriptional activation of the heme oxygenase gene by heme and cadmium in mouse hepatoma cells. J Biol Chem. 1989;264:6371–6375. [PubMed] [Google Scholar]
- 19.Nath KA, Balla G, Vercellotti GM, Balla J, Jacob HS, Levitt MD, Rosenberg ME. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest. 1992;90:267–270. doi: 10.1172/JCI115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauer M, Bauer I. Heme oxygenase-1: Redox regulation and role in the hepatic response to oxidative stress. Antioxid Redox Signal. 2002;4:749–758. doi: 10.1089/152308602760598891. [DOI] [PubMed] [Google Scholar]
- 21.Naughton P, Foresti R, Brains SK, Hoque M, Green CJ, Motterlini R. Induction of heme oxygenase-1 by nitrosative stress. A role for nitroxyl anion. J Biol Chem. 2002;277:40666–40674. doi: 10.1074/jbc.M203863200. [DOI] [PubMed] [Google Scholar]
- 22.Ryter SW, Choi AMK. Heme-oxygenase-1: Molecular mechanisms of gene expression in oxygen related stress. Antioxid Redox Signal. 2002;4:625–632. doi: 10.1089/15230860260220120. [DOI] [PubMed] [Google Scholar]
- 23.Suttner DM, Dennery PA. Reversal of HO-1 related cytoprotection with increased expression is due to reactive iron. FASEB J. 1999;13:1800–1809. doi: 10.1096/fasebj.13.13.1800. [DOI] [PubMed] [Google Scholar]
- 24.Ryter SW, Tyrrell RM. The heme synthesis and degradation pathways: Role in oxidant sensitivity. Free Radic Biol Med. 2000;28:289–309. doi: 10.1016/s0891-5849(99)00223-3. [DOI] [PubMed] [Google Scholar]
- 25.Hemdan S, Almazan G. Iron contributes to dopamine-induced toxicity in oligodendrocyte progenitors. Neuropath Appl Neurobiol. 2006;32:428–440. doi: 10.1111/j.1365-2990.2006.00757.x. [DOI] [PubMed] [Google Scholar]
- 26.Chen S, Khan ZA, Barbin Y, Chakrabarti S. Pro-oxidant role of heme oxygenase in mediating glucose-induced endothelial cell damage. Free Radic Res. 2004;38:1301–1310. doi: 10.1080/10715760400017228. [DOI] [PubMed] [Google Scholar]
- 27.Khan ZA, Barbin YP, Cukiernik M, Adams PC, Chakrabarti S. Heme-oxygenase-mediated iron accumulation in the liver. Can J Physiol Pharmacol. 2004;82:448–456. doi: 10.1139/y04-052. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Dore S. Heme oxygenase-1 exacerbates early brain injury after intracerebral haemorrhage. Brain. 2007;130:1643–1652. doi: 10.1093/brain/awm095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu X, Kannan S, Sadagopa Ramanujam VM, Khan MF. Iron release and oxidative DNA damage in splenic toxicity of aniline. J Toxicol Environ Health, Part A. 2005;68:657–666. doi: 10.1080/15287390590921757. [DOI] [PubMed] [Google Scholar]
- 30.Khan MF, Wu X, Wang J. Up-regulation of transforming growth factor-β1 in the spleen of aniline treated rats. Toxicol Appl Pharmacol. 2003;187:22–28. doi: 10.1016/s0041-008x(02)00041-8. [DOI] [PubMed] [Google Scholar]
- 31.Khan MF, Kannan S, Wang J. Activation of transcription factor AP-1 and mitogen-activated protein kinases in aniline-induced splenic toxicity. Toxicol Appl Pharmacol. 2006;210:86–93. doi: 10.1016/j.taap.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Ma H, Wang J, Abdel-Rehman SZ, Boor PJ, Khan MF. Oxidative DNA damage and its repair in rat spleen following subchronic exposure to aniline. Toxicol Appl Pharmacol. 2008;233:247–253. doi: 10.1016/j.taap.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J, Kannan S, Li H, Khan MF. Cytokine gene expression and activation of NF-kappa B in aniline-induced splenic toxicity. Toxicol Appl Pharmacol. 2005;15:36–44. doi: 10.1016/j.taap.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Wang G, Ansari GAS, Khan MF. Activation of oxidative stress-responsive signaling pathways in early splenotoxic response of aniline. Toxicol Appl Pharmacol. 2008;230:227–234. doi: 10.1016/j.taap.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simpson DA, Feeney S, Boyle C, Stitt AW. Retinal VEGF mRNA measured by SYBR green I fluorescence: a versatile approach to quantitative. PCR Mol Vision. 2000;6:178–183. [PubMed] [Google Scholar]
- 36.Rajeevan MS, Ranamukhaarachchi DG, Vernon SD, Unger ER. Use of realtime quantitative PCR to validate the results of cDNA array and differential display PCR technologies. Methods. 2001;25:443–4451. doi: 10.1006/meth.2001.1266. [DOI] [PubMed] [Google Scholar]
- 37.Khan MF, Wu X, Tipnis UR, Ansari GAS, Boor PJ. Protein adducts of malondialdehyde and 4-hydroxynonenal in livers of iron loaded rats:quantitation and localization. Toxicology. 2002;173:193–201. [PubMed] [Google Scholar]
- 38.Alcock NW. A hydrogen peroxide digestion system for tissue trace metal analysis. Biol Trace Elem Res. 1987;13:363–370. doi: 10.1007/BF02796647. [DOI] [PubMed] [Google Scholar]
- 39.Klausner RD, Rouault TA, Harford JB. Regulating the fate of mRNA: the control of cellular iron metabolism. Cell. 1993;72:19–28. doi: 10.1016/0092-8674(93)90046-s. [DOI] [PubMed] [Google Scholar]
- 40.Reif DW. Ferritin as a source of iron for oxidative damage. Free Radic Biol Med. 1992;12:417–427. doi: 10.1016/0891-5849(92)90091-t. [DOI] [PubMed] [Google Scholar]
- 41.Aust SD. Ferritin as a source of iron and protection from iron-induced toxicities. Toxicol Lett. 1995;82-83:941–944. doi: 10.1016/0378-4274(95)03605-9. [DOI] [PubMed] [Google Scholar]
- 42.Rokushima M, Omi K, Imura K, Arari A, Furukawa N, Itoh F, Miyazaki M, Yamamoto J, Rokushima M, Okada M, Torii M, Kato I, Ishizaki J. Toxicogenomics of drug-induced hemolytic anemia by analyzing gene expression profiles in the spleen. Toxicol Sci. 2007;100:290–302. doi: 10.1093/toxsci/kfm216. [DOI] [PubMed] [Google Scholar]
- 43.Bradbear RA, Bain C, Siskind V, Schofield FD, Webb S, Azelsen EM, Halliday JW, Bassett ML, Powell LW. Cohort study of internal malignancy in genetic hemochromatosis and other chronic non-alcoholic liver diseases. J Natl Cancer Ins. 1985;75:81–84. [PubMed] [Google Scholar]
- 44.Toyokuni S. Iron-induced carcinogenesis: The role of redox regulation. Free Radic Biol Med. 1996;20:553–566. doi: 10.1016/0891-5849(95)02111-6. [DOI] [PubMed] [Google Scholar]
- 45.Halliwell B, Gutteridge JMC. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1985;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferrali M, Signorini C, Sugherini L, Pompella A, Lodovici M, Caciotti B, Ciccolit L, Comporti M. Release of free, redox-active iron in the liver and DNA oxidative damage following phenylhydrazine intoxication. Biochem Pharmacol. 1997;53:1743–1751. doi: 10.1016/s0006-2952(97)82456-2. [DOI] [PubMed] [Google Scholar]
- 47.Stocker R. Induction of haem oxygenase as a defence against oxidative stress. Free Radic Res Commun. 1990;9:101–112. doi: 10.3109/10715769009148577. [DOI] [PubMed] [Google Scholar]
- 48.Gutteridge JMC. Iron promoters of the Fenton reaction and lipid peroxidation can be released from haemoglobin by peroxides. FEBS Lett. 1986;201:291–295. doi: 10.1016/0014-5793(86)80626-3. [DOI] [PubMed] [Google Scholar]
- 49.Crichton RR, Charloteaux-Wauters M. Iron transport and storage. Eur J Biochem. 1987;164:485–506. doi: 10.1111/j.1432-1033.1987.tb11155.x. [DOI] [PubMed] [Google Scholar]
- 50.Ceccarelli D, Gallesi D, Giovannini F, Ferrali M, Masini A. Relationship between free iron level and rat liver mitochondrial dysfunction in experimental dietary iron overload. Biochem Biophys Res Commun. 1995;209:53–59. doi: 10.1006/bbrc.1995.1469. [DOI] [PubMed] [Google Scholar]
- 51.Ghio AJ, Turi JL, Madden MC, Dailey LA, Richards JD, Stonehuerner JG, Morgan DL, Singleton S, Garrick LM, Garrick MD. Lung injury after ozone exposure is iron dependent. Am J Physiol Lung Cell Mol Physiol. 2007;292:L134–L143. doi: 10.1152/ajplung.00534.2005. [DOI] [PubMed] [Google Scholar]
- 52.Mori N, Hirayama K. Long-term consumption of a methionine-supplemented diet increases iron and lipid peroxide levels in rat liver. J Nutr. 2000;130:2349–2355. doi: 10.1093/jn/130.9.2349. [DOI] [PubMed] [Google Scholar]
- 53.Malaguarnera L, Madeddu R, Palio E, Arena N, Malaguarnera M. Heme oxygenase-1 levels and oxidative stress-related parameters in non-alcoholic fatty liver disease patients. J Hepatol. 2005;42:585–591. doi: 10.1016/j.jhep.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 54.Kassab-Chekir A, Laradi S, Ferchichi S, Haj Khelil A, Feki M, Amri F, Selmi H, Bejaoui M, Miled A. Oxidant, antioxidant status and metabolic data in patients with beta-thalassemia. Clin Chim Acta. 2003;338:79–86. doi: 10.1016/j.cccn.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 55.Agrawal R, Sharma P, Rao GS. Release of iron from ferritin by metabolites of benzene and superoxide radical generating agents. Toxicology. 2001;168:223–230. doi: 10.1016/s0300-483x(01)00412-7. [DOI] [PubMed] [Google Scholar]
- 56.Brennan RJ, Schiestl RH. Aniline and its metabolites generate free radicals in yeast. Mutagenesis. 1997;12:215–220. doi: 10.1093/mutage/12.4.215. [DOI] [PubMed] [Google Scholar]


