Abstract
Autism spectrum disorders (ASD) are associated with disturbances of neural connectivity. Functional connectivity between neural structures is typically examined within the context of a cognitive task, but also exists in the absence of a task (i.e., “rest”). Connectivity during rest is particularly active in a set of structures called the default network, which includes the posterior cingulate cortex (PCC), retrosplenial cortex, lateral parietal cortex/angular gyrus, medial prefrontal cortex, superior frontal gyrus, temporal lobe, and parahippocampal gyrus. We previously reported that adults with ASD relative to controls show areas of stronger and weaker connectivity within the default network. The objective of the present study was to examine the default network in adolescents with ASD. Sixteen adolescents with ASD and 15 controls participated in a functional MRI study. Functional connectivity was examined between a PCC seed and other areas of the default network. Both groups showed connectivity in the default network. Relative to controls, adolescents with ASD showed widespread weaker connectivity in nine of the eleven areas of the default network. Moreover, an analysis of symptom severity indicated that poorer social skills and increases in restricted and repetitive behaviors and interests correlated with weaker connectivity, whereas poorer verbal and non-verbal communication correlated with stronger connectivity in multiple areas of the default network. These findings indicate that adolescents with ASD show weaker connectivity in the default network than previously reported in adults with ASD. The findings also show that weaker connectivity within the default network is associated with specific impairments in ASD.
Keywords: Functional Magnetic Resonance Imaging, development, symptom severity, Asperger’s syndrome, pervasive developmental disorder not otherwise specified
1. INTRODUCTION
Three core features define autism spectrum disorders (ASD): impairments in social functioning, difficulties in communication, and restricted and repetitive behaviors and/or interests (APA 1994). Converging lines of evidence indicate that ASD is a disorder of brain connectivity (Belmonte et al. 2004; Just et al. 2007). First, individuals with ASD show marked disturbances in cortical organization as evidenced by narrower and more densely packed columns of neuronal cells (Casanova et al. 2006). These morphological differences suggest an alteration in structural connectivity which could in turn have an impact on the functional connectivity between brain structures. Second, individuals with ASD have increases in white matter volume that are present in outer zones and not inner zones of white matter, such as the corpus callosum (Herbert et al. 2004). This suggests that individuals with ASD have a greater number of short to medium range intrahemispheric connections and fewer longer range interhemispheric connections (Herbert et al. 2003; Herbert et al. 2004). In addition, diffusion tensor imaging (DTI) techniques show that white matter integrity is compromised in ASD (Alexander et al. 2007; Barnea-Goraly et al. 2004). Third, individuals with ASD have abnormalities in functional connectivity within regions of the brain. Specifically, some studies found that individuals with ASD relative to controls show stronger connectivity (Mizuno et al. 2006; Noonan et al. 2009; Turner et al. 2006) and others report weaker connectivity (Just et al. 2007; Kana et al. 2006; Kleinhans et al. 2008; Koshino et al. 2008; Villalobos et al. 2005; Welchew et al. 2005; Wicker et al. 2008). Thus, both structural and functional evidence suggest that there is profound disruption in brain connectivity in ASD.
The functional connectivity studies reported above were carried out in the context of a cognitive task. However, the brain is highly active even when participants are not engaged in any task (i.e., at rest). The default network is a set of structures that is known to be particularly active when participants are at rest (Fox et al. 2005; Greicius et al. 2003; Shulman et al. 1997). The brain regions that form the default network are the posterior cingulate cortex (PCC), retrosplenial, lateral parietal/angular gyrus, medial prefrontal cortex, superior frontal gyrus, regions of the temporal lobe, and finally the parahippocampal gyrus (Fox et al. 2005; Greicius et al. 2003; Shulman et al. 1997). Because of the tremendous amount of energy that this activation consumes (Raichle and Mintun 2006), many have speculated that the default network’s function may extend beyond thought processes and encompass the role of maintaining homeostasis between excitatory and inhibitory neuronal responses (Biswal et al. 1995; Laughlin and Sejnowski 2003). Others have argued that the default network is active when contemplating scenarios and events, mind wandering, or lower-level observations of the individual’s external surroundings (Buckner et al. 2008; Christoff et al. 2009; Mason et al. 2007; Raichle and Snyder 2007). Thus, although the precise role of the default network remains unclear, investigators suggest that it may be involved in fundamental aspects of nervous system functioning (Raichle and Snyder 2007).
The majority of default network studies have focused on typical adults (Fox et al. 2005; Greicius et al. 2003; Greicius et al. 2009). Recently, investigations have examined functional connectivity within the default network in individuals with ASD (Cherkassky et al. 2006; Kennedy and Courchesne 2008; Monk et al. 2009). The results of these three studies are not entirely consistent. In the first study, Cherkassky et al. (2006) found broad-based weaker connectivity. For the second study, Kennedy and Courchesne (2008) identified selective areas of reduced connectivity. Finally, in the third study, we found weaker connectivity between the PCC and the superior frontal gyrus and stronger connectivity between the PCC and two other regions in the default network (Monk et al. 2009). The three studies employed different data collection and analytic procedures, which might explain the differential findings. In addition, the age ranges in each study was very broad and this might contribute to inconsistent findings. In the present study, we closely followed the procedures of our study of adults and we also selectively examined an adolescent age group. No known published study has focused solely on the default network in adolescents with ASD.
Dramatic anatomical changes occur within the brain during adolescence (Giedd 2008; Sowell et al. 2003). These anatomical changes are accompanied by functional alterations within specific brain regions (Booth et al. 2001; Guyer et al. 2008; Koch et al. 2002; Monk et al. 2003; Thomas et al. 2001). Moreover, functional connectivity of the default network changes between childhood and adulthood (Fair et al. 2008; Stevens et al. 2009). In the study by Stevens et al., (2009), when they examined three of the networks that overlap closely with the default network structures, they reported that the strength of connectivity decreased with increasing age. A reduction in functional connectivity between adolescence and adulthood was also found in a study in which participants were engaged in a cognitive task involving empathy (Burnett and Blakemore 2009). Similar to their typically developing counterparts, adolescents with ASD are likely to show age-related changes within the default network. Since adolescence is a key period for the development of social interactions along with corresponding changes in brain function (Nelson et al. 2005) and ASD is a life-long condition of severe social impairment that begins early in life, it is important to better understand how brain function is different during this stage. Examining the default network in adolescents with ASD allows for a fuller characterization of connectivity during this critical period of brain development.
The goals of this study were to examine the default network in adolescents with ASD relative to controls and to associate abnormalities in the default network with core symptoms of ASD (social impairments, communication deficits, and restricted and repetitive behaviors and interests). To evaluate default network functional connectivity, we followed the procedures we previously used with ASD adults (Monk et al. 2009). Specifically, we monitored the default network for 10 minutes in adolescents with ASD and controls as they passively viewed a fixation cross. Moreover, as in our previous study, we used a seed in the posterior cingulate cortex (PCC) to examine pair-wise couplings in the default network. Not only is the PCC highly active at rest (Raichle et al. 2001), but this region also exhibits robust connectivity with other default network regions during resting state (Fransson and Marrelec 2008). Past studies have used this seed successfully in adults (Buckner et al. 2008; Fox et al. 2005; Shulman et al. 1997) and children (Thomason et al. 2008) to reveal connectivity in the default network.
Based on evidence that adults with ASD have regions of weaker connectivity in the default network (Cherkassky et al. 2006; Kennedy and Courchesne 2008; Monk et al. 2009) and findings that typical adolescents show heightened connectivity (Burnett and Blakemore 2009; Stevens et al. 2009), our first hypothesis was that adolescents with ASD, relative to adolescent controls, would show multiple regions of weaker connectivity in the default network. In addition, based on our previous reports that resting connectivity in the default network was associated with core ASD symptoms in adults (Monk et al. 2009), our second hypothesis was that severity of symptoms within the ASD adolescents would correlate with connectivity within the network.
2. RESULTS
Default network within each group
At the threshold of p < 0.05 (small volume corrected for the default network), both the control group as well as the ASD group showed functional connectivity that was similar to previous reports on the default network in typical adult populations (Fox et al. 2005; Greicius et al. 2003; Shulman et al. 1997). Specifically, both groups showed functional connectivity between the PCC seed and regions in the retrosplenial, left/right angular gyrus, left/right medial prefrontal, left/right temporal lobe, left/right superior frontal gyrus, left/right parahippocampal gyrus (Table 2).
Table 2.
A. Functional connectivity in the ASD group between the PCC and other default network regions. B. Functional connectivity in the control group between the PCC and other default network regions. The threshold was set at p < 0.05 (small volume-corrected for the eleven default network regions combined) in both tables. Brodmann’s areas for this and subsequent tables were derived from WFU pickatlas, (http://www.fmri.wfubmc.edu/(Maldjian) et al. 2002).
| A. | ||||||
|---|---|---|---|---|---|---|
| Region | Brodmann’s | Cluster size | t | MNI Coordinates | ||
| Area | x | y | z | |||
| Retrosplenial | 30 | 36 | 10.68 | 4 | −46 | 20 |
| 30 | 29 | 10.33 | −2 | −46 | 18 | |
| 30 | 162 | 8.75 | 12 | −54 | 6 | |
| 30 | 70 | 7.84 | −18 | −42 | −4 | |
| 30 | 165 | 7.63 | −12 | −64 | 10 | |
| Left angular gyrus | 39 | 396 | 7.86 | −44 | −60 | 34 |
| Right angular gyrus | 40 | 390 | 8.76 | 54 | −62 | 36 |
| Left medial prefrontal | 10 | 2486 | 11.37 | −22 | 58 | −6 |
| Right medial prefrontal | 10 | 2463 | 9.02 | 4 | 58 | 8 |
| Left temporal lobe | 21 | 14507 | 10.29 | −14 | −60 | −20 |
| Right temporal lobe | 20 | 13137 | 10.68 | 54 | −20 | −22 |
| Left superior frontal gyrus | 10 | 5237 | 11.50 | −22 | 58 | −4 |
| Right superior frontal gyrus | 9 | 5097 | 11.69 | 36 | 34 | 38 |
| 6 | 23 | 3.80 | 8 | 20 | 68 | |
| Left parahippocampal gyrus | 28 | 1889 | 8.64 | −24 | −26 | −10 |
| Right parahippocampal gyrus | 30 | 1704 | 7.99 | 16 | −38 | −6 |
| B. | ||||||
|---|---|---|---|---|---|---|
| Region | Brodmann’s | Cluster size | t | MNI Coordinates | ||
| Area | x | y | z | |||
| Retrosplenial | 30 | 36 | 14.08 | 8 | −54 | 16 |
| 30 | 29 | 12.86 | −2 | −54 | 20 | |
| 30 | 162 | 11.77 | 8 | −54 | 6 | |
| Left angular gyrus | 39 | 394 | 9.36 | −54 | −72 | 30 |
| Right angular gyrus | 40 | 390 | 11.50 | 52 | −60 | 34 |
| Left medial prefrontal | 10 | 2486 | 12.11 | −22 | 60 | 2 |
| Right medial prefrontal | 10 | 2333 | 12.63 | 4 | 58 | 8 |
| Left temporal lobe | 19 | 14437 | 11.02 | −18 | −48 | −2 |
| Right temporal lobe | 21 | 13420 | 15.90 | 52 | −18 | −22 |
| Left superior frontal gyrus | 10 | 5469 | 14.25 | −22 | 56 | 2 |
| Right superior frontal gyrus | 8 | 5628 | 15.89 | 18 | 28 | 50 |
| Left parahippocampal gyrus | 27 | 1926 | 11.85 | −10 | −36 | −2 |
| Right parahippocampal gyrus | 30 | 1714 | 10.71 | 16 | −38 | −8 |
Group differences in the default network
To assess our first hypothesis that adolescents with ASD relative to controls would show weaker connectivity between the PCC and areas in the default network, we performed a random effects analysis in SPM2. At the threshold of p < 0.05 (small volume corrected for the default network), we found weaker connectivity in the ASD group relative to the control group in 9 of the 11 areas of the default network (Table 3, Figure 1).
Table 3.
Functional connectivity between the PCC and other regions of the default network where controls showed stronger connectivity relative to the ASD group. Statistical threshold was set at p < 0.05 small volume-corrected for the ROI of the eleven default network regions combined. There were no regions in the default network where the ASD group showed stronger connectivity than the control group.
| Region | Brodmann’s | Cluster size | t | MNI coordinates | ||
|---|---|---|---|---|---|---|
| Area | x | y | z | |||
| Restro-splenial | 30 | 1 | 3.41 | 6 | −52 | 20 |
| Left medial prefrontal | 32 | 8 | 3.67 | −18 | 46 | 10 |
| Right medial prefrontal | 32 | 3 | 4.10 | 16 | 24 | 42 |
| Left temporal lobe | 21 | 10 | 3.75 | −48 | 8 | −40 |
| 38 | 43 | 3.35 | −38 | 4 | −16 | |
| Right temporal lobe | 22 | 703 | 5.68 | 42 | −52 | 16 |
| 20 | 222 | 4.71 | 46 | −8 | −24 | |
| 37 | 13 | 3.82 | 40 | −44 | −18 | |
| 13 | 3 | 3.59 | 42 | −22 | 8 | |
| Left superior frontal gyrus | 10 | 71 | 4.35 | −20 | 56 | 2 |
| 6 | 24 | 4.04 | −10 | −16 | 74 | |
| 11 | 23 | 3.73 | −18 | 54 | −12 | |
| 8 | 1 | 3.51 | −8 | 50 | 48 | |
| 6 | 1 | 3.35 | −10 | −2 | 68 | |
| Right superior frontal gyrus | 8 | 96 | 4.77 | 14 | 26 | 48 |
| 9 | 16 | 3.91 | 16 | 34 | 40 | |
| Left parahippocampal gyrus | 34 | 19 | 4.36 | −16 | 0 | −16 |
| 37 | 44 | 4.07 | −34 | −42 | −10 | |
| 27 | 2 | 3.40 | −20 | −34 | −4 | |
| Right parahippocampal gyrus | 28 | 32 | 4.50 | 18 | −12 | −22 |
| 36 | 12 | 3.88 | 30 | −28 | −24 | |
| 34 | 8 | 3.76 | 16 | −4 | −16 | |
Figure 1.
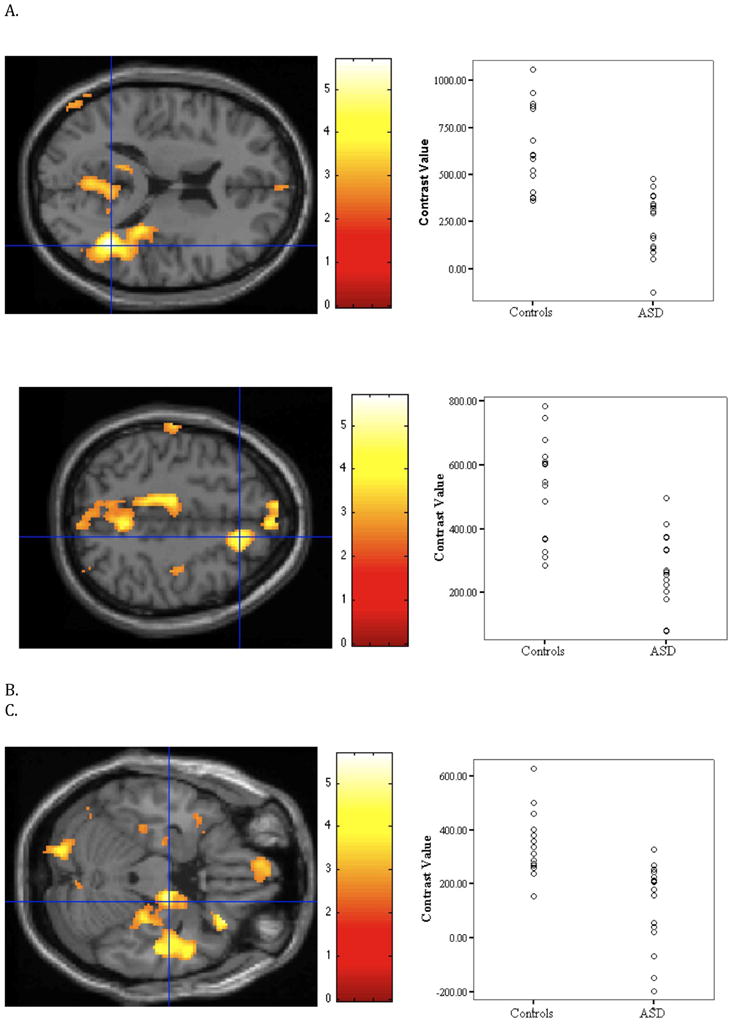
Relative to the ASD group, the control group showed stronger connectivity in nine of the eleven areas of the default network (small volume-corrected). The figure illustrates three of the areas where group differences were found. Images on the left side depict the area of activation and the scatterplots on the right side show the strength of connectivity of each individual in each group. (A) Right temporal lobe t(29) = 5.68, xyz = 42 −52 16. (B) Right superior frontal gyrus, t(29) = 4.77, xyz = 14 26 48. (C) Right parahippocampal gyrus, t(29) = 4.50, xyz = 18 −12 −22. For illustration purposes, the threshold was set at p < 0.01 with a cluster size of k > 10 voxels for all images. To depict activation for each subject, contrast values were extracted from a 4mm sphere around the peak.
Correlations of default network connectivity and symptom severity
To assess the relationship between strength of functional connectivity and measures of severity of symptoms within the ASD group, as reported in the Autism Diagnostic Interview-Revised (ADI-R) (Lord et al. 1994), we performed regression analyses. As described in the methods, we corrected for multiple comparisons by dividing the p value of 0.05 by eleven (the number of default network regions) to obtain a threshold of p = 0.0045. Only clusters greater than 20 voxels are reported. Poorer social functioning was associated with weaker connectivity between the PCC and multiple regions, including the superior frontal gyri, the temporal lobes and the parahippocampal gyri (Table 4; Figure 2). More severe restricted and repetitive behaviors were associated with weaker connectivity between the PCC and the following regions: medial prefrontal cortex, the temporal lobes and the superior frontal gyri (Table 5). Poorer verbal communicative ability was associated with stronger connectivity between the PCC and the temporal lobes as well as the PCC and the right parahippocampal gyrus (Table 6). Poorer non-verbal communicative ability was associated with stronger connectivity between the PCC and the temporal lobes, the superior frontal gyri and the right parahippocampal gyrus (Table 7).
Table 4.
Clusters in default network where social symptom severity was negatively associated with degree of functional connectivity, using a PCC seed. Social symptom severity was measured by the ADI-R “ever” symptoms total. Threshold was set at p < .0045. Only clusters of 20 or more voxels in the default network are reported.
| Region | Brodmann’s | Cluster size | t | MNI Coordinates | ||
|---|---|---|---|---|---|---|
| Area | x | y | z | |||
| Left temporal lobe | 22 | 122 | 4.29 | −56 | 4 | 0 |
| Right temporal lobe | 21 | 34 | 3.89 | 64 | −16 | −6 |
| Left superior frontal gyrus | 8 | 41 | 4.21 | −24 | 42 | 50 |
| Left superior frontal gyrus | 6 | 27 | 4.08 | −2 | −10 | 74 |
| Right superior frontal gyrus | 9 | 88 | 4.47 | 14 | 48 | 38 |
| Left parahippocampal gyrus | 30 | 65 | 5.98 | −12 | −42 | −4 |
| Right parahippocampal gyrus | 30 | 110 | 4.35 | 8 | −42 | 2 |
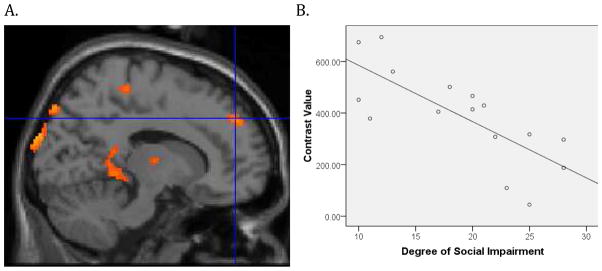
Figure 2.
Within the ASD group, social functioning was based on the ADI-R measure of total reciprocal social interaction and was negatively correlated with functional connectivity in the superior frontal gyrus, t (14)= 4.47, p < 0.001, xyz coordinates 14 48 38 (Figure 2A). To illustrate the association, contrast values were extracted from a 4mm sphere around the peak activation and plotted with the ADI-R measure of social function for each subject (Figure 2B). For illustration purposes the threshold figure A was set at p = 0.005 with a cluster size k = 10.
Table 5.
Clusters in default network where restricted and repetitive symptom severity was negatively associated with degree of functional connectivity, using a PCC seed. Restricted and repetitive symptom severity was measured by the ADI-R “ever” symptoms total. Threshold was set at p < 0.0045. Only clusters of 20 or more voxels in the default network are reported.
| Region | Brodmann’s | Cluster size | t | MNI Coordinates | ||
|---|---|---|---|---|---|---|
| Area | x | y | z | |||
| Left medial prefrontal | 32 | 47 | 4.72 | −20 | 38 | 18 |
| Left medial prefrontal | 10 | 39 | 3.95 | −46 | 56 | 8 |
| Left medial prefrontal | 32 | 21 | 3.87 | −16 | 22 | 40 |
| Right medial prefrontal | 32 | 26 | 4.25 | 10 | 34 | 18 |
| Left temporal lobe | 38 | 566 | 7.94 | −50 | 12 | −14 |
| Right temporal lobe | 39 | 521 | 5.44 | 50 | −58 | 8 |
| Right temporal lobe | 38 | 25 | 4.15 | 38 | 16 | −22 |
| Right temporal lobe | 22 | 157 | 3.96 | 62 | 8 | −6 |
| Right temporal lobe | 38 | 21 | 3.57 | 34 | 2 | −14 |
| Left superior frontal gyrus | 9 | 84 | 5.33 | −24 | 42 | 18 |
| Left superior frontal gyrus | 6 | 53 | 3.68 | −16 | 18 | 56 |
| Right superior frontal gyrus | 8 | 141 | 5.61 | 12 | 22 | 50 |
| Right superior frontal gyrus | 9 | 298 | 5.37 | 30 | 46 | 32 |
| Right superior frontal gyrus | 6 | 151 | 4.93 | 8 | 10 | 70 |
| Right superior frontal gyrus | 9 | 52 | 4.76 | 14 | 48 | 36 |
Table 6.
Clusters in default network where verbal communication symptom severity was positively related to degree of functional connectivity, using a PCC seed. Verbal communication symptom severity was measured by the ADI-R “ever” symptoms total. Threshold was set at p < 0.0045. Only clusters of 20 or more voxels in the default network are reported.
| Region | Brodmann’s | Cluster size | t | MNI Coordinates | ||
|---|---|---|---|---|---|---|
| Area | x | y | z | |||
| Right temporal lobe | 37 | 51 | 5.97 | 42 | −44 | −14 |
| Left temporal lobe | 20 | 79 | 4.18 | −50 | −26 | −18 |
| Right parahippocampal gyrus | 36 | 29 | 4.15 | 30 | −36 | −6 |
Table 7.
Clusters in default network where non-verbal communication symptom severity is positively related to degree of functional connectivity, using a PCC seed. Non-verbal communication symptom severity was measured by the ADI-R “ever” symptoms total. Threshold was set at p < 0.0045. Only clusters of 20 or more voxels in the default network are reported.
| Region | Brodmann’s | Cluster size | t | MNI Coordinates | ||
|---|---|---|---|---|---|---|
| Area | x | y | z | |||
| Left temporal lobe | 20 | 98 | 4.68 | −52 | −26 | −20 |
| Left temporal lobe | 37 | 41 | 4.35 | −48 | −60 | −18 |
| Left temporal lobe | 20 | 35 | 4.12 | −64 | −50 | −16 |
| Right temporal lobe | 37 | 46 | 4.99 | 42 | −44 | −16 |
| Right temporal lobe | 39 | 40 | 3.92 | 52 | −74 | 8 |
| Left superior frontal gyrus | 6 | 47 | 3.98 | −6 | 10 | 62 |
| Right superior frontal gyrus | 6 | 40 | 4.26 | 30 | 10 | 56 |
| Right parahippocampal gyrus | 34 | 26 | 3.71 | 16 | −6 | −20 |
Effects of group differences in non-verbal cognitive functioning
The ASD group had higher non-verbal cognitive functioning scores than the control group (Table 1). Therefore, in order to determine if the level of non-verbal cognitive functioning accounted for group differences in default network, we covaried for the non-verbal cognitive functioning scores and found that the ASD group relative to the control group continued to show weaker connectivity between the PCC and the same nine areas of the default network where we found group differences (p < .05) small volume corrected for the entire default network.
Table 1.
Subject characteristics
| ASD | Control | t (df) | p value | |
|---|---|---|---|---|
| Age, mean (SD) | 15 (1.45) | 16 (1.44) | 1.21 (29) | 0.24 |
| Age range | 13 – 17 | 13 – 18 | ||
| Male to female ratio | 14:2 | 14:01 | ||
| Verbal cognitive functioning mean (SD) | 114 (18.58) | 113 (14.10) | 0.21 (29) | 0.83 |
| Nonverbal cognitive functioning mean (SD) | 117 (13.82) | 106 (12.53) | 2.38 (29) | 0.024 |
| Handedness left to right ratio | 1:15 | 2:13 | ||
| Social Responsiveness Scale | 75 (10.41) | 45 (8.10) | 8.81 | <0.001 |
| ADI-R Social Total | 19 (6.22) | |||
| ADI-R Social Current | 8.62 (4.21) | |||
| ADI-R Verbal Communication Total | 16 (3.66) | |||
| ADI-R Verbal Communication Current | 9 (3.72) | |||
| ADI-R Nonverbal Communication Total | 9 (2.67) | |||
| ADI-R Nonverbal Communication Current | 4 (2.66) | |||
| ADI-R Rigidity, Repetitive Behaviors Total | 6 (2.78) | |||
| ADI-R Rigidity, Repetitive Behaviors Current | 5 (2.06) |
Measures of co-occurring symptoms
Co-occurring symptoms of mental conditions, including depression, anxiety and attention-deficit hyperactivity disorder (ADHD) are common in ASD (Ghaziuddin et al. 1992; Lainhart and Folstein 1994; Leyfer et al. 2006). To quantify these symptoms, the Children’s Depression Inventory (CDI) (Kovacs 1992), the Spence Children’s Anxiety Scale (SCAS) (Spence 1997), and the Child Behavior Checklist (CBCL) (Achenbach and Edelbrock 1981) were administered to the parents. The CDI is a questionnaire that assesses cognitive, affective and behavioral signs of depression in children and adolescents. The SCAS provides a quantitative measure of anxiety symptoms and it has been effectively used to measure anxiety in ASD (Russell and Sofronoff 2005; Sofronoff et al. 2005). The CBCL assesses a broad range of behaviors and symptoms of mental disorders, including aggression, attention problems, defiance and social problems.
For the measure of depression (CDI) the ASD group had higher ratings than the control group, t (29)=2.52, p =0.02. The ASD group had a mean on the CDI of 8.50 (SD=5.29) and the control group had a mean of 4.73 (SD=2.43). For the measure of anxiety (SCAS), there were no significant differences between groups, t (29)= 1.35, p =0.188. The ASD group had a mean of 20.69 (SD=13.10) and the control group had a mean of 15.80 (SD=5.16). Based on the CBCL symptom profile total score, the ASD group had more behavioral problems than the control group, t (25)=4.54, p <0.001 (the CBCL was not collected from four subjects in the ASD group). The ASD group had a mean CBCL total score of 62 (SD=10.72) and the control group had a mean score of 44 (SD=9.75). Finally, for the CBCL subscore of attention problems, the ASD group were more impaired than the control group, t(25)=3.85, p =0.001. The ASD group had a mean attention subscale score of 65 (SD=12.25) and the control group had mean score of 53 (SD=3.33).
To evaluate whether symptoms that differed between groups accounted for the group differences in default network connectivity, we covaried for the CDI and CBCL attention subscores in separate analyses and found that the ASD group relative to the control group continued to show weaker connectivity between the PCC and the same nine areas of the default network where we found group differences. However, group differences were only significant at p < .05 uncorrected. Since the CBCL total score captures symptoms that are also core features of ASD, it was not warranted to perform a covariate analysis with this measure.
Effects of medication
In order to assess whether medications influenced the results, we followed a previous study by Kennedy and Courchesne (2008) on resting connectivity in adults with ASD and excluded the 10 out of the 16 adolescents with ASD who were on medications. When the remaining 6 adolescents with ASD who were not on any psychotropic medication were compared to the 15 controls using the same procedures as described above (a random effects analysis in SPM2), there was a similar pattern of results as described above. Specifically, as in the full sample, the adolescents with ASD, who were not on medication relative to the controls showed reduced connectivity across the same nine default network structures (p< 0.05 uncorrected) where group differences were found in the full sample.
3. DISCUSSION
We examined functional connectivity of the default network in adolescents with ASD and typical adolescents in the absence of a cognitive task. Consistent with our first hypothesis, the ASD group relative to the control group showed weaker connectivity between the PCC and nine of the eleven areas of the default network. In support of our second hypothesis, poorer social impairment was associated with weaker connectivity between the PCC and the superior frontal gyri, the PCC and the temporal lobes, as well as the PCC and the parahippocampal gyri. Additionally, more severe restricted and repetitive behaviors were associated with weaker connectivity between the PCC and the medial prefrontal cortex, the PCC and the temporal lobes, as well as the PCC and the superior frontal gyri. Finally, poorer verbal and non-verbal communicative ability was associated with stronger connectivity between the PCC and the right parahippocampal gyrus as well as the PCC and the temporal lobes. Non-verbal communication was also associated with stronger connectivity between the PCC and the superior frontal gyri.
Using the same procedures as in the present study, we recently reported that adults with ASD relative to controls showed weaker connectivity between the PCC and one other structure of the default network (Monk et al. 2009). In addition, in the one other study to examine the default network in ASD during extended periods of rest, the ASD relative to the control group showed weaker connectivity between three default network seed regions and two other default network regions (Kennedy and Courchesne 2008). Notably, the work by Kennedy and Courchesne involved a broad age range that encompassed both adults and adolescents. Across these studies a developmental pattern emerges in which group differences in default network connectivity decrease between adolescence and adulthood. Further work is necessary to understand how these developmental changes in the default network in ASD are associated with changes in function, symptoms and adaptation.
There is strong evidence for age-related changes in the default network during normative development as well (Fair et al. 2008; Fair et al. 2009; Fair et al. 2007; Stevens et al. 2009; Supekar et al. 2009). These studies consistently found that development from childhood to adulthood was associated with decreased connectivity across networks and increased connectivity within networks, including the default network. This pattern may represent increased segregation and strengthening of networks in development (Stevens et al. 2009). Thus, relating this developmental pattern to our adolescent and adult ASD findings, adolescents with ASD may undergo a more protracted development of the default network. This is consistent with the present finding that adolescents with ASD show widespread weaker functional connectivity of the default network relative to adolescent controls whereas adults with ASD only evidence weaker connectivity in one structure (Monk et al. 2009).
In the present study, we found that social impairment in ASD was associated with strength of connectivity in multiple regions of the default network. Specifically, the greater the degree of social impairment the weaker the connectivity between the PCC and the superior frontal gyri, the PCC and the temporal lobes, as well as the PCC and the parahippocampal gyri. In contrast, adults with ASD only showed this negative relationship between the PCC and right superior frontal gyrus (Monk et al. 2009). This suggests that at younger ages, weaker connectivity throughout the default network relates to social impairment whereas in adulthood, the impairment is more specific to a subset of structures. Consistent with the suggestion that individuals with ASD may undergo a more protracted development of the default network, the symptom findings indicate that those with the worst social functioning have multiple areas of weak connectivity. With development into adulthood, these regions that are associated with social impairment are reduced, leaving just the PCC-superior frontal gyrus consistently related to social functioning across development.
In addition, more severe restricted and repetitive behaviors were associated with weaker functional connectivity between the PCC and the medial prefrontal gyri, the PCC and the temporal lobes, as well as the PCC and the parahippocampal gyri. There were two differences between the findings reported here and our study of adults with ASD (Monk et al. 2009). First, in the adult ASD study, more severe restricted and repetitive behaviors were associated with stronger connectivity in the ASD group. Therefore, the direction of the relationship differed between studies. Second, unlike the present work, the adult study reported that the positive association was present in only one pair-wise coupling, namely the PCC and the parahippocampal gyrus. This suggests that the relationship between the default network and repetitive behaviors phenotype changes dramatically between adolescence and adulthood. In our study of adults, we suggested that either t he hyper-connectivity may give rise to the behavioral abnormalities of restricted and repetitive behaviors or that the connectivity patterns may be a consequence of the restricted and repetitive behaviors (e.g., greater connectivity may be a result of a constant effort to control these behaviors). Our adolescent and adult findings in conjunction with a study indicating that restricted and repetitive behaviors lessen between adolescence and adulthood (Seltzer et al. 2003), suggests that the developmental switch from weak connectivity to strong connectivity being associated with these symptoms may serve a compensatory purpose in adulthood.
Furthermore, poorer verbal communicative ability in the ASD group was associated with stronger connectivity between the PCC and the right parahippocampal gyrus as well as the PCC and the temporal lobes in adolescents with ASD. In addition, poorer non-verbal communicative ability was associated with stronger connectivity between the PCC and the superior frontal gyri in addition to the right parahippocampal gyrus and temporal lobes. In our study of adults, no associations were noted between communicative ability and functional connectivity in the default network. Since the ASD subjects had overall weaker connectivity than controls, the finding that worse communicative ability was associated with stronger connectivity in the ASD sample is counter-intuitive. Replication of this finding is particularly important before inferences are made. Moreover, it is noteworthy that the patterns of connectivity associated with verbal and non-verbal communication were distinct from the patterns of connectivity related to social function. Social functioning and communication impairments often correlate in ASD. Thus, these initial findings suggest that although these symptoms correlate with one another, they may not share common brain patterns within the default network.
To summarize the findings of symptom severity and brain connectivity, across adolescence and adulthood, ASD social impairment was associated with weaker connectivity between the PCC and superior frontal gyrus. Beyond that, the findings were not consistent across the age groups. Overall, ASD symptom severity correlated with functional connectivity to a greater extent in the adolescents than the adults.
The precise function of the default network is unclear (Christoff et al. 2009; Gilbert et al. 2007; Mason et al. 2007). One explanation is that activation of the default network at rest represents intrinsic, physiological functioning of the nervous system. Evidence for this position comes from default network activity in nonhuman primates (Vincent et al. 2007) as well as humans during light sleep (Horovitz et al. 2008) and vegetative state (Boly et al. 2008). Another conceptualization is that the default network is related to current or recent cognitive processing, such as memory consolidation, mind wandering or comprehension. Support for this view comes from studies showing an association between activation in the default network and mind wandering (Mason et al. 2007; Christoff et al 2009) and evidence that default network functioning is influenced by demands of a language comprehension task (Hasson et al. 2009).
The present findings may be consistent with both views. Since we found that the default network is associated with diverse symptoms and other studies report that it is altered across a multitude of disorders (Castellanos et al. 2008; Garrity et al. 2007; Grimm et al. 2009; Sheline et al. 2009; Zhou et al. 2007), the default network may subserve fundamental processes that underlie many functions. This is consistent with the view that the default network reflects intrinsic physiological functioning. Alternatively, the present findings could indicate that the default network is affected by many different symptoms and conditions. This possibility is consistent with the view that the default network reflects current or recent mental events. For example, differences in the degree, quality or content of mind wandering may contribute to group differences in the present study. In typical individuals, mind wandering might involve imagining social situations that occurred in the past or are hypothetical. In ASD, such thoughts may be qualitatively different or the content might be less socially oriented. If the default network activation reflects thought processes, the present findings suggest that these inner thought processes differ in ASD and this difference is pronounced in adolescence.
Limitations
The limitations of this study are as follows. First, resting connectivity is vulnerable to noise as well as motion and physiological artifacts (cardiac and respiratory signals). However, in addition to performing physiological correction on the data, we covaried out the six head motion parameters in our regression analysis. This enabled us to ameliorate the effects of artifacts. Second, our sample size was relatively small (N=31). Therefore, more work is needed to ascertain if the pattern of results reported in this study can be replicated in future studies. Third, although all the participants in the ASD group met criteria for an autism spectrum diagnosis, they also had higher ratings of depression and attention problems. This is consistent with previous work that examined co-occurring symptoms of ASD (Ghaziuddin et al. 1992; Lainhart and Folstein 1994; Leyfer et al. 2006). Nevertheless, although the pattern of group differences in default network connectivity remained when depression and attention problems were controlled for by treating them as nuisance covariates, the present study is insufficiently powered to adequately examine this. Therefore, group differences in depression and attention problems may have contributed to the overall results. To effectively examine contributions of these types of covariates, it is necessary to collect large samples. The present work may help to provide justification for such an undertaking. Fourth, 10 of the 16 participants were on psychotropic medication. Although the use of psychotropic medication is extremely common in the ASD population (Oswald and Sonenklar 2007), they may influence the present results. To reduce the possibility that medications impacted our results, we carried out a follow-up analysis by excluding those participants who were on any psychotropic medication and the same pattern of results remained. However, use of medication also suggests other symptoms may be masked. Thus, further work is necessary to disentangle the roles of ASD, co-occurring symptoms and medication on resting connectivity. Fifth, since we only explored resting connectivity in 11 regions, this might have limited the scope of our study and prevented us from exploring other regions that showed group differences outside the default network.
Future directions
As stated above, future work is needed to examine if adolescence in normative development is marked by magnified differences in functional connectivity as compared to pre- and post adolescent periods. In addition, future studies may consider studying younger age ranges and include lower functioning individuals with ASD so as to obtain a more complete picture of connectivity during rest. This will help us to clarify how connectivity relates to symptoms severity. Furthermore, studies examining the distribution, density and properties of white matter tracts between regions in the default network through diffusion tensor imaging (DTI) techniques will help to elucidate the anatomical bases of the abnormal functional connectivity. Finally, the use of experience sampling techniques in conjunction with acquisition of default network activation (Christoff et al. 2009) would be useful for helping to identify what mental processes underlie this form of activation and how these processes might differ in those with ASD.
Conclusions
To our knowledge, this is the first study to explore resting connectivity within the default network selectively in adolescents with ASD. These findings extend previous reports of abnormal patterns of connectivity in ASD when participants are not engaged in a cognitive task. In this study, adolescents with ASD relative to controls showed weaker functional connectivity across nine of the eleven regions of the default network. Moreover, in the ASD group, poorer social functioning and more severe restricted and repetitive behaviors and interests were associated with weaker connectivity in the default network. Conversely, poorer verbal and non-verbal communicative ability was associated with stronger connectivity in the default network. Further clarification of how these core features of ASD relate to functional connectivity in the default network at different stages of development will be crucial to understanding brain correlates of ASD.
4. EXPERIMENTAL PROCEDURES
Participants
Twenty high functioning adolescents with ASD (IQ>85) and 17 healthy controls participated in the study. Due to movement that was greater than 1 voxel, 2 participants were removed (1 ASD and 1 control). In addition, 3 adolescents with ASD did not complete the resting connectivity scan due to discomfort. Lastly, 1 control was excluded due to technical complications that arose during data preprocessing. The final set consisted of 16 adolescents (14 males, 2 females) with ASD and 15 (14 males, 1 female) controls. Of the 16 adolescents with ASD, 6 were diagnosed with autism, 2 were diagnosed with Asperger’s syndrome and 8 were diagnosed with pervasive developmental disorder not otherwise specified (PDD-NOS). All of these conditions fall under the larger category of ASD and share deficits in the social domain that meet diagnostic criteria cutoffs. According to the DSM-IV, PDD-NOS differs from autism in that these individuals do not meet the criteria or cut-off for autism. Individuals with autism must have at least two deficits in the social domain together with at least one deficit in the communication, and one deficit in the restricted and repetitive domain for a total of six deficits overall. However, individuals with PDD-NOS need only have deficits in the social domain in the presence of communication and/or restricted and repetitive behaviors.
Adolescents with ASD were recruited through the University of Michigan Autism and Communication Disorders Center (UMACC) and received their diagnosis based on the Autism Diagnostic Observation Schedule (ADOS) (Lord et al. 2000), the Autism Diagnostic Interview-Revised (ADI-R) (Lord et al. 1994) and confirmed by clinical consensus. Verbal and non-verbal cognitive functioning was obtained by administering the Peabody Picture Vocabulary Test (Dunn and Dunn 1997), the Differential Ability Scales (DAS), the Wechsler Intelligence Scale for Children, the Stanford-Binet Intelligence Scales, or the Ravens Progressive Matrices (Raven 1960). Controls were recruited through posted flyers and advertisements, and were excluded if diagnosed with any mental or neurological condition.
For controls, verbal and non-verbal cognitive functioning was measured with the Peabody Picture Vocabulary Test (Dunn and Dunn 1997) and the Ravens Progressive Matrices (Raven 1960). Both the ASD and controls groups were also administered the Social Responsiveness Scale (SRS) (Constantino et al. 2003). The SRS is comprised of 65 items and each item is rated on a 4-point scale. Scores greater than 76 are in the severe range, 60–75 is in the mild to moderate range and 59 or less is considered to be normal. (Table 1 includes results from the SRS and ASD symptom scores from the ADI-R.) Each participant verbally indicated his/her handedness. In the ASD group, 10 of the 16 ASD participants were taking psychotropic medication (4 were on selective serotonin reuptake inhibitors, 7 were on stimulants, 3 were on neuroleptics, 1 was on atomoxetine and 1 was on an anticonvulsant). Post-hoc analysis was carried out by re-running the random effects analysis with the 6 unmedicated individuals with ASD and the 15 controls (all unmedicated), to determine if the pattern of group differences in activation remained when the subjects on medication were removed (see Results). There were no significant group differences in age, verbal cognitive functioning, gender, and handedness (Table 1). The ASD group had higher non-verbal cognitive functioning scores than the control group. Therefore, a post hoc analysis was carried out to determine if these scores contributed to group differences (see Results).
Procedures
The University of Michigan Institutional Review Board approved all procedures. All participants underwent an initial phone screening to ensure that none of the participants had surgeries in which metal was placed in the body. In addition, we did not recruit adolescents who wore braces as the metal can interfere with fMRI acquisition.
Participants were scheduled for an initial visit prior to the fMRI scan visit. During the first visit, parents signed consent forms and completed questionnaires and the adolescents signed assent forms and completed self-report questionnaires to gain a better understanding of overall functioning. Finally, participants were familiarized to the fMRI procedures by having them lie in a mock MRI for several minutes. During the second visit, participants underwent an fMRI scan at the University of Michigan fMRI Lab. During this visit, participants were screened for the presence of metal in their bodies prior to entering the MRI. The scan lasted approximately 45 minutes.
fMRI Data Acquisition
Participants lay supine in the fMRI scanner and wore glasses with built-in mirrors (VisuaStim XGA, Resonance Technologies) in order to view the projected stimuli inside the scanner. A black fixation cross on a white background was displayed in the center of the screen for 10 minutes. Participants were instructed to keep their eyes open and fixed on the cross. In addition, participants were told explicitly to “let their minds wander freely” and to not dwell on anything in particular. A pulse oximeter was attached to the participant’s finger in order to obtain their cardiac response. In addition, a pressure belt was worn around the participant’s abdomen in order to obtain their respiratory response. Both the cardiac and respiratory signals were synchronized to the fMRI data and were collected so that these physiological variations could be removed in a regression analysis (Glover et al. 2000).
Imaging was performed on a long bore 3T GE signa scanner operating on a 12.0 platform at the University of Michigan’s Functional MRI laboratory. A GE quad head coil was used. For the functional data, a total of 300 T2* weighed BOLD images were acquired using a reverse spiral sequence (Glover and Law 2001). Whole brain coverage was obtained with 40 contiguous 3mm axial slices (TR=2000 ms, TE=30 ms, flip angle=90°, FOV=22 cm, 64×64 matrix). Each slice was acquired parallel to the AC-PC line. Structural data included two T1 weighted images. The first was a 3D T1 axially acquired anatomical localizer 3D (TR=8.9, TE=1.8, flip angle=15°, FOV=26 cm, slice thickness=1.4 mm, 124 slices; matrix=256 ×160). The second was a sagitally acquired high-resolution spoiled gradient- recalled acquisition in steady state (SPGR) image (flip angle=15°, FOV=26cm, 1.4mm slice thickness, 110 slices).
Preprocessing of fMRI data
An initial series of preprocessing steps was carried out. First, we removed k-space outliers in raw data that were two standard deviations away from the mean and substituted them with the average value from neighboring voxels. Next, a field map correction was performed on the reconstructed images to remove the distortions that resulted from magnetic field inhomogenity. The variance due to physiological responses was removed using a regression analysis (Glover et al. 2000). The cardiac signal was not collected for one participant due to pulse oximeter malfunction. However, an examination of the standard deviation maps revealed that the susceptibility region for cardiac responses was in an area outside the default network and therefore, did not affect our data. The data were then slice-time corrected using local sinc interpolation (Oppenheim et al. 1999) and realigned using MCFLIRT in FSL (Jenkinson et al. 2002).
After initial preprocessing steps were carried out, the functional images were first examined to exclude cases with head movement greater than 1 voxel in any motion parameter. Additional preprocessing and image analysis were performed in SPM2 (Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk). First we coregistered the high-resolution T1 images to the functional images. Second, T1 images were normalized to the scalped T1 template in SPM2 and the functional volumes were normalized using a similar transformation matrix. Third, images were smoothed using an isotropic 8mm full- width-half maximum (FWHM) Gaussian kernel.
Data Analysis
A regression analysis was performed prior to generating the functional connectivity maps in order to reduce the noise related to movement. This was done by entering the 6 motion parameters as nuisance covariates for each individual subject. In order to create functional connectivity maps, the data were passed through three in-house batch scripts implemented in MATLAB 7.0 (The Mathworks Inc. Natick, MA). The first script was used to low-pass filter the data at 0.08 Hz to remove higher frequency sources of noise (Biswal et al. 1995). The second script, placed a seed region in the posterior cingulate cortex that was centered at −5 −53 41 (MNI). This seed region was employed following previous publications (Fox et al. 2005; Monk et al. 2009; Shulman et al. 1997). The seed region that was used in these studies were reported in Talairach-Tournoux space and corresponded to −5 −49 40. The BOLD timecourses from the 4-voxel square that was centered around the seed was averaged to form a single waveform. The third script then correlated this reference waveform with all other voxels to generate functional connectivity maps for each individual subject.
We performed a group-level random effects analysis in SPM2. For the within and between group analyses, we used a threshold of p < 0.05 small volume corrected for multiple comparisons within the entire default network using false discovery rate (FDR). The region of interest (ROI) was comprised of the whole default network. Following seminal studies (Fair et al. 2008; Fox et al. 2005), the default network ROI was defined as the aggregate of the bilateral retrosplenial/BA30; left lateral parietal/angular gyrus; right lateral parietal/angular gyrus; left medial prefrontal/BA32 and BA10 combined; right medial prefrontal/BA32 and BA10 combined; left superior frontal gyrus; right superior frontal gyrus; left temporal lobe; right temporal lobe; left parahippocampal gyrus and the right parahippocampal gyrus. These regions were defined from the WFU Pickatlas toolbox (http://www.fmri.wfubmc.edu/) (Maldjian et al. 2002).
Finally, in order to examine how symptom severity of core features related to functional connectivity, we focused on the eleven default network regions. The core domains that we used in the study were the amount of total reciprocal social interaction, communication deficits (verbal and non-verbal separately) and presence of restricted and repetitive behaviors and interests. These measures were obtained from the ADI-R scores (Ever). The ADI-R has also been used in several MRI studies (Boddaert et al. 2009; Hollander et al. 2005; Kleinhans et al. 2008). Since this analysis only focused on the ASD group, power was limited. Therefore, instead of using a threshold of p < 0.05 (FDR), a more lenient threshold was selected. Specifically, in order to control for multiple comparisons, we followed our previous work (Monk et al. 2009) and divided the p-value of 0.05 by the number regions within the default network (eleven), which yielded a corrected p-value of 0.0045.
Acknowledgments
This research was supported in part by an Autism Speaks grant (to C.S.M.) and the National Institutes of Health (U19 HD035482 to C.L. and MH066496 to C.L.). Drs. Lord and Risi receive royalties from a publisher of diagnostic instruments described in this paper. They give all profits generated by the University of Michigan Autism and Communication Disorders Center (UMACC) in regards to this paper and all other UMACC projects to a charity.
We thank the families who participated. We also thank Dr. D. Noll for methodological advice, and N. Kurapati, K. Newnham and C. Hammond for technical support
Footnotes
Data from this study were presented at the International Meeting for Autism Research, Chicago, IL, May 2009.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach TM, Edelbrock CS. Behavioral problems and competencies reported by parents of normal and disturbed children aged four through sixteen. Monogr Soc Res Child Dev. 1981;46:1–82. [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Boudos R, DuBray MB, Oakes TR, Miller JN, Lu J, Jeong EK, McMahon WM, Bigler ED, Lainhart JE. Diffusion tensor imaging of the corpus callosum in Autism. Neuroimage. 2007;34:61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry. 2004;55:323–6. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24:9228–31. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Boddaert N, Zilbovicius M, Philipe A, Robel L, Bourgeois M, Barthelemy C, Seidenwurm D, Meresse I, Laurier L, Desguerre I, Bahi-Buisson N, Brunelle F, Munnich A, Samson Y, Mouren MC, Chabane N. MRI findings in 77 children with non-syndromic autistic disorder. PLoS One. 2009;4:e4415. doi: 10.1371/journal.pone.0004415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M, Phillips C, Tshibanda L, Vanhaudenhuyse A, Schabus M, Dang-Vu TT, Moonen G, Hustinx R, Maquet P, Laureys S. Intrinsic brain activity in altered states of consciousness: how conscious is the default mode of brain function? Ann N Y Acad Sci. 2008;1129:119–29. doi: 10.1196/annals.1417.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Van Santen FW, Harasaki Y, Gitelman DR, Parrish TB, Marsel Mesulam MM. The development of specialized brain systems in reading and oral-language. Child Neuropsychol. 2001;7:119–41. doi: 10.1076/chin.7.3.119.8740. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Burnett S, Blakemore SJ. Functional connectivity during a social emotion task in adolescents and in adults. Eur J Neurosci. 2009;29:1294–301. doi: 10.1111/j.1460-9568.2009.06674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, van Kooten IA, Switala AE, van Engeland H, Heinsen H, Steinbusch HW, Hof PR, Trippe J, Stone J, Schmitz C. Minicolumnar abnormalities in autism. Acta Neuropathol. 2006;112:287–303. doi: 10.1007/s00401-006-0085-5. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, Shehzad Z, Di Martino A, Biswal B, Sonuga-Barke EJ, Rotrosen J, Adler LA, Milham MP. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–7. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17:1687–90. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci U S A. 2009;106:8719–24. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, Metzger LM, Shoushtari CS, Splinter R, Reich W. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord. 2003;33:427–33. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody Picture Vocabulary Test. Circle Pines, MN: American Guidance Services; 1997. [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. The maturing architecture of the brain’s default network. Proc Natl Acad Sci U S A. 2008;105:4028–32. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A. 2007;104:13507–12. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage. 2008;42:1178–84. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–7. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- Ghaziuddin M, Tsai L, Ghaziuddin N. Comorbidity of autistic disorders in children and adolescents. Eur Child Adolesc Psychiatry. 1992;1:209–213. doi: 10.1007/BF02094180. [DOI] [PubMed] [Google Scholar]
- Giedd JN. The teen brain: insights from neuroimaging. J Adolesc Health. 2008;42:335–43. doi: 10.1016/j.jadohealth.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Dumontheil I, Simons JS, Frith CD, Burgess PW. Comment on “Wandering minds: the default network and stimulus-independent thought”. Science. 2007;317(43) doi: 10.1126/science.317.5834.43. author reply 43. [DOI] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46:515–22. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44:162–7. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–8. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Boesiger P, Beck J, Schuepbach D, Bermpohl F, Walter M, Ernst J, Hell D, Boeker H, Northoff G. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology. 2009;34:932–843. doi: 10.1038/npp.2008.81. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, Fromm SJ, Leibenluft E, Pine DS, Ernst M. A developmental examination of amygdala response to facial expressions. J Cogn Neurosci. 2008;20:1565–82. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Nusbaum HC, Small SL. Task-dependent organization of brain regions active during rest. Proc Natl Acad Sci U S A. 2009;106:10841–6. doi: 10.1073/pnas.0903253106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, O’Brien LM, Lange N, Bakardjiev A, Hodgson J, Adrien KT, Steele S, Makris N, Kennedy D, Harris GJ, Caviness VS., Jr Dissociations of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys. Brain. 2003;126:1182–92. doi: 10.1093/brain/awg110. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, Filipek PA, Kemper TL, Normandin JJ, Sanders HA, Kennedy DN, Caviness VS., Jr Localization of white matter volume increase in autism and developmental language disorder. Ann Neurol. 2004;55:530–40. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- Hollander E, Anagnostou E, Chaplin W, Esposito K, Haznedar MM, Licalzi E, Wasserman S, Soorya L, Buchsbaum M. Striatal volume on magnetic resonance imaging and repetitive behaviors in autism. Biol Psychiatry. 2005;58:226–32. doi: 10.1016/j.biopsych.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Horovitz SG, Fukunaga M, de Zwart JA, van Gelderen P, Fulton SC, Balkin TJ, Duyn JH. Low frequency BOLD fluctuations during resting wakefulness and light sleep: a simultaneous EEG-fMRI study. Hum Brain Mapp. 2008;29:671–82. doi: 10.1002/hbm.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cereb Cortex. 2007;17:951–61. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129:2484–93. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008;39:1877–85. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, Greenson J, Dawson G, Aylward E. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131:1000–12. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- Koch MA, Norris DG, Hund-Georgiadis M. An investigation of functional and anatomical connectivity using magnetic resonance imaging. Neuroimage. 2002;16:241–50. doi: 10.1006/nimg.2001.1052. [DOI] [PubMed] [Google Scholar]
- Koshino H, Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. fMRI investigation of working memory for faces in autism: visual coding and underconnectivity with frontal areas. Cereb Cortex. 2008;18:289–300. doi: 10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M. Children’s Depression Inventory (CDI) Manual. North Tonawanda, NY: Multi-Health Systems; 1992. [Google Scholar]
- Lainhart JE, Folstein SE. Affective disorders in people with autism: a review of published cases. J Autism Dev Disord. 1994;24:587–601. doi: 10.1007/BF02172140. [DOI] [PubMed] [Google Scholar]
- Laughlin SB, Sejnowski TJ. Communication in neuronal networks. Science. 2003;301:1870–4. doi: 10.1126/science.1089662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J, Tager-Flusberg H, Lainhart JE. Comorbid Psychiatric Disorders in Children with Autism: Interview Development and Rates of Disorders. J Autism Dev Disord. 2006 doi: 10.1007/s10803-006-0123-0. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–23. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Maldjian J, Laurienti P, Burdette J, Kraft R. An Automated Method for Neuroanatomic and Cytoarchitectonic Atlas-based Interrogation of fMRI Data Sets. NeuroImage. 2002;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–5. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno A, Villalobos ME, Davies MM, Dahl BC, Muller RA. Partially enhanced thalamocortical functional connectivity in autism. Brain Res. 2006;1104:160–74. doi: 10.1016/j.brainres.2006.05.064. [DOI] [PubMed] [Google Scholar]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, Charney DS, Ernst M, Pine DS. Adolescent Immaturity in Attention-Related Brain Engagement to Emotional Facial Expressions. Neuroimage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Monk CS, Peltier SJ, Wiggins JL, Weng SJ, Carrasco M, Risi S, Lord C. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage. 2009;47:764–772. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol Med. 2005;35:163–74. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Noonan SK, Haist F, Muller RA. Aberrant functional connectivity in autism: evidence from low-frequency BOLD signal fluctuations. Brain Res. 2009;1262:48–63. doi: 10.1016/j.brainres.2008.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim A, Schafer R, Buck J. Discrete-time signal processing. Upper Saddle River, NJ: Prentice Hall; 1999. [Google Scholar]
- Oswald DP, Sonenklar NA. Medication use among children with autism spectrum disorders. J Child Adolesc Psychopharmacol. 2007;17:348–55. doi: 10.1089/cap.2006.17303. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–76. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1083–90. doi: 10.1016/j.neuroimage.2007.02.041. discussion 1097–9. [DOI] [PubMed] [Google Scholar]
- Raven JC. Guide to using the Standard Progressive Matrices. London, UK: Lewis; 1960. [Google Scholar]
- Russell E, Sofronoff K. Anxiety and social worries in children with Asperger syndrome. Aust N Z J Psychiatry. 2005;39:633–8. doi: 10.1080/j.1440-1614.2005.01637.x. [DOI] [PubMed] [Google Scholar]
- Seltzer MM, Krauss MW, Shattuck PT, Orsmond G, Swe A, Lord C. The symptoms of autism spectrum disorders in adolescence and adulthood. J Autism Dev Disord. 2003;33:565–81. doi: 10.1023/b:jadd.0000005995.02453.0b. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009;106:1942–7. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. Journal of Cognitive Neuroscience. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Sofronoff K, Attwood T, Hinton S. A randomised controlled trial of a CBT intervention for anxiety in children with Asperger syndrome. J Child Psychol Psychiatry. 2005;46:1152–60. doi: 10.1111/j.1469-7610.2005.00411.x. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–15. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Spence SH. Structure of anxiety symptoms among children: a confirmatory factor-analytic study. J Abnorm Psychol. 1997;106:280–97. doi: 10.1037//0021-843x.106.2.280. [DOI] [PubMed] [Google Scholar]
- Stevens MC, Pearlson GD, Calhoun VD. Changes in the interaction of resting-state neural networks from adolescence to adulthood. Hum Brain Mapp. 2009;30:2356–66. doi: 10.1002/hbm.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Pearlson GD, Calhoun VD. Changes in the interaction of resting-state neural networks from adolescence to adulthood. Hum Brain Mapp. 2009 doi: 10.1002/hbm.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLoS Biol. 2009;7:e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, Casey BJ. Amygdala response to facial expressions in children and adults. Biol Psychiatry. 2001;49:309–16. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Chang CE, Glover GH, Gabrieli JD, Greicius MD, Gotlib IH. Default-mode function and task-induced deactivation have overlapping brain substrates in children. Neuroimage. 2008;41:1493–503. doi: 10.1016/j.neuroimage.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner KC, Frost L, Linsenbardt D, McIlroy JR, Muller RA. Atypically diffuse functional connectivity between caudate nuclei and cerebral cortex in autism. Behav Brain Funct. 2006;2:34. doi: 10.1186/1744-9081-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos ME, Mizuno A, Dahl BC, Kemmotsu N, Muller RA. Reduced functional connectivity between V1 and inferior frontal cortex associated with visuomotor performance in autism. Neuroimage. 2005;25:916–25. doi: 10.1016/j.neuroimage.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–6. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Welchew DE, Ashwin C, Berkouk K, Salvador R, Suckling J, Baron-Cohen S, Bullmore E. Functional disconnectivity of the medial temporal lobe in Asperger’s syndrome. Biol Psychiatry. 2005;57:991–8. doi: 10.1016/j.biopsych.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Wicker B, Fonlupt P, Hubert B, Tardif C, Gepner B, Deruelle C. Abnormal cerebral effective connectivity during explicit emotional processing in adults with autism spectrum disorder. Soc Cogn Affect Neurosci. 2008;3:135–43. doi: 10.1093/scan/nsn007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Tian L, Wang K, Hao Y, Liu H, Liu Z, Jiang T. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res. 2007;97:194–205. doi: 10.1016/j.schres.2007.05.029. [DOI] [PubMed] [Google Scholar]