Abstract
Cancer cells within solid tumors live in a unique microenvironment containing barriers impairing the penetration of antibodies, immunoconjugates and immunotoxins. SS1P is an immunotoxin composed of the Fv portion of a mesothelinspecific antibody fused to a bacterial toxin and is now undergoing Phase II testing in mesothelioma. We describe a new approach to study the targeting process of SS1P in tumors. A flow cytometry-based method (FC method) was developed to quantify the uptake of SS1P by individual tumor cells, and a gel filtration assay was developed to study shed mesothelin (sMSLN), a barrier for SS1P therapy. The cellular uptake of SS1P in tumor cells peaked several hours after blood SS1P was cleared, reflecting the underlining intra-tumor distribution process of SS1P independent of its blood supply. With this approach, we demonstrated Taxol improved the penetration of SS1P in the tumor, associated with a reduced sMSLN barrier in tumor extracellular fluid. Our study provides a mechanistic basis for the combined use of SS1P with cytotoxic drugs and helps explain the increased anti-tumor activity when chemotherapy and antibody-based therapies are combined. We also showed that the FC method can quantify the tumor cell uptake of Herceptin and an immunotoxin targeting HER2/neu.
Keywords: solid tumor, shed antigen, tumor microenvironment, interstitial fluid, drug barrier
Introduction
Solid tumors remain a major therapeutic problem despite the large number of treatments available. Antibody based therapies have shown great promise, but a major challenge is delivering sufficient amounts of antibody, immuno-conjugate or immunotoxin to all malignant cells within large tumor masses. To kill cells, these agents must be distributed to the tumor by blood vessels, escape from capillaries, and diffuse through the interstitial space to reach cells in the interior of the tumor. Several barriers to antibody penetration have been identified including defective vasculature (1), a binding site barrier (2), high interstitial fluid pressure (3), high collagen composition (4) and the presence of high levels of shed antigen in the tumor (5).
Recombinant immunotoxins are genetically engineered proteins composed of the Fv portion of an antibody fused to a toxic protein designed to kill cancer cells (6). Two of these, Ontak (7) and BL22 (8) are very active in hematological malignancies, but immunotoxins are less active against solid tumors (9, 10).
Immunotoxin SS1P, currently in a Phase II clinical trial, targets solid tumors with mesothelin expression. It is a 63 kDa protein with a half-life of 20 min in mice. The large molecular size and short half-life as well as its systemic toxicity limit its entry to tumor tissue. In addition, mesothelin is actively shed by tumors. Shed mesothelin (sMSLN) is present in tumor extracellular fluid (ECF) at a very high concentration and acts as a decoy receptor to prevent SS1P from targeting tumor cells (5). These factors make enhancing drug delivery into tumors critical to improve the therapeutic effect of SS1P.
In this study, we describe a new approach to study the targeting process of SS1P in tumors. Because only internalized SS1P can kill cancer cells, we developed a flow cytometry-based method (FC method) to measures both the percentage of cells in a tumor that accumulate SS1P and the average amount of SS1P internalized by these cells. A gel filtration assay was also developed to study the formation of SS1P/mesothelin complexes in tumor ECF. This approach showed that Taxol treatment improved SS1P penetration into tumors and increased the fraction of tumor cells that accumulate SS1P. We conclude that this effect of Taxol contributes to the remarkable synergy obtained when the two agents are used together. We also showed this new method can be used to study the uptake of Herceptin and an immunotoxin targeting HER2/neu.
Materials and Methods
Reagents
Immunotoxins SS1P, SS1P(E553D) and Erb38 were prepared as previously described (11). SS1P(E553D) is an ADP-ribosylating mutant of SS1P, in which glutamic acid 553 of Pseudomonas exotoxin A was substituted by aspartic acid. It is a nontoxic form of SS1P, retaining full binding capacity (12). The Alexa labeling was done with Alexa Fluor™ Protein Labeling Kit (Invitrogen, Carlsbad, CA). SS1P was biotinylated with Sulfo-NHS-Biotinylation (Pierce, Rockford, IL). Rat anti-mouse CD16/32 and PE-conjugated mouse anti-human EGF receptor (EGFR) are from BD (San Jose, CA).
Cell culture
A431/H9 is a human mesothelin transfected A431 cell line. There are 5×106 mesothelin molecules for binding on the surface of each A431/H9 cell. NIH-3T3/HER-2 is a gift from Dr. Peter L. Choyke (NIH) (13). It is a human HER-2 transfected NIH-3T3 cell line, with high HER-2 expression on the cell surface. KB and A431/H9 cells are grown in DMEM with 10% FBS. NIH-3T3/HER-2 is maintained in RPMI1640 (10% FBS).
Tumor dissociation and cell labeling analysis
Alexa labeled immunotoxin or antibody was given i.v. in 200 μL of 0.9% NaCl with 0.2 mg/mL BSA. Xenografted A431/H9 tumors were removed and minced. Tumor dissociation was performed with 0.2 U/mL Liberase III (Roche, Indianapolis, IN) and 0.1 mg/mL DNase I in Hank's Buffered Salt Solution. The incubation was at 37°C for 30 min of constant mixing. The cell suspension was passed through cell strainer (50 μm). A431/H9 cells were identified by the staining with PE-labeled anti-human EGFR. Rat anti-mouse CD16/32 (10 μg/mL) was used to block non-specific binding to Fc receptor. When antibody was injected, tumor perfusion was performed to remove circulating antibody in the blood before tumor was harvested (14).
The number of incorporated SS1P molecules
One A431/H9 cell has 5 × 106 sites for SS1P-Alexa binding. A fluorescence intensity after saturation by SS1P-Alexa of culture cells was used as a standard. In tumor experiments, mice received an injection of SS1P-Alexa. The MFI was measured for EGFR positive cell population in tumor cell suspension. The MFI was compared to that of mice which received only saline treatment. The average number of cell-associated SS1P-Alexa was then calculated. Since surface SS1P-Alexa was completely removed during tumor digestion, the number actually represented internalized SS1P-Alexa molecules by A431/H9 tumor cells.
Tumor experiments
Tumor experiments were done as previously described (11). NIH-3T3/HER-2 (2.0 × 106 cells) were used for implantation. The animal protocol was approved by the National Cancer Institute Animal Care and Use Committee.
Mesothelin preparation
Mesothelin was expressed and purified as a rabbit Fc fusion protein with a His tag (15). The fusion protein has a thrombin cleavage site between the rabbit IgG and the extra-cellular domain of mesothelin. After purification of Fc-mesothelin over Protein A sepharose (Amersham, Piscataway, NJ), Fc-mesothelin was dialyzed against immobilized affinity chromatography (IMAC) A buffer (50 mM NaPO4H2, 10 mM Imidazole, 500 mM NaCl; pH 7.5) in the presence of thrombin (200 units; GE Healthcare, Piscataway, NJ) at room temperature overnight. The cleaved mesothelin product was then purified from the remaining Fc protein by IMAC on Ni Sepharose™ High Performance resin (GE Healthcare) in a 2 mL column.
ELISA assay for SS1P-biotin
The concentration of SS1P-biotin was measured by ELISA. Briefly, microtiter plates were coated with 4 μg/mL goat anti-mouse IgG. After blocking, mouse anti-PE mAb IP57 (4 μg/mL) was added for incubation. After 4 washes, SS1P-biotin samples with proteinase inhibitor cocktail III (Calbiochem, La Jolla, CA) was added and incubated overnight at 4°C, followed by 45 min-incubation with streptavidin-HRP (100 ng/mL). The color was developed by tetramethylbenzidine substrate (Pierce). The assay can detect SS1P-biotin concentration as low as 0.1 ng/mL.
The characterization of SS1P and mesothelin in tumor ECF
Tumor ECF was obtained by nylon mesh basket method as previously described (16). To analyze the formation of SS1P/mesothelin complex in tumor ECF, gel filtration studies were performed with a TSK G2000sw column (30 cm × 7.8 mm; TOSOH, Tokyo, Japan). Briefly, 100 μL of isolated ECF (diluted 1:10 in PBS) was loaded onto column. PBS was applied to the column at the flow rate of 0.6 mL/min. Fractions of 0.2 mL were collected. SS1P and mesothelin concentrations in each fraction were measured by ELISA.
Statistics
All data are presented as mean ± SD. Statistical differences between groups were measured by Student's t test with 2-tailed distribution. A P value of less than 0.05 was considered significant.
Results
FC method for in vivo mesothelin targeting
To measure SS1P uptake by cells in a tumor, we developed the FC method, which we designed to measure the percentage of tumor cells that accumulate SS1P in vivo. SS1P was labeled by a small and stable fluorophore, Alexa 488. The resulting conjugate, SS1P-Alexa488, has the same activity in cell killing assays and anti-tumor activity in mice as SS1P. It is also very stable after uptake by tumor cells with a half-life of 40 h (data not shown).
SS1P-Alexa488 was given i.v. to mice bearing A431/H9 tumors (150 mm3). Tumors were removed 3 h later, and a single cell suspension was prepared as described in Materials and Methods. The fluorescent signal of SS1P-Alexa488 on these cells was measured by flow cytometry. A431/H9 cells express a high level of mesothelin and EGFR on their cell surface. EGFR is used to identify and gate tumor cells, because of its resistance to enzymatic digestion (Fig. 1A). Several different types of cells within the tumor were identified, including tumor cells (50%), macrophages (30%), endothelial cells (2%) and others.
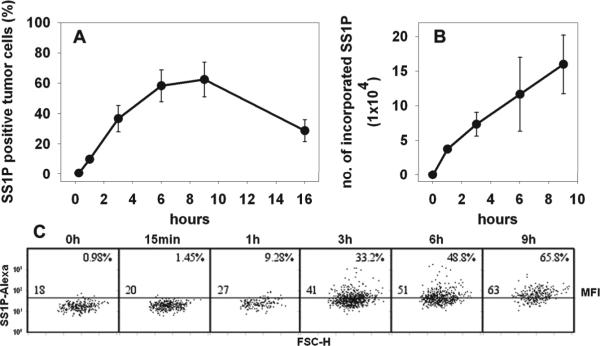
Figure 1.
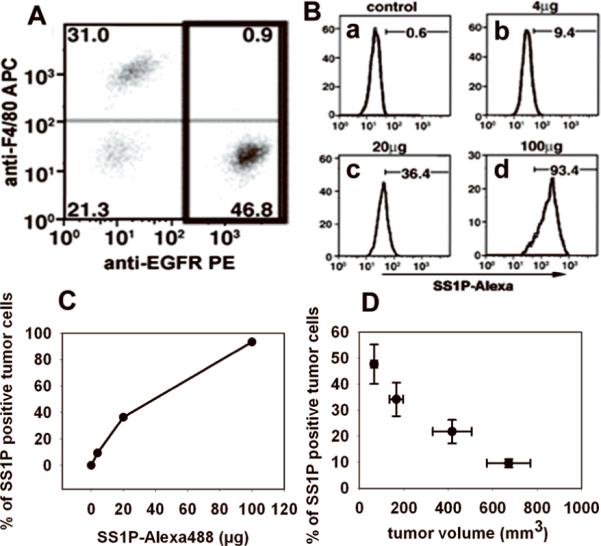
In vivo tumor cell staining by SS1P-Alexa. A, Cell composition of tumor cell suspension. Tumor cell suspension prepared by digestion was stained by anti-EGFR PE and anti-F4/80 APC to distinguish A431/H9 cells and mouse macrophages. B, The staining of A431/H9 tumors. Control mice received saline (Ba). Mice bearing A431/H9 tumors received SS1P-Alexa488 of different doses, 4 μg (Bb), 20 μg (Bc) and 100 μg (Bd). After 3 h, the staining of SS1P-Alexa on tumor cells (gated population in A) was analyzed. Numbers denote the percentage of SS1P-Alexa positive tumor cells as total of tumor cells. C, Summary of tumor staining by increased doses of SS1P-Alexa. Data from Ba, Bb, Bc and Bd were plotted. D, The effect of tumor size on tumor cell staining. Mice bearing A431/H9 tumors of various size (70, 170, 400 and 650 mm3) received 20 μg of SS1P-Alexa. Tumor cell staining after 3 h was measured (n = 5).
The EGFR-positive tumor cell population was gated for the analysis of SS1P staining. Because the digesting enzymes completely removed surface bound SS1P, this method specifically measured internalized SS1P. When 4 μg of SS1P-Alexa488 was given, 9.4% of tumor cells became SS1P positive. When 20 and 100 μg were given, the positive tumor cells rose to 36% and 93%. There is only a 0.6% background in the assay (Fig. 1B). Thus the fraction of positive tumor cells is positively correlated with the dose of SS1P-Alexa488 (Fig. 1C).
In addition, we injected 20 μg of Alexa488 labeled immunotoxin BL22 that binds to human CD22, which is not expressed on A431/H9 cells (17). We also injected SS1PAlexa488 (20 μg) into mice bearing A431 tumors that do not express mesothelin. No Alexa-positive tumor cells were detected in these experiments (data not shown). These results show that SS1P-Alexa488 uptake by A431/H9 cells is specifically mediated by mesothelin binding.
The effect of tumor size on SS1P uptake was evaluated by injecting 20 μg of SS1PAlexa into mice with different size tumors. Figure 1D shows that the percentage of cells labeled at 3 h is inversely related to tumor size with the highest uptake (48%) for the smallest tumor (75 mm3). The values fell to 31% for 170 mm3 tumors, to 22% for 400 mm3 tumors and to 10% for 650 mm3. Tumor cell staining in vivo by SS1P-Alexa is strongly dependent on tumor size.
FC method for in vivo HER-2 targeting
We also applied the FC method to study the in vivo targeting process of Herceptin, a humanized antibody targeting HER2/neu expressing tumors. We labeled it with Alexa647, which is structurally similar to Alexa488 but brighter. At a dose of 30 μg, 24% of NIH-3T3/HER2 tumor cells became positive for Herceptin. Rituxan-Alexa647 was used as a negative control, and only 0.6% positive tumor cells were observed (Fig. 2A). We also applied FC method to the anti-HER2/neu immunotoxin - Erb38 (18); 33% of tumor cells were labeled at 20 μg of Erb38-Alexa647 and 98% at 100 μg. Only 1.5% of cells were labeled with immunotoxin HA22 targeting CD22 (Fig. 2B). These studies demonstrate that the FC method can have a wide-range of applications in the study of antibody-based therapies.
Figure 2.
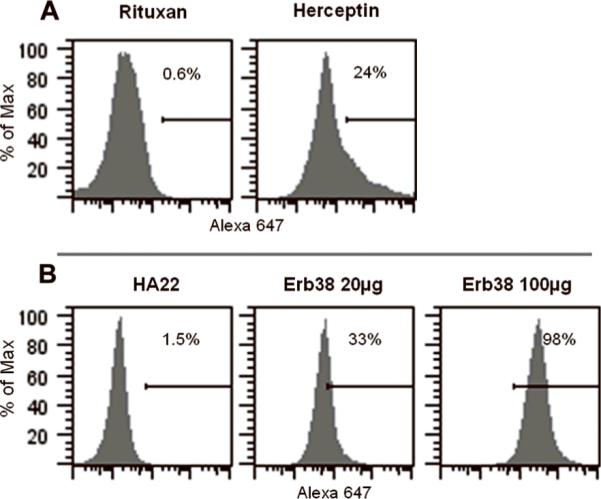
In vivo tumor cell staining by anti-HER-2 antibody (Herceptin) and immunotoxin (Erb38). Mice with NIH-3T3/HER2 tumors (about 200 mm3) received either Herceptin (30 μg) (A) or Erb38 (B). Tumor cells were defined as the CD45-/CD31-population. The staining was measured 24 h after Herceptin administration and 3 h after Erb38.
The kinetics of cellular uptake of SS1P in tumor
The tumor digestion step in this FC method completely removes surface-bound SS1P and measures only internalized SS1P. To examine the kinetics of cellular accumulation of SS1P in tumors independent of tumor cell killing, we used an inactive SS1P mutant SS1P(E553D)-Alexa. A 20 μg dose was given to mice with 150 mm3 tumors. Figure 3A shows that the percentage of SS1PAlexa positive cells increases with time, peaking at 60% between 6 and 9 h. The uptake fell to 30% at 16 h, which reflects a combination of degradation and dilution by cell division.
Figure 3.
In vivo time course for internalized SS1P in tumor cells. SS1P(E553D)-Alexa (20 μg) was given to mice bearing A431/H9 tumors. Tumors were removed at different time points, 15 min, 1, 3, 6, 9 and 16 h (n = 3). The staining of tumor cells by SS1P(E553D)-Alexa was analyzed. The percentage of SS1P(E553D)-Alexa positive tumor cells was shown in A. The number of SS1P-Alexa molecules incorporated into tumor cells was shown in B. A representative result from each time point was shown in C. The number at the upper right corner of each time point denotes the percentage of SS1P(E553D)-Alexa positive tumor cells. The number in the left above the middle line is the MFI of tumor cell population.
The FC method allows one to calculate the average amount of SS1P taken up per cell by using the MFI and converting it to number of molecules per cell (Materials and Methods). Figure 3B shows that at 1 h, when 20% of cells contain SS1P, there are 37,000 SS1P molecules per cell, and this rises to 110,000 molecules per cell at 6 h (p<0.01), when 60% of cells contain SS1P. Between 6 and 9 h there is a further increase. Representative SS1P staining of tumor cells at each time point is shown in Figure 3C. These studies demonstrate that the FC method has an advantage over other methods because it quantifies the amount of study drug targeting tumor cells on a cellular basis.
Synergy of Taxol and immunotoxin SS1P on mesothelin-expressing tumors
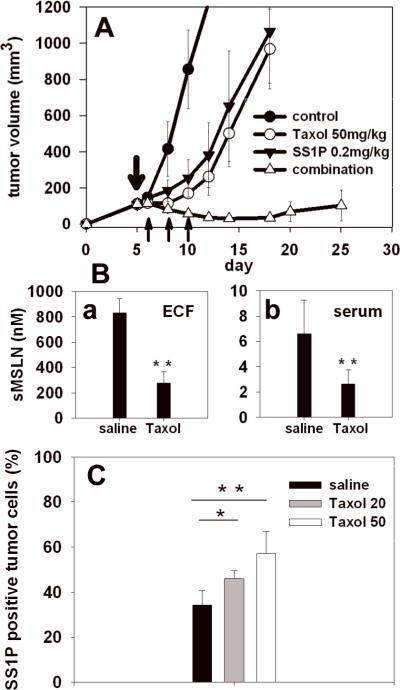
We previously showed that Taxol and SS1P synergize to cause the regression or eradication of mesothelin-expressing A431/K5 and KB tumors (11, 16). Taxol was shown to significantly decrease the sMSLN level in tumor ECF, which constitutes a barrier for SS1P therapy. Similar results were also identified with the tumor model used in the current study. When one dose of Taxol or three doses of SS1P alone delayed tumor growth for about five days, combination therapy caused dramatic regressions on tumor growth with a nadir on day 15 (Fig. 4A). Taxol treatment reduced the sMSLN levels in both tumor ECF and serum about 3-fold (Fig. 4Ba and 4Bb).
Figure 4.
Synergistic anti-tumor effect of Taxol and SS1P. A, Treatments were begun when A431/H9 tumors reached 120 mm3 (n = 5). Taxol was given at 50 mg/kg 5 days after implantation. SS1P was given at 0.2 mg/kg on days 6, 8 and 10. Controls received saline. (●), control; (◯), Taxol alone; (▾), SS1P alone; (▵), combination treatment. B, Shed mesothelin levels in tumor ECF (Ba) and serum (Bb) were measured 4 days after Taxol treatment (n = 5). C, The effect of Taxol on SS1P-Alexa staining of A431/H9 tumors. Mice with A431/H9 tumors (150 mm3) were treated with Taxol at 20 or 50 mg/kg (n = 5). SS1P-Alexa (20 μg) was injected. The control group received saline. Black, saline; grey, Taxol at 20 mg/kg; white, Taxol at 50 mg/kg. *, P < 0.05; ** P < 0.01.
We investigated whether reducing the sMSLN barrier by Taxol was associated with an improvement of cellular uptake of SS1P in tumors with the FC method. Mice with tumors received SS1P-Alexa alone or SS1P-Alexa proceeded by Taxol (Fig. 4C). Without Taxol, 35% of the tumor cells are positive for SS1P at 3 h. The percentage increased to 43% three days after one dose of 20 mg/kg Taxol (P<0.05) and to 57% after 50 mg/kg Taxol (P<0.01). Taxol did not increase the cell surface expression of mesothelin (data not shown). We conclude that the increased cellular uptake of SS1P after Taxol administration results from improved SS1P penetration in tumor.
Formation of SS1P/mesothelin complexes in tumor extracellular space
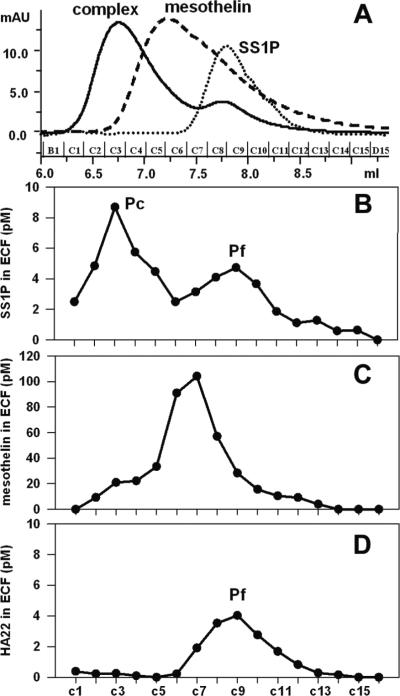
To provide direct evidence that sMSLN can act as a barrier in tumors, we developed a gel filtration method to determine if SS1P in tumors was free, or bound to mesothelin. SS1P entering a tumor is expected to form a complex with sMSLN. Figure 5A shows that the TSK column can resolve mesothelin, SS1P and the complex. As standards, SS1P alone, purified mesothelin alone and preformed SS1P/mesothelin complexes were each run over the column. The SS1P peak emerged at 7.8 mL, corresponding to 61 kDa. Mesothelin eluted at 7.2 mL, corresponding to an apparent MW of 73 kDa. Although this is much larger than the expected MW of 42 kDa (19), analysis by dynamic light scattering shows mesothelin is a monomer (data not shown) and behaves aberrantly on the column. The mesothelin-SS1P complex was prepared by mixing in a 1:1 molar ratio. A major peak was detected at 6.76 mL.
Figure 5.
The identification of SS1P/mesothelin complex in tumor ECF. TSKG2000 column was used to separate free SS1P and SS1P in complex. A, The overlapped chromatograms of standards, 20 μg of SS1P-biotin (dot line), 40 μg of mesothelin (dash line) and a mixture of 20 μg of SS1P-biotin and 25 μg of mesothelin (solid line). B, SS1P-biotin distribution in fractions of tumor ECF. Ten microgram of SS1P-biotin was injected. C, Mesothelin distribution in fractions of tumor ECF. Mice received 10 μg of SS1P-biotin. D, HA22-biotin distribution in fractions of tumor ECF. Twenty microgram of biotin-labeled HA22 was injected.
Tumor ECF was collected at 3 h after SS1P-biotin injection, and analyzed on the TSK column. Two SS1P peaks, Pc and Pf, were identified (Fig. 5B), corresponding to the complex and free SS1P. Further analysis showed SS1P in Pf is damaged and unable to, to bind to mesothelin (data not shown). Two sMSLN peaks were identified, a large peak of free mesothelin and a smaller peak in the position of the complex (Fig. 5C). This is the expected result, because the concentration of free mesothelin greatly exceeds that of SS1P in the tumor extra-cellular space. We demonstrated that the complex peak is SS1Pmediated by using HA22 as a negative control. ECF from HA22-biotin treated tumors has only one peak at the position of free SS1P (Fig. 5D). This study provides direct evidence that sMSLN in tumor ECF binds mesothelin which can serve as a barrier to SS1P therapy.
Discussion
We have developed a new flow cytometry based approach to study the targeting process of immunotoxins and antibodies within solid tumors. In this approach, the immunotoxin or antibody labeled with a fluorescent dye, is injected into mice bearing solid tumors. After various time periods, the tumor is removed, separated into single tumor cells and their content of immunotoxin analyzed by flow cytometry. We initially applied this method to study the uptake of the SS1P immunotoxin targeting mesothelin, but subsequently have shown that the method works for both the Herceptin antibody and an immunotoxin targeting Her2/neu.
We also studied the formation of SS1P/sMSLN complexes in the tumor ECF by gel filtration chromatography. We then used these methods to examine the mechanism of synergy observed when mesothelin-expressing tumors are treated with Taxol and SS1P. We demonstrated that prior Taxol treatment increases the percentage of tumor cells that accumulate SS1P and propose that this effect accounts for the synergy observed when the agents are used together.
Because solid tumors have no lymphatics, there is a major defect in perfusion and drugs can only enter by diffusion, a much slower process. In addition the high interstitial fluid pressure in tumors further impairs drug entry and this is especially important for antibody based drugs, which diffuse slowly because of their large size (20). Drug is also consumed by binding to ligands along the path of entry. Because immunotoxins have a relatively short life in the circulation (20 min in mice and 2–8 h in humans), and because they cannot be given at very high doses due to nonspecific side effects, the time that the tumor is exposed to high immunotoxin concentrations is relatively short. Hence improving penetration should have an important impact on their therapeutic effect.
FC method for in vivo targeting
Previously uptake of antibodies and immunotoxins by tumors has been investigated using radiolabeling or by immuno-histochemistry, methods which are difficult to quantify at a cellular level. In this study, we used Alexalabeled immunotoxin. The Alexa molecules employed are small (less than 1 kDa), stable across a broad pH range and emit a strong fluorescent signal upon excitation. These properties make these fluorochromes ideal for tumor staining studies and enabled us to measure both the percentage of cells in the tumor taking up the immunotoxin and the amount taken up by single cells.
One feature of the method is that an enzymatic digestion step is used to dissociate tumor cells. This treatment completely removes mesothelin and SS1P bound to it from the cell surface and allowed us to quantify the number of molecules internalized, which are critical for immunotoxin activity. Other methods measure both bound and internalized molecules as well as molecules trapped in the ECF or by other cell types. In addition, when multicolor staining is used, cell populations, other than tumor cells, can be simultaneously studied for their contributions to the drug distribution.
Gel filtration study
Mesothelin is actively shed from the cell surface so that sMSLN exists in tumor ECF at high concentrations (16), where it should be able to form complexes with SS1P, preventing it from binding to tumor cells or diffusing further into the tumor. In this study, we analyzed the nature of SS1P in the tumor ECF by size exclusion chromatography and showed it was present in complexes with mesothelin. The complex is a result of SS1P distribution between cellular components of tumor and ECF. Several factors affects its formation in the tumor, including the level of SS1P in the tumor, the time and distance SS1P penetrates and the sMSLN level in ECF. Thus, the level of complex is closely associated with SS1P targeting process.
SS1P redistribution in tumor
In mice, the half-life of SS1P in the circulation is about 20 min, so that after 2 h the immunotoxin is essentially gone from the circulation and not available for tumor uptake. We were surprised to find that tumor cell labeling increased for up to 6 h. There is a clear discrepancy between the kinetics of SS1P in blood and tumor cell uptake. This finding indicates that significant amounts of the immunotoxin are being redistributed within the tumor after blood levels have fallen to insignificant levels. Because the immunotoxin we measure is within the cell, some of the increase observed at 6 h could have been due to SS1P on the cell surface, which is subsequently internalized. To investigate this we measured the rate of internalization of surface bound SS1P into A431/H9 cells in vitro, and found the internalization is complete within 1 h (data not shown). We think it is unlikely that internalization is much slower in vivo. Our data suggests that there may be a reservoir of SS1P in the tumor that supplies SS1P to the tumor cells for several hours after SS1P in the blood has been cleared. This is in accord with the model of Jain and colleagues who predicted that under some circumstances a “drug reservoir” is present in the tumor that allows drug to spread within the tumor independent of blood supply (21).
Contribution of attenuated sMSLN barrier to Taxol and SS1P synergy
Based on the results from the FC method and gel filtration study, we illustrate in Figure 6 how reduction of the sMSLN level in ECF and reduction of the site density barrier after Taxol treatment contributes to the improved anti-tumor effect (Fig. 6). The injection of 20 μg of SS1P gives a blood level of about 20 μg per ml. SS1P immediately enters the tumor because of the large concentration gradient. Entry continues for 1–2 h until the blood concentration falls to insignificant levels (0.5 μg/mL). During this period, SS1P molecules move out of capillary and into the tumor compartment where they initially bind to tumor cells closest to blood vessels. As SS1P molecules penetrate further, they reach more tumor cells. Along the path, they bind to sMSLN in tumor ECF, forming complexes. Because of the very large amounts of sMSLN in the ECF, the distance SS1P can penetrate is limited. ECF complex formation is a function of sMSLN levels and penetration distance. After Taxol treatment, sMSLN is decreased, allowing SS1P to reach more tumor cells.
Figure 6.
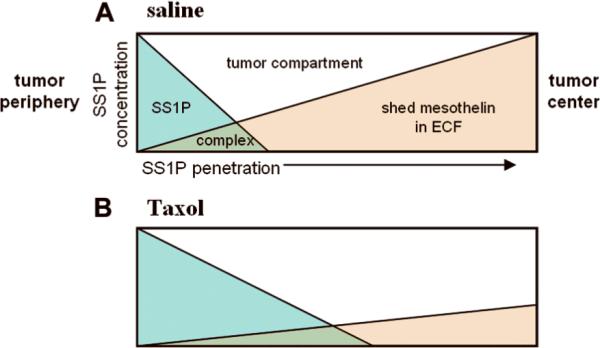
Formation of SS1P/mesothelin complex in tumor. A, Injected SS1P (blue) is transported into tumor tissue. It distributes between tumor cells and tumor extracellular space. Excess SS1P in tumor ECF penetrates to reach more tumor cells. At the same time, it forms complex (green) with shed mesothelin (light brown) over that area. Tumor periphery has highest SS1P concentration because of rich vasculature. And tumor center has lowest one. B, When Taxol is given, shed mesothelin level in tumor ECF is lowered. More excess SS1P in ECF is available for penetration. A larger fraction of tumor cells are stained by SS1P.
Overcome barriers by Taxol
Raising the SS1P dose to 100 μg overcame the barriers to entry so that over 90% of the cells were labeled by 3 h. Unfortunately, due to nonspecific liver damage, the maximum single dose of SS1P that can be safely given to a mouse is about 20 μg. One way to increase the number of cells that can be reached by SS1P is to pretreat animals with Taxol. Taxol has several useful effects. One is to lower sMSLN levels, removing the decoy barrier (16). A second is to disrupt the close packing of tumor cells, reducing the site barrier (2), and a third is to lower interstitial pressure, which allows for more rapid entry of macromolecules like SS1P (3, 22). One reason for the fall in mesothelin levels is cessation of production, due to tumor cell death. A second is more rapid transfer from the tumor into the blood due to disruption of the tumor. This is consistent with the suggestion that the void space produced by cancer cell death enhances the spreading of oncolytic herpes simplex virus (23). Thus, Taxol treatment could be creating an environment that facilitates the diffusion of sMSLN.
Implications for human studies
We conducted this study to determine how to increase immunotoxin activity in solid tumors in humans. Based on our preclinical studies demonstrating increased anti-tumor activity of immunotoxins when given with chemotherapy, a Phase II trial of SS1P in patients with mesothelioma has opened. In this trial, patients with pleural mesothelioma are treated with chemotherapy (Cisplatin and Pemetrexed) followed by immunotoxin SS1P. We have measured mesothelin expression (24), the sensitivity of mesothelioma cells from patients to SS1P (25) and the soluble mesothelin levels in several human mesotheliomas (unpublished data); all these values are similar to the values we found in KB cells or KB tumors. Since KB tumors are effectively treated with a combination of chemotherapy and SS1P, we believe our combinational approach will be of value in some of these patients.
Acknowledgements
We thank Dr. Xing Du and Dr. Francis Mussai for providing Alexa labeled BL22 and HA22. We also thank Dr. Peter L. Choyke and Dr. Hisataka Kobayashi for NIH-3T3/HER-2 cells and Dawn A. Walker for reading the manuscript.
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Disclosure of Potential Conflicts of Interest No potential conflicts of interest
References
- 1.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Juweid M, Neumann R, Paik C, et al. Micropharmacology of monoclonal antibodies in solid tumors: direct experimental evidence for a binding site barrier. Cancer Res. 1992;52:5144–53. [PubMed] [Google Scholar]
- 3.Jain RK. Transport of molecules, particles, and cells in solid tumors. Annu Rev Biomed Eng. 1999;1:241–63. doi: 10.1146/annurev.bioeng.1.1.241. [DOI] [PubMed] [Google Scholar]
- 4.Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60:2497–503. [PubMed] [Google Scholar]
- 5.Zhang Y, Pastan I. High shed antigen levels within tumors: an additional barrier to immunoconjugate therapy. Clin Cancer Res. 2008;14:7981–6. doi: 10.1158/1078-0432.CCR-08-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer. 2006;6:559–65. doi: 10.1038/nrc1891. [DOI] [PubMed] [Google Scholar]
- 7.Olsen E, Duvic M, Frankel A, et al. Pivotal phase III trial of two dose levels of denileukin diftitox for the treatment of cutaneous T-cell lymphoma. J Clin Oncol. 2001;19:376–88. doi: 10.1200/JCO.2001.19.2.376. [DOI] [PubMed] [Google Scholar]
- 8.Kreitman RJ, Wilson WH, Bergeron K, et al. Efficacy of the anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemia. N Engl J Med. 2001;345:241–7. doi: 10.1056/NEJM200107263450402. [DOI] [PubMed] [Google Scholar]
- 9.Hassan R, Bullock S, Premkumar A, et al. Phase I study of SS1P, a recombinant antimesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–9. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 10.Pai LH, Wittes R, Setser A, Willingham MC, Pastan I. Treatment of advanced solid tumors with immunotoxin LMB-1: an antibody linked to Pseudomonas exotoxin. Nat Med. 1996;2:350–3. doi: 10.1038/nm0396-350. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Xiang L, Hassan R, et al. Synergistic antitumor activity of taxol and immunotoxin SS1P in tumor-bearing mice. Clin Cancer Res. 2006;12:4695–701. doi: 10.1158/1078-0432.CCR-06-0346. [DOI] [PubMed] [Google Scholar]
- 12.Douglas CM, Collier RJ. Exotoxin A of Pseudomonas aeruginosa: substitution of glutamic acid 553 with aspartic acid drastically reduces toxicity and enzymatic activity. J Bacteriol. 1987;169:4967–71. doi: 10.1128/jb.169.11.4967-4971.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urano Y, Asanuma D, Hama Y, et al. Selective molecular imaging of viable cancer cells with pH-activatable fluorescence probes. Nat Med. 2009;15:104–9. doi: 10.1038/nm.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakahara T, Norberg SM, Shalinsky DR, Hu-Lowe DD, McDonald DM. Effect of inhibition of vascular endothelial growth factor signaling on distribution of extravasated antibodies in tumors. Cancer Res. 2006;66:1434–45. doi: 10.1158/0008-5472.CAN-05-0923. [DOI] [PubMed] [Google Scholar]
- 15.Kaneko O, Gong L, Zhang J, et al. A binding domain on mesothelin for CA125/MUC16. J Biol Chem. 2009;284:3739–49. doi: 10.1074/jbc.M806776200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Xiang L, Hassan R, Pastan I. Immunotoxin and Taxol synergy results from a decrease in shed mesothelin levels in the extracellular space of tumors. Proc Natl Acad Sci USA. 2007;104:17099–104. doi: 10.1073/pnas.0708101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kreitman RJ, Margulies I, Stetler-Stevenson M, Wang QC, FitzGerald DJ, Pastan I. Cytotoxic activity of disulfide-stabilized recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) toward fresh malignant cells from patients with B-cell leukemias. Clin Cancer Res. 2000;6:1476–87. [PubMed] [Google Scholar]
- 18.Pai-Scherf LH, Villa J, Pearson D, et al. Hepatotoxicity in cancer patients receiving erb-38, a recombinant immunotoxin that targets the erbB2 receptor. Clin Cancer Res. 1999;5:2311–5. [PubMed] [Google Scholar]
- 19.Ho M, Onda M, Wang QC, Hassan R, Pastan I, Lively MO. Mesothelin is shed from tumor cells. Cancer Epidemiol Biomarkers Prev. 2006;15:1751. doi: 10.1158/1055-9965.EPI-06-0479. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt MM, Wittrup KD. A modeling analysis of the effects of molecular size and binding affinity on tumor targeting. Mol Cancer Ther. 2009;8:2861–71. doi: 10.1158/1535-7163.MCT-09-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baxter LT, Jain RK. Transport of fluid and macromolecules in tumors. II. Role of heterogeneous perfusion and lymphatics. Microvasc Res. 1990;40:246–63. doi: 10.1016/0026-2862(90)90023-k. [DOI] [PubMed] [Google Scholar]
- 22.Taghian AG, Abi-Raad R, Assaad SI, et al. Paclitaxel decreases the interstitial fluid pressure and improves oxygenation in breast cancers in patients treated with neoadjuvant chemotherapy: clinical implications. J Clin Oncol. 2005;23:1951–61. doi: 10.1200/JCO.2005.08.119. [DOI] [PubMed] [Google Scholar]
- 23.Nagano S, Perentes JY, Jain RK, Boucher Y. Cancer cell death enhances the penetration and efficacy of oncolytic herpes simplex virus in tumors. Cancer Res. 2008;68:3795–802. doi: 10.1158/0008-5472.CAN-07-6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res. 2004;10:3937–42. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- 25.Hassan R, Lerner MR, Benbrook D, et al. Antitumor activity of SS(dsFv)PE38 and SS1(dsFv)PE38, recombinant antimesothelin immunotoxins against human gynecologic cancers grown in organotypic culture in vitro. Clin Cancer Res. 2002;8:3520–6. [PubMed] [Google Scholar]