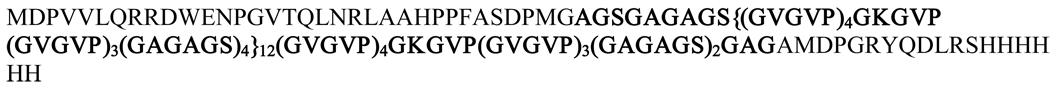Abstract
Vector dissemination, transient gene expression and rapid clearance are major obstacles to successful human gene therapy. In this study, we investigated the effect of silk-elastinlike protein polymer (SELP) hydrogels, on biodistribution and anticancer efficacy of adenoviral gene therapy in a head and neck cancer model. Transcriptional activities of adenovirus carrying β-galactosidase (Ad-LacZ) and luciferase (Ad-Luc) reporter genes were evaluated in (nu/nu) mice with head and neck cancer, as a function of polymer concentration. Antitumor efficacy of thymidine kinase encoding adenovirus (Ad-Tk) and ganciclovir (GSV) combination was also evaluated. 4 wt% SELP matrices localized viral release, minimized dissemination to liver and enhanced reporter gene expression levels by 4–8 fold compared to virus alone. SELP- Ad-Tk with GSV reduced tumor volume significantly compared to the virus alone. SELPs provide a means for temporal and spatial control of viral gene delivery to head and neck tumors.
Keywords: Gene Delivery, Adenovirus, Protein Polymers, Hydrogels, Head and Neck Tumors, Matrix-Mediated, Silk-elastinlike
INTRODUCTION
Head and neck squamous cell carcinomas (HNSCC) are among the leading causes of cancer related mortality in the United States1. The low five-year survival rate in advanced HNSCC is largely due to the failure of the current treatment modalities to suppress tumor progression in the locoregional area for prolonged periods of time2,3. Local therapy approaches through intratumoral injection of adenoviral vectors loaded with various therapeutic genes have shown great promise in achieving significant anticancer therapeutic efficacy. However, progress in the field is severely undermined by the transient expression of the adenoviral transgene(s), vector dissemination and associated toxicities4–7. Matrix-mediated localized delivery can address these issues and enhance the safety and efficacy of adenoviruses while minimizing their toxicities.
Several polymeric matrices of natural and synthetic origins have been used for localized controlled delivery of bioactive agents with varying degrees of success8–10. Silk-elastinlike protein polymers (SELPs) are a unique class of genetically engineered block copolymers consisting of peptide repeats of silk-like (GAGAGS) and elastin-like (GVGVP) units11. Silk units in these polymers allow formation of hydrogen bonds (physical crosslinks) and render mechanical strength to the formed hydrogels. Elastin units on the other hand confer aqueous solubility, elasticity and allow biodegradation12. By precisely controlling the ratio(s) and length(s) of silk and elastin units using recombinant techniques, the material properties of SELPs can be tailored to control spatio-temporal release of viruses and subsequent gene transfection13,14. The potential of SELPs for matrix-mediated gene delivery to solid tumors is reviewed elsewhere15. SELP-47K a copolymer with four silk and eight elastin blocks (including one lysine-substituted elastin - VPGKG) (Figure 1) undergoes irreversible sol-to-gel transition at body temperature16,17. This allows the minimally invasive intratumoral injection of aqueous polymer-virus solutions that transform into solid hydrogel matrices within minutes in the body. The formed hydrogels provide localized, controlled release of viruses at the site of administration.
Figure 1.
The amino acid sequence of SELP-47K (molecular weight: 69,814 Da). The protein polymer is composed of head and tail portions and a series of silk (GAGAGS) and elastin-like (GVGVP) repeats with the primary repetitive sequences shown in bold.
SELP-47K has been shown to localize and prolong transgene expression of green fluorescent protein (GFP) in a murine xenograft model of head and neck tumor, serving as a depot for enhanced viral supply and transfection14. However, quantitative expression levels of the reporter gene in the tumor and distant organs and subsequent efficacy in reducing tumor size are unknown. Reported here is the influence of SELP polymer concentration on the duration and extent of localized expression using two reporter genes (Ad-LacZ, and Ad-Luc). Also reported is the therapeutic efficacy of SELP-mediated localized controlled release of adenovirus encoding thymidine kinase (Tk) in a murine xenograft model of head and neck cancer.
MATERIALS AND METHODS
Materials
SELP-47K a protein block co-polymer, with 12 repeating units, and molecular weight of 69,814 Da was obtained as a 12 wt% solution from Protein Polymer Technologies, Inc. (San Diego, CA). The amino acid sequence of SELP-47K is shown in Figure 1. Replication defective human adenovirus (Ad) Type 5 with E1/E3 deletion, under the control of the CMV promoter, encoding for either β-galactosidase (β-gal) or firefly luciferase (Luc) reporter genes, was purchased from Vector Biolabs (Philadelphia, PA). Adenovirus expressing thymidine kinase gene (Ad-Tk) was propagated in HEK 293 cells and harvested. JHU-012 oral cavity cancer cell line was a kind gift from Professor David Sidransky of Johns Hopkins University (Baltimore, MD). For establishing tumor xenografts, JHU-012 cells were cultured in Roswell Park Memorial Institute (RPMI 1640) medium containing 0.1 mg/ mL streptomycin, 100 IU/ mL penicillin, and 10% fetal calf serum (Gibco, Carlsbad, CA) in a humidified atmosphere with 5% CO2 at 37°C. Chlorophenol red-β-D-galactopyranoside (CPRG) beta-gal quantitative kit was purchased from Imgenex (San Diego, CA), and β-Galactosidase Reporter Gene Staining Kit from Sigma-Aldrich, Inc (St. Louis, MO). Luciferase assay system was purchased from Promega (Madison, WI). iScript cDNA synthesis kit and iQ SYBR green quantification kit was purchased from Bio-Rad (Hercules, CA). 6 week old female athymic (nu/nu) mice (Charles River Laboratories, Davis, CA) were used for the animal studies in accordance with the Institutional Animal Care and Use Committees (IACUC) of the University of Utah and the University of Pennsylvania Health System.
Reporter (β-gal) gene delivery
Head and neck cancer xenografts were established by subcutaneously injecting 2×106 JHU-012 cells suspended in 200 ul phosphate buffer saline (PBS) on each hind flank of athymic nude (nu/nu) mice. Tumors were allowed to grow for two weeks to reach an average diameter of 7 mm. A dose of 5 × 108 PFU of Ad-LacZ was administered to mice with 4, 8, and 10.8 wt% SELP-47K or physiological saline. Virus-polymer solutions were prepared by thawing SELP and virus stocks and mixing them gently with physiological saline. Mice were anesthetized using 4% isofluorane mixed with oxygen, and intratumorally injected with 50 uL of the polymer-virus solutions. On day 7, 14 and 21 mice were euthanized and tumor and liver tissues were isolated.
Chlorophenol red-β-D-galactopyranoside (CPRG) β-gal colorimetric assay was carried out as described previously18. Briefly, fresh tissue samples (tumor and liver) were collected and snap-frozen in liquid nitrogen. Subsequently tissues were ground with mortar and pestle, re-suspended in 1 mL lysis buffer (50 mM HEPES, 5mM CHAPS, pH 7.4), and sonicated on ice in 10–15 second bursts for a total of one minute utilizing Virsonic-475 ultrasonic homogenizer with 475 W output, at 50% power, (Virtis, Gardiner, NY). Lysates were then centrifuged for 45 minutes at 14,000 rpm and the supernatant was analyzed using CPRG quantitative kit as per manufacturer’s instructions. Colorimetric measurements at 570 nm were carried out using a Universal Spectrum Max micro plate reader (Molecular Devices, Sunnyvale, CA).
Tissue/tumor sections were analysed using β-galactosidase reporter gene staining kit. Briefly, tissue sections were fixed for 3 minutes in fixation buffer, then rinsed twice with PBS and stained with X-gal staining solution overnight at 37°C. Sections were then rinsed with PBS and observed under a Nikon Optiphot-2 light microscope to determine the extent of gene expression. Cells expressing the β-gal transgene were stained indigo blue.
Bioluminescence imaging
Tumor xenografts in nude mice were induced as described above. Tumors were grown to an average of 7 mm and injected with SELP mixed with adenovirus encoding luciferase (Ad-Luc) or Ad-Luc alone. To collect images, animals were injected intraperitoneally with 0.2 ml luciferin potassium salt. Twenty five minutes following injection, animals were anesthetized and placed in the imaging chamber of Xenogen IVIS100 Bioluminescent Imager (Hopkinton, MA). Peak luminescence was found to be 30 minutes post injection for 30 seconds exposure times. Data were analyzed by Living Image 2.2 software (Caliper LifeSciences, Hopkinton, MA) coupled to the bioluminescence imaging system.
Tumor suppressor gene delivery
Tumor xenografts were induced as described above. Mice were anaesthetized with avertin (tribromoethanol-tert-amyl alcohol), and the skin flaps on the flanks were raised to expose the tumors for measurement and intratumoral injection. Each group was injected intratumorally with 50 uL of either dPBS, SELP-47K (4wt% polymer concentration), Ad-DL312 (control), Ad-Tk (thymidine kinase), or Ad-Tk in 4wt% SELP-47K. Ganciclovir (GSV) was administered intraperitoneally on a daily basis to the animals receiving the Ad-Tk treatments. On days 3, 7, and 14 animals were euthanized, tumor size measured and harvested. Subsequently, tissue homogenates were used for quantification of transgene expression levels. mRNA was isolated and cDNA synthesized according to iScript cDNA synthesis kit (Bio-Rad). The following PCR protocol was utilized, 5 minutes at 25°C, 30 minutes at 42°C, and 5 minutes at 85°C. Quantitative PCR (qPCR) of the cDNA was performed with iCycler system according to the manufacture’s instruction for iQ SYBR green quantification Kit(Bio-Rad). Quantification was carried out using forward (5’-CTGCGG GT TTA TA TAGACGG-3’) and reverse (5’-CATTGTTATCTGGGCGCT-3’) primers for the target Tk gene. Expression levels were normalized using amplification obtained with control gene β-2 microglobulin with forward (5’-TAGCTGTGCTCGCGC TACTC-3’) and reverse (5’- TTCACA CGGCAGGCATACTC-3’) primers. PCR conditions were 5 minutes at 95 °C, followed by 45 cycles of 15 sec at 95 °C, and 30 sec at 60 °C.
Statistical analysis
Changes in tumor volume were analyzed using ANOVA and Mann-Whitney’s test at an α =0.05. The results are expressed as the mean ± SE. Student’s T test was used to analyze the significance of difference.
RESULTS AND DISCUSSION
Controlled expression of reporter adenovirus
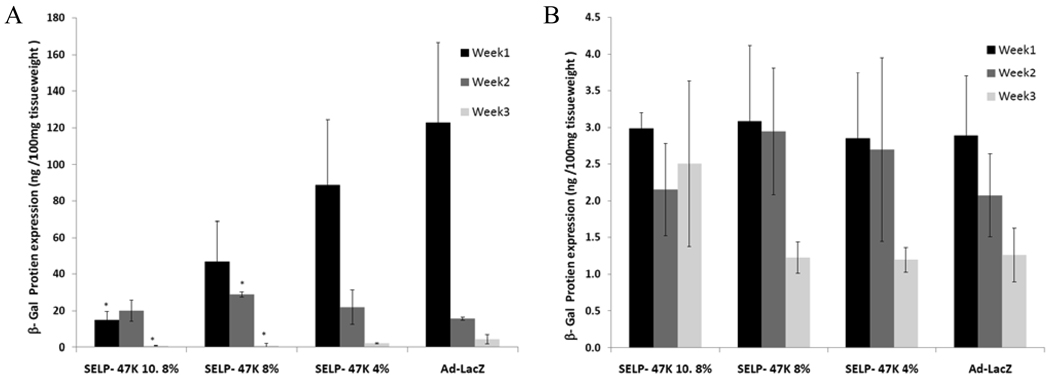
Despite promising outcomes for the treatment of various cancers, clinical application of adenoviral vectors remains a challenge. Acute liver toxicity, transient expression at the tumor site, and immunogenicity have severely undermined the efficacy of high dose adenovirus administration19,20. The limitation of long-term sequential intratumoral injections of adenoviruses such as inadequate intratumoral distribution and vector dissemination to liver, have emphasized the need for localized controlled delivery21. SELP-47K matrices have previously shown to localize and prolong green florescent protein (GFP) expression within the tumor, however, quantitative expression levels of the delivered transgenes remain to be measured14. The current study investigates the influence of SELP-47K polymer concentration on the magnitude and duration of gene expression in the tumor as well as liver tissues in nude mice. Results indicate that among the SELP-47K compositions transfection levels of β-gal increased with decrease in polymer concentration during the first week (Figure 2A). The high levels of transfection observed at lower polymer concentration can be explained by the lower crosslinking density that results in increased viral release13.
Figure 2.
β-galactosidase expression levels (ng/100mg tissue weight) in tumor (A), and liver (B) from 5 × 108 PFU of Ad-LacZ in10.8, 8, and 4wt% SELP-47K compared to free Ad-LacZ over 3 weeks, * p <0.05.
Further, the extent of localization by SELP-mediated delivery was evaluated by determining the β-gal expression levels in the liver (Figure 2B). Transfection levels in the liver over a 3 week period were comparable among the 3 SELP concentrations tested as well as free Ad-LacZ, with no statistically significant difference.
As shown in figure 2A, we compared the β-gal gene expression in tumors injected with free adenovirus to that administered with 4 wt% SELP-47K. At week 1 Ad- LacZ produced 1.5 fold higher transfection level than when delivered along with 4 wt% SELP-47K. However by week 2 the expression levels in the virus alone group decreased to levels slightly lower than the polymer-virus group. Further, 3 weeks post injection the expression levels were reduced in all groups and the difference was not statistically significant between SELP-47K 4 wt% and Ad-LacZ. Based on these results 4 wt% SELP-47K was selected for matrix-mediated delivery of the therapeutic gene vector (Ad-Tk).
Expression levels of the reporter gene Luciferase was also compared between animals injected with virus alone and in combination with 4 wt% SELP-47K using bioluminescence imaging (Figure 3). Animals injected with Ad-Luc in saline alone displayed a higher expression during the first week, however, when injected along with the SELP, Luciferase had 4-fold and 8-fold higher expression levels in the second and third weeks respectively (p<0.05). It is noteworthy that after week 1, both SELP group and free adenoviral group showed a second area of gene transfection. Further analysis confirmed that this area corresponds to transmitted light from the liver. During the first week the ratios of tumor/liver expression were high enough for the software algorithm to consider liver expression as background level. In the second and third weeks, expression was high enough to interfere with image of the tumor Luc gene expression.
Figure 3.
Typical time course tracking of athymic nude mice with JHU-012 xenografts intratumorally injected with Ad-Luc by bioluminescence (Luciferin/Luciferase assay system). Group on left received an intratumoral injection of Ad-Luc in saline. Mice on right received injection of Ad-Luc in 4 wt% SELP-47K. Luciferase expression one week (A), two weeks (B) and three weeks (C) post injection. Graphs show photon counts from each group. * p <0.05.
This data along with similar pattern of β-gal expression can possibly be due to controlled release of adenoviral vector from SELP matrix compared to instantaneous distribution of free adenoviral particles into the tumor tissues in case of virus injected in saline. However, in case of free adenovirus injection in saline, the virus moved beyond tumor tissues before that in SELP matrix, as can be extrapolated from the significantly higher expression level of Luciferase in SELP group at second and third week post injection.
Compared to β-gal colorimetric assay bioluminescence has the advantage that gene expression can be observed in the same animal over the course of the study period. It is interesting that in comparing results of gene expression using colorimetric assay to that of bioluminescence detection, results showed much higher sensitivity of bioluminescence imaging, which detected million photon counts even after 3 weeks. Even though bioluminescence imaging has higher sensitivity, the method has inherent limitations related to light attenuation, reflection, and transmission. Results of bioluminescence imaging however confirm that matrix-mediated adenoviral gene delivery localizes and prolongs transfection.
Typical adenoviral gene expression usually lasts for 7–11 days upon local administration22. However, in our study, animals injected with adenoviruses in saline showed extended expression over a three week period. This prolonged expression of reporter genes from adenovirus could be related to poorer than expected clearance from the JHU-012 tumors due to aberrant vasculature23. In addition, the density of collagen fibers and basement membrane in this specific tumor type could also limit the dissemination free virus24. We examined the spatial distribution of adenovirus transfection by staining tumor tissue sections specifically for β-gal expression. SELP-47K effectively localized transfection during the first week (Figure 4A). During the second week transfection began to be observed in more distant places of the tumor beyond the boundary of the injected SELP (Figure 4B). Overall the localization was observed throughout the 3 week period (Figure 4A–C). However, in the case of intratumoral injection of adenovirus alone a quick distribution of the viruses occurred resulting in minimal staining over a 3 week period (Figure 4D–F). The histological data further confirms that SELP-47K effectively localizes the release of virus in the tumor tissue and prolongs viral transfection over a greater period compared to virus injected in saline. Taken together these results along with the bioluminescence and β-gal colorimetric assays demonstrate the benefits of localization and prolonged adenoviral gene expression using 4 wt% SELP-47K as a matrix for controlled release.
Figure 4.
Patterns of Ad-LacZ expression in 4 wt% SELP-47K (A, B, and C) and Ad-LacZ in saline (D, E, and F). β-gal expression is highlighted with white arrows. Note the localizing effect of SELP at week 1 (A) and the spread of β-gal expression throughout the tumor tissue in weeks 2 (B) and 3 (C). Virus in saline shows wide distribution of expression in week one (D), reduction in week two (E) and negligible expression in week three (F).
Antitumor efficacy of SELP-mediated Ad-Tk therapy
Gene-directed enzyme prodrug therapy (GDEPT) using Ad-Tk in conjunction with ganciclovir (GSV) is efficacious in tumor management 25, 26. Therapeutic efficacy of Ad-Tk in JHU-012 is mediated by the conversion of an inactive prodrug (GSV) to the phosphorylated cytotoxic agent in the presence of the Thymidine Kinase (Tk-1) transgene, producing a targeted anticancer effect that is further augmented by the strong “bystander effect” 25, 26. However, transient expression levels of Tk-1 have limited the progress of these therapeutic vectors in the clinic. We have used the Tk-1/GCV combination as a model virus/drug system to demonstrate the therapeutic utility of matrix-mediated adenoviral gene delivery using SELP-47K (4 wt%).
Localized injection of 4 wt% SELP-47K with Ad-Tk into JHU-012 tumors was followed by daily IP administration of ganciclovir (25 mg/kg body weight). Tumor volumes increased in the control groups without Ad-Tk (saline and 4 wt% SELP-47K only) throughout the study. Tumor volumes decreased in the Ad-Tk injected alone and in combination with 4wt% SELP-47K on days 3, 7 and 14, compared to control (PBS and non-therapeutic adenovirus DL312) groups (Figure 5A) (p<0.05). The tumor volume reduction on day 14 observed with Ad-Tk in SELP-47K was significantly less compared to Ad-Tk injected in saline alone (p<0.05). This observation, coupled with the data of the reporter gene distribution, suggests that Ad-Tk when administered without the polymer is cleared from the site of injection more rapidly than when it is incorporated in the hydrogel.
Figure 5.
Antitumor efficacy of 4 wt% SELP-47K. A. Antitumor efficacy of SELP-mediated controlled delivery of therapeutic adenoviruses (Ad-Tk) in a Xenograft model of head and neck cancer (JHU-012). Tumor volumes of groups receiving Ad-Tk injections showed significant reduction in volume when compared to PBS and Ad-DL312 treated groups. The SELP-47K + Ad-Tk group prolonged the antitumor effect longer than the Ad-Tk group and at day 14 tumor volume was significantly less with SELP-47K than without. (P<0.05 in all cases); B. Efficacy of SELP-mediated controlled delivery of Ad-TK in nude mice having JHU-012 as measured by RT-PCR of mRNA isolated from the tumors. At day 14, the SELP-47K + Ad-TK group had significantly higher levels of mRNA expression than the Ad-Tk group. The Ad-TK group showed a continuous decrease over the time course of the experiment while SELP-47K sustained the expression of Ad-Tk with a maximum around day 7.
Real-time PCR (RT-PCR) of mRNA isolated from the tumors show higher Tk expression levels in the 4 wt% SELP-47K + Ad-Tk group compared to Ad-Tk alone on Days 7 and 14, reaching statistical significance on Day 14 (p<0.05) (Figure 5B). Results indicate that adenoviruses when delivered alone produce high levels of transfection initially (Day 3) which tapers down over the two week period (Day 14). Expression observed in the virus alone group is much higher than that of polymer-virus compositions up to day 3 (Figure 5B). However with time transfection levels decline sharply in the Ad-Tk group compared to polymer-virus group. Beyond day 7 (up to 14 days) viruses delivered with 4 wt% SELP-47K produced the highest transfection levels compared to other treatment groups including the virus alone group. These results corroborate with the observations made in the β-gal and luciferase expression studies, further demonstrating the value of SELP in terms of augmenting and prolonging gene expression locally (Figure 2–Figure 4). It is noteworthy that in all adenovirus models measured for assessment of transfection efficiency (β-gal, Luc, and Tk), free virus injections show higher transfection initially, then at time points beyond the first week SELP incorporated virus shows higher transfection efficiency. These results demonstrate the ability of SELP to control gene delivery over longer periods compared to the burst activity followed by dissemination beyond tumor margins in case of free virus injection. In the case of β-gal and Luc delivery with SELP, gene expression was extended up to 3 weeks and up to 2 weeks in the case of Ad-Tk. The earlier activity and advantages of Ad-TK with SELP system in conjunction with GCV may be attributed to the cytotoxic nature of this gene system, compared to non-cytotoxic β-gal and Luc genes. With Ad-Tk delivery tumor cell death can result in higher division rate and more protein synthesis in surviving cells27. This may account for the early higher Tk expression in SELP group compared with β-gal and Luc expression.
CONCLUSIONS
The current study demonstrates that silk-elastinlike polymers can localize and prolong the duration of reporter gene expression from injected therapeutic adenoviruses up to 21 days in head and neck xenograft models. It also demonstrates that adenoviral gene expression levels in the tumor are significantly increased with the use of the SELP matrix. 4 wt% SELP-47K enhanced the anti-tumor efficacy of Ad-Tk/GSV combination compared with Ad-Tk/GSV alone. Together, these studies suggest the potential of recombinant silk-elastinlike matrix systems for controlled delivery of adenoviral vectors to head and neck tumors. These results coupled with the ability to tailor-make SELP copolymers using genetic engineering techniques, show promise for the design and development of novel matrices for delivery of viral vectors to treat head and neck and other tumors.
ACKNOWLEDGMENT
This research was supported by the National Institutes of Health grants (R01-CA107621 (HG) and ED014562 (BO)) and the Utah Science Technology and Research (USTAR) Initiative.
REFERENCES
- 1.Ragin CC, Modugno F, Gollin SM. The epidemiology and risk factors of head and neck cancer: a focus on human papillomavirus. J Dent Res. 2007;86(2):104–114. doi: 10.1177/154405910708600202. [DOI] [PubMed] [Google Scholar]
- 2.Deshpande AM, Wong DT. Molecular mechanisms of head and neck cancer. Expert Rev Anticancer Ther. 2008;8(5):799–809. doi: 10.1586/14737140.8.5.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chin D, Boyle GM, Theile DR, Parsons PG, Coman WB. Molecular introduction to head and neck cancer (HNSCC) carcinogenesis. Br J Plast Surg. 2004;57(7):595–602. doi: 10.1016/j.bjps.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Araki K, Ahmad SM, Li G, Bray DA, Jr., Saito K, Wang D, Wirtz U, Sreedharan S, O'Malley BW, Jr., Li D. Retinoblastoma RB94 enhances radiation treatment of head and neck squamous cell carcinoma. Clin Cancer Res. 2008;14(11):3514–3519. doi: 10.1158/1078-0432.CCR-07-4538. [DOI] [PubMed] [Google Scholar]
- 5.Li D, Guang W, Abuzeid WM, Roy S, Gao GP, Sauk JJ, O'Malley BW., Jr. Novel adenoviral gene delivery system targeted against head and neck cancer. Laryngoscope. 2008;118(4):650–658. doi: 10.1097/MLG.0b013e3181613aba. [DOI] [PubMed] [Google Scholar]
- 6.Kim SY, Yang YS, Hong KH, Jang KY, Chung MJ, Lee DY, Lee JC, Yi HK, Nam SY, Hwang PH. Adenovirus-mediated expression of dominant negative c-myb induces apoptosis in head and neck cancer cells and inhibits tumor growth in animal model. Oral Oncol. 2008;44(4):383–392. doi: 10.1016/j.oraloncology.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Verma IM, Somia N. Gene therapy-promises, problems and prospects. Nature. 1997;389(6648):239–242. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]
- 8.Doukas J, Chandler LA, Gonzalez AM, Gu D, Hoganson DK, Ma C, Nguyen T, Printz MA, Nesbit M, Herlyn M, Crombleholme TM, Aukerman SL, Sosnowski BA, Pierce GF. Matrix immobilization enhances 1 the tissue repair activity 2 of growth factor gene therapy vectors. Hum Gene Ther. 2001;12(7):783–798. doi: 10.1089/104303401750148720. [DOI] [PubMed] [Google Scholar]
- 9.Bellocq NC, Kang DW, Wang X, Jensen GS, Pun SH, Schluep T, Zepeda ML, Davis ME. Synthetic biocompatible cyclodextrin-based constructs for local gene delivery to improve cutaneous wound healing. Bioconjug Chem. 2004;15(6):1201–1211. doi: 10.1021/bc0498119. [DOI] [PubMed] [Google Scholar]
- 10.Wang D, Molavi O, Lutsiak ME, Elamanchili P, Kwon GS, Samuel J. Poly(D,L-lactic-co-glycolic acid) microsphere delivery of adenovirus for vaccination. J Pharm Pharm Sci. 2007;10(2):217–230. [PubMed] [Google Scholar]
- 11.Cappello J, Crissman J, Dorman M, Mikolajczak M, Textor G, Marquet M, Ferrari F. Genetic engineering of structural protein polymers. Biotechnol Prog. 1990;6(3):198–202. doi: 10.1021/bp00003a006. [DOI] [PubMed] [Google Scholar]
- 12.Hwang D, Moolchandani V, Dandu R, Haider M, Cappello J, Ghandehari H. Influence of polymer structure and biodegradation on DNA release from silk-elastinlike protein polymer hydrogels. Int J Pharm. 2009;368(1–2):215–219. doi: 10.1016/j.ijpharm.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dandu R, Cappello J, Ghandehari H. Characterization of structurally related adenovirus-laden silk-elastinlike hydrogels. J Bioact Compat Polym. 2008;(23):5–19. [Google Scholar]
- 14.Hatefi A, Cappello J, Ghandehari H. Adenoviral gene delivery to solid tumors by recombinant silk-elastinlike protein polymers. Pharm Res. 2007;24(4):773–779. doi: 10.1007/s11095-006-9200-5. [DOI] [PubMed] [Google Scholar]
- 15.Dandu R, Ghandehari H. Delivery of bioactive agents from recombinant polymers. Prog in Polym Sci. 2007;(32):1008–1030. [Google Scholar]
- 16.Cappello J, Crissman JW, Crissman M, Ferrari FA, Textor G, Wallis O, Whitledge JR, Zhou X, Burman D, Aukerman L, Stedronsky ER. In-situ self-assembling protein polymer gel systems for administration, delivery, a nd release of drugs. J Control Release. 1998;53(1–3):105–117. doi: 10.1016/s0168-3659(97)00243-5. [DOI] [PubMed] [Google Scholar]
- 17.Dinerman AA, Cappello J, Ghandehari H, Hoag SW. Swelling behavior of a genetically engineered silk-elastinlike protein polymer hydrogel. Biomaterials. 2002;23(21) doi: 10.1016/s0142-9612(02)00164-3. 4203-1420. [DOI] [PubMed] [Google Scholar]
- 18.Buller CJ, Zang XP, Howard EW, Pento JT. Measurement of beta-galactosidase tissue levels in a tumor cell xenograft model. Methods Find Exp Clin Pharmacol. 2003;25(9):713–716. doi: 10.1358/mf.2003.25.9.793338. [DOI] [PubMed] [Google Scholar]
- 19.Swisher SG, Roth JA, Nemunaitis J, Lawrence DD, Kemp BL, Carrasco CH, Connors DG, El-Naggar AK, Fossella F, Glisson BS, Hong WK, Khuri FR, Kurie JM, Lee JJ, Lee JS, Mack M, Merritt JA, Nguyen DM, Nesbitt JC, Perez-Soler R, Pisters KM, Putnam JB, Jr., Richli WR, Savin M, Schrump DS, Shin DM, Shulkin A, Walsh GL, Wait J, Weill D, Waugh MK. Adenovirus-mediated p53 gene transfer in advanced non-small-cell lung cancer. J Natl Cancer Inst. 1999;91(9):763–771. doi: 10.1093/jnci/91.9.763. [DOI] [PubMed] [Google Scholar]
- 20.Mahasreshti PJ, Kataram M, Wang MH, Stockard CR, Grizzle WE, Carey D, Siegal GP, Haisma HJ, Alvarez RD, Curiel DT. Intravenous delivery of adenovirus-mediated soluble FLT-1 results in liver toxicity. Clin Cancer Res. 2003;9(7):2701–2710. [PubMed] [Google Scholar]
- 21.Adams JY, Johnson M, Sato M, Berger F, Gambhir SS, Carey M, Iruela-Arispe ML, Wu L. Visualization of advanced human prostate cancer lesions in living mice by a targeted gene transfer vector and optical imaging. Nat Med. 2002;8(8):891–897. doi: 10.1038/nm743. [DOI] [PubMed] [Google Scholar]
- 22.Fan W, Plaut K, Bramley AJ, Barlow JW, Mischler SA, Kerr DE. Persistency of adenoviral-mediated lysostaphin expression in goat mammary glands. J Dairy Sci. 2004;87(3):602–608. doi: 10.3168/jds.S0022-0302(04)73202-6. [DOI] [PubMed] [Google Scholar]
- 23.Greish K, Fang J, Inutsuka T, Nagamitsu A, Maeda H. Macromolecular therapeutics: advantages and prospects with special emphasis on solid tumour targeting. Clin Pharmacokinet. 2003;42(13):1089–1105. doi: 10.2165/00003088-200342130-00002. [DOI] [PubMed] [Google Scholar]
- 24.Yun CO. Overcoming the extracellular matrix barrier to improve intratumoral spread and therapeutic potential of oncolytic virotherapy. Curr Opin Mol Ther. 2008;10(4):356–361. [PubMed] [Google Scholar]
- 25.Goebel EA, Davidson BL, Graham SM, Kern JA. Tumor reduction in vivo after adenoviral mediated gene transfer of the herpes simplex virus thymidine kinase gene and ganciclovir treatment in human head and neck squamous cell carcinoma. Otolaryngol Head Neck Surg. 1998;119(4):331–336. doi: 10.1016/S0194-5998(98)70073-7. [DOI] [PubMed] [Google Scholar]
- 26.Van der Most RG, Robinson BW, Nelson DJ. Gene therapy for malignant mesothelioma: beyond the infant years. Cancer Gene Ther. 2006;13(10):897–904. doi: 10.1038/sj.cgt.7700935. [DOI] [PubMed] [Google Scholar]
- 27.Valeriote F, van Putten L. Proliferation-dependent cytotoxicity of anticancer agents: a review. Cancer Res. 1975;35:2619–2630. [PubMed] [Google Scholar]