Abstract
Reactive oxygen species mediate a decrease in nitric oxide (NO) bioavailability and endothelial dysfunction, with secondary oxidized and nitrated byproducts of these reactions contributing to the pathogenesis of numerous vascular diseases. While oxidized lipids and lipoproteins exacerbate inflammatory reactions in the vasculature, in stark contrast the nitration of polyunsaturated fatty acids and complex lipids yield electrophilic products that exhibit pluripotent anti-inflammatory signaling capabilities acting via both cGMP-dependent and -independent mechanisms. Herein we report that nitro-oleic acid (OA-NO2) treatment increases expression of endothelial nitric oxide synthase (eNOS) and heme oxygenase 1 (HO-1) in the vasculature, thus transducing vascular protective effects associated with enhanced NO production. Administration of OA-NO2 via osmotic pump results in a significant increase in eNOS and HO-1 mRNA in mouse aortas. Moreover, HPLC-MS/MS analysis showed that NO2-FAs are rapidly metabolized in cultured endothelial cells (ECs) and treatment with NO2-FAs stimulated the phosphorylation of eNOS at Ser1179. These post-translational modifications of eNOS, in concert with elevated eNOS gene expression, contributed to an increase in endothelial NO production. In aggregate, OA-NO2-induced eNOS and HO-1 expression by vascular cells can induce beneficial effects on endothelial function and provide a new strategy for treating various vascular inflammatory and hypertensive disorders.
Keywords: nitrated fatty acids, nitroalkenes, eNOS, HO-1, endothelial cells, NO, electrophiles
Introduction
Nitro-fatty acids (NO2-FA) are byproducts of oxidative reactions between unsaturated fatty acids and both nitric oxide and nitrite (NO2−)-derived species. Current data support that NO2-FA are pluripotent anti-inflammatory cell signaling mediators acting via both cGMP-dependent and -independent mechanisms. While nitro derivatives of oleic and linoleic acid (nitro-oleic acid, OA-NO2; nitro-linoleic acid, LNO2), can decay via a Nef reaction to liberate NO and mediate cGMP-dependent vascular smooth muscle cell relaxation, this reaction is inhibited by both protein reactions and organization into lipid membranes and micelles [1]. Proteomic analysis reveals that NO2-FA derivatives are also electrophilic and readily undergo reversible reaction with GSH, Cys, and His residues of proteins [2, 3]. This electrophilic reaction results in post-translational modifications that alter both protein function and distribution, thus manifesting cell signaling actions via cGMP-independent mechanisms [3]. For example, LPS-induced cytokine and expression of other pro-inflammatory genes by macrophages is attenuated via the NO2-FA adduction of critical thiols in the p65 subunit of NFκB, suppressing p65 DNA binding and downstream expression of NFκB regulated genes [4]. Also, NO2-FA display robust PPARγ ligand activity, a property that promotes the expression of predominantly adaptive and anti-inflammatory genes [5, 6]. Thus, in contrast to peroxidized lipids and lipoproteins that exert pro-inflammatory reactions in the vasculature, all current data support that nM concentrations of NO2-FA exert broad anti-inflammatory and cardioprotective actions [7–10].
One of the hallmarks of inflammatory vascular diseases is a loss of NO bioavailability. This highly lipophilic and readily-diffusible free radical species regulates blood flow and serves important anti-inflammatory actions by inhibiting platelet aggregation, leukocyte adhesion, and expression of pro-inflammatory mediators within the arterial wall [11]. Reactions that contribute to impaired NO signaling include radical-radical interactions [superoxide (O2•−) and peroxyl radical (LOO•) reaction with NO], eNOS uncoupling, and tetrahydrobiopterin (BH4) oxidation [12–14].
Multiple clinical strategies can enhance NO bioavailability, including the use of organic nitrates, sodium nitroprusside [15], and statins [16]. Of relevance, numerous physiological and pathophysiological stimuli can also increase endogenous NO formation by activating eNOS catalytic activity and gene expression. This regulation of eNOS can occur transcriptionally, post-transcriptionally, and post-translationally, resulting in 2- to 3-fold changes in the production of NO that exerts a significant downstream physiological impact. For example, shear stress and other mechanical stimuli increase both the transcription and stability of eNOS mRNA [17–19]. Moreover, shear stress-induced phosphorylation of serine residues of eNOS via the stimulation of Akt and protein kinase A-dependent signaling results in increased eNOS activity [20–22]. These phosphorylation-dependent mechanisms of eNOS regulation do not require a sustained increase in Ca2+, thus representing a Ca2+-independent mode of eNOS activation.
Another mechanism whereby NO-derived species can exert anti-inflammatory signaling actions in the vasculature is via the transcriptional activation of heme oxygenase 1 (HO-1) expression [23, 24]. Heme oxygenase 1 is a 32-kDa enzyme that is the rate limiting step in heme degradation, catalyzing the cleavage of the heme ring yielding equimolar amounts of biliverdin, carbon monoxide (CO), and ferrous iron. Biliverdin is subsequently converted to bilirubin by biliverdin reductase, with these products of heme catabolism limiting vascular inflammation through multiple antioxidant, anti-proliferative, and anti-apoptotic signaling actions [25]. The inhibition of either eNOS or HO-1 expression increases systemic arterial pressure [26–28] and conversely, enhanced eNOS or HO-1 expression normalizes blood pressure in hypertensive animals [29–31]. In aggregate, these studies demonstrate that eNOS- and HO-1-catalzyed production of NO and CO, respectively, can regulate blood pressure via cGMP-dependent mechanisms and lend cytoprotection to vascular inflammatory insults by both cGMP-dependent and –independent mechanisms.
Recently, NO2-FA derivatives have been observed to be produced at high nM concentrations by activated inflammatory cells and both cardiac tissue and mitochondria following ischemia-reperfusion events [8–10, 32]. In light of the ability of this class of signaling mediators to induce anti-inflammatory actions, we evaluated the potential impact of NO2-FA on the vascular cell expression and activity of eNOS- and HO-1 both in vitro and in vivo. We observed that these enzymes were upregulated and thus can serve to transduce the vascular signaling actions of nitroalkene fatty acid derivatives.
Materials and Methods
Materials and chemicals
Anti-HO-1 antibody (SPA-896) was obtained from Stressgen Biotechnologies (Vancouver, Canada); monoclonal anti-eNOS, polyclonal anti-phospho-eNOS (Ser1177/9) were from Transduction Laboratories (Lexington, KY); and anti-phospho-Akt (Ser473), -phospho-p38, and -phospho-ERK p42/44) were from and Cell Signaling Technology (Beverly, MA); anti-actin Ab and the secondary Ab were from Sigma (St. Louis, MO). Nitro-oleic acid (9- and 10-nitro-9-cis-octadecaenoic acid; OA-NO2) and the corresponding internal standard [13C18]-OA-NO2 were synthesized and purified as previously [5].
Cell culture systems
Bovine aortic endothelial cells (BAECs) were isolated from descending thoracic aortas [33] and maintained in Medium 199 (Cellgro; Herndon, VA) containing 10% fetal bovine serum (Hyclone; Logan, UT), 10 μM thymidine (Gibco; Carlsbad, CA), and antibiotic-antimycotic solution (Cellgro). Cells were monitored visually for typical cobblestone morphology indicative of endothelial cells by staining for von Willebrand factor expression and were not passaged for more than eight cycles. Human coronary aortic endothelial cells (HCAECs) were obtained from Lonza Group Ltd (Basel, Switzerland) and were maintained according to manufacturer’s directions. Studies were performed on confluent monolayers of endothelial cells (ECs) over a range of three to seven passages. All cells were grown at 37°C in 95% air and 5% CO2. For all treatments, ECs were serum deprived (for cell synchronization) for 16 h and then treated with NO2-FA, the corresponding native fatty acid, or vehicle (methanol) added to complete media containing 10% serum at indicated time points.
In vivo administration of OA-NO2
All animal studies were approved by the University of Pittsburgh Institutional Animal Care and Use Committee (Approval 0710454). Osmotic mini-pumps (7 d delivery, ALZET®, Durect Corporation, CA, USA) containing either OA-NO2 (3 mg/kg/d) or the parent fatty acid oleic acid (OA, 3 mg/kg/d) were subcutaneously implanted in C57BL/J6 male mice, 8–10 wks of age (Jackson Laboratories, Bar Harbor, ME). Briefly, mice received a pain-relief injection, Buprenex (0.05 mg/kg, SQ) prior to surgical implantation and were anesthetized using isoflurane. A small longitudinal incision was made for subcutaneous implantation of the osmotic mini-pump in the nape of the neck of the mouse. The pump was inserted and the skin incision was closed using Stoelting 9mm staples (Wood Dale, IL).
Lipid extraction of serum
Blood was collected from the saphenous vein of mice, transferred to a Microtainer® Brand Serum Separator Tube (Becton Dickinson and Company, Franklin Lakes, NJ), and allowed to clot for 2 h at room temperature. The serum fraction was obtained by centrifugation at 6000 × g at room temperature for 5 min. Serum (10–20 μl) was combined with cold acetonitrile (1:4, −20°C) and centrifuged at 2500 rpm for 15 min at 4°C to obtain lipid extracts. A final concentration of 0.5 ng/ml of internal standard ([13C]9- and 10-nitro-octadenoic acid, termed [13C]-OA-NO2, m/z 344) was added during extraction to correct for losses due to sample preparation. In the preparation of this internal standard and the use of more accurate gravimetric determination of concentration, it was appreciated that plasma OA-NO2 concentrations determined with previous internal standard preparations of [13C]-OA-NO2 led to a 3.2-fold underestimation of endogenous blood and tissue concentrations of OA-NO2 (in particular [10, 34, 35]).
Endothelial cell metabolism of OA-NO2
BAECs were grown to confluence on 6-well-plates and incubated at 37°C for 90 min with 5 μM oleic acid, OA-NO2 or [13C]-OA-NO2 in 2 ml Hank’s buffered salt solution (HBSS). Cell solution was collected at baseline and after 5, 15, 30, 60, and 90 min. After each time point, cells were rinsed twice with HBSS and scrape harvested in HBSS. HBSS (from cells at indicated time points), cell lysate and a control HBSS solution (treatment with lipids at 37°C for indicated time point) were deproteinized and the lipids extracted with acetonitrile (ACN) as mentioned above. Metabolite analyses were performed by high performance liquid chromatography-electrospray (HPLC, Shimadzu CBM20A, Japan) ionization mass spectrometry (HPLC-ESI MS/MS) using a hybrid triple quadrupole mass spectrometer (API 5000, Applied Biosystems/MDS Sciex, Thornhill, ON, Canada) as described previously for lipid extraction of blood [34]. For ECs, to characterize OA-NO2 and/or its metabolites, reverse-phase HPLC was used to resolve the NO2-FAs using a 20 × 2-mm Gemini C18 3μm Mercury MS column (Phenomenex, Torrance, CA, USA) at a flow rate of 0.75 ml/min using a gradient elution with 0.1% acetic acid in water as solvent A and 0.1% acetic acid in 100% ACN as solvent B. For [13C18]-OA-NO2, elution was carried out with the following gradient profile: 0–0.5 min 11% of B, 0.5–4 min of 11–99% B, 4–5 min 99% of B, 5–5.1 min 99–11% of B, 5.1–7 min 11% of B; and for [13C18]-OA-NO2 metabolites, the gradient profile consisted of: 0–1 min 11% of B, 1–9 min of 11–100% B, 9–11 min 100% of B, 11–11.1 min 100–11% of B, 11.1–14 min 11% of B. Enhanced product ion (EPI) analysis was performed in the negative-ion mode to generate characteristic and identifying fragmentation patterns of adducted eluting species with precursor masses of m/z 344.3, [13C18]-OA-NO2; 346.2, specific saturated 9-nitro-octadecanoic acid (9-SA-NO2, where SA represents stearic acid) represented as [13C18]-SA-NO2; and 362.3, 9-nitro-10-hydroxyoctadecanoic acids or 10-nitro-9-hydroxyoctadecanoic acids, represented as [13C18]-OA-(OH)-NO2.
RNA isolation and real time PCR analysis
Mouse aorta and total cellular RNA (from BAECs and HCAECs) were isolated using RNeasy kit (Qiagen, Valencia, CA). Reverse transcription to cDNA was performed using iScript cDNA Synthesis Kit (BioRad) according to manufacturer’s instructions. Real-time quantitative PCR analysis was performed with a StepOne Real-Time PCR System (Applied Biosystems, Foster City, CA). Primer-probe sets were purchased from Qiagen (Hs_NOS3_1_FAM QuantiTect Gene Expression) and Applied Biosystem’s “Assays-on-demand”. Expression was normalized to actin.
Western blotting analysis
Both BAECs and HCAECs were rinsed with ice cold phosphate buffer saline (PBS) and homogenized in lysis buffer consisting of 1% Triton X-100, 10 mM NaF, 1 mM vanadate, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin in Tris-buffer saline at pH 7.5. The cells were disrupted by sonication on ice and then centrifuged at 12,000 × g for 5 min at 4 °C. The protein concentration was determined by the BCA protein assay kit (Pierce, Rockford, IL). Protein was denatured by boiling, resolved by SDS-PAGE, and transferred to nitrocellulose (BioRad, Hercules, CA). Membranes were probed with antibodies to eNOS at 1:2000, HO-1 at 1:5000, all phospho-antibodies (eNOS, Akt, ERK1/2, p38) at 1:1000 overnight and then washed with TTBS, incubated with horseradish peroxidase-conjugated antibodies (Sigma) at 1:25000 dilution. Immunoreactive bands were detected using chemiluminescence (Amersham, Piscataway, NJ). To verify protein loading, membranes were subsequently stripped and reprobed with mouse monoclonal antibodies against β-actin (Sigma) at a dilution of 1:1000.
Assessment of NO production
NO was determined by the accumulation of nitrite (NO2−), the stable oxidation product of NO, in aqueous medium. NO was detected by chemiluminescence following the reduction of NO2− with KI and CuSO4, according to manufacturer’s instructions (Sievers). BAECs and HCAECs were grown to confluency in six-well plates in 10% fetal bovine serum medium. The medium was removed and cells were washed twice with HBSS at 37°C prior to incubation with 0.75 ml of HBSS containing 25 μM L-arginine plus the addition of NO2-FA, native FAs, or vehicle (MeOH) for 5–120 min. Following treatment, the HBSS plus treatment was collected from each well and centrifuged at 1,000 × g for 10 min. The resulting supernatant was then injected (50 μl via Hamilton syringe) into the Sievers nitric oxide analyzer (NOA). Each treatment was performed in triplicate and normalized to cell protein content. A serial dilution of nitrite standards were prepared for each experiment using a freshly prepared NaNO2 solution. Background levels of NO2− concentrations were determined using samples of dH2O and HBSS containing L-arginine.
Statistical analysis
Results are from at least three independent experiments and data are expressed as mean ± SEM. Statistical analysis was performed with GraphPad Prism and the data were analyzed by either Student’s t test or 1-way ANOVA with Student Newman-Keuls post hoc comparisons. All results are considered significant at p < 0.05.
Results
Serum OA-NO2 levels
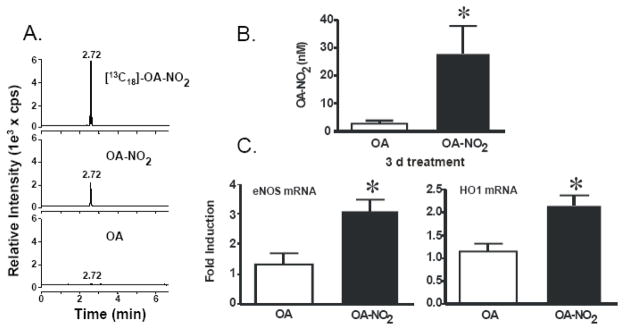
To investigate the actions of OA-NO2 on eNOS and HO-1 expression in vivo, osmotic mini-pumps containing OA or OA-NO2 were implanted subcutaneously in C57Bl/J6 mice. Representative chromatographs of lipids extracted from serum of control and OA-NO2 treated mice (MRM, m/z=326/46) revealed identical retention times to that of internal standard (MRM, m/z = 344/46 for [13C]OA-NO2) as detected using a 13C isotope dilution by reverse-phase HPLC with electrospray ionization triple quadrupole mass spectrometry (ESI MS/MS) in the negative ion mode (Fig 1A). Administration of OA-NO2 (3 mg/kg/d for 3 d) resulted in a significant increase in plasma levels of OA-NO2 (28.3±9.6 nM) compared to OA-treated mice (2.72±0.96 nM) (Fig. 1B).
Figure 1.
OA-NO2 levels increased in mice treated subcutaneously with OA or OA-NO2 via osmotic mini pump for three days accompanies enhanced eNOS and HO-1 gene expression. Blood was collected from the treated mice. The serum was analyzed by HPLC ESI MS/MS in the negative ion mode using [13C]OA-NO2 as an internal standard by acquiring MRM transitions consistent with the loss of the nitro functional group (46 amu corresponds to the mass of NO2): m/z 326/46 and m/z 344/46 for OA-NO2 and [13C]OA-NO2 respectively. Representative chromatographs of lipids extracted from serum [13C18] OA-NO2 (top panel), OA-NO2 (middle panel), and OA (bottom panel) are shown (A). Free OA-NO2 levels (nM) were determined using ANALYST 1.4 quantitation software (B). Real time PCR analysis was performed for eNOS and HO-1 from aortas of mice with the osmotic mini pump for 3 d. Administration of OA-NO2 (3 mg/kg/d for 3 d) increased eNOS (left) and HO-1(right) mRNA levels compared to OA-treated mice. eNOS and HO-1 mRNA levels were normalized to Actin (C). Data are expressed from 6–8 mice per group and expressed as mean ± SEM.
OA-NO2 induces in vivo eNOS and HO-1 expression in mouse aorta
There was a 3.0-fold increase in eNOS mRNA expression in mouse aorta induced by OA-NO2 administration, compared to native fatty acid (OA)-treated mice. The administration of OA-NO2 also significantly upregulated HO-1 mRNA expression (1.9-fold) in the mouse aorta as determined by multiplex real time PCR analysis (Fig. 1C).
HO-1 induction by OA-NO2 in cultured vascular cells
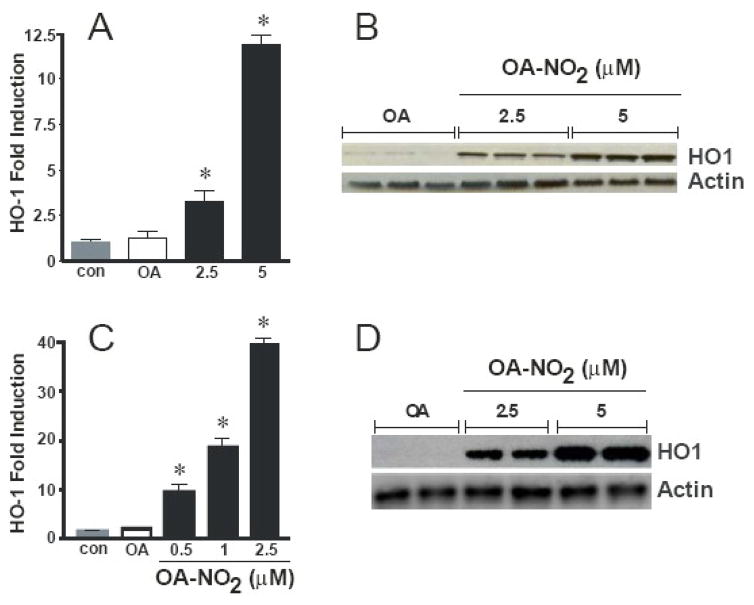
Previous observations have shown that the majority of the NO2-FAs are rapidly adducted to plasma lipoproteins, proteins, and low molecular weight thiols in vivo and in vitro. Thus, experimental conditions were conducted in complete media containing 10% serum (which acts a sink for adduction) therefore the treatment concentrations ranged from 1–5 μM to ensure sufficient quantities of non-adducted, free OA-NO2. Incubation of endothelial cells with OA-NO2 for 6 h significantly induced HO-1 mRNA expression in a dose-dependent manner with a 3.3- and 11.9-fold increase over controls at 2.5 and 5 μM, respectively. There was no change in HO-1 mRNA expression upon treatment with similar concentrations of native OA, as detected by multiplex real time analysis (Fig. 2A). Similar results were obtained by treating ECs under the same conditions with primers specific for HO-1 and 18S using a SYBR green method detection system (not shown). Western blot analysis revealed a robust induction of HO-1 protein expression after cell treatment with 1 and 2.5 μM OA-NO2 for 24 h (Fig. 2B).
Figure 2.
OA-NO2 induces HO-1 mRNA and protein in endothelial and vascular smooth muscle cells. Endothelial cells were incubated with 2.5 and 5 μM OA-NO2 or the native fatty acid OA for 16 hrs and real time PCR analysis was performed (A). Endothelial cells were treated with the same concentrations of OA-NO2 or OA for 24 hr and Western blotting was performed (B). VSMCs were treated with the indicated concentrations of OA-NO2 or OA for 2.5 hr and 24 hr and real time PCR analysis (C) and Western blotting (D) was performed, respectively. The Western blot is a representative of 5–7 separate experiments. The real time PCR analysis results are derived from at least five independent experiments and data are expressed as mean ± SEM.
The OA-NO2-mediated induction of HO-1 expression occurred not only in cultured endothelial vascular cells but also in rat aortic smooth muscle cell (RASMC) cultures. Exposure to OA-NO2 for 2.5 h to concentrations of 1 and 2.5 μM OA-NO2 resulted in an induction of HO-1 mRNA expression of 7.4- and 14.2-fold, respectively (Fig. 2C). Moreover, Western blot analysis showed HO-1 protein expression was substantially increased compared to controls 24 h after treatment with the same concentrations of OA-NO2 (Fig. 2D).
OA-NO2 increases eNOS expression and NO release in cultured endothelial cells
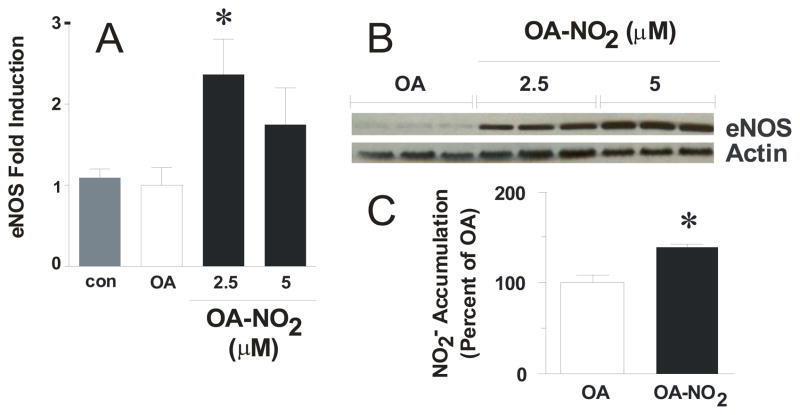
Cells were treated with 1 and 2.5 μM OA-NO2 for 16 h. OA-NO2 significantly induced eNOS mRNA expression over controls and native OA, as determined by multiplex real time PCR analysis (Fig. 3A). Western blot analysis showed a dose-dependent increase in eNOS protein expression following treatment with 2.5 and 5 μM OA-NO2 for 24 h, respectively (Fig. 3B). Additionally, OA-NO2-mediated increases in eNOS expression resulted in a significant increases in cellular NO generation as determined by NO2− accumulation (basal NO2− = 326.5±8.7 pmol/mg protein) in the media which was normalized to cell protein content and reported as a percent of the OA control (Fig 3C).
Figure 3.
Endothelial cells increase eNOS following OA-NO2 treatment. Cells were incubated for 16 hr with indicated concentrations of OA-NO2 or OA and real time PCR analysis was performed (A) and 24 hr for Western blotting (B). OA-NO2 treatment for 16 hr stimulates NO release from ECs. NO generation was determined in OA- and 2.5 μM OA-NO2-treated confluent ECs for 16 hrs using Sievers nitric oxide analyzer (NOA). The levels of nitrite accumulation have been normalized to protein content of the ECs and are reported as a percent of the OA control. Basal levels of NO2− = 326.5 ± 8.7 pmoles/mg protein. Results are derived from at least five independent experiments and data are expressed as mean ± SEM.
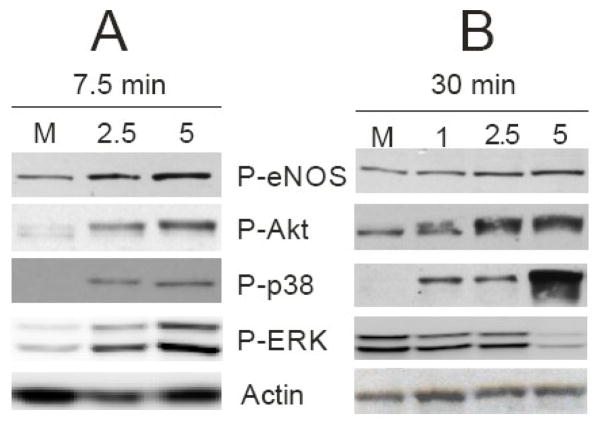
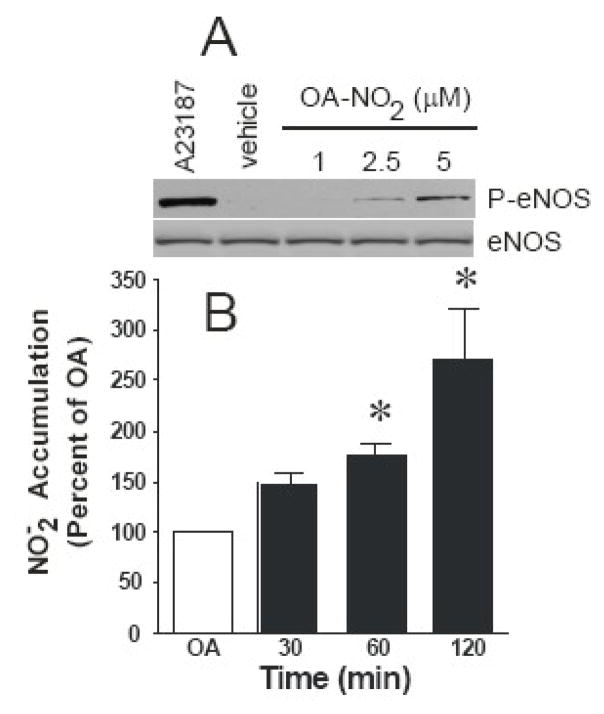
A critical eNOS regulatory event occurs by phosphorylation of serine1177/9, resulting in a 2- to 3-fold increase in eNOS distribution, activity, and NO generation, therefore the link between OA-NO2-induced NO production and activation of eNOS signaling cascades was explored. Incubation of cells with OA-NO2 for 7.5 min resulted in the phosphorylation of eNOS at Ser1179 (Fig. 4A). Potential upstream pathways of eNOS activation include the phosphorylation of Akt, p38, and ERK [36–38]. Treatment with OA-NO2 stimulated phosphorylation of both Akt (at Ser473) and p38 in a dose-dependent manner at 7.5 min (Fig. 4A), a reaction that was still sustained at 30 min (Fig. 4B). ERK activation by OA-NO2 peaked at approximately 7.5 min and then decreased rapidly below basal levels at 15 min (data not shown) and 30 min (Fig. 4B).
Figure 4.
OA-NO2 treatment stimulates phosphorylation of eNOS, Akt, ERK, and p38. Confluent ECs were stimulated with 2.5 and 5 μM OA-NO2 for 7.5 (A) and 30 min (B) and Western blotted for P-eNOS, P-Akt, P-p38, P-ERK, and actin. The results are a representative of 5–7 separate experiments.
The ability of OA-NO2 to stimulate eNOS phosphorylation at Ser1179 led to an increase in NO production over time in BAECs. The media was collected and analyzed for NO-derived NO2− levels after treating cells with 2.5 μM OA-NO2 for 30, 60, and 120 min. This NO2− accumulation in the media was normalized for cell protein content (basal NO2− = 4.8 ±0.2 pmol/min/mg protein) and reported as a percent of OA control. No changes in NO2− accumulation were observed for untreated, vehicle and native fatty acid treated cells at the same sampling times (not shown). Additional control studies revealed that the addition of 2.5 μM OA-NO2 to culture medium for 30, 60, and 120 min did not liberate detectable NO levels, in the absence of cells (not shown). Exposure of cells to OA-NO2 increased NO2− accumulation 1.5-, 1.8-, and 2.7-fold over control at 30, 60, and 120 min, respectively (Fig. 5B).
Figure 5.
OA-NO2 treatment stimulates NO release from ECs. NO generation was determined in OA- and 2.5 μM OA-NO2-treated confluent ECs for the indicated times using Sievers nitric oxide analyzer (NOA). The levels of nitrite have been normalized to protein concentration (basal NO2− = 4.8 ± 0.2 pmoles/min/mg protein) and are reported as a percent of control at each individual time point (30, 60, and 120 min). Results are derived from at least five independent experiments and data are expressed as mean ± SEM.
OA-NO2 is metabolized by ECs
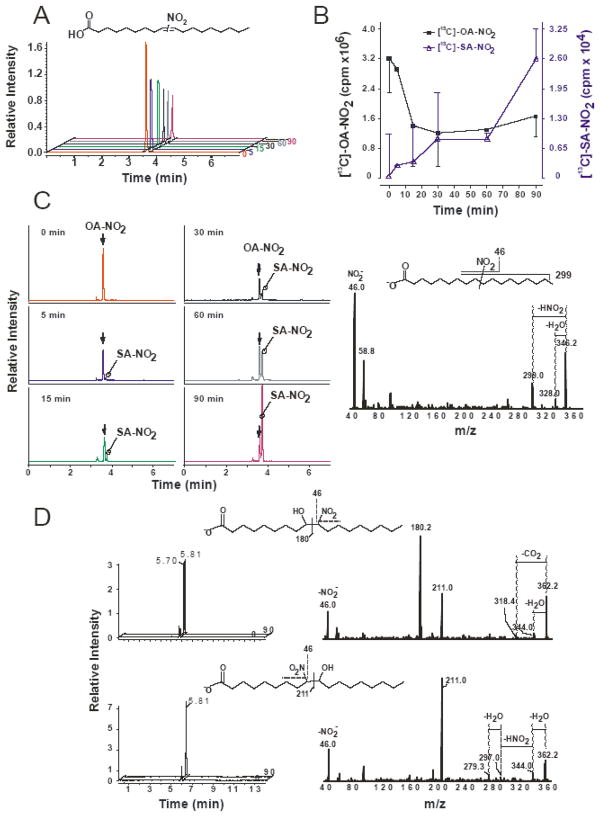
NO2-FA metabolism may either inactivate or form bioactive species. To test the ability of ECs to metabolize OA-NO2 without interference coming from endogenous NO2-FAs and their metabolites, ECs were rinsed with HBSS (to remove complications of protein adduction with serum in the media) and supplemented with a mixture of 9- and 10-nitro regio-isomers of [13C18]-OA-NO2 (Fig 6A, inset) in HBSS. These [13C18]-OA-NO2 metabolism studies were conducted by examining cell lysate and the extracellular release from the ECs by collecting the HBSS over the indicated time course. Only in the presence of ECs did [13C18]-OA-NO2 undergo decay in HBSS (~35% remaining after 30 min) as evidenced by the representative HPLC-MS/MS analysis of [13C18]-OA-NO2 over time (0–90 min) (Fig. 6A). The decreases in [13C18]-OA-NO2 concentration could be partially due to a minimal non-enzymatic decay resulting in the formation of nitro-hydroxy products, a key reaction in the absence of cells [1]. Additionally, electrophilic adduction with nucleophilic residues (i.e.- Cys and His) form a ‘reversible sink’-the nitroalkene moiety present in [13C18]-OA-NO2 is rapidly saturated to a nitroalkane forming nitro-stearic acid ([13C18]-SA-NO2, 18:0-NO2). This product is no longer electrophilic and becomes detectable in the HBSS in a time-dependent manner (Fig. 6C, left panel). The [13C18]-SA-NO2 metabolite was detectable in HBSS as early as 5 min after cell treatment and yielded increasing concentrations over 90 min (Fig 6C, left panel). There is a corresponding loss of the [13C18]-OA-NO2 (filled squares) and the formation of [13C18]-SA-NO2 (open triangle) over the 90 min time course (Fig 6B). The identity of [13C18]-SA-NO2 was confirmed by the analysis of [13C18]-OA-NO2 by HPLC ESI MS/MS in the negative ion mode by performing product ion analyses concurrent to MRM detection (Fig 6C, right panel). In addition, the non-electrophilic nature of [13C18]-SA-NO2 was confirmed by not reacting with high concentrations of β-mercaptoethanol (not shown) [32]. The spectra shows the major expected fragments from [13C18]-SA-NO2, which are indicative of native fatty acids and NO2-FA derivatives, including the neutral loss of a HNO2 (loss of 47 amu) and H2O (loss of 18 amu) corresponding to m/z 299 and 328, respectively. The HBSS from ECs supplemented with [13C18]-OA-NO2 also contained nitro-hydroxyoctadecanoic acid derivatives ([13C18]-OA-(OH)-NO2) (Fig 6D, left panel). The presence of [13C18]-OA-(OH)-NO2 was confirmed using HPLC ESI MS/MS concurrent with MRM detection [5]. Two [13C18]-OA-(OH)-NO2 derivatives, the 9- and 10-nitro regio-isomers were measured corresponding to the m/z 362 to 211 and to 180 respectively (Fig. 6D, right panel). The main fragmentation products of [13C18]-OA-(OH)-NO2 are shown in Fig. 6D (insets). The same distribution profiles were observed from cell lysates as detected in the HBSS (not shown).
Figure 6.
OA-NO2 is rapidly metabolized by ECs. BAEC were grown to confluence and treated with a mixture of 9- and 10-nitro regio-isomers of [13C18]-OA-NO2 (5 μM) in HBSS over a period of 90 minutes. Equimolar distribution of the 9- and 10-nitro regio-isomers of OA-NO2 is depicted (A, inset) as previously determined ([7], online supplement). The HBSS solution was collected and ECs were scrape harvested at the indicated time points. HBSS, corresponding cell extracts and control HBSS treatments (with [13C18]-OA-NO2 at 37°C at each indicated time point) were extracted using ACN precipitation as described in Materials and Methods and supernatants were analyzed by LC-MS-MS. [13C18]-OA-NO2 was detected following a m/z 344/46 for [13C18]-OA-NO2 and a MRM transition corresponding to the detection of the nitro group (NO2−) with peak at 3.6 min. A continuous decay in peak area was observed for [13C18]-OA-NO2 over the 90 min time course (A). A composite of the chromatograms shows the loss of the [13C18]-OA-NO2 (filled square) and the concomitant formation of [13C18]-SA-NO2 (open triangle) over the period of 90 min (B). [13C18]-OA-NO2 is rapidly saturated to a nitro-stearic acid ([13C18]-SA-NO2). [13C18]-SA-NO2 was detected following a m/z 346/46 MRM transition (C, left panel). Arrow indicates a peak corresponding to the contribution of heavy isotopes from [13C18]-OA-NO2. Fragmentation patterns of product ion analysis (peak at 3.73 min) confirmed the formation of [13C18]-SA-NO2. The major expected fragments from [13C18]-SA-NO2 were detected, corresponding to the formation of 46 (NO2−): m/z 299 (neutral loss of HNO2,-47 amu) and 328 (the loss H2O, −18 amu) (C, right panel). Scheme showing the main fragmentation products (C, inset right panel). The two isomers of [13C18]-OA-(OH)-NO2 were detected following a formation of specific breakdown product ions. The MRM transition m/z 362/180 corresponds to the fragmentation of 9-(OH)-10NO2 (peak at 5.7 and 5.8 min) (D, upper panels), and transition m/z 362/211 corresponds to the fragmentation of 10-(OH)-9NO2 (peak at 5.8 min) (D, lower panels). Fragmentation patterns of [13C18]-OA-(OH)-NO2 isomers (D, right panels) show characteristic fragmentation for vicinal nitro-hydroxy fatty acids (180 and 193 ions) and formation of NO2− (46 ion).
Discussion
This study reveals that subcutaneous administration of nM concentrations of the nitrated fatty acid OA-NO2 to C57Bl/J6 significantly induces vascular eNOS and HO-1 expression. In vitro, OA-NO2 treatment induces a robust increase in eNOS and HO-1 gene expression in cultured vascular cells and activates eNOS by phosphorylation of Ser1179. In aggregate, these responses result in increased NO production by cells and encourage a conferral of greater anti-inflammatory character to the cardiovascular system. Of note, this electrophilic fatty acid nitroalkene derivative is rapidly metabolized in cultured ECs to the non electrophilic metabolites SA-NO2 and OA-(OH)-NO2 (Fig. 6). The SA-NO2 and OA-(OH)-NO2 metabolites of OA-NO2 detected in BAECs represented ~1–2% of initial administered OA-NO2. This low yield of byproducts suggested that these OA-NO2 metabolites are not responsible for the signaling actions of OA-NO2. To test this hypothesis, ECs were treated with supra-physiological concentrations of SA-NO2 (50 μM) or OA-(OH)-NO2 (20 μM), which resulted in no significant increases in eNOS and HO-1 expression (not shown). This indicates that the true active signaling molecule mediating the effects on HO-1 and eNOS is either free or protein-adducted OA-NO2 and not either of these two key metabolites. The intravenous injection of OA-NO2 in mice leads to ~40% conversion of OA-NO2 to SA-NO2 within 5 min [34], indicating significant differences between in vivo and vascular EC metabolism of OA-NO2 to SA-NO2.
Nitro derivatives of unsaturated fatty acids are mainly generated by NO and NO2−-derived reactive species that are produced at increased rates during oxidative inflammatory conditions [9, 10, 12, 39]. These lipid signaling mediators exhibit pluripotent anti-inflammatory actions, acting via both cGMP-dependent and -independent mechanisms. For example, NO2-FA derivatives bind to and activate all three peroxisome proliferator-activated receptor (PPAR) isotypes [5, 6], which have been mediate broad metabolic and anti-inflammatory signaling actions in the vasculature. In addition, NO2-FA derivatives inhibit neutrophil and macrophage activation, inhibit platelet aggregation and suppress both TNFα-induced VCAM-1 expression and monocyte rolling and adhesion to vascular endothelium [4]. When added to neutral aqueous buffer at concentrations below the critical micellar concentration of NO2-FA, these species can undergo a slow Nef reaction to release low yields of •NO [1, 40]. Under biologically relevant conditions that contain plasma lipoproteins, membranes, proteins and low molecular weight thiols, •NO release from NO2-FAs, through the Nef reaction, is inhibited and does not participate in •NO-mediated signaling [41]. Rather, the electron-withdrawing NO2 group renders the vicinal olefinic carbon of unsaturated moieties strongly electrophilic. This in turn facilitates the adduction of nucleophiles such as the amino acids cysteine, lysine, and histidine [2, 3].
In contrast to the predominantly pro-inflammatory reactions mediated by oxidized lipids in inflammatory diseases, electrophilic NO2-FAs-exert pluripotent anti-inflammatory cell signaling responses in both vascular and nonvascular tissues that contrast with many oxidized lipid species (e.g., hydroperoxyl, hydroxyl, aldehyde and keto derivatives) [1, 6, 42, 43]. Recent data ranging from in vitro to clinical studies supports that many electrophilic species are adaptive signaling mediators that act, at least in part, by modifying key thiols and other nucleophilic moieties of transcription factors and signaling proteins. The nitroalkylation of proteins is unique, in that NO2-FA are generated by oxidative inflammatory reactions, are highly thiol-reactive, and undergo post-translational protein modifications (PTM) at rate constants 10–1000 times faster than most other biological electrophiles. These PTMs not only alter protein function but also distribution within the cell, since the addition of a fatty acid moiety to a protein can significantly influence catalytic activity, structure, and protein hydrophobicity [1, 3]. Finally, the ability of electrophilic NO2-FA to undergo reversible PTMs is encouraging from a toxicological perspective, since the reversibility of electrophile-biomolecule reactions appears to correlate with a lack of toxicity [44].
The PTM of proteins by redox-derived electrophiles, particularly by NO2-FAs, can be expected to impact multiple cell signaling pathways [45–47]. For example, NO2-FAs inhibit NFkB activity by nitroalkylating the p65 subunit, thereby suppressing NFkB-mediated pro-inflammatory cytokine and adhesion protein expression [4]. Moreover, NO2-FA also upregulate phase II gene expression via electrophile responsive element (EpRE), also known as the antioxidant responsive element (ARE) [48, 49]. This signaling mechanism is activated by electrophiles such as NO2-FA, 4-hydroxy-2-nonenal (4-HNE), and 4-oxononenal (4-ONE), alkylating critical cysteine residues on Kelch ECH associating protein 1 (Keap1). This results in the liberation of nuclear transcription factor erythroid 2-related factor 2 (Nrf2), translocation to the nucleus, and the transactivation of ARE genes which encode phase-II enzymes responsible for detoxification and cell protection [47, 50].
HO-1 is an adaptive inflammatory mediator that catalyzes the degradation of hemoglobin to ferric iron (Fe2+), CO, and biliverdin, which is subsequently oxidized to bilirubin. Biliverdin, bilirubin, and CO display anti-inflammatory properties- with the latter species sharing many signaling characteristics with NO. For example, CO is highly diffusible, has a longer tissue half-life than NO, ligates the metal center of many Feheme proteins and activates soluble guanylyl cyclase. Moreover, CO elicits other diverse anti-inflammatory, antioxidant, metabolic, and anti-apoptotic signaling actions which may modulate vascular tone [51–54].
During inflammatory conditions HO-1 acts as an adaptive mediator, attenuating vascular pathologies such as ischemia-reperfusion injury, atherogenesis and neointimal hyperplasia [25]. In all of these instances, an early manifestation of vascular dysfunction is decreased bioactivity of eNOS, resulting in impaired NO-dependent vessel relaxation, increased smooth muscle cell proliferation, elevated peroxynitrite (ONOO−) production and enhanced cytokine and cell adhesion protein expression [55]. Oxidative stress, resulting from excess production of ROS and NO-derived species, leads to vascular inflammation. The upregulation of HO-1 and eNOS have shown to protect against oxidative stress-mediated tissue injuries by altering redox balance, influencing cell function, and gene regulation. Consequently, eNOS and HO-1 expression and activity induced by NO2-FAs can exert broad protective effects within the vasculature that suppresses pro-oxidant and pro-inflammatory mediators.
HO-1 is typically regulated at the level of transcription. Several key regulatory binding domains exist in the 5′-flanking region covering over 10-kb of the HO-1 gene including AREs and cyclic AMP response elements (CRE) [25]. Previous data demonstrated LNO2–mediated transcriptional upregulation of HO-1 in human aortic endothelial cells [41]. New evidence supports that LNO2-mediated HO-1 induction is strongly dependent on CRE, NF-E2/AP-1, and proximal E-box sequences of the HO-1 promoter and is not acting solely through activation of Nrf2-dependent transcriptional regulation [56].
Unlike HO-1, eNOS does not appear to be regulated at the level of transcription, as there was no observable difference in promoter activity following treatment of cells transfected with the human eNOS promoter (1625 bp) with either OA-NO2 or LNO2 (not shown). Endothelial-derived NO maintains an anti-proliferative and anti-apoptotic environment in the vessel wall as well as inhibits platelet aggregation, expression of adhesion molecules, and leukocyte adhesion. eNOS expression is regulated by both transcriptional and posttranscriptional mechanisms by numerous physiological stimuli such as shear stress and inflammatory mediators [17, 22, 57, 58]. Both eNOS mRNA and protein induction result in an increase in NO production and more importantly NO bioavailability [18, 59, 60]. Notably, eNOS is regulated by post-translational modifications and protein/protein interactions that are important factors in regulating eNOS activity, expression, and localization. Three events can regulate eNOS via covalent modification- myristoylation, palmitoylation, and phosphorylation. Covalent modifications of eNOS include the co-translational and irreversible myristoylation of the N-terminal Gly and the post-translational and reversible palmitoylation of multiple Cys residues [61, 62]. These covalent modifications are both required for efficient targeting of eNOS to the caveolae of endothelial cells [63]. Nitroalkylation of eNOS can conceivably compete with or supplement other thiol or amino-targeted eNOS lipid modifications, such as S-palmitoylation and N-myristoylation, thereby changing the dynamic regulation of eNOS activity by its subcellular localization. Similar to previous studies with GAPDH [3], NO2-FA may also change the intrinsic function of eNOS by altering lipid-dependent cell membrane protein modifications through myristoylation, palmitoylation, and now nitroalkylation reactions. There are also numerous critical Cys residues that play a significant role in the regulation of eNOS [64]. Nitroalkylation by NO2-FAs may thus also affect eNOS trafficking and function to increase NO bioavailability.
In addition to the above-mentioned PTMs, several phosphorylation sites also influence eNOS activity [65]. The phosphorylation of eNOS at Ser1179 by the redox-sensitive PI3K/Akt, p38, or ERK results in a 2-fold increase in NO production and decreased sensitivity to Ca2+/CaM [20, 36, 37, 66]. Also, agonist-induced activation of eNOS is also regulated, in part, by the dephoshorylation of Thr497 [67, 68] which coincides with phosphorylation at Ser1179 and increased NO production. Herein, OA-NO2 treatment did not significantly alter the phosphorylation status of Thr497 (not shown) but did stimulate eNOS phosphorylation at Ser1179, leading to an increase in NO production.
The upregulation of eNOS and HO-1 activity can contribute to the impact of chronic OA-NO2 administration, via subcutaneous osmotic mini-pumps, on the inhibition of atherosclerotic lesion formation in apoE−/− mice (unpublished). Related to this, HO-1 inhibits atherosclerotic lesion formation in both LDLR−/− [69] and apoE−/− mice [70]. Additionally, gene transfer of eNOS regressed atherosclerosis in rabbits fed a high cholesterol diet [71]. Finally, the induction of eNOS and HO-1 expression in the vasculature by OA-NO2 may result in improved vascular function. This is reinforced by the observation that ex vivo infection of eNOS to atherosclerotic rabbit aortic rings improves vasorelaxation in response to acetylcholine [72].
Another cardiovascular problem, vessel wall injury-induced neointimal hyperplasia, is attenuated by the induction of HO-1 [73, 74] and its metabolite CO [75–77]. We have previously reported that LNO2 induces HO-1 expression in HAECs and rat aortic segments ex vivo [41]. This current study adds new insight, in that OA-NO2 also increases HO-1 expression in VSMCs in vitro and throughout the endothelial and intimal cells of vessels in vivo. Relevant to this, subcutaneous delivery of OA-NO2 also inhibits neointimal hyperplasia following wire-induced vascular injury, with upregulation of HO-1 activity vital in OA-NO2-mediated vascular protection (unpublished). Adenovirus eNOS delivery also markedly inhibits neointima proliferation in the denuded rabbit carotid artery by enhancing endothelial regeneration [78, 79]. Thus, the upregulation of both eNOS and HO-1 may have profound effects on inhibiting atherogenesis and restenosis following angioplasty of occluded vessels.
In summary, the administration of OA-NO2 to mice in vivo can induce multiple signaling actions by increasing eNOS and HO-1 expression, events that are mirrored in cultured vascular endothelial and smooth muscle cells, both in vitro and in vivo. Moreover, NO2-FA derivatives are further metabolized and in part affect eNOS activity and NO availability via PI3K-, p38-, and MAPK-dependent signaling. These events can in turn mediate the anti-inflammatory actions of NO and nitrite-derived fatty acid signaling mediators.
Acknowledgments
This work was supported in part by National Institutes of Health Grants HL8115 and HL64937 (to B.A.F.) and AHA #0525330B (to N.K.H.K). B.A.F. acknowledges a financial interest in Complexa, Inc.
Abbreviations
- ACN
acetonitrile
- AP1
activator protein-1
- apoE−/−
apolipoprotein E knockout
- ARE
antioxidant responsive element
- BAECs
bovine aortic endothelial cells
- BH4
tetrahydrobiopterin
- CO
carbon monoxide
- CRE
cyclic AMP response elements
- CVD
cardiovascular diseases
- EC
endothelial cell
- eNOS
endothelial nitric oxide synthase
- EPI
enhanced product ion
- EpRE
electrophile responsive element
- ERK
extracellular signal-regulated kinase
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HBSS
Hank’s buffered salt solution
- HCAECs
human coronary aortic endothelial cells
- HO-1
heme oxygenase 1
- HPLC-ESI MS/MS
high-performance liquid chromatography electrospray ionization triple quadrupole mass spectrometry
- Keap1
Kelch ECH associating protein 1
- LDL
low-density lipoprotein
- LDLR−/−
low-density lipoprotein receptor knockout
- LNO2
nitro-linoleic acid
- LOO•
peroxyl radical
- m/z
mass-to-charge ratio
- MRM
multiple reaction monitoring
- NFkB
nuclear factor kappa B
- NO
nitric oxide
- NO2−
nitrite
- NO2-FA
nitro-fatty acids
- Nrf2
nuclear transcription factor erythroid 2-related factor 2
- O2•−
superoxide
- OA-(OH)-NO2
nitrohydroxy allylic derivative
- OA
oleic acid
- OA-NO2
nitro-oleic acid
- p38
p38 mitogen-activated protein kinase
- PAGE
polyacrylamide gel electrophoresis
- PBS
phosphate buffer saline
- PI3K
phosphoinositide 3-kinases
- PPARγ
peroxisome proliferator-activated receptor γ
- PTM
post-translational protein modifications
- RASMCs
rat aortic smooth muscle cells
- ROS
reactive oxygen species
- SA-NO2
nitro-stearic acid derivative
- SDS
sodium dodecyl sulfate
- TTBS
tween 20 tris-buffered saline
- 4-HNE
4-hydroxy-2-nonenal
- 4-ONE
4-oxononenal
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schopfer FJ, Baker PR, Giles G, Chumley P, Batthyany C, Crawford J, Patel RP, Hogg N, Branchaud BP, Lancaster JR, Jr, Freeman BA. Fatty acid transduction of nitric oxide signaling. Nitrolinoleic acid is a hydrophobically stabilized nitric oxide donor. J Biol Chem. 2005;280:19289–19297. doi: 10.1074/jbc.M414689200. [DOI] [PubMed] [Google Scholar]
- 2.Baker LM, Baker PR, Golin-Bisello F, Schopfer FJ, Fink M, Woodcock SR, Branchaud BP, Radi R, Freeman BA. Nitro-fatty acid reaction with glutathione and cysteine. Kinetic analysis of thiol alkylation by a Michael addition reaction. J Biol Chem. 2007;282:31085–31093. doi: 10.1074/jbc.M704085200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batthyany C, Schopfer FJ, Baker PR, Duran R, Baker LM, Huang Y, Cervenansky C, Branchaud BP, Freeman BA. Reversible post-translational modification of proteins by nitrated fatty acids in vivo. J Biol Chem. 2006;281:20450–20463. doi: 10.1074/jbc.M602814200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui T, Schopfer FJ, Zhang J, Chen K, Ichikawa T, Baker PR, Batthyany C, Chacko BK, Feng X, Patel RP, Agarwal A, Freeman BA, Chen YE. Nitrated fatty acids: Endogenous anti-inflammatory signaling mediators. J Biol Chem. 2006;281:35686–35698. doi: 10.1074/jbc.M603357200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker PR, Lin Y, Schopfer FJ, Woodcock SR, Groeger AL, Batthyany C, Sweeney S, Long MH, Iles KE, Baker LM, Branchaud BP, Chen YE, Freeman BA. Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands. J Biol Chem. 2005;280:42464–42475. doi: 10.1074/jbc.M504212200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schopfer FJ, Lin Y, Baker PR, Cui T, Garcia-Barrio M, Zhang J, Chen K, Chen YE, Freeman BA. Nitrolinoleic acid: an endogenous peroxisome proliferator-activated receptor gamma ligand. Proc Natl Acad Sci USA. 2005;102:2340–2345. doi: 10.1073/pnas.0408384102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman BA, Baker PR, Schopfer FJ, Woodcock SR, Napolitano A, d’Ischia M. Nitro-fatty acid formation and signaling. J Biol Chem. 2008;283:15515–15519. doi: 10.1074/jbc.R800004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trostchansky A, Souza JM, Ferreira A, Ferrari M, Blanco F, Trujillo M, Castro D, Cerecetto H, Baker PR, O’Donnell VB, Rubbo H. Synthesis, isomer characterization, and anti-inflammatory properties of nitroarachidonate. Biochemistry. 2007;46:4645–4653. doi: 10.1021/bi602652j. [DOI] [PubMed] [Google Scholar]
- 9.Nadtochiy SM, Baker PR, Freeman BA, Brookes PS. Mitochondrial nitroalkene formation and mild uncoupling in ischaemic preconditioning: implications for cardioprotection. Cardiovasc Res. 2009;82:333–340. doi: 10.1093/cvr/cvn323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudolph V, Rudolph TK, Schopfer FJ, Bonacci G, Woodcock SR, Cole MP, Baker PR, Ramani R, Freeman BA. Endogenous generation and protective effects of nitro-oleate in a murine model of focal cardiac ischemia and reperfusion. Cardiovasc Res. 2009 doi: 10.1093/cvr/cvp275. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ignarro LJ. Haem-dependent activation of cytosolic guanylate cyclase by nitric oxide: a widespread signal transduction mechanism. Biochem Soc Trans. 1992;20:465–469. doi: 10.1042/bst0200465. [DOI] [PubMed] [Google Scholar]
- 12.O’Donnell VB, Freeman BA. Interactions between nitric oxide and lipid oxidation pathways: implications for vascular disease. Circ Res. 2001;88:12–21. doi: 10.1161/01.res.88.1.12. [DOI] [PubMed] [Google Scholar]
- 13.Harrison DG. Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest. 1997;100:2153–2157. doi: 10.1172/JCI119751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vasquez-Vivar J, Kalyanaraman B, Martasek P. The role of tetrahydrobiopterin in superoxide generation from eNOS: enzymology and physiological implications. Free Radic Res. 2003;37:121–127. doi: 10.1080/1071576021000040655. [DOI] [PubMed] [Google Scholar]
- 15.Napoli C, Ignarro LJ. Nitric Oxide Releasing Drugs. Annual Review of Pharmacology and Toxicology. 2003;43:97–123. doi: 10.1146/annurev.pharmtox.43.100901.140226. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Gonzalez J, Badimon L. Influence of statin use on endothelial function: from bench to clinics. Curr Pharm Des. 2007;13:1771–1786. doi: 10.2174/138161207780831220. [DOI] [PubMed] [Google Scholar]
- 17.Cai H, McNally JS, Weber M, Harrison DG. Oscillatory shear stress upregulation of endothelial nitric oxide synthase requires intracellular hydrogen peroxide and CaMKII. J Mol Cell Cardiol. 2004;37:121–125. doi: 10.1016/j.yjmcc.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Drummond GR, Cai H, Davis ME, Ramasamy S, Harrison DG. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression by hydrogen peroxide. Circ Res. 2000;86:347–354. doi: 10.1161/01.res.86.3.347. [DOI] [PubMed] [Google Scholar]
- 19.Weber M, Hagedorn CH, Harrison DG, Searles CD. Laminar Shear Stress and 3′ Polyadenylation of eNOS mRNA. Circ Res. 2005;96:1161–1168. doi: 10.1161/01.RES.0000170651.72198.fa. [DOI] [PubMed] [Google Scholar]
- 20.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 21.Boo YC, Hwang J, Sykes M, Michell BJ, Kemp BE, Lum H, Jo H. Shear stress stimulates phosphorylation of eNOS at Ser(635) by a protein kinase A-dependent mechanism. Am J Physiol Heart Circ Physiol. 2002;283:H1819–H1828. doi: 10.1152/ajpheart.00214.2002. [DOI] [PubMed] [Google Scholar]
- 22.Boo YC, Sorescu G, Boyd N, Shiojima I, Walsh K, Du J, Jo H. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: role of protein kinase A. J Biol Chem. 2002;277:3388–3396. doi: 10.1074/jbc.M108789200. [DOI] [PubMed] [Google Scholar]
- 23.Durante W, Kroll MH, Christodoulides N, Peyton KJ, Schafer AI. Nitric Oxide Induces Heme Oxygenase-1 Gene Expression and Carbon Monoxide Production in Vascular Smooth Muscle Cells. Circ Res. 1997;80:557–564. doi: 10.1161/01.res.80.4.557. [DOI] [PubMed] [Google Scholar]
- 24.Motterlini R, Foresti R, Intaglietta M, Winslow RM. NO-mediated activation of heme oxygenase: endogenous cytoprotection against oxidative stress to endothelium. Am J Physiol Heart Circ Physiol. 1996;270:H107–114. doi: 10.1152/ajpheart.1996.270.1.H107. [DOI] [PubMed] [Google Scholar]
- 25.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 26.Gamboa A, Shibao C, Diedrich A, Choi L, Pohar B, Jordan J, Paranjape S, Farley G, Biaggioni I. Contribution of endothelial nitric oxide to blood pressure in humans. Hypertension. 2007;49:170–177. doi: 10.1161/01.HYP.0000252425.06216.26. [DOI] [PubMed] [Google Scholar]
- 27.Johnson RA, Lavesa M, Askari B, Abraham NG, Nasjletti A. A heme oxygenase product, presumably carbon monoxide, mediates a vasodepressor function in rats. Hypertension. 1995;25:166–169. doi: 10.1161/01.hyp.25.2.166. [DOI] [PubMed] [Google Scholar]
- 28.Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, Sherman PA, Sessa WC, Smithies O. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 1996;93:13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao CX, Xu X, Cui Y, Wang P, Wei X, Yang S, Edin ML, Zeldin DC, Wang DW. Increased endothelial nitric-oxide synthase expression reduces hypertension and hyperinsulinemia in fructose-treated rats. J Pharmacol Exp Ther. 2009;328:610–620. doi: 10.1124/jpet.108.143396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sacerdoti D, Escalante B, Abraham NG, McGiff JC, Levere RD, Schwartzman ML. Treatment with tin prevents the development of hypertension in spontaneously hypertensive rats. Science. 1989;243:388–390. doi: 10.1126/science.2492116. [DOI] [PubMed] [Google Scholar]
- 31.Escalante B, Sacerdoti D, Davidian MM, Laniado-Schwartzman M, McGiff JC. Chronic treatment with tin normalizes blood pressure in spontaneously hypertensive rats. Hypertension. 1991;17:776–779. doi: 10.1161/01.hyp.17.6.776. [DOI] [PubMed] [Google Scholar]
- 32.Schopfer FJ, Batthyany C, Baker PR, Bonacci G, Cole MP, Rudolph V, Groeger A, Rudolph TK, Nadtochiy S, Brookes PS, Freeman BA. Detection and Quantification of Protein Adduction by Electrophilic Fatty Acids: Mitochondrial Generation of Fatty Acid Nitroalkene Derivatives. Free Radic Biol Med. 2009 doi: 10.1016/j.freeradbiomed.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poss WB, Huecksteadt TP, Panus PC, Freeman BA, Hoidal JR. Regulation of xanthine dehydrogenase and xanthine oxidase activity by hypoxia. Am J Physiol. 1996;270:L941–946. doi: 10.1152/ajplung.1996.270.6.L941. [DOI] [PubMed] [Google Scholar]
- 34.Rudolph V, Schopfer FJ, Khoo NK, Rudolph TK, Cole MP, Woodcock SR, Bonacci G, Groeger AL, Golin-Bisello F, Chen CS, Baker PR, Freeman BA. Nitro-fatty acid metabolome: saturation, desaturation, beta-oxidation, and protein adduction. J Biol Chem. 2009;284:1461–1473. doi: 10.1074/jbc.M802298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cole MP, Rudolph TK, Khoo NKH, Motanya UN, Golin-Bisllo F, Wertz JW, Schopfer FJ, Woodcock SR, Ali MS, Rudolph V, Zhang J, Chen YE, Agarwal A, Freeman BA, Bauer PM. Nitro-Fatty Acid Inhibition of Neointima Formation After Endoluminal Vessel Injury. Circ Res. 2009 doi: 10.1161/CIRCRESAHA.109.199075. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anter E, Thomas SR, Schulz E, Shapira OM, Vita JA, Keaney JF., Jr Activation of endothelial nitric-oxide synthase by the p38 MAPK in response to black tea polyphenols. J Biol Chem. 2004;279:46637–46643. doi: 10.1074/jbc.M405547200. [DOI] [PubMed] [Google Scholar]
- 38.Chen Z, Yuhanna IS, Galcheva-Gargova Z, Karas RH, Mendelsohn ME, Shaul PW. Estrogen receptor alpha mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J Clin Invest. 1999;103:401–406. doi: 10.1172/JCI5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferreira AM, Ferrari M, Trostchansky A, Batthyany C, Souza JM, Alvarez MN, Lopez GV, Baker PR, Schopfer FJ, O’Donnell V, Freeman BA, Rubbo H. Macrophage activation induces formation of the anti-inflammatory lipid cholesteryl-nitrolinoleate. Biochem J. 2008 doi: 10.1042/BJ20080701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim DG, Sweeney S, Bloodsworth A, White CR, Chumley PH, Krishna NR, Schopfer F, O’Donnell VB, Eiserich JP, Freeman BA. Nitrolinoleate, a nitric oxide-derived mediator of cell function: synthesis, characterization, and vasomotor activity. Proc Natl Acad Sci U S A. 2002;99:15941–15946. doi: 10.1073/pnas.232409599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wright MM, Schopfer FJ, Baker PR, Vidyasagar V, Powell P, Chumley P, Iles KE, Freeman BA, Agarwal A. Fatty acid transduction of nitric oxide signaling: nitrolinoleic acid potently activates endothelial heme oxygenase 1 expression. Proc Natl Acad Sci USA. 2006;103:4299–4304. doi: 10.1073/pnas.0506541103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coles B, Bloodsworth A, Clark SR, Lewis MJ, Cross AR, Freeman BA, O’Donnell VB. Nitrolinoleate inhibits superoxide generation, degranulation, and integrin expression by human neutrophils: novel antiinflammatory properties of nitric oxide-derived reactive species in vascular cells. Circ Res. 2002;91:375–381. doi: 10.1161/01.res.0000032114.68919.ef. [DOI] [PubMed] [Google Scholar]
- 43.Coles B, Bloodsworth A, Eiserich JP, Coffey MJ, McLoughlin RM, Giddings JC, Lewis MJ, Haslam RJ, Freeman BA, O’Donnell VB. Nitrolinoleate inhibits platelet activation by attenuating calcium mobilization and inducing phosphorylation of vasodilator-stimulated phosphoprotein through elevation of cAMP. J Biol Chem. 2002;277:5832–5840. doi: 10.1074/jbc.M105209200. [DOI] [PubMed] [Google Scholar]
- 44.Lin D, Saleh S, Liebler DC. Reversibility of Covalent Electrophile-Protein Adducts and Chemical Toxicity. Chemical Research in Toxicology. 2008;21:2361–2369. doi: 10.1021/tx800248x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ceaser EK, Moellering DR, Shiva S, Ramachandran A, Landar A, Venkartraman A, Crawford J, Patel R, Dickinson DA, Ulasova E, Ji S, Darley-Usmar VM. Mechanisms of signal transduction mediated by oxidized lipids: the role of the electrophile-responsive proteome. Biochem Soc Trans. 2004;32:151–155. doi: 10.1042/bst0320151. [DOI] [PubMed] [Google Scholar]
- 46.Ishii T, Tatsuda E, Kumazawa S, Nakayama T, Uchida K. Molecular basis of enzyme inactivation by an endogenous electrophile 4-hydroxy-2-nonenal: identification of modification sites in glyceraldehyde-3-phosphate dehydrogenase. Biochemistry. 2003;42:3474–3480. doi: 10.1021/bi027172o. [DOI] [PubMed] [Google Scholar]
- 47.Villacorta L, Zhang J, Garcia-Barrio MT, Chen XL, Freeman BA, Chen YE, Cui T. Nitro-linoleic acid inhibits vascular smooth muscle cell proliferation via the Keap1/Nrf2 signaling pathway. Am J Physiol Heart Circ Physiol. 2007;293:H770–H776. doi: 10.1152/ajpheart.00261.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Talalay P, De Long MJ, Prochaska HJ. Identification of a common chemical signal regulating the induction of enzymes that protect against chemical carcinogenesis. Proc Natl Acad Sci USA. 1988;85:8261–8265. doi: 10.1073/pnas.85.21.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prestera T, Talalay P. Electrophile and antioxidant regulation of enzymes that detoxify carcinogens. Proc Natl Acad Sci USA. 1995;92:8965–8969. doi: 10.1073/pnas.92.19.8965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rachakonda G, Xiong Y, Sekhar KR, Stamer SL, Liebler DC, Freeman ML. Covalent modification at Cys151 dissociates the electrophile sensor Keap1 from the ubiquitin ligase CUL3. Chem Res Toxicol. 2008;21:705–710. doi: 10.1021/tx700302s. [DOI] [PubMed] [Google Scholar]
- 51.Liu XM, Peyton KJ, Mendelev NN, Wang H, Tulis DA, Durante W. YC-1 stimulates the expression of gaseous monoxide-generating enzymes in vascular smooth muscle cells. Mol Pharmacol. 2009;75:208–217. doi: 10.1124/mol.108.048314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ndisang JF, Wu L, Zhao W, Wang R. Induction of heme oxygenase-1 and stimulation of cGMP production by hemin in aortic tissues from hypertensive rats. Blood. 2003;101:3893–3900. doi: 10.1182/blood-2002-08-2608. [DOI] [PubMed] [Google Scholar]
- 53.Wang R. Resurgence of carbon monoxide: an endogenous gaseous vasorelaxing factor. Can J Physiol Pharmacol. 1998;76:1–15. doi: 10.1139/cjpp-76-1-1. [DOI] [PubMed] [Google Scholar]
- 54.Freitas A, Alves-Filho JC, Secco DD, Neto AF, Ferreira SH, Barja-Fidalgo C, Cunha FQ. Heme oxygenase/carbon monoxide; biliverdin pathway down regulates neutrophil rolling, adhesion and migration in acute inflammation. British Journal of Pharmacology. 2006;149:345–354. doi: 10.1038/sj.bjp.0706882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas SR, Witting PK, Drummond GR. Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2008;10:1713–1765. doi: 10.1089/ars.2008.2027. [DOI] [PubMed] [Google Scholar]
- 56.Wright MM, Kim J, Hock TD, Leitinger N, Freeman BA, Agarwal A. Human heme oxygenase-1 induction by nitro-linoleic acid is mediated by cyclic AMP, AP-1, and E-box response element interactions. Biochem J. 2009 doi: 10.1042/BJ20090339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anderson HD, Rahmutula D, Gardner DG. Tumor necrosis factor-alpha inhibits endothelial nitric-oxide synthase gene promoter activity in bovine aortic endothelial cells. J Biol Chem. 2004;279:963–969. doi: 10.1074/jbc.M309552200. [DOI] [PubMed] [Google Scholar]
- 58.Bulotta S, Barsacchi R, Rotiroti D, Borgese N, Clementi E. Activation of the endothelial nitric-oxide synthase by tumor necrosis factor-alpha. A novel feedback mechanism regulating cell death. J Biol Chem. 2001;276:6529–6536. doi: 10.1074/jbc.M006535200. [DOI] [PubMed] [Google Scholar]
- 59.Li JM, Shah AM. Mechanism of endothelial cell NADPH oxidase activation by angiotensin II. Role of the p47phox subunit. J Biol Chem. 2003;278:12094–12100. doi: 10.1074/jbc.M209793200. [DOI] [PubMed] [Google Scholar]
- 60.Ramasamy S, Drummond GR, Ahn J, Storek M, Pohl J, Parthasarathy S, Harrison DG. Modulation of expression of endothelial nitric oxide synthase by nordihydroguaiaretic acid, a phenolic antioxidant in cultured endothelial cells. Mol Pharmacol. 1999;56:116–123. doi: 10.1124/mol.56.1.116. [DOI] [PubMed] [Google Scholar]
- 61.Michel T. Targeting and translocation of endothelial nitric oxide synthase. Braz J Med Biol Res. 1999;32:1361–1366. doi: 10.1590/s0100-879x1999001100006. [DOI] [PubMed] [Google Scholar]
- 62.Liu J, Garcia-Cardena G, Sessa WC. Biosynthesis and palmitoylation of endothelial nitric oxide synthase: mutagenesis of palmitoylation sites, cysteines-15 and/or -26, argues against depalmitoylation-induced translocation of the enzyme. Biochemistry. 1995;34:12333–12340. doi: 10.1021/bi00038a029. [DOI] [PubMed] [Google Scholar]
- 63.Shaul PW, Smart EJ, Robinson LJ, German Z, Yuhanna IS, Ying Y, Anderson RG, Michel T. Acylation targets emdothelial nitric-oxide synthase to plasmalemmal caveolae. J Biol Chem. 1996;271:6518–6522. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- 64.Dudzinski DM, Igarashi J, Greif D, Michel T. The regulation and pharmacology of endothelial nitric oxide synthase. Annu Rev Pharmacol Toxicol. 2006;46:235–276. doi: 10.1146/annurev.pharmtox.44.101802.121844. [DOI] [PubMed] [Google Scholar]
- 65.Fulton D, Gratton JP, Sessa WC. Post-translational control of endothelial nitric oxide synthase: why isn’t calcium/calmodulin enough? . J Pharmacol Exp Ther. 2001;299:818–824. [PubMed] [Google Scholar]
- 66.Davis ME, Grumbach IM, Fukai T, Cutchins A, Harrison DG. Shear stress regulates endothelial nitric-oxide synthase promoter activity through nuclear factor kappaB binding. J Biol Chem. 2004;279:163–168. doi: 10.1074/jbc.M307528200. [DOI] [PubMed] [Google Scholar]
- 67.Harris MB, Ju H, Venema VJ, Liang H, Zou R, Michell BJ, Chen ZP, Kemp BE, Venema RC. Reciprocal phosphorylation and regulation of endothelial nitric-oxide synthase in response to bradykinin stimulation. J Biol Chem. 2001;276:16587–16591. doi: 10.1074/jbc.M100229200. [DOI] [PubMed] [Google Scholar]
- 68.Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr(495) regulates Ca(2+)/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res. 2001;88:E68–E75. doi: 10.1161/hh1101.092677. [DOI] [PubMed] [Google Scholar]
- 69.Ishikawa K, Sugawara D, Wang X, Suzuki K, Itabe H, Maruyama Y, Lusis AJ. Heme oxygenase-1 inhibits atherosclerotic lesion formation in ldl-receptor knockout mice. Circ Res. 2001;88:506–512. doi: 10.1161/01.res.88.5.506. [DOI] [PubMed] [Google Scholar]
- 70.Juan SH, Lee TS, Tseng KW, Liou JY, Shyue SK, Wu KK, Chau LY. Adenovirus-mediated heme oxygenase-1 gene transfer inhibits the development of atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2001;104:1519–1525. doi: 10.1161/hc3801.095663. [DOI] [PubMed] [Google Scholar]
- 71.Hayashi T, Sumi D, Juliet PAR, Matsui-Hirai H, Asai-Tanaka Y, Kano H, Fukatsu A, Tsunekawa T, Miyazaki A, Iguchi A, Ignarro LJ. Gene transfer of endothelial NO synthase, but not eNOS, plus inducible NOS regressed atherosclerosis in rabbits. Cardiovasc Res. 2004;61:339–351. doi: 10.1016/j.cardiores.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 72.Mozes G, Kullo IJ, Mohacsi TG, Cable DG, Spector DJ, Crotty TB, Gloviczki P, Katusic ZS, O’Brien T. Ex vivo gene transfer of endothelial nitric oxide synthase to atherosclerotic rabbit aortic rings improves relaxations to acetylcholine. Atherosclerosis. 1998;141:265–271. doi: 10.1016/s0021-9150(98)00180-4. [DOI] [PubMed] [Google Scholar]
- 73.Duckers HJ, Boehm M, True AL, Yet SF, San H, Park JL, Clinton Webb R, Lee ME, Nabel GJ, Nabel EG. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat Med. 2001;7:693–698. doi: 10.1038/89068. [DOI] [PubMed] [Google Scholar]
- 74.Tulis DA, Durante W, Liu X, Evans AJ, Peyton KJ, Schafer AI. Adenovirus-mediated heme oxygenase-1 gene delivery inhibits injury-induced vascular neointima formation. Circulation. 2001;104:2710–2715. doi: 10.1161/hc4701.099585. [DOI] [PubMed] [Google Scholar]
- 75.Kim HP, Wang X, Nakao A, Kim SI, Murase N, Choi ME, Ryter SW, Choi AM. Caveolin-1 expression by means of p38beta mitogen-activated protein kinase mediates the antiproliferative effect of carbon monoxide. Proc Natl Acad Sci USA. 2005;102:11319–11324. doi: 10.1073/pnas.0501345102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tulis DA, Durante W, Peyton KJ, Evans AJ, Schafer AI. Heme oxygenase-1 attenuates vascular remodeling following balloon injury in rat carotid arteries. Atherosclerosis. 2001;155:113–122. doi: 10.1016/s0021-9150(00)00552-9. [DOI] [PubMed] [Google Scholar]
- 77.Tulis DA, Keswani AN, Peyton KJ, Wang H, Schafer AI, Durante W. Local administration of carbon monoxide inhibits neointima formation in balloon injured rat carotid arteries. Cell Mol Biol (Noisy-le-grand) 2005;51:441–446. [PMC free article] [PubMed] [Google Scholar]
- 78.Cooney R, Hynes SO, Sharif F, Howard L, O’Brien T. Effect of gene delivery of NOS isoforms on intimal hyperplasia and endothelial regeneration after balloon injury. Gene Ther. 2006;14:396–404. doi: 10.1038/sj.gt.3302882. [DOI] [PubMed] [Google Scholar]
- 79.Sharif F, Hynes SO, Cooney R, Howard L, McMahon J, Daly K, Crowley J, Barry F, O’Brien T. Gene-eluting Stents: Adenovirus-mediated Delivery of eNOS to the Blood Vessel Wall Accelerates Re-endothelialization and Inhibits Restenosis. Mol Ther. 2008 doi: 10.1038/mt.2008.165. [DOI] [PubMed] [Google Scholar]