Abstract
Wnt5A has been implicated in melanoma metastasis, and the progression of other cancers including pancreatic, gastric, prostate and lung cancers. Assays to test motility and invasion include both in vivo assays, and in vitro assays. The former assays include the use of tail vein or footpad injections of metastatic cells, and are often laborious and expensive. In vitro invasion assays provide quick readouts that can help to establish conditions that either activate or inhibit melanoma cell motility, and to assess whether the conditions in question are worth translating into an in vivo model. Here we describe two standard methods for assaying motility and invasion in vitro including wound healing assays and Matrigel invasion assays (Boyden chamber assays). In addition, we and several other laboratories have previously shown that melanoma cells require MMP-2 for their invasion, and have recently shown that Wnt5A treatment can increase the levels of this enzyme in melanoma cells, as demonstrated by gelatin zymography. The use of these techniques can help to assess the migratory capacity of melanoma cells in response to Wnt treatment.
Keywords: Melanoma, Wnt5A, Matrigel, Boyden chamber, invasion, wound-healing assays
1. Introduction
Tumor cell invasion occurs via three steps: Attachment of cells to the basement membrane, proteolytic dissolution of the basement membrane, and finally movement through it, into the bloodstream (intravasation). Extravasation at the site of distant metastasis occurs via the homing of tumor cells due to the expression both on tumor and endothelial cell of molecules such as CD44, and the release of proteolytic enzymes such as the matrixmetalloproteinases, which allow the tumor cells to digest their way out of the blood vessels into distant organs (1). This complex process is governed by a delicate balance between angiogenic or pro-metastatic factors, such as VEGF, and CD44 (2, 3), and metastasis suppressors such as Kiss-1 and nm-23 (4), and between matrix metalloproteinases, and inhibitors of these enzymes (5, 6), or tissue inhibitors of metalloproteinases (TIMPs).
We have previously demonstrated that Wnt5A signaling can increase the invasive ability of melanoma cells, and the increased expression of Wnt5A correlates to increased malignancy in melanoma patients. A recent study from our lab has indicated that Wnt5A is able to mediate these effects via the increase of tumor-associated antigens such as CD44, and the decrease of metastasis suppressors such as Kiss-1 (7). Using recombinant Wnt5A we are able to increase the motility of non-invasive melanoma cells, upregulate the expression of CD44 and downregulate the expression of Kiss-1. Conversely, we can inhibit the invasion of highly invasive melanoma cells using siRNA against Wnt5A (7) and Figure 1A, downregulate the expression of CD44, and upregulate the expression of Kiss-1, indicating that Wnt5A is able to regulate the metastatic phenotype of melanoma cells. These effects can be mimicked by regulating PKC, where, for example, inhibiting PKC in highly metastatic cells can inhibit motility in a wound healing assay (Figure 1B). Other laboratories have confirmed the importance of Wnt5A in melanoma progression (8–10), and Wnt5A has also been implicated in the invasion of several other cancers, including pancreatic cancer (11), gastric cancer (12), prostate cancer (13) and non-small cell lung cancer (14). Interestingly, in some cancers such as breast cancer, and colon cancer, increased Wnt5A expression signifies a positive outcome for patients (15, 16), indicating that Wnt5A may be acting as tumor suppressor in these patients. Here, we provide detailed protocols for assaying the invasion potential of cancer cells, including wound-healing assays, Matrigel (Boyden Chamber) invasion assays, and zymography.
Figure 1. Inhibiting PKC and Wnt5A in melanoma cells results in an inhibition of melanoma cell motility in a wound-healing assay.
A. UACC647 melanoma cells (highly invasive, high Wnt5A) were treated with either a vehicle control or a PKC inhibitor, and subjected to a wound-healing assay. 24 hours post-treatment, vehicle controls had healed the wound, while PKC-inhibited cells could not. B. Treating highly invasive Wnt5A-high UACC903 melanoma cells with an siRNA against Wnt5A results in a decrease in the ability of these cells to close a wound.
2. Materials
2.1. Scratch Assay
-
2.1.1
Collagen IV and Fibronectin coated 24 well plates or slide chambers (BD Biosciences, San Jose CA). See Note 1.
-
2.1.2
200μl yellow p200 tips.
-
2.1.3
Light microscope with imaging capabilities.
2.2. Matrigel Invasion Assay
-
2.2.1
Fluoroblok HTS transwell filters, 12mm, either 3 micron, or 8 micron, available from Fisher Scientific (Rocehster, NY), depending on the size of the cell. Nuclear diameter is the most critical measurement and must be smaller than the pore size selected.
-
2.2.2
Reconstituted basement membrane (Matrigel ®), from BD Biosciences (San Jose, CA).
-
2.2.3
Phosphate buffered saline (PBS) pH 7.4.
-
2.2.4
24-well tissue culture plates.
-
2.2.5
Calcein-AM (Molecular Probes, Salem, OR).
2.3. Gelatin Zymography
-
2.3.1
10% Tris-Glycine gel with 0.1% gelatin (Invitrogen, Carlsbad, CA).
-
2.3.2
4X Zymogram Sample Buffer (To make 50mLs add: 6.25mLs of 2M Tris.Cl pH 6.8, 25mLs of glycerol, 5g of SDS, 2.5mLs of beta-mercaptoethanol and 5 mgs of Bromophenol Blue).
-
2.3.3
10X Zymogram Developing Buffer (BioRad, Hercules, CA).
-
2.3.4
10X Zymogram Renaturing Buffer (BioRad, Hercules, CA).
-
2.3.5
Coomassie Blue Stain (10% glacial acetic acid, 30% Methanol, 0.25% Coomassie Brilliant Blue).
-
2.3.6
Destaining solution (10% glacial acetic acid, 10% Methanol).
-
2.3.7
Recombinant MMP-2 (R&D Systems, Minneaopolis, MN).
3. Methods
3.1 Wound Healing Assays
-
3.1.1
Transfect or treat cells as described in Chapter, Section 3.1.2. Briefly, seed melanoma at 0.5×105 in fibronectin or collagen IV 24 well plates and grow to 60% confluence for transfection, or 80% confluence for treatment (see Note 2).
-
3.1.2
Once the culture has reached complete confluency, seal a 200μl plastic pipette tip using a blue flame (see Note 3). Take the cooled pipette tip, and make two scratches in each well, one horizontal and one vertical (see Note 4, 5).
-
3.1.3
Allow the cells to sit for 30 minutes in the incubator and then begin imaging, treating first image as 0h (Figure 1B). Wounds can heal in as little as 12–24 hours for highly metastatic cells, or may take up to 72 hours for less metastatic cells (see Note 6).
-
3.1.4
Using the intersection of the two lines, select a spot to follow. Take images of the same field at 0, 12, 24, 36, 48, and 72 hours using phase contrast light microscopy. It is advisable to select one spot to follow, but to also assess the overall rate of closure of the entire wound. To do this we image at both 2.5X and 10X.
3.2. Matrigel Invasion Assays
These in vitro invasion assays, also known as Boyden chamber assays provide an assessment of the three basic steps of tumor cell metastasis- attachment to a basement membrane, proteolytic dissolution of this membrane, and migration through it. Older versions of this assay required that researchers remove the filter from the chamber, scrape off the Matrigel, stain the filter and assess the number of cells left on the underside of the filter. This method was highly prone to human error. The advent of new filters (Fluorblok ®) that have patented membranes which do not permit fluorescence to pass through them allow for a much more accurate assessment of this process, as cells can be stained with fluorescent markers such as Calcein-AM. The amount of fluorescence that accumulates in the bottom chamber due to migrating cells can then be assessed using a fluorescent plate reader and assays can be followed in real time, in a more quantitative fashion. An added benefit of using Calcein-AM is that it requires a cell to be alive and actively cleaving the ester (AM) in order to produce fluorescence.
-
3.2.1
Two days prior to the assay, place filters, Matrigel, PBS pH 7.4 and pipette tips at 4°C to chill.
-
3.2.2
The day before the assay take the filters out of the 4°C, and place them immediately on ice- be sure to keep them either in their plastic container, or place them immediately in a 24-well dish that you will use for the assay. Place pipette tips on parafilm on ice (See Note 7).
-
3.2.3
Dilute the Matrigel to 80μg/mL in ice-cold PBS. Use ice-cold tips. Pipette in the Matrigel first, then use a small volume 200μL or less to really resuspend it before adding the rest of the PBS- make sure it is completely dissolved.
-
3.2.4
Pipette 150μL of Matrigel (80μg/mL) onto each filter- be careful not to touch the tip to the filter- get rid of air bubbles in the solution, by lightly running the tip over the solution (See Note 8).
-
3.2.5
Place filters at 37°C for 2 hours, then allow to dry overnight in the tissue culture hood. Examine filters for even dispersement of the Matrigel. Dispersement is even if the surface looks smooth and slightly cloudy, with no dark holes or gaps in the cloudy layer. Filters can be stored at 4°C for up to one week after this. Make enough filters for the assay, with a few extra in case of uneven coating (see Note 9).
-
3.2.6
Place cells in serum-free or low serum the day before the assay (so that they have been exposed to low serum for 16 hours by the time of assay). This allows them to respond better to the chemoattractant and also allows them to cycle together. Treat or transfect the cells such that the ideal time point for the treatment coincides with the start of the assay.
-
3.2.7
Two hours prior to commencing the assay place cells in Calcein-AM. Determine to which concentration of Calcein-AM the cells in question best respond. Typically it is a good idea to test a range of Calcein-AM from 2μM to 10μM and time ranging from 45 minutes to 2 hours. A typical concentration is 5μM for 1 hour for melanoma cells (see Note 10).
-
3.2.8
Place 800μL of chemoattractant in the bottom of a 24 well plate- this chemoattractant can vary, e.g., Conditioned media from 3T3 cells, media containing chemokines, etc-for basic assessments of invasion, we use standard culture media with 20% FBS.
-
3.2.9
Gently place the transwell chamber with the filter on top of this solution (in the well) - again make sure there are no air bubbles. Overlay the filter with 150μL of serum-free or low-serum containing medium– the same solution that the cells were placed in the night before medium. Place in the 37°C incubator until you are ready to add cells to the filter. At least 30 minutes is required for efficient reconstitution of the Matrigel.
-
3.2.10
After incubation in Calcein-AM, count the cells, and seed 50,000 cells per filter. Bring the volume of cells in the transwell chamber up to 800μL using no/low serum medium (this takes into account the 150μL that is already in the chamber from the Matrigel reconstitution).
-
3.2.11
Allow to sit for 15 minutes at 37°C, then take the first reading. We use a Cytofluor-4000 from Perkin-Elmer, but any bottom reading fluorescence reader will do. Use an excitation/emission of 480/530 and a gain of 50, using bottom read fluorescence only (see Note 11).
-
3.2.12
Take readings every hour for four to six hours. Invasion can be assessed by subtracting the average of the first read for each condition from the last read. Background fluorescence can be assessed and subtracted from these measurements using a well with only the chemoattractant media in it. Results can be measured in one of three ways-A) as a fold increase over time zero for each condition. B) As a fold increase over a control sample (e.g., the least invasive cell line) or C) The amount of fluorescence/cell can be quantitated by plating a serial dilution of Calcein-AM labelled cells directly into the bottom well, and calculating fluorescence units per cell. However, due to the fact that the levels of Calcein-AM appear to change over the 6 hour period, perhaps due to breakdown within the cells, we find method B to be the most informative when assessing changes in invasion. A bar graph is usually the best way to represent the results.
3.3. Zymography
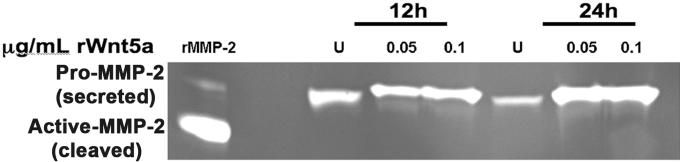
We, and others have demonstrated the importance of MMP-2 in melanoma cell motility (17, 18). We have also shown that Wnt5A treatment increases MMP-2 secretion in melanoma cells with low motility, and also increases their invasion in a scratch assay (7) and Figure 2. Assaying increases in MMP-2 secretion, or inhibition of MMP-2 is another method to assess changes in the invasive ability of melanoma cells and is assyed using zymography. Zymography can be used to detect and characterize metalloproteinases, collagenases, and various other proteases. The type and quantity of metalloproteinases expressed by tumor cells may allow determination of the metastatic potential of the tumor. Areas of proteolytic activity appear as clear bands against a dark blue background due to proteolytic digestion of the gelatin protein (see Note 12).
-
3.3.1
Mix one part sample (conditioned medium from cells- see Notes13, 14- with three parts Zymogram Sample Buffer (4x) and let stand 10 minutes at room temperature. Do not heat.
-
3.3.2
Apply samples (typically 10–25μl containing 30–50μg) and run the gel with 1x Tris-Glycine SDS Running Buffer according to the following running conditions: Voltage: 125V constant, Run time: Approximately 90 minutes (see Note 15).
-
3.3.3
After running, dilute the Zymogram Renaturing Buffer (10X) 1:9 and incubate the gel in the buffer (100mL for 2 gels) with gentle agitation for 30 minutes at room temperature.
-
3.3.4
Decant the Zymogram Renaturing Buffer and replace with 1X Zymogram Developing Buffer (100mL for 2 gels). Equilibrate the gel for 30 minutes at room temperature with gentle agitation then replace with fresh 1X Zymogram Developing Buffer and incubate at 37°C for at least 4 hours (see Note 16). The optimal result can be determined empirically by varying the sample load or incubation time.
-
3.3.5
Stain with Coomassie Blue solution for 30 minutes to1 hour. Gels should be destained with Destaining solution. Change destain every 30 minutes for the first 2 hours. Areas of protease activity will show up as clear bands. Stained gels can be wrapped in plastic and stored at 4°C for several months.
Figure 2. Treatment of melanoma cells with recombinant Wnt5A increases MMP-2 secretion as determined by gelatin zymography.
Wnt5A-low UACC1273EV and G361 cells were treated with recombinant Wnt5A at different concentrations, and subjected to gelatin zymography. Wnt5A treatment increases the secretion of MMP-2.
Acknowledgements
We thank Dr. Michel Bernier and Dr. Paritosh Ghosh for helpful comments on this manuscript. Any data represented in this chapter was generated with the support of funds from the Intramural Research Program of the National Institute on Aging, Baltimore, MD.
Footnotes
The type of plate used will affect the motile and invasive capability of the cells. We use plates that have some sort of a matrix on them, usually Collagen IV or fibronectin. The researcher should be aware that the same cell line may exhibit marked differences between the different matrices, depending most likely upon their integrin profile. Matrigel plates cannot be used as a scratch cannot be performed without disrupting the gel. Further, some cells are not suitable for the scratch assay as, once the scratch has been made, the entire layer of cells peels off. Other cells are not suitable because they cannot form a confluent monolayer. Obviously, this assay can only be used for adherent cells. Therefore, when performing a scratch assay a range of plates need to be tested for the cell line being used.
Because cells have to be completely confluent for the assay, plan transfections and experiments very carefully around the doubling time of each specific cell. We transfect siRNAs at a slightly higher cell confluency because transfection is still efficient at higher confluencies. Wounds can then be inflicted within 24 hours onto a confluent monolayer. Transfecting DNA is slightly more tricky, as a lower confluency is required for efficient transfection but a confluent monolayer is required for an efficient assay, so these conditions should be optimized for each cell line.
The sealing of the tip needs to be complete and clean. When sealing the tip, it needs to be placed very briefly in the blue part of the flame and a complete seal has occurred once the end of the tip is smooth and round. The exposure to the flame must be short but efficient-an overly long exposure will cause bubbling of the tip, resulting in an overly thick tip, which will inflict too wide a scratch. Overexposure to the flame, or exposure to the yellow part of the flame will also result in charring (evident by a smoky residue on the tips), which we find causes cell death around the scratch, perhaps due to toxins that are released when the plastic burns. It is imperative to attempt to just lightly seal the tip, as that will keep the size of the tips consistent, such that scratches do not vary widely in width. A tip that is not completely sealed will result in cells left behind in the center of the scratch. Although images will still be able to be taken whilst the scratch is open, once it begins to close an inaccurate result may develop as the scratch may appear to be closed due to the unscratched cells left in the center of the scratch.
The pressure applied to make the scratch must be firm and even across the entire well. A scratch that has been made using too little pressure results in live cells left in the center of the scratch and leads to the problems associated with this, as mentioned above. A scratch made using too much pressure will scrape the bottom of the well and leave grooves in the matrix. This can be visualised under the microscope as large black lines seen in the middle of the scratch. Often when this occurs the cells take longer to migrate across these grooves, causing an inaccurate result, although highly metastatic cells may remain unaffected by this.
It is important to make two scratches in each well, one horizontal and one vertical. This results in a cross that can be seen under the microscope and helps orient the investigator to the same field. The same field must be used when taking images as the size of the scratch will vary slightly across the well. Therefore, choosing a similar size scratch across samples and using the same field for imaging is very important. Often cells may die around the scratch, leaving some areas less confluent, which will affect the ability of the cells to invade. Thus, even though one area is followed, it is important to assess invasion across the entire scratch, to get an idea of whether that portion of the scratch is truly representative of the whole field.
The media level used must be sufficient to last the entire duration of the wound-healing assay. Changes of media can disrupt the cells at the edges of the scratch leaving debris and causing an inaccurate measure of invasiveness. Therefore, if using a 24 well plate, add at least 1ml of media.
It is crucial to keep everything ice-cold for the Matrigel invasion assay. We suggest pre-chilling tips, filters etc, and using parafilm laid over ice in a ice-bucket in the hood as a surface on which to place filters, tips etc.
A significant source of air bubbles can be avoided by not ejecting the solution out all the way, just to the end of the first ejection.
For each condition you are testing it is advisable to do triplicates in each experiment, and repeat each invasion assay three times, as the standard error bars can be quite large.
It is advisable to do a trypan blue viability assay to ensure that Calcein-AM is not affecting the viability of the cells.
If you do not have a bottom-reading fluorescent reader, it is possible to get data by removing the transwell, and assaying only the plate. This will count the fluorescent cells that have migrated all the way through, although not the ones that have migrated through the filter, but are still attached to the underside. Cell counts will be smaller but still representative. This can also be done if Fluorblok filters are not readily available and regular transwell migration chambers are used.
As an added confirmation to zymography we also stain for cell-associated MMP-2, using goat-anti-MMP-2 (R&D Systems), and assess membrane associated MMP-2 using confocal microscopy. For examples, see Leotlela et al, 2006, and Dissanayake et al, 2007 (7, 18).
It is advisable to use serum-free medium where possible, and to concentrate the medium.
Phenol-red free medium should be used as Phenol-Red can interfere with colorimetric quantitation in a BCA assay.
Because the gels used are so small, make sure to run them for an extended period of time to fully separate the secreted and active band for better visualization.
Many protocols state that zymograms can be developed for four hours: we find this to be far too short a period of time for most cells examined.
References
- 1.Fidler IJ. Critical determinants of metastasis. Semin Cancer Biol. 2002;12:89–96. doi: 10.1006/scbi.2001.0416. [DOI] [PubMed] [Google Scholar]
- 2.Bourguignon LY. CD44-mediated oncogenic signaling and cytoskeleton activation during mammary tumor progression. J Mammary Gland Biol Neoplasia. 2001;6:287–97. doi: 10.1023/a:1011371523994. [DOI] [PubMed] [Google Scholar]
- 3.Agnantis NJ, Goussia AC, Batistatou A, Stefanou D. Tumor markers in cancer patients. an update of their prognostic significance. Part II. In Vivo. 2004;18:481–8. [PubMed] [Google Scholar]
- 4.Steeg PS, Ouatas T, Halverson D, Palmieri D, Salerno M. Metastasis suppressor genes: basic biology and potential clinical use. Clin Breast Cancer. 2003;4:51–62. doi: 10.3816/cbc.2003.n.012. [DOI] [PubMed] [Google Scholar]
- 5.Hofmann UB, Westphal JR, Van Muijen GN, Ruiter DJ. Matrix metalloproteinases in human melanoma. J Invest Dermatol. 2000;115:337–44. doi: 10.1046/j.1523-1747.2000.00068.x. [DOI] [PubMed] [Google Scholar]
- 6.Hofmann UB, Houben R, Brocker EB, Becker JC. Role of matrix metalloproteinases in melanoma cell invasion. Biochimie. 2005;87:307–14. doi: 10.1016/j.biochi.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Dissanayake SK, Wade MS, Johnson CE, O'Connell MP, Leotlela PD, A.D. F, et al. The Wnt5a/Pkc Pathway Mediates Motility In Melanoma Cells Via The Inhibition Of Metastasis Suppressors, And Initiation Of An Epithelial To Mesenchymal Transition. J Biol Chem. 2007 doi: 10.1074/jbc.M700075200. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis TB, Robison JE, Bastien R, Milash B, Boucher K, Samlowski WE, et al. Molecular classification of melanoma using real-time quantitative reverse transcriptase-polymerase chain reaction. Cancer. 2005;104:1678–86. doi: 10.1002/cncr.21372. [DOI] [PubMed] [Google Scholar]
- 9.Bachmann IM, Straume O, Puntervoll HE, Kalvenes MB, Akslen LA. Importance of P-cadherin, beta-catenin, and Wnt5a/frizzled for progression of melanocytic tumors and prognosis in cutaneous melanoma. Clin Cancer Res. 2005;11:8606–14. doi: 10.1158/1078-0432.CCR-05-0011. [DOI] [PubMed] [Google Scholar]
- 10.Rodolfo M, Daniotti M, Vallacchi V. Genetic progression of metastatic melanoma. Cancer Lett. 2004;214:133–47. doi: 10.1016/j.canlet.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 11.Ripka S, Konig A, Buchholz M, Wagner M, Sipos B, Kloppel G, et al. WNT5A - target of CUTL1 and potent modulator of tumor cell migration and invasion in pancreatic cancer. Carcinogenesis. 2007 doi: 10.1093/carcin/bgl255. [DOI] [PubMed] [Google Scholar]
- 12.Kurayoshi M, Oue N, Yamamoto H, Kishida M, Inoue A, Asahara T, et al. Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res. 2006;66:10439–48. doi: 10.1158/0008-5472.CAN-06-2359. [DOI] [PubMed] [Google Scholar]
- 13.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–25. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 14.Huang CL, Liu D, Nakano J, Ishikawa S, Kontani K, Yokomise H, et al. Wnt5a expression is associated with the tumor proliferation and the stromal vascular endothelial growth factor--an expression in non-small-cell lung cancer. J Clin Oncol. 2005;23:8765–73. doi: 10.1200/JCO.2005.02.2871. [DOI] [PubMed] [Google Scholar]
- 15.Dejmek J, Dejmek A, Safholm A, Sjolander A, Andersson T. Wnt-5a protein expression in primary dukes B colon cancers identifies a subgroup of patients with good prognosis. Cancer Res. 2005;65:9142–6. doi: 10.1158/0008-5472.CAN-05-1710. [DOI] [PubMed] [Google Scholar]
- 16.Jonsson M, Dejmek J, Bendahl PO, Andersson T. Loss of Wnt-5a protein is associated with early relapse in invasive ductal breast carcinomas. Cancer Res. 2002;62:409–16. [PubMed] [Google Scholar]
- 17.Bartolome RA, Molina-Ortiz I, Samaniego R, Sanchez-Mateos P, Bustelo XR, Teixido J. Activation of Vav/Rho GTPase signaling by CXCL12 controls membrane-type matrix metalloproteinase-dependent melanoma cell invasion. Cancer Res. 2006;66:248–58. doi: 10.1158/0008-5472.CAN-05-2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leotlela PD, Wade MS, Duray PH, Rhode MJ, Brown HF, Rosenthal DT, et al. Claudin-1 overexpression in melanoma is regulated by PKC and contributes to melanoma cell motility. Oncogene. 2006 doi: 10.1038/sj.onc.1210155. epub ahead of print. [DOI] [PubMed] [Google Scholar]