Abstract
Purpose
The prevalences of diabetes, hypertension, and high cholesterol all decrease with increased levels of physical activity and cardiorespiratory fitness. Whether these reductions extend beyond contemporary guideline activity levels and whether fitness affects medication use independent of activity, remains unclear.
Methods
Cross-sectional analyses of 62,291 male and 45,041 female runners, of whom 496 used antidiabetic, 3738 used antihypertension, and 2360 used low-density lipoprotein cholesterol (LDL-C)–lowering medications. Cardiorespiratory fitness was reported as speed (m/s) during a 10-km foot race.
Results
Medication use was significantly inversely associated with activity and fitness (P < 0.001, except LDL-C–lowering versus women’s fitness). Compared with ≤ 16 km/wk (guideline levels), the odds in men and women who ran > 64 km/wk were, respectively, 69% and 55% lower for antidiabetic, 48% and 52% lower for antihypertension, and 64% and 51% lower for LDL-C–lowering medication use. Compared with the least-fit men (< 3.25 m/s) and women (< 2.8 m/s), the odds for those who were most fit (men > 4.75 m/s; women > 4.0 m/s) were 58% and 65% lower for antidiabetic, and 76% and 55% lower for antihypertensive medication use. Odds for LDL-C–lowering medication use was 87% lower in the fittest versus the least-fit men. Adjustment for activity only moderately diminished the inverse relationships of fitness with medication use.
Conclusion
Among individuals who exceed current guideline levels, antidiabetic, antihypertension, and LDL-C–lowering medications are inversely related to vigorous physical activity and cardiorespiratory fitness. Lower odds of medication use with higher fitness occur independently of physical activity.
INTRODUCTION
Nearly 100 million American adults have total blood cholesterol values ≥ 200 mg/dL, and among these about 34.5 million have levels ≥ 240 mg/dL [16]. Hypertension affects approximately 50 million U.S. adults [5]. The risk of becoming hypertensive increases with age, such that the lifetime risk of becoming hypertensive exceeds 80% [5]. More than one half of those 60–69 yr old and three quarters of those ≥ 70 are hypertensive [5]. Approximately 18 million Americans are estimated to have diabetes, and this number is expected to increase to 30 million by 2030 [10,15,17].
Clinical trials have demonstrated a 30% reduction in coronary heart disease (CHD) incidence, a 29% reduction in CHD mortality, and 27–31% reductions in strokes by lowering low-density lipoprotein cholesterol (LDL-C) with statins (HMG-CoA reductase inhibitors) [16]. This success has led to LDL-C–lowering drugs being typically recommended for patients with total blood cholesterol levels > 200 mg/dL [16]. Elevated blood pressure is estimated to cause 62% of cerebrovascular disease and 49% of ischemic heart disease [5]. Clinical trials show significant reductions in CHD and stroke risk when lowering blood pressure by antihypertensive drugs [5]. Type II diabetes accounts for about 90% of diabetic cases [17], which may be treated by oral antidiabetic agents, insulin, or both. Improved glucose control through treatment leads to reduced risks for proliferative retinopathy, amputations, ischemic heart disease, and cardiovascular mortality [15].
For some individuals, therapeutic lifestyle changes may postpone or eliminate the need for cardioprotective medications, thereby both improving their quality of life and reducing the economic cost to society. With respect to high cholesterol, hypertension, and diabetes, modified lifestyle alternatives include eating low-fat diets rich in fruits and vegetables, maintaining ideal weight, and exercising regularly [5,16]. Guidelines for the promotion of physical activity have generally focused on moderate amounts of moderate-intensity activity [19,23]. This report examines relationships of varying levels of self-reported physical activity and cardiorespiratory fitness with reported medication use to lower LDL-C and blood pressure and to manage diabetes in a large, vigorously active cohort of men and women runners. The hypotheses to be tested are whether beyond current guideline levels [19,23], higher doses of physical activity and greater cardiorespiratory fitness are associated with reduced medication use, and whether the effects of exercise and fitness are statistically independent of each other.
METHODS
The design and baseline characteristics of the National Runners’ Health Study have been reported previously [25-29]. Briefly, a two-page questionnaire, distributed nationally at races and to subscribers of the nation’s largest running magazine (Runner’s World, Emmaus, PA), solicited information on demographics (age, race, education), running history (age when began running at least 12 miles per week, average weekly mileage, number of marathons run during the preceding 5 yr, and best marathon and 10-km times), weight history (greatest and current weight, weight when began running, least weight as a runner, circumferences of the chest, waist, and hips), diet (vegetarianism and the current weekly intakes of alcohol, red meat, fish, fruit, vitamin C, vitamin E, and aspirin), current and past cigarette use, prior history of heart attacks and cancer, and medications for blood pressure, thyroid conditions, cholesterol levels, or diabetes. Recruitment took place during two phases, between 1991 and 1993 and between 1998 and 1999. Runners were excluded from these analyses if they smoked or followed strict vegetarian diets. All participants signed a statement of informed consent in accordance with the study protocol approved by the University of California, Berkeley review board.
Height and weight were determined by asking the participant, ‘‘What is your current height (in inches, without shoes)?’’ and ‘‘What is your current weight (prepregnancy weight if pregnant)?’’ Running distances were reported in miles run per week and body weights in pounds, which were converted to kilometers and kilograms for this report. Body mass index (BMI) was calculated as the weight in kilograms divided by the height in meters squared. Previously, we reported strong correlations between repeated questionnaires for self-reported running distances (r = 0.89) [26], between self-reported and clinically measured heights (r = 0.96) and weights (r = 0.96) [26], and for self-reported running distances versus self-reported BMI and body circumferences in cross-sectional analyses [25-29]. Running was the predominant physical activity of the study population. Although other leisure-time physical activities were not recorded for many in this cohort, data from runners recruited after 1998 (when the question was introduced in the survey) show that running represents (mean ± SD) 91.5 ± 19.1% and 85.2 ± 24.0% of all vigorously intense activity in men and women, respectively, and 73.5 ± 23.7% and 69.4% ± 25.7% of total leisure-time physical activity. Use of antidiabetic, antihypertensive, or LDL-C–lowering medications was reported by the participants as part of their baseline survey.
For this report, cardiorespiratory fitness is defined as speed (m/s) during the participant’s best 10-km race during the previous 5 yr. The positive correlation between walk–run endurance performance and aerobic capacity was initially reported by Balke and Ware [2] when they suggested relating laboratory-determined maximal oxygen consumption (VO2max) either to the distance covered in a given time period or to the time required to run a given distance. Using previously published data regarding the energy cost of progressive running speeds [13], Hellerstein [9] accurately estimated the time in minutes to complete a marathon race, assuming that 70% of the VO2max was used. Cooper [6] studied 115 U.S. Air Force male officers and airmen (mean age = 22 yr) who were evaluated by a 12-min walk–run test for distance and a treadmill VO2max test. The correlation of the walk–run test data with the laboratory-determined VO2max was 0.90. Collectively, these findings suggest that the 10-km performance time provides a good estimate of treadmill-determined VO2max in well-motivated subjects, and vice versa.
Statistical analyses
Table 1 presents means ± SD for all variables assessed; all other statistics are expressed as means ± SE or coefficients ± SE. Least-squares regression was used to estimate the adjusted differences in running distances and 10-km race performance times, and logistic regression was used to estimate the dose–response relationships of medication use with distances run and fitness. The logistic regression models were computed with the log odds of medication users as linear functions of running distances and fitness, and as functions of coefficients of distance and fitness intervals. We also included covariates for age and the reported intakes of meat, fish, fruit, and alcohol in all analyses because age is a strong determinant for the risk of all three conditions and because dietary and alcohol intake are also known to affect risk. Results are included with and without adjusting for BMI because of its strong relationships to running distance, 10-km performance time, and the risks for diabetes, hypertension, and hypercholesterolemia. The adjustment for age and BMI included quadratic terms to allow for nonlinear relationships.
Table 1.
Baseline characteristics of runners by reported medication use
| Anti-diabetic medications |
Anti-hypertensive medications |
LDL-cholesterol lowering medications |
||||
|---|---|---|---|---|---|---|
| Users | Nonusers | Users | Nonusers | Users | Nonusers | |
| Men | ||||||
| Sample (N) | 357 | 61,934 | 2893 | 59,398 | 2,069 | 60,222 |
| Age (years) | 48.9± 12.0§ |
44.4± 10.9 |
53.4± 9.9§ |
44.0± 10.7 |
53.2± 9.0§ |
44.1± 10.8 |
| Education (years) | 16.5± 2.5 |
16.4± 2.5 |
16.4± 2.6 |
16.4± 2.5 |
16.7± 2.6§ |
16.4± 2.5 |
| BMI current (kg/m2) |
25.1± 3.4§ |
24.1± 2.8 |
25.3± 3.1§ |
24.1± 2.8 |
25.0± 3.0§ |
24.1± 2.8 |
| BMI starting (kg/m2) |
24.9± 3.9† |
24.3± 3.8 |
25.6± 3.4§ |
24.2± 3.8 |
25.3± 3.2§ |
24.2± 3.8 |
| Running distance (km/wk) |
30.9± 21.2§ |
37.2± 22.6 |
31.1± 19.3§ |
37.4± 22.4 |
30.7± 18.5‡ |
37.4± 22.7 |
| 10 km performance (min) |
47.4± 9.5§ |
43.8± 7.2 |
48.4± 8.1§ |
43.6± 7.1 |
48.6± 8.7§ |
43.7± 7.1 |
| Years run | 12.9± 9.2 |
12.6± 8.3 |
14.4± 9.1§ |
12.5± 8.3 |
14.3± 9.3§ |
12.6± 8.3 |
| Women | ||||||
| Sample (N) | 139 | 44,902 | 845 | 44,196 | 291 | 44,750 |
| Age (years) | 41.8± 11.8§ |
38.2± 10.1 |
50.3± 10.6§ |
38.0± 10.0 |
50.0± 12.6§ |
38.2± 10.1 |
| Education (years) | 15.5± 2.8† |
16.1± 2.6 |
15.7± 2.6‡ |
16.1± 2.6 |
15.8± 3.3 |
16.1± 2.6 |
| BMI current (kg/m2) |
25.1± 6.3§ |
21.6± 2.7 |
23.5± 4.6§ |
21.6± 2.7 |
23.5± 3.9§ |
21.6± 2.7 |
| BMI starting (kg/m2) |
23.8± 4.8§ |
21.5± 3.0 |
22.6± 3.5§ |
21.4± 3.0 |
22.9± 3.9§ |
21.5± 3.0 |
| Running distance (km/wk) |
24.2± 20.8§ |
33.6± 21.0 |
27.2± 19.2§ |
33.7± 21.0 |
26.6± 19.9§ |
33.6± 21.0 |
| 10 km performance (min) |
55.8± 18.1‡ |
50.5± 9.2 |
55.5± 11.0§ |
50.4± 9.2 |
53.8± 11.7§ |
50.5± 9.2 |
| Years run | 8.7± 6.8 |
9.2± 6.7 |
11.5± 7.9§ |
9.2± 6.7 |
11.6± 7.8§ |
9.2± 6.7 |
Significance levels for differences are designated by
for P<0.05
for P<0.01
for P<0.001
for P<0.0001.
RESULTS
There were 62,291 nonsmoking men and 45,041 nonsmoking women who provided complete data on age, current weekly running distance, height, and weight as part of the National Runners’ Health Study. Included among these were 49,870 men (72.3%) and 30,239 women (67.1%) who had provided their best 10-km performance times during the past 5 yr. On average, this subset was similar to the complete sample in age (males, mean ± SD: 44.33 ± 10.55 vs. 44.43 ± 10.86; females: 38.48 ± 9.63 vs. 38.22 ± 10.11 yr) and BMI (males: 24.00 ± 2.71 vs. 24.15 ± 2.82 kg/m2; females: 21.42 ± 2.45 vs. 21.64 ± 2.72 kg/m2), but they ran somewhat farther (males: 39.22 ± 22.46 vs. 37.15 ± 22.60 km/wk; females: 36.92 ± 20.97 vs. 33.53 ± 21.01 km/wk) and for more years if female (males: 12.68 ± 8.09 vs. 12.63 ± 8.34 yr, females: 9.74 ± 6.59 vs. 9.21 ± 6.73 yr) than the complete sample. When adjusted for age, 10-km performance speeds were significantly (P < 0.0001), albeit only moderately, correlated with weekly running distance in men (r = 0.44) and women (r = 0.39).
There were 496 who reported using antidiabetic medications, 3738 who reported using antihypertension medications, and 2360 who reported using LDL-C–lowering medications. As shown in Table 1, the pharmacologically treated were significantly older and significantly more overweight both before and after starting to run. Being older, medication users might be expected to have run more years, as shown.
All age-adjusted mean physical activity and fitness differences between those on and off these medications were statistically significant at P < 0.0001, with one exception: the female fitness difference associated with using LDL-C– lowering medications (P = 0.24 with adjustment). Age accounted for approximately one third of the mean differences in weekly running distance between users and nonusers of antihypertensive or LDL-C–lowering medications. Age accounted for about one half of fitness differences between users and nonusers of antihypertensive medications. Age accounted for less than one quarter of the difference in physical activity and fitness between men reporting and not reporting antidiabetic medication use, and 7% of these differences in women. Table 2 presents the distribution medication users and nonusers by weekly running distance and 10-km performance speeds. All of the results to follow are age adjusted.
Table 2.
Reported medication use by categories of physical activity and cardiorespiratory fitness.
| Sample size | Anti-diabetic medications |
Anti- hypertensive medications |
LDL-cholesterol lowering medications |
|||||
|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | Males | Females | |
|
Physical activity-all (Weekly running distance, km/wk) | ||||||||
| 0-15 | 11,505 | 10,322 | 102 | 59 | 697 | 274 | 505 | 95 |
| 16-31 | 21,939 | 16,755 | 123 | 46 | 1197 | 325 | 843 | 122 |
| 32-47 | 15,723 | 10,486 | 83 | 21 | 640 | 148 | 487 | 46 |
| 48-63 | 7,762 | 4,820 | 36 | 7 | 232 | 75 | 164 | 19 |
| ≥64 | 5,362 | 2,658 | 13 | 6 | 127 | 23 | 70 | 9 |
|
Physical activity-fitness subset (Weekly running distance, km/wk) | ||||||||
| 0-15 | 7,557 | 5,079 | 58 | 19 | 396 | 97 | 287 | 30 |
| 16-31 | 17,025 | 11,080 | 83 | 29 | 875 | 194 | 611 | 74 |
| 32-47 | 13,552 | 8,020 | 68 | 11 | 523 | 110 | 391 | 36 |
| 48-63 | 6,963 | 3,878 | 31 | 4 | 204 | 62 | 147 | 13 |
| ≥64 | 4,773 | 2,182 | 11 | 6 | 103 | 22 | 62 | 8 |
|
Cardiorespiratory fitness (10 km performance) | ||||||||
| slowest | 5,885 | 3,596 | 60 | 17 | 609 | 132 | 432 | 32 |
| slow | 13,998 | 7,025 | 85 | 22 | 811 | 134 | 583 | 48 |
| intermediate | 16,740 | 9,557 | 70 | 15 | 495 | 132 | 361 | 44 |
| fast | 9,407 | 6,470 | 23 | 9 | 156 | 65 | 110 | 25 |
| fastest | 3,840 | 3,591 | 13 | 6 | 30 | 22 | 12 | 12 |
1. Cardiovascular fitness categories are defined as slowest (males: <3.25 m/s; females: <2.8 m/s); slow (males: 3.25 to 3.74 m/s; females: 2.8 to 3.19 m/s); intermediate (males: 3.75 to 4.24 m/s; females: 3.20 to 3.59 m/s); fast (males: 4.25 to 4.74 m/s; females: 3.6 to 3.99 m/s); fastest (males: >4.75 m/s; females: >4.00 m/s ).
Antidiabetic medications
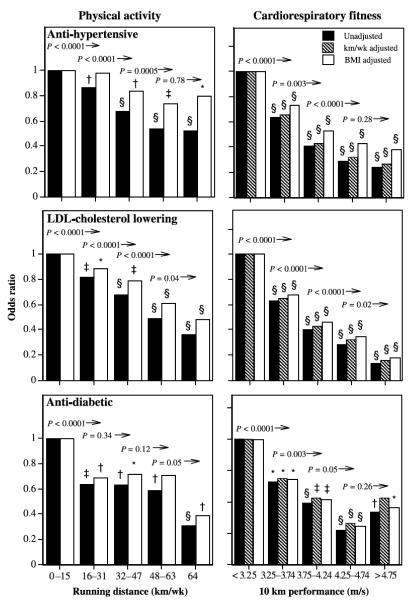
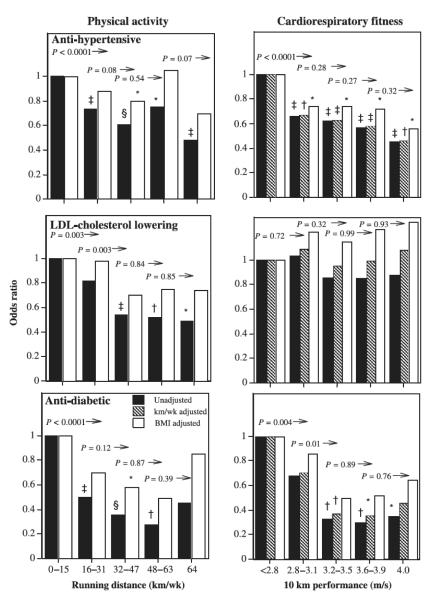
The logistic regression analyses of Table 3 show that the proportions on antidiabetic medications were significantly lower among those with higher levels of physical activity and fitness in both men and women. Adjustment for BMI reduced the significance of the associations, but, with the exception of fitness in women, all remain statistically significant. These relationships are illustrated in Figures 1 and 2 for men and women. Symbols above the bars designate significance differences from the least-active or least-fit men, and numerical probabilities above each bar denote significant differences of the interval compared with all higher levels. The proportion using each type of medication is lower in a stepped, linear manner comparing across fitness levels, especially in men. The odds for antidiabetic medication use is at least 36% lower in men who ran 16–31 km/wk compared with shorter-distance runners, and 69% lower for ≥ 64 km/wk versus < 16 km/wk. The men’s reductions in odds are attenuated modestly when adjusted for BMI. The odds reductions also decline in association with cardiorespiratory fitness, with antidiabetic medication use being 58% less in men who ran ≥ 4.75 m/s compared with < 3.25 m/s. Each 0.5-m/s increment in fitness through 4.25 m/s is associated with a significant reduction in the odds ratio. Among women, odds of antidiabetic medication use is lower by one half in those running 16–32 km/wk and by nearly three quarters among those running > 48 km/wk compared with women running less than 16 km/wk, and these odds reductions are diminished by about 15% when adjusted for BMI. The lower odds with higher fitness are weakened only slightly when adjusted for BMI in men, and more so in women.
Table 3.
Logistic regression analyses of hypertension, high cholesterol, and diabetes in relation to physical activity and cardiorespiratory fitness
| Males | Females | |||||
|---|---|---|---|---|---|---|
| Intercept | Regression coefficients±SE |
Intercept | Regression coefficients±SE |
|||
| Physical activity (km/wk) |
Cardio- Respiratory Fitness (m/s) |
Physical activity (km/wk) |
Cardio- Respiratory Fitness (m/s) |
|||
| Anti-diabetic medications | ||||||
| All | ||||||
| Activity, unadjusted |
−4.929 | −0.012 ±0.003§ |
−5.195 | −0.025 ±0.005§ |
||
| Activity, BMI adjusted |
−5.093 | −0.009 ±0.003† |
−5.785 | −0.012 ±0.005* |
||
| Fitness subset | ||||||
| Activity, unadjusted |
−5.051 | −0.011 ±0.003‡ |
−5.623 | −0.016 ±0.007* |
||
| Activity, BMI adjusted |
−5.142 | −0.009 ±0.003† |
−6.030 | −0.008 ±0.007 |
||
| Fitness, unadjusted |
−2.799 | −0.697 ±0.118§ |
−3.492 | −0.809 ±0.226‡ |
||
| Fitness, BMI adjusted |
−2.943 | −0.664 ±0.127§ |
−4.799 | −0.453 ±0.239 |
||
| Activity & Fitness, unadjusted |
−2.879 | −0.004 ±0.004 |
−0.634 ±0.129§ |
−3.566 | −0.009 ±0.007 |
−0.692 ±0.245† |
| Activity & Fitness, BMI adjusted |
−2.992 | −0.004 ±0.004 |
−0.611 ±0.136§ |
−4.795 | −0.005 ±0.007 |
−0.401 ±0.248 |
| Anti-hypertension medications | ||||||
| All | ||||||
| Activity, unadjusted |
−2.927 | −0.011 ±0.001§ |
−4.208 | −0.012 ±0.002§ |
||
| Activity, BMI adjusted |
−3.206 | −0.005 ±0.001§ |
−4.485 | −0.005 ±0.002† |
||
| Fitness subset | ||||||
| Activity, unadjusted |
−3.022 | −0.011 ±0.001§ |
−4.472 | −0.004 ±0.003 |
||
| Activity, BMI adjusted |
−3.289 | −0.005 ±0.001§ |
−4.623 | −0.001 ±0.003 |
||
| Fitness, unadjusted |
−0.535 | −0.757 ±0.045§ |
−3.171 | −0.435 ±0.093§ |
||
| Fitness, BMI adjusted |
−1.454 | −0.529 ±0.049§ |
−3.669 | −0.294 ±0.096† |
||
| Activity & Fitness, unadjusted |
−0.597 | −0.004 ±0.001† |
−0.705 ±0.049§ |
−3.175 | −0.001 ±0.003 |
−0.428 ±0.099§ |
| Activity & Fitness, BMI adjusted |
−1.462 | −0.001 ±0.001 |
−0.516 ±0.051§ |
−3.671 | −0.001 ±0.003 |
−0.307 ±0.100† |
| LDL-cholesterol lowering medications | ||||||
| All | ||||||
| Activity, unadjusted |
−3.185 | −0.013 ±0.001§ |
−5.107 | −0.014± 0.003§ |
||
| Activity, BMI adjusted |
−3.349 | −0.009 ±0.001§ |
−5.399 | −0.007 ±0.003* |
||
| Fitness subset | ||||||
| Activity, unadjusted |
−3.281 | −0.013 ±0.001§ |
−5.265 | −0.009± 0.004* |
||
| Activity, BMI adjusted |
−3.441 | −0.009 ±0.001§ |
−5.475 | −0.004 ±0.004 |
||
| Fitness, unadjusted |
−0.608 | −0.825 ±0.052§ |
−5.186 | −0.117 ±0.153 |
||
| Fitness, BMI adjusted |
−1.107 | −0.697 ±0.057§ |
−5.964 | −0.100 ±0.150 |
||
| Activity & Fitness, unadjusted |
−0.687 | −0.005 ±0.002† |
−0.756 ±0.057§ |
−5.227 | −0.009 ±0.005* |
−0.012 ±0.159 |
| Activity & Fitness, BMI adjusted |
−1.134 | −0.004 ±0.002* |
−0.654 ±0.060§ |
−5.924 | −0.005 ±0.005 |
−0.143 ±0.152 |
All coefficients adjusted to the mean age, and intakes of red meat, fish, fruit, and alcohol. The coefficients were also adjusted for BMI where indicated. Significance levels are coded:
P<0.05
P<0.01
P<0.001
P<0.0001.
The probability of medication use at any running distance or fitness level is given by the formulas 1/(1+exp(-intercept-distance coefficient*km/wk-fitness coefficient*m/s)).
FIGURE 1.
Odds ratios for men’s medication use by physical activity (weekly distance run) or cardiorespiratory fitness (10-km performance speed) relative to the least-active or least-fit men. The odds ratios are adjusted for age and intakes of meat, fish, fruit, and alcohol. The odds ratios were also adjusted for BMI where indicated. Symbols above the bars designate significance levels of the interval relative to the least-active or least-fit interval: * P < 0.05; † P < 0.01; ‡ P < 0.001; § P < 0.0001 (the absence of a symbol designates nonsignificance). The numeric probabilities above the bars represent the significance of the interval relative to all greater levels without adjustment for BMI.
FIGURE 2.
Odds ratios for women’s medication use by physical activity (weekly distance run) or cardiorespiratory fitness (10-km performance speed) relative to the least-active or least-fit women. The odds ratios are adjusted for age and intakes of red meat, fish, fruit, and alcohol. The odds ratios were also adjusted for BMI where indicated. Symbols above the bars designate significance levels of the interval relative to the least-active or least-fit interval: * P < 0.05; † P < 0.01; ‡ P < 0.001; § P < 0.0001 (the absence of a symbol designates nonsignificance). The numeric probabilities above the bars represent the significance of the interval relative to all greater levels without adjustment for BMI.
Table 3 also assesses the relative contributions of physical activity and fitness on the probability of using diabetic medications. For comparison, the logistic regression analyses for running distance versus diabetic medication use are repeated for the subset of participants with fitness data. In men, distance run per week was unrelated to medication use when adjusted for fitness, whereas cardiorespiratory fitness was significantly related to the probability of using these medications when adjusted for activity, with or without adjustment for BMI. Female antidiabetic medication use was more strongly associated with fitness than activity; however, the differences were less apparent when adjusted for BMI.
Antihypertensive medications
The logistic regression analyses of Table 3 suggest that in both sexes, the proportions using antihypertensive medications are significantly lower with higher levels of physical activity and fitness. Adjustment for BMI attenuated the significance of the associations, but the lower odds associated with longer running distances remained significant, albeit weakened in women. Figure 1 shows that the odds ratio for men’s antihypertensive medication use decreased significantly with each 16-km/wk increment in running distance through 48 km/wk. The apparent decline in the odds ratios are even more pronounced in association with cardiorespiratory fitness, with the odds for medication use being 76% less in men who ran > 4.75 m/s compared with < 3.25 m/s. These odds reductions are attenuated slightly when adjusted for BMI, but for both sexes they remain significantly less than the odds for the least fit. When both fitness and activity are considered simultaneously (Table 3), greater cardiorespiratory fitness in both sexes is associated with significantly less medication use when adjusted for physical activity, with or without adjustment for BMI. Antihypertensive medication use is associated with the quantity of physical activity when adjusted for fitness in men only, and only without adjustment for BMI.
LDL-C–lowering medications
In both sexes, the proportions on cholesterol-lowering medications were significantly lower among those with higher levels of physical activity, including when adjusted for BMI (Table 3). The odds for LDL-C lowering-medication use is 64% less for men who run more than 64 km/wk compared with those who run < 16 km/wk, and at shorter distances the odds increase in a stepped fashion with lower self-reported running distances (Fig. 1). BMI adjustment weakens the odds reductions and corresponding significance levels. The odds reduction for individual distance categories in women is less consistent (Fig. 2) and does not persist when adjusted for BMI. In men, but not women, the odds of using LDL-C– lowering medications decreases significantly in association with greater cardiorespiratory fitness (Table 3). For men, the plot of Figure 1 shows lower odds with higher fitness, with significance achieved above 3.25 m/s, with or without BMI adjustment. When both cardiorespiratory fitness and physical activity are used simultaneously to predict men’s medication use, fitness is the stronger predictor, but both are significant, except when adjusted for BMI; then, fitness is strongly predictive, and physical activity is not.
DISCUSSION
Dose–response relationships to physical activity
During the past decade, physical activity guidelines of the Centers for Disease Control and Prevention, National Institutes of Health and the American Heart Association have emphasized the health benefits of walking 2 miles (3.2 km) briskly on most days of the week (the energy equivalent of running 8–12 km/wk) [19,23]. This corresponds to the referent activity levels of Figures 1 and 2. These guidelines acknowledge that additional benefits may accrue for longer, more intense activity. Consistent with this premise, our findings indicate that the odds of self-reported use of LDL-C–lowering, antihypertensive, and antidiabetic medications are lower among those reporting longer running distances. Compared with ≤ 16 km/wk (guideline levels), the odds in men and women who ran > 64 km/wk were, respectively, 69% and 55% lower for antidiabetic, 48% and 52% lower for antihypertension, and 64% and 51% lower for LDL-C–lowering medication use. Runners with levels of activity greater than the recommended levels but less than 64 km/wk (40 miles) had odds of medication use that were lower than the referent group of 0–15 miles per week, but greater than for those reporting the highest weekly running distances.
The 107,332 runners reported here represent the largest cross-sectional study of LDL-C–lowering, antihypertension, and antidiabetic medications in vigorously active men and women. These associations complement randomized clinical trials of exercise training by establishing associations across a range of activity levels generally unattainable in trials. As with all cross-sectional associations, it is not possible to distinguish the causal direction of the relationship— that is, whether physical activity reduces the need for medications, or, contrariwise, whether medications reduce cardiorespiratory fitness and impair exercise capacity. For example, mild musculoskeletal symptoms such as cramps, stiffness, and tendonitis are reported in about 20% of patients using LDL-C–lowering medications [8]. We also cannot exclude the possibility that the frequency of medical checkups might differ by running distance or race performance, thus affecting the opportunity for diagnosis.
We suspect, however, that the associations depicted in the figures are unlikely to have been appreciably influenced by medications affecting running ability. This is because these associations are consistent with the declines in blood pressure and LDL-C reported previously for a minor subset of the National Runners’ Health Study. These earlier analyses showed that among 7059 male and 1837 female runners who did not use medications for the treatment of blood pressure, cholesterol level, or diabetes, physician-reported systolic and diastolic blood pressure, respectively, were lower with greater running distance (−0.040 ± 0.009 and −0.027 ± 0.006 mm Hg/km per week run in men, and −0.06 ± 0.02 and −0.028 ± 0.013 mm Hg/km per week run in women [27]). Men’s plasma LDL-C and plasma glucose concentrations were also significantly lower among those with greater self-reported running distance (−0.002 ± 0.001 mg/dL per kilometer per week and −0.001 ± 0.000 mg/dL per kilometer per week), whereas women’s LDL-C was not (−0.03 ± 0.04 mg/dL) [27]. To our knowledge, LDL-C and blood pressure do not affect the ability to run longer distances. We abandoned our intentions to retrieve cholesterol, blood pressure, and fasting glucose concentrations from the medical records of the entire 107,332 runners reported herein after legislation that changed the consent requirement and increased the potential liability to medical entities that might make errors in sharing this information.
Obesity increases the risk for elevated LDL-C, hypertension, and diabetes [11], and there is a strong, inverse association between body weight and running distance [28,29]. Table 1 shows that each medication was associated with significantly higher BMI, and although the differences between users and nonusers were not great among men, they were more substantial among women. Table 3 presents the logistic regression coefficients for the effects of running distance on medication use, with and without adjustment for BMI. In all cases, 1) the coefficients for BMI are statistically significant, and 2) the coefficients for distance run are reduced but remain significant when adjusted for BMI. Figure 2 shows further that the reduction in medication use remains significant when adjusted for BMI, albeit slightly diminished. These statistical adjustments are presented with the caveat that the underlying statistical model may not strictly apply (i.e., the relationship of running distance to the 95th, 90th, or 80th BMI percentile are substantially greater than the corresponding relationships at the 5th, 10th, or 25th percentile) [28,29]. Our analyses are consistent with meta-analyses showing that aerobic exercise decreases systolic (3.8 mm Hg) and diastolic blood pressure (2.6 mm Hg) without a significant reduction in body weight [24].
Dose–response relationship to cardiorespiratory fitness
In both sexes, the proportion using antidiabetic and antihypertensive drugs was lower among those with higher levels of cardiorespiratory fitness, independently of the physical activity dose (kilometers run per week). Similarly, LDL-C–lowering drug use was lower in association with higher cardiorespiratory fitness in men but not women, with the association being largely independent of weekly distance run. The difference in the men’s odds of medication use were the same or greater across categories of fitness than across categories of physical activity (Fig. 1). Although beta-blocker therapy may decrease physical work capacity and/or the ability to sustain submaximal exercise [30], cardiorespiratory fitness is generally unaffected by diuretics, angiotensin converting enzyme inhibitors, hypoglycemic medications, and antihyperlipidemic agents [1]. Our previous report has shown that men’s blood pressures and LDL-C levels both declined significantly with greater cardiorespiratory fitness [26], substantiating the lower odds of medication use reported here.
The Aerobics Center Longitudinal Study (ACLS) 5-yr follow-up of 4884 normotensive women showed that compared with rates in the least-fit cohort, women in the intermediate-fit category had a 60% lower risk of becoming hypertensive, and those in the highest fitness category had a 79% lower risk of becoming hypertensive. From this, the authors inferred that physical activity lowers the risk of developing hypertension [3]. Our analyses fully support the association that the ACLS reported between fitness and hypertension but also suggest that the ACLS results may not necessarily be attributable to physical activity. Table 2 suggests that lower odds of antihypertensive medication use with fitness could not be attributed to activity per se. Previously, we reported that lower physician-reported blood pressure with higher fitness remain strongly significant when adjusted for physical activity [26]. Cardiorespiratory fitness is known to reflect a substantial hereditary component [4], and genetic factors associated with higher fitness levels might also reduce the risks for becoming hypertensive.
If the fitness–hypertension relations reported by us and the ACLS were attributable to physical activity, then we might anticipate that vigorous exercise would produce a greater reduction in blood pressure than would moderate-intensity exercise. This is because cardiorespiratory fitness is increased to a greater extent by vigorous physical activity (> 6 METs) as compared with moderate-intensity activity (3–6 METs) [21]. In fact, separate meta-analyses by Whelton et al. [24] and Fagard [7] suggest that clinical trials generally report similar blood pressure reductions by moderate-intensity exercise as compared with vigorous exercise. In a detailed analysis by Swain and Franklin [22] of selected studies where total energy expenditure was held constant, trials employing more vigorous exercise (typically ≥ 60% aerobic capacity) generally have reported greater improvements in diastolic blood pressure as compared with moderate-intensity exercise, but they have found no intensity effect on improvements in systolic blood pressure. Epidemiological studies that report lower blood pressures among more vigorously active than moderately active individuals [14] and reduced incidences of hypertension among men engaged in activities requiring at least 4.5 METs than those engaged in less-intense activities [18] may also reflect a tendency for those endowed with greater cardiorespiratory fitness to choose to exercise more vigorously.
Summary
We have demonstrated cross-sectionally the association between daily dose of vigorous exercise and lower odds of use of antidiabetic, antihypertensive, and LDL-C–lowering medications. Larger doses of exercise than are currently recommended are associated with reduced odds of taking these medications. Unfortunately, we do not have data available from this cohort on the injury rates associated with running distance, and, therefore, we cannot estimate the costs and interruptions associated with running longer distances. Injuries could limit the adoption of physical activity for doses and intensities that exceed guideline levels. We also acknowledge that the higher-mileage runners of this cohort may be genetically different from those who can only achieve shorter distances. Our analyses of self-selection shows that whereas the leanness of men and women who run faster 10-km races can be attributed entirely to self-selection based on pre-exercising weight, self-selection based on pre-exercising weights accounts for only a portion (26% in men and 58% in women) of the leanness associated with running longer distances [25]. We also acknowledge that we have no information on the training regimen preceding their fastest recent 10-km race, which may affect performance time. However, our expectation is that usual activity levels, rather than exceptional periods of training, are likely to influence health most directly.
Currently, 27% of U.S. women and 34% of U.S. men meet or exceed the more general exercise recommendations for health benefits [20]. However, this percentage may better be assessed relative to the success of other treatment alternatives (e.g., use of adjunctive pharmacotherapies). Only one half of those for whom LDL-C–lowering medications are indicated take these drugs, despite their few side effects [16]. An advantage of physical activity over medications is that this intervention may be advocated and more readily embraced at a much earlier age. This is important because LDL-C–lowering medications produce substantially greater CHD risk reductions when started earlier in life [12]. Cross-sectional data from this large study support the need for further research to understand the relative costs of more widely promoting exercise, the costs of the injuries resulting from the high doses of exercise, and the costs of prescription medications.
Acknowledgments
Supported in part by grants HL-45652, HL-072110, and DK066738 from the National Heart Lung and Blood Institute, and the National Institute of Diabetes and Digestive and Kidney Diseases, and conducted at the Ernest Orlando Lawrence Berkeley National Laboratory (Department of Energy DE-AC03-76SF00098 to the University of California).
REFERENCES
- 1.American College of Sports Medicine . In: ACSM’s Guidelines for Exercise Testing and Prescription. 6th ed Franklin B, editor. Lippincott Williams & Wilkins; Baltimore, MD: 2007. [Google Scholar]
- 2.Balke B, Ware RW. An experimental study of physical fitness of Air Force personnel. US Armed Forces Med. J. 1959;10:875–888. [PubMed] [Google Scholar]
- 3.Barlow CE, Lamonte MJ, Fitzgerald SJ, Kampert JB, Perrin JL, Blair SN. Cardiorespiratory fitness is an independent predictor of hypertension incidence among initially normotensive healthy women. Am. J. Epidemiol. 2006;163:142–150. doi: 10.1093/aje/kwj019. [DOI] [PubMed] [Google Scholar]
- 4.Bouchard C, Lesage R, Lortie G, et al. Aerobic performance in brothers, dizygotic and monozygotic twins. Med. Sci. Sports Exerc. 1986;18:639–646. [PubMed] [Google Scholar]
- 5.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 6.Cooper KH. A means of assessing maximal oxygen intake: correlation between field and treadmill testing. JAMA. 1968;203:201–204. [PubMed] [Google Scholar]
- 7.Fagard RH. Exercise characteristics and the blood pressure response to dynamic physical training. Med. Sci. Sports Exerc. 2001;33(Suppl):S484–S492. doi: 10.1097/00005768-200106001-00018. [DOI] [PubMed] [Google Scholar]
- 8.Franc S, Dejager S, Bruckert E, Chauvenet M, Giral P, Turpin G. A comprehensive description of muscle symptoms associated with lipid-lowering drugs. Cardiovasc. Drugs Ther. 2003;17:459–465. doi: 10.1023/b:card.0000015861.26111.ab. [DOI] [PubMed] [Google Scholar]
- 9.Hellerstein HK. Limitations of marathon running in the rehabilitation of coronary patients: anatomic and physiologic determinants. Ann. N.Y. Acad. Sci. 1977;301:484–494. doi: 10.1111/j.1749-6632.1977.tb38224.x. [DOI] [PubMed] [Google Scholar]
- 10.Hogan P, Dall T, Nikolov P. Economic costs of diabetes in the US in 2002. Diabetes Care. 2003;26:917–932. doi: 10.2337/diacare.26.3.917. [DOI] [PubMed] [Google Scholar]
- 11.Institute of Medicine . Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients) The National Academies Press; Washington, DC: 2005. [Google Scholar]
- 12.Law MR. Lowering heart disease risk with cholesterol reduction: evidence from observational studies and clinical trials. Eur. Heart J. Suppl. 1999;1(S):S3–S8. [Google Scholar]
- 13.Margaria R, Cerretelli P, Aghemo P, Sassi G. Energy cost of running. J. Appl. Physiol. 1963;18:367–370. doi: 10.1152/jappl.1963.18.2.367. [DOI] [PubMed] [Google Scholar]
- 14.Mensink GB, Heerstrass DW, Neppelenbroek SE, Schuit AJ, Bellach BM. Intensity, duration, and frequency of physical activity and coronary risk factors. Med. Sci. Sports Exerc. 1997;29:1192–1198. doi: 10.1097/00005768-199709000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Minshall ME, Roze S, Palmer AJ, et al. Treating diabetes to accepted standards of care: a 10-yr projection of the estimated economic and health impact in patients with type 1 and type 2 diabetes mellitus in the United States. Clin. Ther. 2005;27:940–950. doi: 10.1016/j.clinthera.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 16.National Cholesterol Education Program Expert Panel On Detection, Evaluation, And Treatment Of High Blood Cholesterol In Adults (Adult Treatment Panel III) National Cholesterol Education Program National Heart, Lung, and Blood Institute National Institutes of Health; NIH Publication No. 02-5215. [Google Scholar]
- 17.Number (in thousands) of persons with diagnosed diabetes, by age, United States, 1980–2002. Diabetes Statistics from the Centers for Disease Control and Prevention for the U.S.A. Available at: http://www.cdc.gov/diabetes/statistics/prey/national/menupersons.htm.
- 18.Paffenbarger RS, Jr, Lee IM. Intensity of physical activity related to incidence of hypertension and all-cause mortality: an epidemiological view. Blood Press. Monit. 1997;2:115–123. [PubMed] [Google Scholar]
- 19.Pate RR, Pratt M, Blair SN, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 20.Schoenborn CA, Barnes PM. Advance Data From Vital and Health Statistics, no. 325. National Center for Health Statistics; Hyattsville, MD: 2002. Leisure-time physical activity among adults: United States, 1997–98. [Google Scholar]
- 21.Swain DP, Franklin BA. VO2 reserve and the minimal intensity for improving cardiorespiratory fitness. Med. Sci. Sports Exerc. 2002;34:152–157. doi: 10.1097/00005768-200201000-00023. [DOI] [PubMed] [Google Scholar]
- 22.Swain DP, Franklin BA. Comparison of cardioprotective benefits of vigorous versus moderate intensity aerobic exercise. Am. J. Cardiol. 2006;97:141–147. doi: 10.1016/j.amjcard.2005.07.130. [DOI] [PubMed] [Google Scholar]
- 23.U.S. Department of Health and Human Services . Physical Activity and Health: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; Atlanta, GA: 1996. [Google Scholar]
- 24.Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann. Intern. Med. 2002;136:493–503. doi: 10.7326/0003-4819-136-7-200204020-00006. [DOI] [PubMed] [Google Scholar]
- 25.Williams PT. Self-selection accounts for inverse association between weight and cardiorespiratory fitness. Obesity (Silver Spring) 2008 Jan;16(1):102–6. doi: 10.1038/oby.2007.5. [DOI] [PubMed] [Google Scholar]
- 26.Williams PT. Relationships of heart disease risk factors to exercise quantity and intensity. Arch. Intern. Med. 1998;158:237–245. doi: 10.1001/archinte.158.3.237. [DOI] [PubMed] [Google Scholar]
- 27.Williams PT. Relationship of distance run per week to coronary heart disease risk factors in 8283 male runners: The National Runners’ Health Study. Arch. Intern. Med. 1997;157:191–198. [PMC free article] [PubMed] [Google Scholar]
- 28.Williams PT, Pate RR. Cross-sectional relationships of exercise and age to adiposity in 60,617 male runners. Med. Sci. Sports Exerc. 2005;37:1329–1337. doi: 10.1249/01.mss.0000174894.05236.45. [DOI] [PubMed] [Google Scholar]
- 29.Williams PT, Satariano WA. Relationships of age and weekly running distance to BMI and circumferences in 41,582 physically active women. Obes. Res. 2005;13:1370–1380. doi: 10.1038/oby.2005.166. [DOI] [PubMed] [Google Scholar]
- 30.Wilmore JH, Freund BJ, Joyner MJ, et al. Acute response to submaximal and maximal exercise consequent to beta-adrenergic blockade: implications for the prescription of exercise. Am. J. Cardiol. 1985;55:135D–141D. doi: 10.1016/0002-9149(85)91070-7. [DOI] [PubMed] [Google Scholar]