Abstract
The immune system is routinely exposed to dead cells during normal cell turnover, injury and infection. Mechanisms must exist to discriminate between different forms of cell death in order to correctly eliminate pathogens and promote healing while avoiding responses to self, which can result in autoimmunity. However, an effective response against host tissue is also often needed to eliminate tumors following treatment with chemotherapeutic agents that trigger tumor cell death. Consequently, a central problem in immunology is to understand how the immune system determines whether cell death is immunogenic, tolerogenic or 'silent'.
Keywords: Apoptosis, immunity, cell death, necrosis, immune tolerance
Introduction
One of the most primitive host defense mechanisms to intracellular infection in animals is for the host cell to die preemptively before the parasite can replicate and kill the cell en route to further infection. It is therefore not surprising that in the vertebrate immune system cell death promotes both innate and adaptive immune responses. However, cell death is not unique to infection; in humans it is estimated that approximately one million cells die per second in the course of normal tissue turnover. In most cases, this does not cause autoreactivity. So, how then does the immune system discriminate between different types of cell death? Historically, this question has been addressed by using two general approaches: by investigating the signals that are produced by dying cells and by investigating the consequences of different forms of cell death.
The first approach is based on the theory that the recognition of pathogen-associated molecular patterns (PAMPs), such as bacterial cell wall components or viral RNA, by immune cells dictates the difference between silent cell death (which occurs in the absence of a pathogen) and immunogenic cell death (which is induced by a pathogen)1. However, the presence or absence of PAMPs cannot be the only criterion that determines immunogenicity because transformed cells (which generally are not infected by foreign organisms) frequently elicit highly effective anti-tumor immune responses as they die2. In addition, debris from non-transformed host cells in some settings can stimulate organ-specific or general autoimmune responses3. Consequently, the concept of damage-associated molecular patterns (DAMPs; Box 1) has been proposed to explain the potential immunogenicity of stressed or dying uninfected cells4. DAMPs are released from dying cells and induce immune responses to cellular antigens whether the cell is a tumor cell, autologous tissue or infected with a pathogen. Therefore, it has been proposed that for cell death to be immunogenic, it must release DAMPs to stimulate immune cells.
Box 1: DAMPs
Potential immunogenic signals emanating from dying cells include proteins that appear at the surface of stressed and dying cells (such as calreticulin and heat shock proteins, HSPs), lipid moieties that flip from the inner plasma membrane leaflet to the outer leaflet (such as phosphatidylserine) 38,60, proteins that are released into the supernatant of cells (such as HMGB1) 51, as well as nucleic acids and their degradation products (oligonucleotides, nucleotides, nucleosides, urate) that appear in the extracellular space 43–45,105. Such damage-associated molecular patterns (DAMPs) released from or exposed at the surface of dying cells then determine the engulfment of apoptotic bodies, DC activation, antigen processing, DC maturation, and T cell activation. Pattern recognition receptors (PRR) present on DC may include endocytic receptors that stimulate antigen uptake (including scavenger receptors and C-type lectin receptors)106. Moreover, PRR present on or within DC include a group of signaling receptors that stimulate the post uptake reactions (including toll-like receptors, NOD-like receptors, and RIG1-like helicases)52,107. One single molecular entity that is exposed or released by dying tumor cells might act on multiple PRRs. Thus, HMGB1 may act on the receptor for advanced glycated endproducts (RAGEs) and possibly the toll-like receptors (TLRs) including TLR2 and TLR4 51,52, although the latter are controversial 53. Similarly, HSPs exposed on or released by tumor cells may act on multiple receptors including CD91, scavenger receptors, TLR2 and TLR4 52. Polynucleotides and oligonucleotides derived from degrading DNA or RNA can act on TLR3, TLR7, TLR8, or TLR9 as well as on intracellular receptors including RIG1, MDA5, or LGP2, while ATP and its degradation products may activate or inhibit distinct classes of purinergic receptors 45,108,109.
The second approach is based on the theory that distinct types of cell death (Box 2) induce different types of immune responses: that physiological cell death (apoptosis) is intrinsically tolerogenic, whereas pathological cell death (necrosis) is inherently immunogenic and elicits inflammatory reactions5. However, in-depth investigations have shown that cells dying by apoptosis can be vigorously immunogenic, whereas necrotic cells can be less immunogenic than cells undergoing an immunogenic form of apoptosis 2,6. In an effort to reconcile these different data, it has been proposed that there are subcategories of cell death (such as immunogenic versus non-immunogenic apoptosis) and that subtle differences in the composition of the cell surface and/or in the products, which could include DAMPs, that are secreted by the dying cells determine whether the death of the cell is immunogenic or not7.
Box 2: Types of cells death
The current nomenclature uses several terms to define morphologically distinct types of cell death 56,110.
Apoptosis is accompanied by rounding-up of the cell, retraction of pseudopods, reduction of cellular volume (pyknosis), chromatin condensation, nuclear fragmentation (karyorrhexis), few or no ultrastructural modifications of cytoplasmic organelles, and plasma membrane blebbing but cell integrity is maintained until the final stages of the process. There are several distinct subtypes of apoptosis that, although morphologically similar, can be triggered through different biochemical routes (for example, through the intrinsic or the extrinsic pathway or with or without caspase activation). Furthermore, the apparent uniformity of apoptotic cell death may conceal heterogeneous functional aspects, such as whether or not dying apoptotic cells are recognized by the immune system 56.
Autophagic cell death occurs in the absence of chromatin condensation but is accompanied by massive autophagic vacuolization of the cytoplasm. Although the term autophagic cell death is a linguistic invitation to believe that cell death is executed by autophagy, the term simply describes cell death that occurs alongside autophagy. In this sense, the term "autophagic cell death" is a misnomer 61.
Necrosis is morphologically characterized by a gain in cell volume (oncosis), swelling of organelles, plasma membrane rupture and subsequent loss of intracellular contents. Necrosis has traditionally been considered merely as an accidental, uncontrolled form of cell death that only occurs in pathological circumstances. Nonetheless, evidence is accumulating that the execution of necrotic cell death may be subtly regulated by a set of signal transduction pathways and catabolic mechanisms 111,112.
Secondary Necrosis is the dissolution of the cell following apoptosis. It is difficult to distinguish from conventional necrosis on morphologic grounds, but the consequences of the previous apoptosis for the immune response are discernable (see text).
Pyroptosis involves the crucial contribution of a particular set of caspase-1 activating complexes, known as the inflammasomes. Although the molecular circuitry of pyroptosis (which is often induced by intracellular bacteria) has been investigated in vitro 107, no data are available on the immunogenicity of pyroptosis in vivo.
Mitotic catastrophe leads to cell death during or after mitosis and is morphologically discernible by multi- or micronucleation and/or mitotic arrest before the cells adopts an apoptotic or necrotic aspects 113.
In animals, cell fate is another important influence on the immune response to dead cells. Dead cells are either shed from bodily surfaces (such as the skin or mucosa) or they are cleared via engulfment by other cells. Those that are shed have little or no influence on the host immune response but those that are engulfed have the potential to affect it. This is particularly true when dying or dead cells are engulfed by antigen-presenting cells, such as dendritic cells (DCs). DC can direct the immune response by presenting antigens that are associated with dying cells to T cells. Whether or not this occurs depends on several factors including the antigen, the DAMPs derived from the dying cell and the type of DC that engulf the dead cell. Therefore, the nature of the immune response to cell death depends on the following considerations: what cell dies, where it dies, how it dies, who eats it, and when (or if) associated antigen has been or will be recognized. Variations in these factors can have consequences that range from effective antipathogen or tumor responses to autoimmune pathology.
In this review, we discuss whether the immune response to cell death results in a measurable response (such as tumor immunity, delayed-type hypersensitivity, antibody response or autoimmunity), in immune tolerance, or in no response. These different responses are linked and in no case do we fully understand the consequences of exposure to dying cells for every aspect of the immune response. Although our challenge is to visualize a global view, we provide here only a snapshot of this rapidly evolving field.
Dying cells affect immunity
The inaugural description of apoptosis as a form of cell death distinct from necrosis (see Box 2) proposed that apoptosis was immunologically silent, whereas necrosis stimulated an immune response8. More than two decades later, a form of immune tolerance was linked to engulfment of apoptotic corpses; cells that were chemically modified with an antigenic hapten induced a state of tolerance when injected into the anterior chamber of the eye, provided that the modified cells undergo apoptosis in this immune privileged site9. In addition, inflammatory cells responding to a viral infection in the eye also underwent apoptosis and induced systemic tolerance to viral antigens 9. Subsequent studies generalized these findings to show that apoptotic cells that are engulfed by DC can produce a state of antigenic tolerance in models of contact hypersensitivity10, autoimmunity11–13, and other immune responses 14–16.
The induction of immune tolerance by apoptotic cells in vivo has been proposed to explain additional phenomena. Intravenous injection of spleen cells that had been coupled to a hapten can induce tolerance in a model of contact hypersensitivity to the hapten17,18, through a mechanism that involves CD95–CD95L (also known as FAS–FASL)-mediated apoptosis of the injected cells10. In addition, graft tolerance induced by the intravenous injection of allogeneic spleen cells19–21, as well as the tolerogenicity of antigens associated with UV-irradiated skin, have been shown to involve the induction of apoptosis and the engulfment of apoptotic cells by the immune system 22,23.
These and related findings promoted the simple idea that apoptosis is tolerogenic or non-immunogenic and necrosis is immunogenic. However, this idea has been successfully challenged by various observations that show that certain types of apoptosis can be immunogenic. For example, the apoptosis of tumor cells induced by chemotherapy can prime an immune response6. Apoptosis was the key event in this study, as priming was dependent on activation of caspases (see below). Other studies have shown that antigens from apoptotic cells can be effectively cross-presented to cytotoxic T cells (CTL) and prime an immune response24,25. Therefore, while dying cells have consequences for immune responses, the dichotomy between necrosis and apoptosis does not predict immunogenicity or tolerance.
Immunogenic cell death
Using several experimental systems (Box 3), it has become clear that various factors work in concert to determine whether cell death is immunogenic or not. These parameters include the intrinsic antigenicity of the cells, the history of activation or stress before cell death, the nature of the cell death inducer, the precise cell death pathway that is engaged, and the availability of cells of the immune system capable of responding (Figure 1).
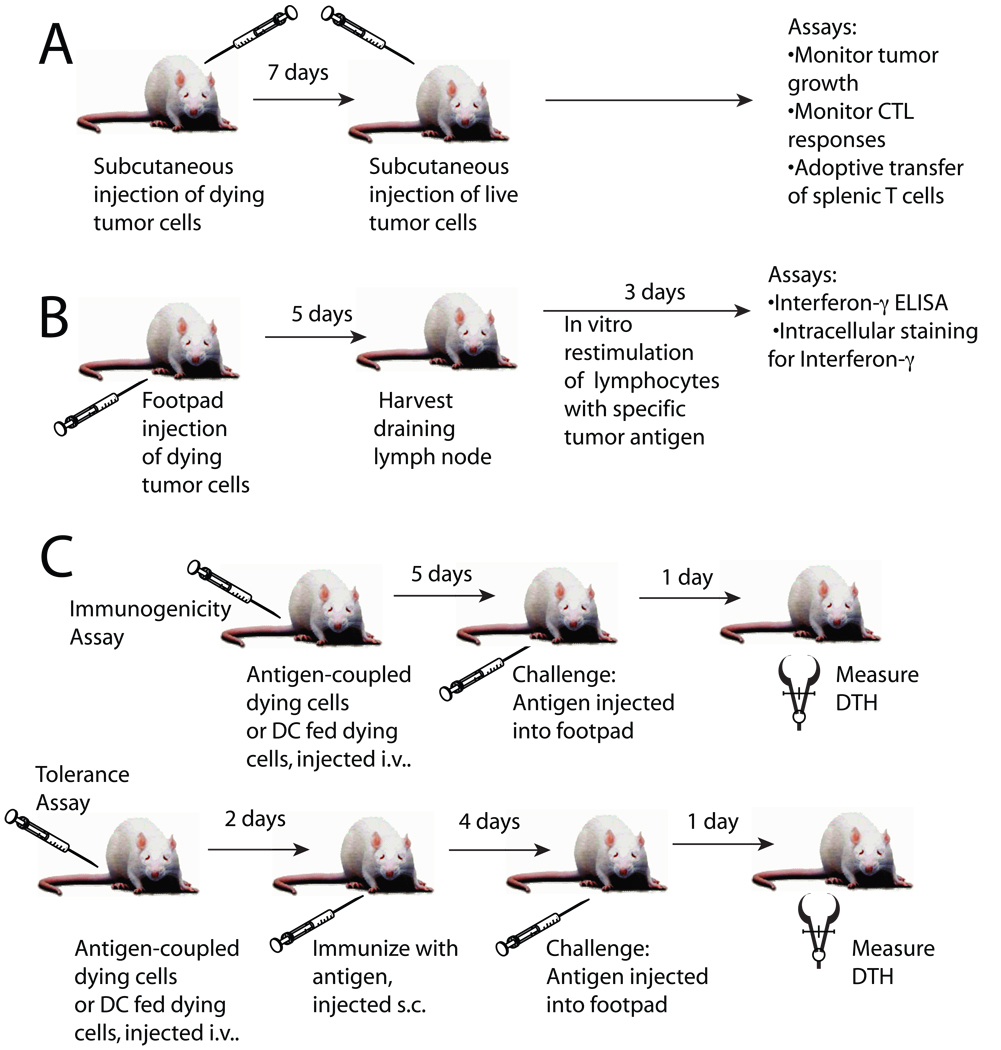
Box 3. Assays for determining immunogenic and tolerogenic cell death
In one widely used assay, cancer cells are treated in vitro to program them for cell death, followed by their s.c. injection into one flank. One week later, live tumor cells are injected s.c. into the opposite flank and tumor growth is monitored. Absence of cancer development is interpreted as the sign of anti-cancer immunity. In this experimental set-up, CTL responses can be evaluated ex vivo/in vitro, by confronting peripheral T cells with cultured tumor cells or anticancer immunity can be adoptively transferred to naive animals. An alternative assay applicable to tumor immunology consists in determining CTL priming with dying tumor cells. Dying cells (or DC pulsed with dying cells) are injected into the foot pad. Several days later, the popliteal lymph node cells are recovered and restimulated in vitro with tumor cell antigens to assess IFNγ production by CD8+ T cells.
In another type of assay, apoptotic cells, often cells from the immune system (such as dying thymocytes, splenocytes or T cells), are coupled with antigen or fed to DC along with antigen and then injected intravenously. Five days later, mice are injected with the same antigen in one footpad and PBS in the other footpad. DTH is assessed 24 hrs later by quantifying antigen-specific footpad swelling. This latter assay can also be adapted for the assessment of immune tolerance. For this, apoptotic cells are coupled with antigen or fed to DC along with antigen and then injected intravenously into mice. Mice are immunized 2 days later by subcutaneous injection of the antigen. Four days later mice are injected with the same antigen in one footpad and PBS in the opposite footpad. DTH is assessed 24 hrs later by measuring the difference in footpad swelling between the antigen and PBS injected footpads with a caliper.
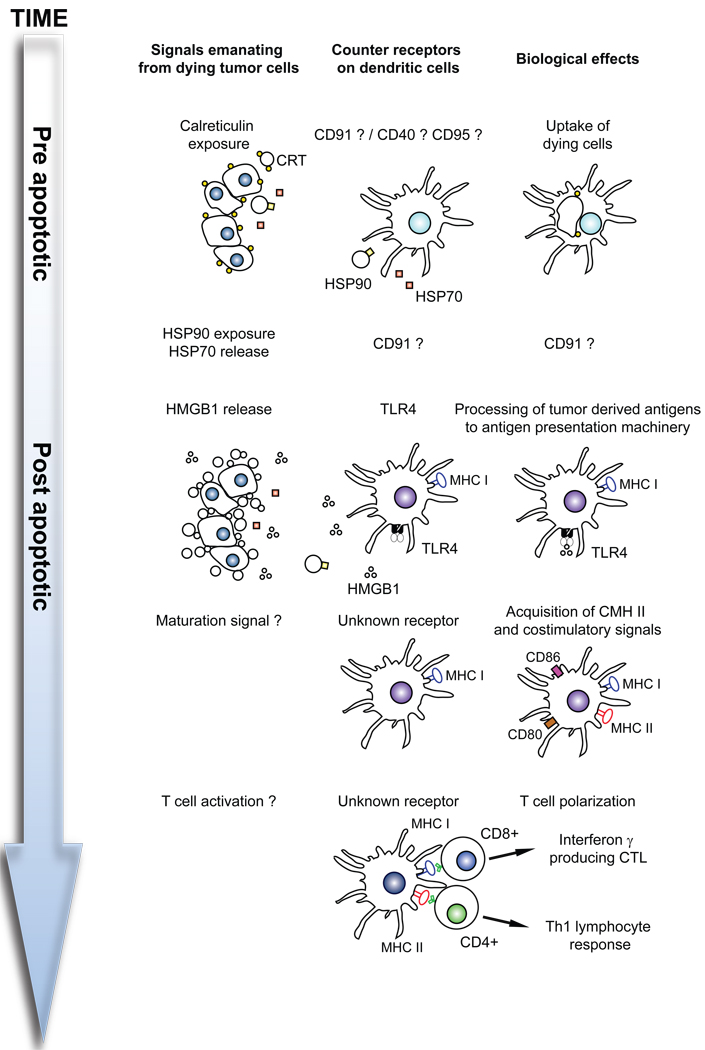
Figure 1. Characteristics of immunogenic cell death.
When cells succumb to the immunogenic variant of apoptosis, they expose calreticulin (CRT) on the cell surface at a pre-apoptotic state. Moreover, they may expose other chaperones including members of the heat shock protein (HSP) family. Later, they release the DAMP HMGB1 as well as other, yet-to-be-characterized factors. The surface-exposed molecules as well as soluble products from dying cells affect the function of DC through the action on specific receptors. For details see main text.
Cell type, activation state and stress
During oncogenesis, tumors can acquire multiple mutations, some of which give rise to altered antigenic peptides that might be recognized by the immune system as altered self 26. Consequently, during radiotherapy or chemotherapy in which the tumor cells are induced to die, immune responses may be biased towards a response against the tumor antigens rather than normal self-antigens, to which the immune system has been tolerized 7,27,28. In addition, the activation state of the cell before the induction of cell death can affect its immunogenicity. Activated but not resting γ-irradiated peripheral blood mononuclear cells can induce the expression of maturation markers (such as CD80, CD83 and CD86) and the secretion of pro-inflammatory cytokines by DCs in vitro29. This suggests that activated apoptotic cells may possess endogenous adjuvant properties. At present, the molecular nature of this activation-dependent immune stimulation is unknown. An interesting contrast is with the effect of T cells that succumb to activation-induced cell death, which promote tolerance through the stimulation of regulatory CD8+ T cells30. Thus, the timing of dead-cell uptake may be important; cells that die at the height of an immune response may promote immunity, whereas cells that die as the response wanes may be tolerogenic.
Stress responses such as the DNA damage response can change the composition of the cellular proteome at several levels. DNA damage of transformed cells induces the expression of surface proteins such as natural-killer group 2, member D (NKG2D) ligands, which act on NGK2D expressed by natural killer (NK) cells and CTL31. Further, DNA damage or activation of oncogenes can induce cellular senescence, through a pathway that relies, in part, on the secretion of pro-inflammatory cytokines such as insulin-like growth factor binding protein-7 (IGFBP7)32 and IL-633, and on the secretion of CXC-chemokine ligand 8 (CXCL8) and other chemokines 33,34, as well as on the expression of the CXCL8 receptor CXC-chemokine receptor 2 (CXCR2) 34. Although the impact of these mediators on the anti-tumour immune response has not been individually evaluated, it is possible that they may condition the local immune response once senescent tumor cells begin to die. Indeed, the induction of senescence by the expression of a p53 transgene in hepatocellular carcinomas can stimulate a vigorous anti-tumor response that is mediated by innate immune cells and is necessary for subsequent tumor clearance35. Together, these examples illustrate that the history of a cell, including its mode of activation and pre-lethal stress, as well as the type of cell that dies, may determine whether cell death is immunogenic or not.
Characteristics of dying cells
Treatment with a specific set of chemotherapeutic agents can induce the exposure of heat-shock proteins (HSPs) on the cell surface. It has been suggested that such chaperones may capture cellular antigens (including tumor antigens) and facilitate their presentation upon uptake by DC 36 or may participate in the physical or functional interaction between the membrane of dying cells and that of DC 37,38. For example, dying myeloma cells expose the chaperone HSP90 on their surface, and this may facilitate their recognition by DC 38. Similarly, in response to some chemotherapeutic agents, such as anthracyclins, but not others, such as mitomycin C or etoposide, tumor cells expose complexes that are formed by the chaperone calreticulin (CRT) and the disulfide isomerase ERp57 on their cell surface. The exposure of this complex occurs at a pre-apoptotic stage and facilitates the uptake of dying cells by DC 7,39. This feature strongly correlates with immunogenicity, and anthracyclin-treated dying tumor cells that expose CRT can be used to vaccinate against cancer, yet lose their immunogenicity upon siRNA-mediated depletion or antibody-mediated neutralization of CRT. Conversely, cells that succumb to non-immunogenic cell death (in response to mitomycin C or etoposide) can be rendered immunogenic by absorbing recombinant CRT protein to their surface7. However, the absorbance of CRT to live tumor cells does not suffice to render them immunogenic 7, meaning that additional factors are essential for immunogenicity.
To be engulfed, dying cells can emit ‘find-me’ signals that attract professional phagocytes and hence accelerate their removal (Box 4). One such signal is lysophosphatidylcholine, which is released through the action of calcium-independent phospholipase A2 (iPLA2) on the membrane glycerolipid phosphatidylcholine, a normal membrane constituent40, but the requirement of lysophosphatidylcholine for immunogenic cell death has not been determined. However, autoimmunity has been observed in mice in which phagocytes lack the G-protein coupled receptor G2A41, which is required for the response to lysophosphatidylcholine 42, and therefore it is likely that such find me signals are more important for tolerance induction than immunity. In contrast, nucleotides such as ATP and UTP that are released from damaged neurons in mice are thought to attract microglial cells (macrophage-like phagocytes in the brain) to sites of tissue damage43. Indeed, ATP, which is released from dying cells and activates macrophages 44, may also be required for the immunogenicity of dying tumor cells (L.Z. and G.K., unpublished observations). Nucleotide products such as uric acid can also function as DAMPs, presumably by activating the inflammasome in APC, thereby stimulating the production of inflammatory cytokines such as interleukin-1β (IL-1β), IL-18 and IL-33, the secretion of which depends on the inflammasome-mediated activation of caspase-145,46. Whether any of these cytokines is required for the immune response against dying cells is an issue of ongoing controversy.
Box 4. Engulfment of Dying Cells
Three classes of event accompany cell death to promote engulfment89,114. First, the cell releases soluble “find me” signals, producing a gradient that attracts phagocytic cells. One such signal is lysophosphatidylcholine 40, which is produced by the action of phospholipase A2 (PLA2). Caspases cleave and thereby activate PLA2, although this is not required for production of the lipid signal. Another such signal is the fractalkine CX3CL1, which recruits phagocytes 115 and induces one of the bridging molecules for detection of apoptotic cells, MFG-E8 116.
Many cells express a “don’t eat me” signal, which must be negated for phagocytosis to occur. Examples of such signals are CD31 117 and CD47 118, although how these molecules are negated by cell death remains unexplored.
As cells die, they expose “eat-me” signals. These include oxidized lipids, changes in carbohydrate composition, and phosphatidylserine (PS). Loss of ATP or permeabilization of the plasma membrane results in diffusion of PS and other asymmetrically oriented phospholipids to both leaflets. During apoptosis, or in response to other signals such as calcium flux, a more rapid “scrambling” of membrane phospholipids occurs, resulting in loss of phospholipids asymmetry including appearance of PS on the outer leaflet. To date, the caspase-dependent mechanisms responsible for phospholipid scrambling have been elusive. Nevertheless, detection of apoptosis using the PS probe Annexin V 119 is a useful marker when paired with agents to detect loss of plasma membrane integrity.
Although a receptor for PS on phagocytes was described, this molecule is now known to be a nuclear factor 120 unlikely to directly recognize PS on dying cells. Instead, a number of bridging molecules have been identified that perform the recognition of this and other eat-me signals. The best characterized of these are the soluble protein MFG-E8 87,121 and the glycolipid-anchored protein Tim-4 88. Evidence also supports roles for Annexin I 122 and Gas-6 123 in recognizing PS on dying cells. Other bridging molecules may include thrombospondin, C1q, and β2-GPI.
On phagocytes, receptors for these bridging molecules are the αvβ3 and αvβ5 integrins (which bind MFG-E8 and thrombospondin), the MER tyrosine kinase family (which binds Gas-6), the scavenger receptors CD36 and SRA (which bind oxidized lipids), and the C1q receptors CD91 and calreticulin. Calreticulin is also expressed on dying cells (see text).
Following permeablization of the plasma membrane, cells can release several proteins including DAMPs that alert the immune system (Box 1). One such DAMP is nuclear protein high-mobility group box 1 protein (HMGB1) 47, a DNA binding protein. Although it was initially thought that HMGB1 is released only from the nucleus during primary necrosis, recent data indicate that HMGB1 can be released during secondary necrosis following apoptosis 27,48–50. It appears that HMGB1 can bind to several pattern recognition receptors (PRRs), including Toll-like receptor 2 (TLR2), TLR4, and receptor for advanced glycosylation end-products (RAGE)51,52 (caution is, of course, urged in interpreting the role of TLRs, as possible contamination with TLR ligands is always an issue). When in a complex with CpG-containing DNA, HMGB1 can also induce the synergistic interaction and activation of RAGE and TLR953. Depletion of HMGB1 from tumor cells undergoing immunogenic apoptosis (induced by anthracyclins, oxaliplatin or ionizing irradiation) abolishes their ability to induce T-cell priming or to protect mice against tumor growth following vaccination27, indicating that this DAMP is required for the immunogenicity of cell death.
A recent study has identified spliceosome-associated protein 130 (SAP130), which is a component of small nuclear ribonucleoprotein, as another DAMP that is released by necrotic and late apoptotic cells 54. SAP130 specifically binds to macrophage-inducible C-type lectin (Mincle/Clec4e), which is an immunoreceptor tyrosine-based activation motif (ITAM)-coupled activating receptor that is mainly expressed by macrophages. Neutralization of Mincle with a specific antibody inhibited the recruitment of neutrophils and pro-inflammatory cytokine production following intraperitoneal injection of dead tumor cells54, indicating a role for SAP130–Mincle in the immunogenicity of dead cells in vivo. However, the expression and function of Mincle on DC has not been directly examined to date.
Dying cells also expose or release another as yet unidentified DAMP which is recognized by a receptor, CLEC9A, present on CD8α+ DC 55. This activates Syk kinase and cross-presentation of associated antigens on class I MHC. As discussed in the next section, such cross-presentation is important not only for the induction of immune responses by dying cells, but also for tolerance induction. Therefore, identification of the CLEC9A ligand will be a valuable advance in our understanding of the immune consequences of cell death.
Caspase activation
The activation of caspases, a specific class of cysteine proteases has multiple effects on the immunogenicity of cell death 56; for example, caspase activity can lead to the proteolytic destruction of immunodominant epitopes57. Conversely, caspase activation can reveal novel epitopes by mobilizing long-lived proteins that are stably anchored in the cytoskeleton and facilitating their access to the cross-presentation pathway in DC58. In addition, cleavage of antigens by caspases can create novel N-termini that target the antigens for proteasomal degradation59, which may facilitate cross-presentation of the resulting peptides. Such caspase-enhanced presentation could be important for the pathogenesis of HIV-1 because the peripheral blood of HIV-1-infected individuals contains a high frequency of effector CD8+ T cells that recognize caspase-cleaved epitopes and correlate with the frequency of apoptotic CD4+ T cells 58.
The inhibition of caspases affects the exposure of find-me signals 40, eat-me signals 40 (Box 4), and the secretion of DAMPs51 from dying cells, as well as the exposure of CRT on anthracyclin-treated cancer cells60. A chemical caspase inhibitor (zVAD-fmk) or transfection with a caspase inhibitor (such as baculovirus p35) destroyed the capacity of anthracyclin-treated cancer cells to induce anti-tumour immune responses following their subcutaneous administration6.
Autophagy
Macroautophagy (also known as autophagy) constitutes an important catabolic mechanism for the sequestration and lysosomal degradation of cytoplasmic material. Although autophagy does not constitute an effector mechanism of cell death61, it often accompanies cell death 62 (Box 2). Autophagy has been shown to be required for the generation of the find-me signal lysophosphatidylcholine and the eat-me signal generated by phosphatidylserine exposure 63 (Box 4), and therefore a failure in autophagy blocks the removal of apoptotic corpses and influences the immunogenicity of cell death. Autophagy might also stimulate the release of HMGB1 64, suggesting another link between autophagy and the immune response to cell death.
In one model of immunogenic cell death, influenza virus-infected Bax−/−Bak−/− mouse embryonic fibroblasts (which cannot engage apoptosis via the mitochondrial pathway) were induced to undergo non-apoptotic cell death accompanied by massive autophagy 65 and immunization with these DNA-damaged Bax−/−Bak−/− cells was found to be more efficient in facilitating the cross-priming of antigen-specific CD8+ T cells than vaccination with wild-type cells undergoing apoptosis66. This gain of immunogenicity was lost upon knockdown of the essential autophagy protein Atg5. Although the exact mechanisms accounting for the immunogenic effect of autophagy remain obscure, these results underscore the importance of catabolic processes in shaping the immune response.
Therefore, a number of factors influence the consequences of dying cells for the induction of immune responses. If the dying cells are not immunogenic, they may be tolerogenic or may have no effect at all. These ideas will be discussed in the following section.
Tolerance induction by dying cells
While dying cells can promote an effective immune response, we know that under different conditions such cells can produce immune tolerance. Consequently, the key to understanding this “immuno-logical” decision is to examine the mechanisms of tolerance induction by dying cells, and then relate them to the mechanisms of immunogenicity discussed above. Ideally, immunogenic cell death should be directed toward tumors and infections, while tolerogenic cell death should be associated with preventing unwanted immune responses to self (for example, autoimmunity) or the protection of organs where excessive inflammation can result in irreversible organ damage (for example, in immune privileged sites such as the eye) 9.
Studies of immune tolerance induced by dying cells have utilized experimental systems such as those described in Box 3. From these studies, factors can be determined that are important for tolerogenic cell death. We outline these below.
The availability of ‘help’
Antigens associated with apoptotic cells and engulfed by DC can be cross-presented by MHC class I molecules to CD8+ T cells to prime CTL. 67 Perhaps paradoxically, tolerance induction by apoptotic cells depends on MHC class I molecules and involves immune suppression by CD8+ T cells10,24,25. Reconciling cross-presentation and “cross-tolerance” provides insights into one mechanism of immune tolerance induction by dying cells.
Following antigen recognition by CD8+ T cells and their development into CTLs, the long-term fate of these cells is determined by additional signals provided by DCs, which must be ‘licensed’ by a prior CD40–CD40L-mediated interaction with activated CD4+ T cells68. Without this additional signal, the activated ‘helpless’ CTL function as primary effector T cells but with a short lifespan69 or die via activation-induced cell death following subsequent exposure to antigen70,71. The latter cell death is mediated by expression of the death ligand TRAIL, which triggers apoptosis in the helpless CTL as well as other activated T cells70,72.
The relationship between these observations and tolerance by apoptotic cells became clear following a recent series of experiments. DC that have engulfed necrotic cells present antigen to both CD4+ and CD8+ T cells, however those that engulf apoptotic cells present antigen to CD8+ T cells but not CD4+ T cells72. As a result, the CD8+ T cells produce TRAIL upon re-exposure to antigen, which acts to inhibit immunization for cell-mediated responses (that is, mediate tolerance). Thus, exposure to apoptotic cells shifts the system from classical “helped” CTL responses to tolerogenic “helpless” CTL that produce TRAIL upon re-exposure to antigen. In support of this, TRAIL-deficient mice were resistant to tolerance induction by intravenously injected apoptotic cells72.
These considerations lead to the following scenario (Figure 2). Upon injection of apoptotic cells, associated antigen is presented to CD8+ CTL in the absence of CD4+ T cell help. The helpless CTL function as primary effector cells and therefore would be observed to be fully functional CTL24,25,67. However, upon re-exposure to antigen, the helpless CTL release TRAIL, resulting in their own deletion and suppression of further CD4+ T cells responses. Studies exploring the function of CTL following exposure to apoptotic cells have not formally tested this idea as yet.
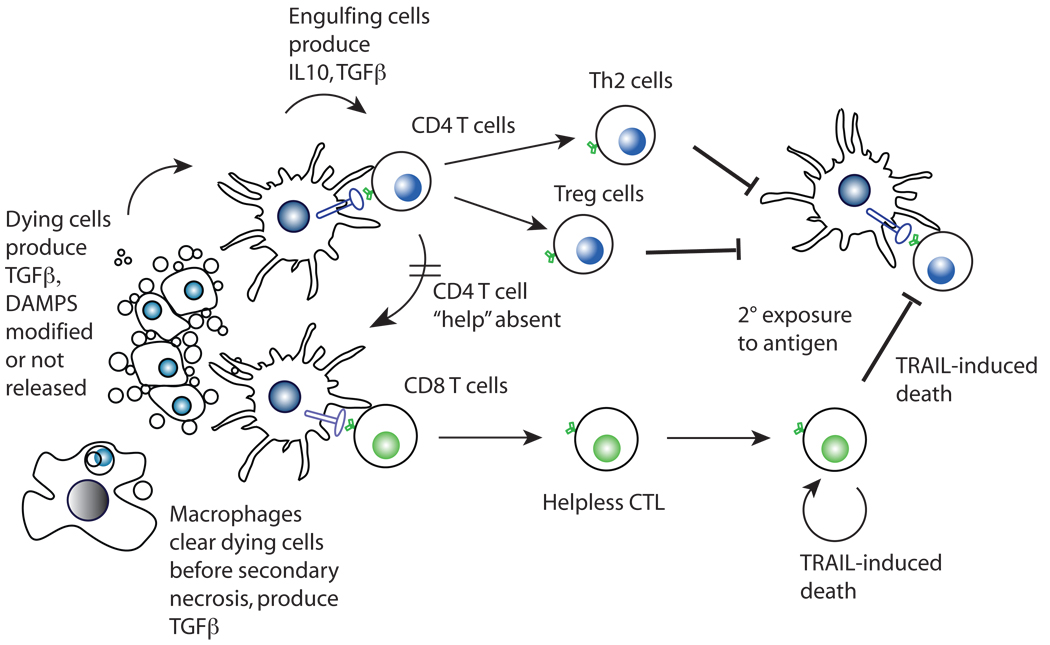
Figure 2. Mechanisms of tolerance induction by apoptotic cells.
Apoptotic cells either do not release DAMPs or modify them (e.g., HMGB1, see text) and are engulfed by DC. TGFβ, produced by the dying cells or the engulfing cells induce the differentiation of inducible Treg, which inhibit immune responses. Alternatively, CD8+ T cells are stimulated in the absence of activated CD4+ T cells, resulting in helpless CTL. These are capable of a primary cytotoxic response, but upon restimulation produce TRAIL. As a result, they themselves die while also inhibiting new immune responses, resulting in tolerance.
Location of dying cells and those that engulf them
In several models of contact hypersensitivity and delayed-type hypersensitivity, antigen-coupled cells injected intravenously produce a state of immune tolerance to the antigen17,18, a process that involves apoptosis of the injected cells10. However, it has long been known that subcutaneous injection of the same cells results in activation of an immune response73,74, and most studies of immunogenic apoptosis involve injection by this route. Subcutaneous injection of cells leads to engulfment by skin derived DC that ultimately traffic to the lymph nodes and lead to immunity. This may mimic the effect of tumors are implanted into subcutaneous sites and which undergo apoptosis following chemotherapy treatment75,76.
Intravenous injection of apoptotic cells leads to their localization in the spleen and it is known that the spleen is critical for the tolerogenic nature of intravenous delivered antigens. For example, splenectomy abolishes tolerance induced by intravenous injection of hapten-modified spleen cells17, as it does to tolerance induced by injection of hapten-modified spleen cells or virus into the anterior chamber of the eye (a system which requires apoptosis of inflammatory cells)9,77. Thus the spleen, as opposed to the lymph nodes, appears to be an organ of tolerance.
Localization of apoptotic cells to the spleen may influence which type of DC engulfs the dying cells, and several observations suggest that distinct DC subsets in this organ can handle stimuli from dead cells differentially78,79. Splenic CD8α+ DCs are potent inducers of tolerance while splenic CD8α− DCs promote immunity10,80. In another study, CD8α+ DC were shown to preferentially phagocytose apoptotic cells, again suggesting a role for distinct DC populations80. It is also noteworthy that there also appears to be a difference in antigen processing intrinsic to these DC subsets that is associated with increased expression of proteins involved in MHC processing 79. CD8α+ DC tend to process antigens for presentation via MHC class I while CD8α− DC present via MHC class II. This suggests that for tolerance CD4+ T cell immunity may be diminished while CD8+ T cell immunity is promoted, resulting in helpless CTL induction as discussed above.
Exceptions should be noted. Bone marrow-derived myeloid DCs (which are predominantly CD8α−), fed with apoptotic cells, effectively induce tolerance when injected intravenously 10,50,72. Also, CD8α+ DC in the skin are potent inducers of anti-viral immunity81, rather than tolerance. Such observations suggest that it may be the location of the uptake of dying cells rather than the type of DC that dictates the outcome. Following intravenous injection of DC that have engulfed apoptotic cells, the DC traffic to the marginal zones of the spleen, where they induce immune tolerance10. If instead, these same DC are injected subcutaneously, immunity is induced10. Interestingly, the spleen is not the only location that appears to be involved in tolerance induction by dying cells. Lymphoid cells that die in vivo tend to accumulate in the liver 82, and this organ has also been implicated as a site for tolerance induction 83.
There are also a number of consequences for the DC following an encounter with apoptotic cells that can have implications for the immune response. It is widely held that DC maturation, as measured by increased MHC class II and co-stimulator (such as CD80 and CD86) expression is critical for the induction of immunity14. DC that are not mature induce immune tolerance. 16,25 One consequence of engulfment of apoptotic cells, as it relates to these observations, is that it can prevent DC maturation in some cases84. In this scenario, apoptotic cells induce tolerance because they fail to promote the maturation of DC. This is a compelling idea that partially explains the tolerogenicity of apoptotic cells, especially in conjunction with the observations on helpless CTL discussed above. However, it should be noted that this is not always the case. There are several reports that mature DC can induce tolerance following engulfment of apoptotic cells10,50,72, and indeed, DC maturation can be required for tolerance induction14. Thus, the maturation state of DC can not be the sole determining factor for tolerance induction, and issues of DC type and localization, as discussed above, are also likely to be important.
Receptors for dying cells
Receptors involved in the recognition and/or engulfment of apoptotic cells (Box 4) can determine the outcome of the immune response to dying cells. Patients with systemic lupus erythematosus (SLE) often have defects in the engulfment of apoptotic cells 3,85, although the cause (and generality) of such defects remain unresolved. Genetic deletion or neutralization of some molecules involved in engulfment, such as MER, MFG-E8, TIM4 or C1q, can compromise self tolerance and induce systemic autoimmune disease86–88. However, for other molecules involved in engulfment, this is not the case. Disruption or blockade of CD14, CD26, β3-integrin, β5-integrin or mannose-binding lectin, all of which are associated with the uptake of dying cells (Box 4), leads to the accumulation of apoptotic bodies but does not result in autoimmunity89. Therefore, it is not a defect in engulfment of apoptotic cells, per se, which causes the disease. Similar to wild-type macrophages, macrophages from mice that lack CD26, β3-integrin or β5-integrin secrete the immunosuppressive cytokine transforming growth factor-β (TGFβ; see below) in response to apoptotic cells90, and this may help to explain why these mice fail to clear apoptotic bodies but do not develop autoimmunity.
Modification of DAMPs
Cells that die release DAMPs, but the process of apoptosis can modify these immunostimulatory molecules in order to promote tolerance rather than immunity. Necrotic cells have been shown to promote antigen-specific immunity through a mechanism that involved the release of HMGB1 when injected intravenously; in the absence of HMGB1, these cells induced tolerance50. Similarly, apoptotic cells can induce an immune response rather than tolerance if caspase activation is blocked or if apoptotic cells lack the expression of the effector caspases caspase-3 and caspase-7 are engulfed by DCs. This seems to contradict the requirement for caspase activation in immunogenic cell death (discussed above) but again, perhaps factors such as the location of the dying cells may apply. In addition, it is clear that events that take place during apoptosis have the capacity to modify the immune response. This would include the production of reactive oxygen species (ROS) and the release of immunosuppressive molecules from dying cells.
Perhaps the best example of this is related to the generation of ROS as a consequence of caspase activation10,50,72. During apoptosis, mitochondria permeabilize to release cytochrome c, which in turn activates caspases; the caspases then cleave a component of complex I in the electron transport chain p75 NDUFS1 (NADH dehydrogenase (ubiquinone) Fe-S protein 1, 75kDa) in the permeabilized mitochondria91. The resulting destruction of complex I function induces the generation of ROS, which oxidizes a key cysteine reside in HMGB1, neutralizing its ability to override tolerance induction and promote immunity 50. Mutation of the caspase cleavage site in p75 NDUFS1 does not block apoptosis, but apoptotic cells that express this mutant promote immunity rather than tolerance, despite releasing HMGB1 upon secondary necrosis (see Box 2). Therefore, caspase activation during apoptosis promotes ROS generation that compromises the immunostimulatory activity of HMGB1 and this leads to tolerance rather than immunity. Thus, one important factor in determining whether tolerance or immunity will result is whether dying cells are capable of modifying HMGB1 and perhaps other DAMPs during apoptosis. One prediction would be that in immunogenic cell death (such as following some cancer chemotherapies) ROS is not produced and DAMPs remain unmodified. This remains to be tested. Similarly, the effects on immune outcome of ROS production during some forms of necrotic cell death have not yet been explored.
Release of immunosuppressive mediators
Another mechanism invoked to explain the tolerogenic effects of apoptotic cells is the release of immunosuppressive cytokines from either the dying cell or the cell that engulfs it. When in contact with macrophages, apoptotic cells induce the secretion of anti-inflammatory cytokines such as TGFβ, IL-10, platelet-activating factor (PAF) and prostaglandin E2 (PGE2) 92–94. Apoptotic cells also stimulate the production of lipid mediators such as 15-lipoxygenase and 15-hydroxyeicosatetraenoic acid, which can participate in the resolution of inflammation95. A role for TGFβ has been shown in a model of lung inflammation in which the administration of apoptotic cells is inhibitory96. In another system, the induction of extensive apoptosis following infection by intracellular trypanosomes promoted the production of TGFβ, which inhibited macrophage responses, thereby permitting survival of the pathogen97. Thus, the interaction of apoptotic cells with macrophages can induce the production of inhibitory cytokines by the latter that restrict immune responses. This effect may simply involve the “eat me” signals on the apoptotic cells since the administration of vesicles containing phosphatidylserine (thus mimicking the “eat me” signal) was shown to inhibit an adaptive immune response in a TGFβ-dependent manner98.
In addition to the production of TGFβ by macrophages, apoptotic cells can also produce immunosuppressive cytokines such as IL-10 99 and TGFβ 100 as they die. These cytokines can influence the type of antigen-specific T-cell response that is induced. Apoptotic T cells released bio-active TGFβ without stimulation of Tgfb transcription; TGFβ was shown to be localized in an intracellular membrane-bound compartment and the cytokine was released into the cytosol following loss of mitochondrial membrane potential. The release of TGFβ from apoptotic T cells inhibited the production of pro-inflammatory cytokines by activated macrophages, resulting in immune suppression100.
Such observations underscore the importance of TGFβ in the effects of apoptotic cells on the immune system. The production of TGFβ by macrophages or DC that have engulfed apoptotic cells can promote the generation of inducible T regulatory (Treg) cells, and administration of apoptotic cells has been shown to generate inducible Treg cells in some settings101,102. Such inducible Treg cells can contribute to the tolerogenic effects of apoptotic cells on the immune system. However, the situation can be more complex; if IL-6 is also present (for example, due to TLR engagement or other inflammation), apoptotic cells can drive the generation of inflammatory TH17 cells rather than Treg cells, a phenomenon seen when apoptosis is caused by bacterial infection.103
Conclusions
The mechanisms our immune system use to deal with dead and dying cells are complex. Understanding (and possibly manipulating) these processes can have important implications for cancer biology, infectious disease, tissue injury and autoimmunity. Dead cells are handled differently depending on a number of factors related to the type of cell death, the cell death pathway, the way corpses are eaten, who eats the corpses, where engulfment takes place, and which cells of the immune system eventually encounter the antigens presented along with the dead cells. All of these issues must be considered when trying to make predictions concerning the impact of death on the immune system.
Although the process of cell death may modify antigens associated with the dying cell, we suspect that it is the effects of DAMPs and other mediators that dictate the impact of cell death on DCs and the immune response. This is not simply due to the presence or absence of costimulatory signals or maturation status of the DC that engulfs the dying cell, but appears to include other signals that have not yet been elucidated. It is likely, however, that this source of intact or altered self antigens in dying cells serves to sustain self tolerance under normal conditions, and can trigger autoimmune responses when the process goes awry.
Why are the effects of dying cells on the immune system so complex? Perhaps this complexity is the price of our highly evolved immune systems. We know that a failure or delay of removing dying cells can have autoimmune consequences, but the mechanisms for removal of corpses is highly conserved in animals that have no adaptive immune systems and do not show anything resembling inflammatory responses to the presence of dead cells that are not removed. When vertebrate immune systems increased their complexity to deal with pathogens, this also increased its chances of eliciting autoimmune reactions. As our immune systems evolved pathogens evolved parallel ways to avoid detection, exploiting this new found complexity. 104 It is plausible that the response of the immune system to dead and dying cells was a relatively recent add-on to the complex and conserved processes of cell death and removal. If dying cells provide the antigens that sustain peripheral tolerance, then parasites that trigger cell death would be cosseted by the immune system, which is clearly untenable. The alternative, that dying cells are always immunogenic, is equally untenable, as the potential for autoimmunity is readily apparent. In response the immune system appears to “evaluate” cell death by a number of criteria trying to stay one step ahead. Like Alice’s Red Queen, it takes all the “running” our evolving immune system can do to “stay in the same place”, with respect to the consequences of dying cells for the immune response.
Supplementary Material
Acknowledgements
The authors declare no competing financial interests. LZ and GK are supported by grants from the Ligue Nationale contre le Cancer, the European Union (ALLOSTEM, DC-THERA for LZ; Active p53, ApoSys, ApopTrain, RIGHT for GK) Fondation pour la Recherche Médicale, Cancéropôle Ile-de-France, Institut National du Cancer and Agence Nationale pour la Recherche. TAF is supported by National Institutes of Health Grants EY06765, EY15570, and the Department of Ophthalmology and Visual Sciences core grant (EY02687). Support was also received from the Foundation for Fighting Blindness (Owings Mills, MD, USA), Research to Prevent Blindness (New York, N.Y., USA), and the Macular Vision Research Foundation (West Conshohocken, PA). DRG is supported by grants from the National Institutes of Health and by the American Lebanese and Syrian Associated Charities.
Glossary
- Activation-induced cell death
(AICD). The apoptotic cell death of activated lymphocytes. It ensures the rapid elimination of effector cells after their antigen-dependent clonal expansion. Defects in AICD result in lymphoproliferative diseases that are associated with autoimmune disorders.
- Apoptosis
A form of cell death, which is also known as intrinsic or programmed cell death. Many physiological and developmental stimuli cause apoptosis, and this mechanism is frequently used to delete unwanted, superfluous or potentially harmful cells, such as those undergoing transformation. Apoptosis involves cell shrinkage, chromatin condensation in the periphery of the nucleus, cell-membrane blebbing and DNA fragmentation into multiples of ~180 base pairs. Eventually, the cell breaks up into many membrane-bound apoptotic bodies, which are phagocytosed by neighbouring cells.
- BCL2 FAMILY
A family of proteins that contains at least one BCL2 homology (BH) region. The family is divided into anti-apoptotic multidomain proteins (such as BCL2, BCL-XL and MCL-1), which contain four BH domains (BH1, BH2, BH3, BH4), pro-apoptotic multidomain proteins (for example, BAX and BAK), which contain BH1, BH2 and BH3, and the pro-apoptotic BH3-only protein family.
- Caspases
A family of cytosolic proteases that cleave their substrate after an aspartic-acid residue. Initiator caspases are typically activated in response to particular stimuli: for example, caspase-8 is activated after cell-death-receptor ligation, caspase-9 after apoptosome activation, and caspase-2 after DNA damage. Effector caspases (such as caspase-3, caspase-6 and caspase-7) are activated by initiator caspases and are particularly important for the ordered dismantling of vital cellular structures.
- CD95L
An apoptosis-inducing ligand (“death ligand”) in the tumor necrosis factor family. Also called “Fas-ligand” (FasL).
- CD95
The receptor for CD95L. A member of the tumor necrosis factor receptor family in the death receptor subfamily. Also called “Fas.”
- Contact hypersensitivity
A T-cell-mediated immune response that is evoked following antigen administration in the skin. It is marked by monocyte and/or macrophage infiltration and activation, and depends on the production of T-helper-1-type cytokines.
- Dendritic cell
(DC) The major antigen presenting cell of the immune system. Engulfment of dying cells by DC leads to tolerance or immunity to associated antigens.
- Cross-presentation
The initiation of a CD8+ T-cell response to an antigen that is not present within antigen-presenting cells (APCs). This exogenous antigen must be taken up by APCs and then re-routed to the MHC-class-I pathway of antigen presentation.
- Damage-associated molecular pattern molecules
(DAMPs). As a result of cellular stress, cellular damage and non-physiological cell death, DAMPs are released from the degraded stroma (for example, hyaluronate), from the nucleus (for example, high-mobility group box 1 protein) and from the cytosol (for example, ATP, uric acid, S100 calcium-binding proteins and heat-shock proteins). Such DAMPs are thought to elicit local inflammatory reactions.
- Hapten
A molecule that can bind antibody but cannot by itself elicit an immune response. Antibodies that are specific for a hapten can be generated when the hapten is chemically linked to a protein carrier that can elicit a T-cell response.
- HMGB1
High-mobility group box protein 1, a DAMP released from dying cells. It can be modified by reactive oxygen species to alter its effects on tolerance induction.
- Inflammasome
A molecular complex of several proteins that upon assembly cleaves pro-IL-1, thereby producing active IL-1.
- Macroautophagy
(Also known as autophagy). The largely non-specific autophagic sequestration of cytoplasm into a double- or multiple-membrane-delimited compartment (an autophagosome) of non-lysosomal origin. Note that certain proteins, organelles and pathogens may be selectively degraded via macroautophagy.
- Necrosis
A form of cell death that frequently results from toxic injury, hypoxia or stress. Necrosis involves cell swelling, dysregulation of cell-membrane ion and water fluxes, mitochondrial swelling and the eventual release of cell contents into the interstitium. This form of cell death usually occurs together with inflammation. Cells that are exposed to the high concentrations of purified perforin that are typically delivered by cytolytic cells, such as natural killer cells and cytotoxic T lymphocyte, usually die by osmotic lysis, a form of necrotic death.
- NKG2D
A lectin-type activating receptor that is expressed on the surface of NK, NKT, gd T cells and some cytolytic CD8+ ab T cells. NKGD recognises the ligands MHC-class-I-polypeptide-related sequence A (MICA) and MICB in humans and retinoic acid early transcript 1 (RAE1) and H60 in mice. Such ligands are generally expressed by infected, stressed or transformed cells.
- p53
An important transcription factor that is activated by numerous genotoxic insults to induce cell cycle arrest, cellular senescence or apoptosis. p53 is frequently mutated or functionally inactivated in cancer.
- Secondary necrosis
The dissolution of cells that die by a mechanism other than conventional necrosis.
- Senescence
A nearly irreversible stage of permanent G1 cell-cycle arrest, linked to morphological changes (flattening of the cells), metabolic changes and changes in gene expression (with expression of senescence-associated β-galactosidase), the induction of which depends on p53 and cell-cycle-blockers such as p21 and p16.
- Tolerance
A term that denotes lymphocyte non-responsiveness to antigen, but implies an active process, not simply a passive lack of response.
- 'Helpless' CTL
CD8+ T cells that have undergone activation without additional stimulation ('help') by CD4+ T cells.
- Reactive oxygen species
(ROS). Oxygen radicals that are produced by the mitochondrial respiratory chain and by other processes. In excess, they can cause intracellular and mitochondrial damage, which promotes cell death. They can also modify DAMPs, such as HMGB1, to influence tolerance and immunity.
Biographies
Douglas R. Green
Douglas R. Green, Ph.D. is chair of the Department of Immunology, St. Jude Children’s Research Hospital, where he holds the Peter C. Doherty Endowed Chair. Prior to taking this position in 2005, he was head of the Division of Cellular Immunology at the La Jolla Institute for Allergy and Immunology in southern California from 1990. He has published over 375 papers, chapters, and books, and is an ISI Highly Cited investigator. His research focuses on apoptotic cell death and related phenomena.
Thomas A. Ferguson
Dr. T. A. Ferguson is a Professor of Ophthalmology and Visual Sciences at Washington University School of Medicine in St. Louis, Missouri USA. He graduated from the University Of Cincinnati College Of Medicine in 1982 with a Ph.D in Microbiology and Immunology. He was a Postdoctoral Fellow at the Howard Hughes Medical Institute in the Department of Pathology at Yale University in the Laboratory of Richard K Gershon from 1982–86. He was as an Assistant Professor at Emory University in Atlanta, Georgia USA from 1986–88. He joined the faculty of Washington University School of Medicine in 1988 where he is currently a Professor. He was the 1988–89 Robert E. McCormick Scholar awarded by Research to Prevent Blindness, and received the 2001–2002 Research to Prevent Blindness Lew Wassermann Award. His current research deals with the effect of apoptotic cells on the immune response as well as the role of death receptors and ligands in the regulation of pathogenic neovascularization.
Laurence Zitvogel
Laurence Zitvogel, MD, PhD, graduated in Medical Oncology from the School of Medicine of the University of Paris in 1992. She started her scientific career when she was at the University of Pittsburgh in the USA in Michael Lotze’s laboratory. She became Research Director at Institut National de la Santé et Recherche Médicale, in a laboratory located at Institut Gustave Roussy, a large cancer Center in Villejuif/France and the Head of the Center for Clinical Investigations for vaccine developments at Villejuif. She has been actively contributing to the field of cancer immunology and immunotherapy, and she brought together basic and translational research, including the design of cancer therapies through combined animal studies and Phase I patient trials. She is internationally recognized for her expertise in dendritic cell and innate effector biology as well as in the design of exosome-based vaccines for tumor therapy.
Guido Kroemer
Guido Kroemer currently serves as a Research Director at the French Medical Research Council (INSERM), in the INSERM Unit 848, located in Villejuif, near to Paris, France. Prior to joining the INSERM (1993), Dr. Kroemer was Senior Scientist of the European Community at the Spanish National Research Council (CSIC), namely at the National Center of Molecular Biology (1990–1992) and at the National Center of Biotechnology (1993). Dr. Kroemer did his post-doctoral training in the Collège de France, Nogent-sur-Marne (1988–1989) and at the University of Innsbruck, Austria, after receiving his Ph.D/M.D. degree at the same University in 1985. He also holds a Ph.D. degree in Biology (Autonomous University of Madrid, 1992). Guido Kroemer is member of EMBO, German Academy of Sciences (Leopoldina), Academia Europaea, and European Academy of Sciences and Arts. He received the 2006 Descartes Prize, the highest scientific distinction of the European Union, for his fundamental discoveries in the field of programmed cell death (apoptosis). He also received one of the Grands Prix from the French Academy of Sciences in 2007, as well as the Carus Medal from the German Academy of Sciences. His current research focuses on the molecular mechanisms of cancer cell death, its pharmacological manipulation and its exploitation for tumor vaccination.
REFERENCES
- 1.Medzhitov R, Janeway CA., Jr. Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 2.Zitvogel L, et al. Immune response against dying tumor cells. Adv Immunol. 2004;84:131–179. doi: 10.1016/S0065-2776(04)84004-5. [DOI] [PubMed] [Google Scholar]
- 3.Gaipl US, et al. Clearance deficiency and systemic lupus erythematosus (SLE) J Autoimmun. 2007;28:114–121. doi: 10.1016/j.jaut.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 5.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 6.Casares N, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202:1691–1701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Obeid M, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. This paper contains information suggesting that subtle differences in the surface characteristics of dying cells can determine how they are handled by dendritic cells and whether an adapative immune response will ensue.
- 8.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Griffith TS, Yu X, Herndon JM, Green DR, Ferguson TA. CD95-induced apoptosis of lymphocytes in an immune privileged site induces immunological tolerance. Immunity. 1996;5:7–16. doi: 10.1016/s1074-7613(00)80305-2. An early paper demonstrating that apoptotic cells induce tolerance to associated antigens.
- 10. Ferguson TA, et al. Uptake of apoptotic antigen-coupled cells by lymphoid dendritic cells and cross-priming of CD8(+) T cells produce active immune unresponsiveness. J Immunol. 2002;168:5589–5595. doi: 10.4049/jimmunol.168.11.5589. One of two papers (with #11) showing that CD8α+ DC cross-present antigen on class I MHC to induce tolerance.
- 11. Belz GT, et al. The CD8alpha(+) dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J Exp Med. 2002;196:1099–1104. doi: 10.1084/jem.20020861. See #10.
- 12.Garza KM, et al. Role of antigen-presenting cells in mediating tolerance and autoimmunity. J Exp Med. 2000;191:2021–2027. doi: 10.1084/jem.191.11.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyake Y, et al. Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell-associated antigens. J Clin Invest. 2007;117:2268–2278. doi: 10.1172/JCI31990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albert ML, Jegathesan M, Darnell RB. Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells. Nat Immunol. 2001;2:1010–1017. doi: 10.1038/ni722. [DOI] [PubMed] [Google Scholar]
- 15.Heath WR, Kurts C, Miller JF, Carbone FR. Cross-tolerance: a pathway for inducing tolerance to peripheral tissue antigens. J Exp Med. 1998;187:1549–1553. doi: 10.1084/jem.187.10.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191:411–416. doi: 10.1084/jem.191.3.411. An important early review on tolerance induction by uptake of apoptotic cells.
- 17. Battisto JR, Bloom BR. Dual immunological unresponsiveness induced by cell membrane coupled hapten or antigen. Nature. 1966;212:156–157. doi: 10.1038/212156a0. Pre-dating most relevant studies by decades, this work showed that injected cells can cause immune tolerance; subsequent studies (e.g., #10) showed that the cells must undergo apoptosis.
- 18.Conlon PJ, Miller SD, Claman HN. The induction of tolerance to DNFB contact sensitivity by using hapten-modified lymphoid cells. III. Effects of hapten concentration on the ability of MLS-disparate cells to induce rapid unresponsiveness. J Immunol. 1980;125:807–813. [PubMed] [Google Scholar]
- 19.Pincott CE, Bainbridge DR. Studies on transplantation immunity. IV. Murine natural immunity to lymphoid cells in vivo. Eur J Immunol. 1980;10:250–257. doi: 10.1002/eji.1830100406. [DOI] [PubMed] [Google Scholar]
- 20.Sun E, et al. Allograft tolerance induced by donor apoptotic lymphocytes requires phagocytosis in the recipient. Cell Death Differ. 2004;11:1258–1264. doi: 10.1038/sj.cdd.4401500. [DOI] [PubMed] [Google Scholar]
- 21.van Twuyver E, et al. Pretransplantation blood transfusion revisited. N Engl J Med. 1991;325:1210–1213. doi: 10.1056/NEJM199110243251704. [DOI] [PubMed] [Google Scholar]
- 22.Pradhan S, et al. A critical role for the proapoptotic protein bid in ultraviolet-induced immune suppression and cutaneous apoptosis. J Immunol. 2008;181:3077–3088. doi: 10.4049/jimmunol.181.5.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwarz A, et al. Ultraviolet light-induced immune tolerance is mediated via the Fas/Fas-ligand system. J Immunol. 1998;160:4262–4270. [PubMed] [Google Scholar]
- 24.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 25.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 26.Segal NH, et al. Epitope landscape in breast and colorectal cancer. Cancer Res. 2008;68:889–892. doi: 10.1158/0008-5472.CAN-07-3095. [DOI] [PubMed] [Google Scholar]
- 27. Apetoh L, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. This paper provides evidence that TLR4 expression on DC dictates whether the immune system can mount a CTL response against antigens from dying tumor cells; one of the ligands of TLR4 released from dying cells is may be HMGB1.
- 28.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 29.Johansson U, Walther-Jallow L, Smed-Sorensen A, Spetz AL. Triggering of dendritic cell responses after exposure to activated, but not resting, apoptotic PBMCs. J Immunol. 2007;179:1711–1720. doi: 10.4049/jimmunol.179.3.1711. [DOI] [PubMed] [Google Scholar]
- 30.Herndon JM, Stuart PM, Ferguson TA. Peripheral deletion of antigen-specific T cells leads to long-term tolerance mediated by CD8+ cytotoxic cells. J Immunol. 2005;174:4098–4104. doi: 10.4049/jimmunol.174.7.4098. [DOI] [PubMed] [Google Scholar]
- 31.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell. 2008;132:363–374. doi: 10.1016/j.cell.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuilman T, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 34.Acosta JC, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 35. Xue W, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. A interesting study in which induction of cellular senescence triggers immune-mediated tumor clearance.
- 36.Srivastava PK. Therapeutic cancer vaccines. Curr Opin Immunol. 2006;18:201–205. doi: 10.1016/j.coi.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Obeid M, et al. Ecto-calreticulin in immunogenic chemotherapy. Immunol Rev. 2007;220:22–34. doi: 10.1111/j.1600-065X.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- 38.Spisek R, et al. Bortezomib enhances dendritic cell (DC)-mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications. Blood. 2007;109:4839–4845. doi: 10.1182/blood-2006-10-054221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Panaretakis T, et al. The co-translocation of ERp57 and calreticulin determines the immunogenicity of cell death. Cell Death Differ. 2008;15:1499–1509. doi: 10.1038/cdd.2008.67. [DOI] [PubMed] [Google Scholar]
- 40.Lauber K, et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113:717–730. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- 41.Le LQ, et al. Mice lacking the orphan G protein-coupled receptor G2A develop a late-onset autoimmune syndrome. Immunity. 2001;14:561–571. doi: 10.1016/s1074-7613(01)00145-5. [DOI] [PubMed] [Google Scholar]
- 42.Peter C, et al. Migration to apoptotic "find-me" signals is mediated via the phagocyte receptor G2A. J Biol Chem. 2008;283:5296–5305. doi: 10.1074/jbc.M706586200. [DOI] [PubMed] [Google Scholar]
- 43.Koizumi S, et al. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature. 2007;446:1091–1095. doi: 10.1038/nature05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanley PJ, et al. Extracellular ATP induces oscillations of intracellular Ca2+ and membrane potential and promotes transcription of IL-6 in macrophages. Proc Natl Acad Sci U S A. 2004;101:9479–9484. doi: 10.1073/pnas.0400733101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 46.Martinon F, Gaide O, Petrilli V, Mayor A, Tschopp J. NALP inflammasomes: a central role in innate immunity. Semin Immunopathol. 2007;29:213–229. doi: 10.1007/s00281-007-0079-y. [DOI] [PubMed] [Google Scholar]
- 47. Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. A key study that identified HMGB1 as a DAMP.
- 48.Bell CW, Jiang W, Reich CF, 3rd, Pisetsky DS. The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol. 2006;291:C1318–C1325. doi: 10.1152/ajpcell.00616.2005. [DOI] [PubMed] [Google Scholar]
- 49.Jiang W, Bell CW, Pisetsky DS. The relationship between apoptosis and high-mobility group protein 1 release from murine macrophages stimulated with lipopolysaccharide or polyinosinic-polycytidylic acid. J Immunol. 2007;178:6495–6503. doi: 10.4049/jimmunol.178.10.6495. [DOI] [PubMed] [Google Scholar]
- 50. Kazama H, et al. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity. 2008;29:21–32. doi: 10.1016/j.immuni.2008.05.013. This study "connects the dots" from caspase activation to ROS production to modification of HMGB1 to immune tolerance.
- 51.Bianchi ME, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev. 2007;220:35–46. doi: 10.1111/j.1600-065X.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 52.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6:823–835. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tian J, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 54.Yamasaki S, et al. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol. 2008;9:1179–1188. doi: 10.1038/ni.1651. [DOI] [PubMed] [Google Scholar]
- 55. Sancho D, et al. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009 doi: 10.1038/nature07750. In press. This paper reports the identification of a novel PRR on dendritic cells that senses the presence of a yet unknown intracellular ligand that becomes accessible in dead cells, after permeabilization of the plasma membrane, and directs antigens for cross-presentation.
- 56.Kroemer G, et al. Classifications of cell death: Recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death & Diff. 2008 doi: 10.1038/cdd.2008.150. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Castiglioni P, et al. Apoptosis-dependent subversion of the T-lymphocyte epitope hierarchy in lymphoma cells. Cancer Res. 2002;62:1116–1122. [PubMed] [Google Scholar]
- 58.Rawson PM, et al. Cross-presentation of caspase-cleaved apoptotic self antigens in HIV infection. Nat Med. 2007;13:1431–1439. doi: 10.1038/nm1679. [DOI] [PubMed] [Google Scholar]
- 59.Tenev T, Ditzel M, Zachariou A, Meier P. The antiapoptotic activity of insect IAPs requires activation by an evolutionarily conserved mechanism. Cell Death Differ. 2007;14:1191–1201. doi: 10.1038/sj.cdd.4402118. [DOI] [PubMed] [Google Scholar]
- 60.Obeid M, et al. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ. 2007;14:1848–1850. doi: 10.1038/sj.cdd.4402201. [DOI] [PubMed] [Google Scholar]
- 61.Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008 doi: 10.1038/nrm2527. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jin S, DiPaola RS, Mathew R, White E. Metabolic catastrophe as a means to cancer cell death. J Cell Sci. 2007;120:379–383. doi: 10.1242/jcs.03349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qu X, et al. Autophagy gene-dependent clearance of apoptotic cells during embryonic development. Cell. 2007;128:931–946. doi: 10.1016/j.cell.2006.12.044. [DOI] [PubMed] [Google Scholar]
- 64.Thorburn J, et al. Autophagy regulates selective HMGB1 release in tumor cells that are destined to die. Cell Death Differ. 2008 doi: 10.1038/cdd.2008.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shimizu S, et al. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol. 2004;6:1221–1228. doi: 10.1038/ncb1192. [DOI] [PubMed] [Google Scholar]
- 66.Uhl M, Kepp O, Jusforgues-Saklani H, Kroemer G, Albert ML. Autophagic death facilitates efficient antigen cross-priming by stimulating type I IFN production by phagocytic dendritic cells. Cell Death Diff. 2009 doi: 10.1038/cdd.2009.8. in press. [DOI] [PubMed] [Google Scholar]
- 67.Albert ML, et al. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 69.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Janssen EM, et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. This studied characterized the production of TRAIL upon restimulation of helpless CTL and its role in killing both the CTL and inhibiting T cell responses.
- 71.Janssen EM, et al. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 72. Griffith TS, et al. Apoptotic cells induce tolerance by generating helpless CD8+ T cells that produce TRAIL. J Immunol. 2007;178:2679–2687. doi: 10.4049/jimmunol.178.5.2679. A study showing that conditions of tolerance induction by apoptotic cells engages helpless CTL and production of TRAIL, required for tolerance.
- 73.Greene MI, Benacerraf B. Studies on hapten specific T cell immunity and suppression. Immunol Rev. 1980;50:163–186. doi: 10.1111/j.1600-065x.1980.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 74.Scott CF, Jr., Tsurufuji M, Benacerraf B, Sy MS. Regulation of the hapten-specific T cell response. I. Preferential induction of hyporesponsiveness to the D-end of the major histocompatibility complex in the hapten-specific cytotoxic T cell response. J Immunol. 1983;131:2184–2189. [PubMed] [Google Scholar]
- 75.Chaput N, et al. Molecular determinants of immunogenic cell death: surface exposure of calreticulin makes the difference. J Mol Med. 2007;85:1069–1076. doi: 10.1007/s00109-007-0214-1. [DOI] [PubMed] [Google Scholar]
- 76.Apetoh L, et al. Immunogenic chemotherapy: discovery of a critical protein through proteomic analyses of tumor cells. Cancer Genomics Proteomics. 2007;4:65–70. [PubMed] [Google Scholar]
- 77.Ferguson TA, Hayashi JD, Kaplan HJ. The immune response and the eye. III. Anterior chamber-associated immune deviation can be adoptively transferred by serum. J Immunol. 1989;143:821–826. [PubMed] [Google Scholar]
- 78.den Haan JM, Lehar SM, Bevan MJ. CD8(+) but not CD8(−) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dudziak D, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 80.Iyoda T, et al. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J Exp Med. 2002;195:1289–1302. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Allan RS, et al. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 82.Huang L, Soldevila G, Leeker M, Flavell R, Crispe IN. The liver eliminates T cells undergoing antigen-triggered apoptosis in vivo. Immunity. 1994;1:741–749. doi: 10.1016/s1074-7613(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 83.Crispe IN, et al. Cellular and molecular mechanisms of liver tolerance. Immunol Rev. 2006;213:101–118. doi: 10.1111/j.1600-065X.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 84.Sauter B, et al. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yamaguchi H, et al. Milk fat globule EGF factor 8 in the serum of human patients of systemic lupus erythematosus. J Leukoc Biol. 2008;83:1300–1307. doi: 10.1189/jlb.1107730. [DOI] [PubMed] [Google Scholar]
- 86.Gaipl US, et al. Inefficient clearance of dying cells and autoreactivity. Curr Top Microbiol Immunol. 2006;305:161–176. doi: 10.1007/3-540-29714-6_8. [DOI] [PubMed] [Google Scholar]
- 87. Hanayama R, et al. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–1150. doi: 10.1126/science.1094359. This paper outlines the functional link between defective clearance of apoptotic cells and systemic autoimmune disease.
- 88.Miyanishi M, et al. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 89.Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7:964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- 90.Lucas M, et al. Requirements for apoptotic cell contact in regulation of macrophage responses. J Immunol. 2006;177:4047–4054. doi: 10.4049/jimmunol.177.6.4047. [DOI] [PubMed] [Google Scholar]
- 91.Ricci JE, et al. Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the p75 subunit of complex I of the electron transport chain. Cell. 2004;117:773–786. doi: 10.1016/j.cell.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 92. Chung EY, et al. Interleukin-10 expression in macrophages during phagocytosis of apoptotic cells is mediated by homeodomain proteins Pbx1 and Prep-1. Immunity. 2007;27:952–964. doi: 10.1016/j.immuni.2007.11.014. A detailed study on the mechanistic link between phagocytosis of dying cells and production of immunosupressive cytokines by macrophages.
- 93.Fadok VA, et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Voll RE, et al. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 95.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 96.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Freire-de-Lima CG, et al. Uptake of apoptotic cells drives the growth of a pathogenic trypanosome in macrophages. Nature. 2000;403:199–203. doi: 10.1038/35003208. [DOI] [PubMed] [Google Scholar]
- 98.Hoffmann PR, et al. Interaction between phosphatidylserine and the phosphatidylserine receptor inhibits immune responses in vivo. J Immunol. 2005;174:1393–1404. doi: 10.4049/jimmunol.174.3.1393. [DOI] [PubMed] [Google Scholar]
- 99.Gao Y, Herndon JM, Zhang H, Griffith TS, Ferguson TA. Antiinflammatory effects of CD95 ligand (FasL)-induced apoptosis. J Exp Med. 1998;188:887–896. doi: 10.1084/jem.188.5.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen W, Frank ME, Jin W, Wahl SM. TGF-beta released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity. 2001;14:715–725. doi: 10.1016/s1074-7613(01)00147-9. [DOI] [PubMed] [Google Scholar]
- 101.Kleinclauss F, et al. Intravenous apoptotic spleen cell infusion induces a TGF-beta-dependent regulatory T-cell expansion. Cell Death Differ. 2006;13:41–52. doi: 10.1038/sj.cdd.4401699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maeda A, et al. Intravenous infusion of syngeneic apoptotic cells by photopheresis induces antigen-specific regulatory T cells. J Immunol. 2005;174:5968–5976. doi: 10.4049/jimmunol.174.10.5968. [DOI] [PubMed] [Google Scholar]
- 103. Torchinsky MB, Garaude J, Martin A, Blander JM. Innate immune recognition of infected apoptotic cells directs TH17 cell differentiation. Nature. doi: 10.1038/nature07781. In press. Engulfment of apoptotic cells by DC induces Treg or Th17 cells, depending on additional signals, relevant to infection.
- 104.Hedrick SM. The acquired immune system: a vantage from beneath. Immunity. 2004;21:607–615. doi: 10.1016/j.immuni.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 105.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 106.Jeannin P, Jaillon S, Delneste Y. Pattern recognition receptors in the immune response against dying cells. Curr Opin Immunol. 2008;20:530–537. doi: 10.1016/j.coi.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 107.Ting JP, Willingham SB, Bergstralh DT. NLRs at the intersection of cell death and immunity. Nat Rev Immunol. 2008;8:372–379. doi: 10.1038/nri2296. [DOI] [PubMed] [Google Scholar]
- 108.Di Virgilio F. Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol Sci. 2007;28:465–472. doi: 10.1016/j.tips.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 109.Beutler B, Moresco EM. The forward genetic dissection of afferent innate immunity. Curr Top Microbiol Immunol. 2008;321:3–26. doi: 10.1007/978-3-540-75203-5_1. [DOI] [PubMed] [Google Scholar]
- 110.Galluzzi L, et al. Cell death modalities: classification and pathophysiological implications. Cell Death Differ. 2007;14:1237–1243. doi: 10.1038/sj.cdd.4402148. [DOI] [PubMed] [Google Scholar]
- 111.Festjens N, Vanden Berghe T, Vandenabeele P. Necrosis, a well-orchestrated form of cell demise: signalling cascades, important mediators and concomitant immune response. Biochim Biophys Acta. 2006;1757:1371–1387. doi: 10.1016/j.bbabio.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 112.Golstein P, Kroemer G. Cell death by necrosis: towards a molecular definition. Trends Biochem Sci. 2007;32:37–43. doi: 10.1016/j.tibs.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 113.Vakifahmetoglu H, Olsson M, Zhivotovsky B. Death through a tragedy: mitotic catastrophe. Cell Death Differ. 2008;15:1153–1162. doi: 10.1038/cdd.2008.47. [DOI] [PubMed] [Google Scholar]
- 114.Erwig LP, Henson PM. Clearance of apoptotic cells by phagocytes. Cell Death Differ. 2008;15:243–250. doi: 10.1038/sj.cdd.4402184. [DOI] [PubMed] [Google Scholar]
- 115.Truman LA, et al. CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood. 2008 doi: 10.1182/blood-2008-06-162404. [DOI] [PubMed] [Google Scholar]
- 116.Miksa M, Amin D, Wu R, Ravikumar TS, Wang P. Fractalkine-induced MFG-E8 leads to enhanced apoptotic cell clearance by macrophages. Mol Med. 2007;13:553–560. doi: 10.2119/2007-00019.Miksa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brown S, et al. Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature. 2002;418:200–203. doi: 10.1038/nature00811. [DOI] [PubMed] [Google Scholar]
- 118.Gardai SJ, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 119.Martin SJ, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wolf A, Schmitz C, Bottger A. Changing story of the receptor for phosphatidylserine-dependent clearance of apoptotic cells. EMBO Rep. 2007;8:465–469. doi: 10.1038/sj.embor.7400956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hanayama R, et al. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 122.Arur S, et al. Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev Cell. 2003;4:587–598. doi: 10.1016/s1534-5807(03)00090-x. [DOI] [PubMed] [Google Scholar]
- 123.Tibrewal N, et al. Autophosphorylation docking site Tyr-867 in Mer receptor tyrosine kinase allows for dissociation of multiple signaling pathways for phagocytosis of apoptotic cells and down-modulation of lipopolysaccharide-inducible NF-kappaB transcriptional activation. J Biol Chem. 2008;283:3618–3627. doi: 10.1074/jbc.M706906200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.