Abstract
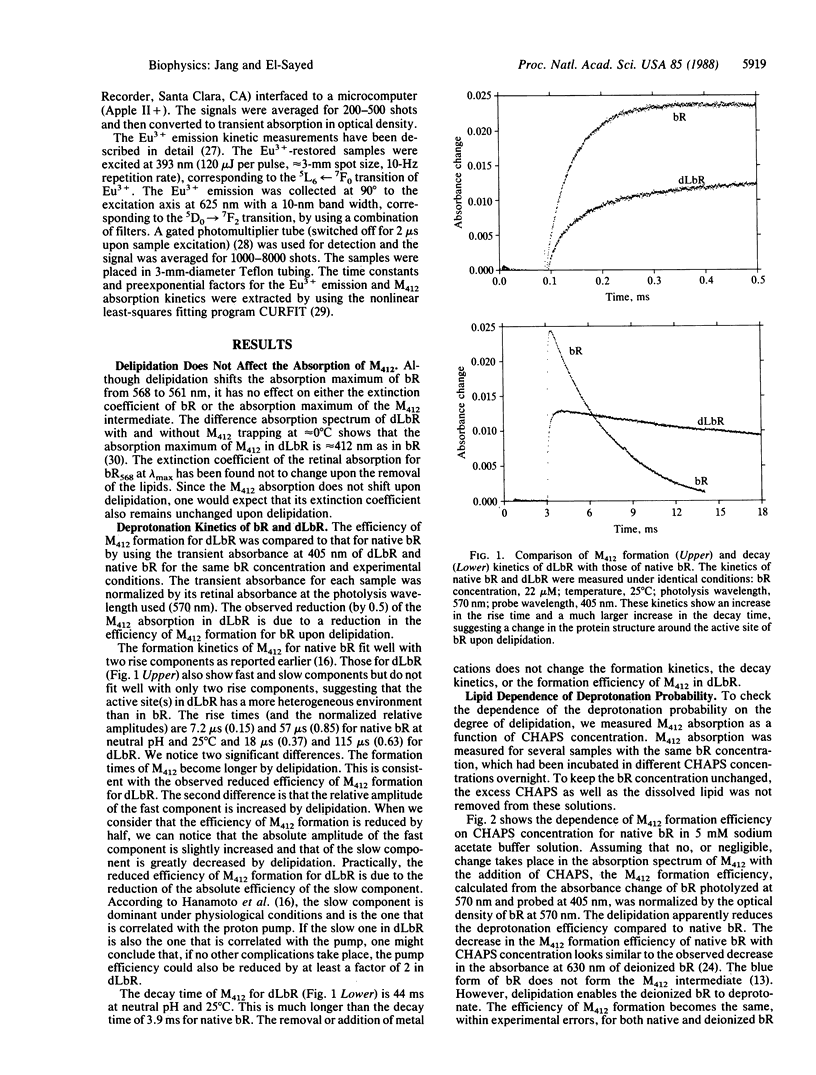
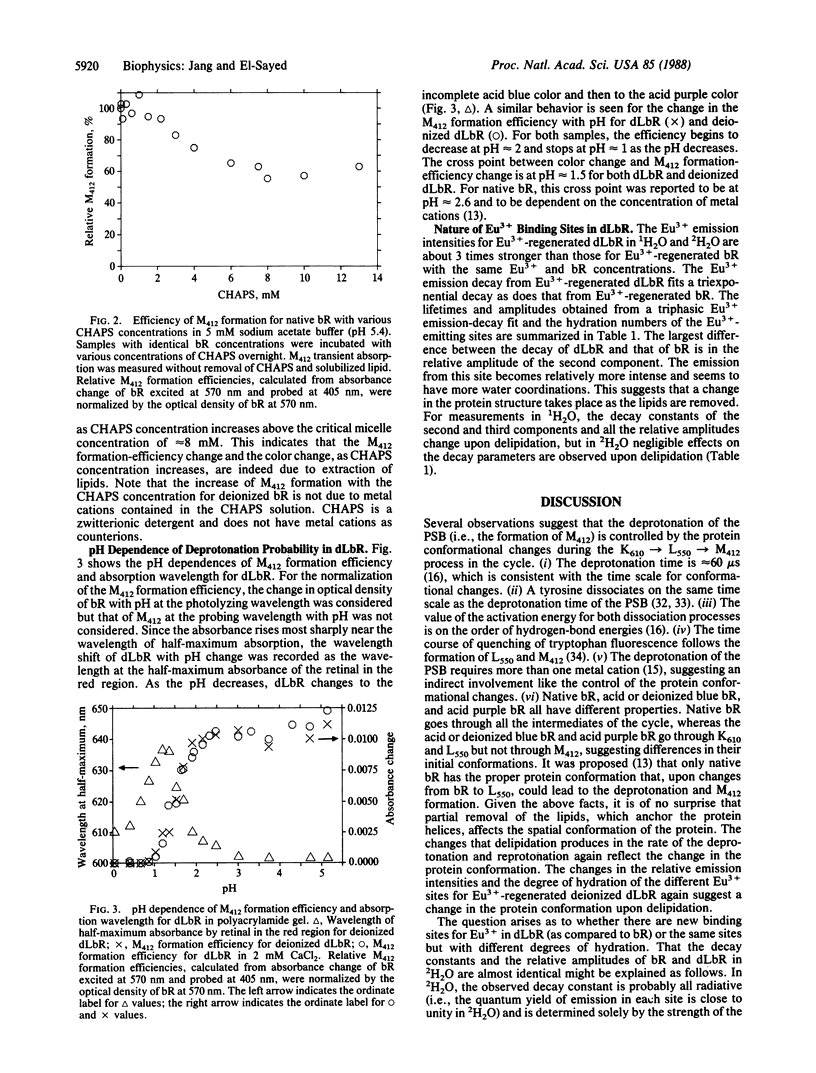
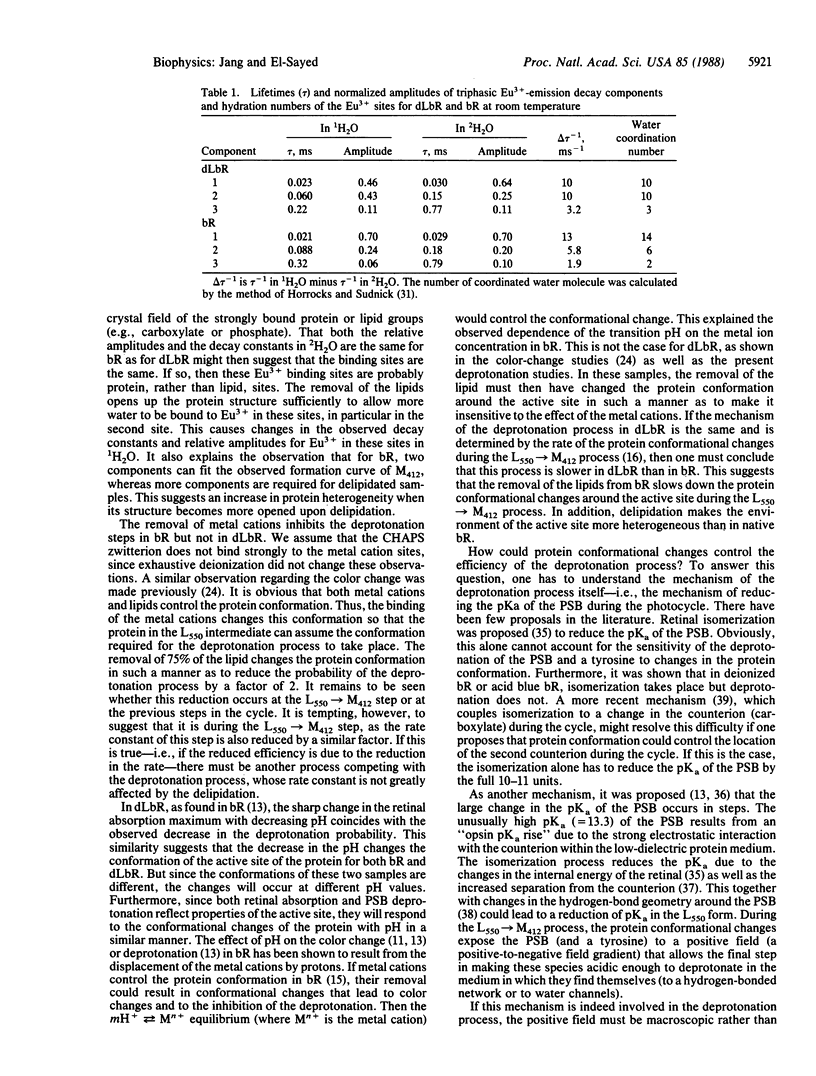
The removal of 75% of the lipid from bacteriorhodopsin caused the following: (i) decreased efficiency and rate of deprotonation of the protonated Schiff base (as monitored by absorption of the M412 intermediate); (ii) increased efficiency of deprotonation of deionized samples; (iii) a decrease by 1 unit in the pH at which deprotonation ceases; (iv) increased intensity of Eu3+ emission in Eu3+-regenerated deionized delipidated samples; (v) increased exposure of the Eu3+ sites to water; and (vi) elimination of the dependence of the deprotonation efficiency on the metal cation concentration. These results are discussed in terms of changes in the protein conformation upon delipidation, which in turn control the deprotonation mechanism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariki M., Lanyi J. K. Characterization of metal ion-binding sites in bacteriorhodopsin. J Biol Chem. 1986 Jun 25;261(18):8167–8174. [PubMed] [Google Scholar]
- Becher B. M., Cassim J. Y. Improved isolation procedures for the purple membrane of Halobacterium halobium. Prep Biochem. 1975;5(2):161–178. doi: 10.1080/00327487508061568. [DOI] [PubMed] [Google Scholar]
- Belliveau J. W., Lanyi J. K. Analogies between respiration and a light-driven proton pump as sources of energy for active glutamate transport in Halobacterium holobium. Arch Biochem Biophys. 1977 Jan 15;178(1):308–314. doi: 10.1016/0003-9861(77)90196-5. [DOI] [PubMed] [Google Scholar]
- Blaurock A. E., Stoeckenius W. Structure of the purple membrane. Nat New Biol. 1971 Sep 29;233(39):152–155. doi: 10.1038/newbio233152a0. [DOI] [PubMed] [Google Scholar]
- Bridgen J., Walker I. D. Photoreceptor protein from the purple membrane of Halobacterium halobium. Molecular weight and retinal binding site. Biochemistry. 1976 Feb 24;15(4):792–798. doi: 10.1021/bi00649a010. [DOI] [PubMed] [Google Scholar]
- Chang C. H., Chen J. G., Govindjee R., Ebrey T. Cation binding by bacteriorhodopsin. Proc Natl Acad Sci U S A. 1985 Jan;82(2):396–400. doi: 10.1073/pnas.82.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronister E. L., Corcoran T. C., Song L., El-Sayed M. A. On the molecular mechanisms of the Schiff base deprotonation during the bacteriorhodopsin photocycle. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8580–8584. doi: 10.1073/pnas.83.22.8580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran T. C., Ismail K. Z., El-Sayed M. A. Evidence for the involvement of more than one metal cation in the Schiff base deprotonation process during the photocycle of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4094–4098. doi: 10.1073/pnas.84.12.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dencher N., Wilms M. Flash photometric experiments on the photochemical cycle of bacteriorhodopsin. Biophys Struct Mech. 1975 May 30;1(3):259–271. doi: 10.1007/BF00535760. [DOI] [PubMed] [Google Scholar]
- Dupuis P., Corcoran T. C., El-Sayed M. A. Importance of bound divalent cations to the tyrosine deprotonation during the photocycle of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3662–3664. doi: 10.1073/pnas.82.11.3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhard M., Gerwert K., Hess B., Kreutz W., Siebert F. Light-driven protonation changes of internal aspartic acids of bacteriorhodopsin: an investigation by static and time-resolved infrared difference spectroscopy using [4-13C]aspartic acid labeled purple membrane. Biochemistry. 1985 Jan 15;24(2):400–407. doi: 10.1021/bi00323a024. [DOI] [PubMed] [Google Scholar]
- Fukumoto J. M., Hopewell W. D., Karvaly B., El-Sayed M. A. Time-resolved protein fluorescence studies of intermediates in the photochemical cycle of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1981 Jan;78(1):252–255. doi: 10.1073/pnas.78.1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaeser R. M., Jubb J. S., Henderson R. Structural comparison of native and deoxycholate-treated purple membrane. Biophys J. 1985 Nov;48(5):775–780. doi: 10.1016/S0006-3495(85)83835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamoto J. H., Dupuis P., El-Sayed M. A. On the protein (tyrosine)-chromophore (protonated Schiff base) coupling in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7083–7087. doi: 10.1073/pnas.81.22.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Hess B., Kuschmitz D. Kinetic interaction between aromatic residues and the retinal chromophore of bacteriorhodopsin during the photocycle. FEBS Lett. 1979 Apr 15;100(2):334–340. doi: 10.1016/0014-5793(79)80364-6. [DOI] [PubMed] [Google Scholar]
- Kimura Y., Ikegami A., Stoeckenius W. Salt and pH-dependent changes of the purple membrane absorption spectrum. Photochem Photobiol. 1984 Nov;40(5):641–646. doi: 10.1111/j.1751-1097.1984.tb05353.x. [DOI] [PubMed] [Google Scholar]
- Kushwaha S. C., Kates M., Martin W. G. Characterization and composition of the purple and red membrane from Halobacterium cutirubrum;. Can J Biochem. 1975 Mar;53(3):284–292. doi: 10.1139/o75-040. [DOI] [PubMed] [Google Scholar]
- Lozier R. H., Bogomolni R. A., Stoeckenius W. Bacteriorhodopsin: a light-driven proton pump in Halobacterium Halobium. Biophys J. 1975 Sep;15(9):955–962. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T. A., Edgerton M. E., Parr G., Greenwood C., Perham R. N. Studies of an acid-induced species of purple membrane from Halobacterium halobium. Biochem J. 1978 May 1;171(2):469–476. doi: 10.1042/bj1710469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowery P. C., Lozier R. H., Chae Q., Tseng Y. W., Taylor M., Stoeckenius W. Effect of acid pH on the absorption spectra and photoreactions of bacteriorhodopsin. Biochemistry. 1979 Sep 18;18(19):4100–4107. doi: 10.1021/bi00586a007. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Hess B. Reversible photolysis of the purple complex in the purple membrane of Halobacterium halobium. Eur J Biochem. 1973 Aug 17;37(2):316–326. doi: 10.1111/j.1432-1033.1973.tb02990.x. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Scheiner S., Hillenbrand E. A. Modification of pK values caused by change in H-bond geometry. Proc Natl Acad Sci U S A. 1985 May;82(9):2741–2745. doi: 10.1073/pnas.82.9.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulten K., Tavan P. A mechanism for the light-driven proton pump of Halobacterium halobium. Nature. 1978 Mar 2;272(5648):85–86. doi: 10.1038/272085a0. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- Szundi I., Stoeckenius W. Effect of lipid surface charges on the purple-to-blue transition of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3681–3684. doi: 10.1073/pnas.84.11.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga F., Ebrey T. The blue membrane: the 3-dehydroretinal-based artificial pigment of the purple membrane. Biochemistry. 1978 May 16;17(10):1915–1922. doi: 10.1021/bi00603a018. [DOI] [PubMed] [Google Scholar]
- Warshel A. Conversion of light energy to electrostatic energy in the proton pump of Halobacterium halobium. Photochem Photobiol. 1979 Aug;30(2):285–290. doi: 10.1111/j.1751-1097.1979.tb07148.x. [DOI] [PubMed] [Google Scholar]



