Abstract
The purpose of this work was to assess the impact of PEGylation on transepithelial transport of anionic poly(amidoamine) dendrimers. Cytotoxicity, uptake and transport across Caco-2 cells of PEGylated G3.5 and G4.5 PAMAM dendrimers were studied. Methoxy polyethylene glycol (750 Da) was conjugated to carboxylic acid-terminated PAMAM dendrimers at feed ratios of 1, 2 and 4 PEG per dendrimer. Compared to the control, PEGylation of anionic dendrimers did not significantly alter cytotoxicity up to a concentration of 0.1 mM. PEGylation of G3.5 dendrimers significantly decreased cellular uptake and transepithelial transport while PEGylation of G4.5 dendrimers led to a significant increase in uptake, but also a significant decrease in transport. Dendrimer PEGylation reduced the opening of tight junctions as evidenced by confocal microscopy techniques. Modulation of the tight junctional complex correlated well with changes in PEGylated dendrimer transport and suggests that anionic dendrimers are transported primarily through the paracellular route. PEGylated dendrimers show promise in oral delivery applications where increased functionality for drug conjugation and release is desired.
Keywords: poly(amidoamine) dendrimers, polyethylene glycol, Caco-2 cells, transport, oral delivery
Introduction
Polymeric drug delivery can improve bioavailability and efficacy of therapeutics with intrinsically poor water solubility and high toxicity [1, 2]. Dendrimers, a class of highly branched polymers, are effective drug delivery vehicles due to their monodispersity and nanoscopic size [3]. With each increase in dendrimer generation, the diameter increases linearly while the number of surface groups increases exponentially. These high density surface groups can be conjugated to drug molecules [4, 5], targeting moieties [6] and imaging agents [7], rendering dendrimers a versatile drug delivery platform [8]. In addition, surface groups on dendrimers can be modified to modulate cytotoxicity [9] and permeation across biological barriers [10, 11].
Polymeric drug delivery systems often fail when applied in oral drug administration, as these systems are generally too large for efficient transport across the epithelial barrier of the intestinal tract. However, previous studies in our laboratory [11-15] and others [16-19] indicate that dendrimers in a specified size and charge window can effectively translocate across gastrointestinal epithelia. In vitro studies suggest that conjugation or complexation of drugs with PAMAM dendrimers improves their transport across the intestinal epithelium. This can enhance the oral bioavailabilty of drugs normally limited to intravenous administration, supporting dendrimers as viable oral drug delivery carriers [20-23]. During their synthesis, PAMAM dendrimers can be produced that are either anionic or cationic in nature, with “full generations” (ie. G1, G2) having amine terminal groups and “half generations” (ie, G0.5, G1.5) possessing carboxylic acid terminal groups. The size and charge of PAMAM dendrimers impact their cytotoxicity and transepithelial transport, with cationic dendrimers showing higher toxicity in vitro [12, 24]. Due to their intrinsically low cytotoxicity and appreciable transepithelial permeation characteristics across Caco-2 monolayers and everted rat intestinal sac models [12, 16], anionic dendrimers show distinct advantages as vehicles for oral drug delivery, with higher generation dendrimers showing the greatest potential because of their large number of modifiable surface groups. The present work aims to further evaluate their potential in facilitating the delivery of drugs across the gastrointestinal tract.
PEGylation of drug delivery systems and bioactive agents is known to reduce toxicity and immunogenicity, influence pharmacokinetics and biodistribution and enhance water solubility [25]. Okuda and colleagues [26, 27] showed that PEGylation of dendritic systems produces desirable biodistribution effects upon intravenous administration; however, the impact of PEGylation on transepithelial transport of PAMAM dendrimers across intestinal cells is presently unknown. In the context of oral drug delivery, polyethylene glycol (PEG) can be used as a surface modifier to modulate the degree and mechanism of transport or can act as drug linker, altering release properties from the polymer backbone. In this paper we report the synthesis and characterization of differentially PEGylated G3.5 and G4.5 anionic PAMAM dendrimers and evaluate their suitability as oral drug delivery carriers by determining their cytotoxicity, cellular uptake and transport across Caco-2 cell monolayers (Scheme 1A). In addition, the effect of PEGylation on transport via the tight junctions was investigated. These studies provide the first evidence of the impact of PEG conjugation on dendrimer transepithelial transport and suggest that PEG could be used as both a surface modifier to modulate transport and uptake properties and as a drug linker for oral drug delivery applications.
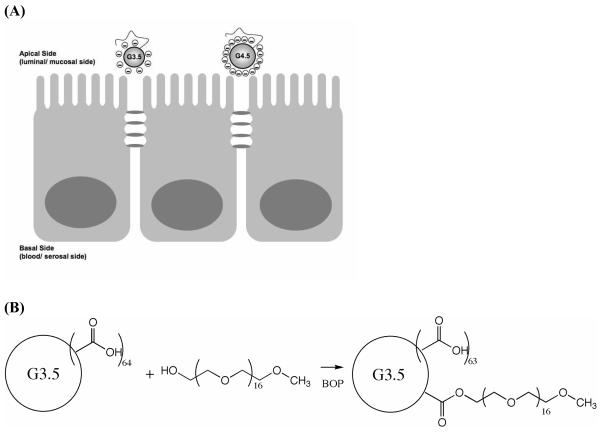
Scheme 1.
(A) Schematics of PEGylated G3.5 and G4.5 dendrimers approaching the apical side of a Caco-2 cell monolayer. (B) PEGylation of G3.5 dendrimer with mPEG750.
Materials and Methods
Materials
PAMAM G3.5 (reported molecular weight=12,931) and PAMAM G4.5 (reported molecular weight 26,258), [14C]-mannitol (specific activity 50mCi/mmol), D2O, and Hank's balanced salt solution (HBSS buffer) salts were purchased from Sigma Aldrich (St. Louis, MO). [3H]-Acetic anhydride was purchased from American Radiolabeled Chemicals (St. Louis, MO). Superose 12 HR 10/300 GL column was purchased from Amersham Pharmacia Biotech (Piscataway, NJ). Caco-2 cells were purchased from American Type Cell Culture (Rockville, MD). WST-1 cell proliferation reagent was purchased from Roche Applied Science (Indianapolis, IN). The BD Biocoat Caco-2 Assay was purchased from BD Biosciences (San Hose, CA). 1H Nuclear Magnetic Resonance (NMR) Spectra were obtained using Varian 500 MHz FT NMR and were processed using Spinworks software (Kirk Marat, University of Mannitoba, Winnipeg, Canada, © 2008).
Conjugation of mPEG750 to PAMAM Dendrimers
PEGylation of PAMAM G3.5 and G4.5 was achieved by formation of ester bonds between surface carboxyl groups of the dendrimers and hydroxyl terminated PEG (Molecular Weight (Mw) 750) using benzotriazole-1-yl-oxy-tris-(dimethylamino)-phosphoniumhexafluorophosphate (BOP) as a coupling agent and methanol as a solvent (Scheme 1B). 1, 2 and 4 equivalents of methoxy polyethylene glycol (mPEG) were reacted with dendrimers to yield samples G3.5-P1, G3.5-P1.4 and G3.5-P2.3, G4.5-P.85, G4.5-P1.9 and G4.5-P2.8 respectively, where G represents the generation number and P represents the number of PEG chains conjugated (Table 1). For each reaction, 75 mg of dendrimer and three equivalents of BOP per equivalent of mPEG were dissolved in anhydrous methanol. mPEG750 was dissolved in methanol to a concentration of 100 mg/mL and the appropriate molar equivalent was added to the mixture. The solution was stirred at room temperature for 72 hours, after which methanol was evaporated to leave the crude product. This product was dissolved in distilled water and purified by dialysis against distilled water using 3500 molecular weight cut off (MWCO) membranes (Spectrum Laboratories, Rancho Dominguez, CA). Subsequently, the product was freeze dried and stored at 4°C.
Table 1.
Characteristics of PAMAM Dendrimer – PEG Conjugates
| Dendrimer Sample |
# mPEG Equivalents |
# mPEG Conjugated* |
Calculated Molecular Weight** |
Specific Activity (mCi/mmol) |
SEC Elution Volume (ml) |
Hydrodynamic Radius (nm) |
|---|---|---|---|---|---|---|
| G3.5 | - | - | 12931 | 1.06 | 13.2 | 1.90 ± 0.00 |
| G3.5-P1 | 1 | 0.98 | 13666 | 3.25 | 13.2 | 1.65 ± 0.07 |
| G3.5-P1.4 | 2 | 1.36 | 13951 | 3.96 | 13.2 | 1.65 ± 0.07 |
| G3.5-P4.5 | 4 | 2.26 | 14626 | 3.91 | 13.2 | 1.70 ± 0.00 |
| G4.5 | - | - | 26258 | 2.64 | 12.8 | 2.35 ± 0.07 |
| G4.5-P.85 | 1 | 0.85 | 26898 | 4.47 | 12.5 | 2.10 ± 0.00 |
| G4.5-P1.9 | 2 | 1.89 | 27673 | 5.11 | 12.6 | 2.15 ± 0.07 |
| G4.5-P2.8 | 4 | 2.83 | 28380 | 4.81 | 12.6 | 2.10 ± 0.00 |
Calculated from NMR data
Calculated based on NMR data and reported (Sigma) dendrimer molecular weights
Characterization of PEGylated G3.5 and G4.5 Dendrimers
Dendrimer-PEG conjugates were characterized by size exclusion chromatography (SEC) using an Acta FPLC system (Acta UPC 900, P-920, INV-907 from GE Healthcare) and phosphate buffered saline (PBS, pH 7.4) with 0.05% sodium azide as the eluent. Samples were injected onto the FPLC system at 5 mg/mL and simultaneously monitored for Ultra-Violet (UV), Refractive Index (RI), Multi-angle Laser Light Scattering (MALLS) and Dynamic Light Scattering (DLS) detection. SEC was used to confirm the absence of low molecular weight impurities and to compare the elution volumes of modified and native dendrimers. In addition, the dendrimer peak was analyzed to determine the hydrodynamic radius using a Wyatt Quasi-Elastic Light Scattering Detector (QELS) and the calculations were performed using the Astra 5.3.4 software. All samples were run in duplicate. 1H NMR was used to determine the number of PEG chains conjugated per dendrimer. NMR samples were prepared at approximately 8 mg/mL in D2O with 0.05 wt % 3-(trimethylsilyl) propionic-2,2,3,3-d4 acid. The number of PEG chains per dendrimer was determined from ratios of integral values for peaks assigned to PEG (3.6-3.7 ppm) and dendrimer (2-3.6). Finally, the zeta potential of PEGylated dendrimers was obtained using the Malvern Nano-ZS system. Dendrimer solutions were prepared at 5 mg/mL in DI water and analyzed in triplicate.
Synthesis of Radiolabeled Dendrimers
Dendrimers and dendrimer-PEG conjugates were radiolabeled using [3H]-acetic anhydride (American Radiolabeled Chemicals, St. Louis, MO) which reacts with internal amines known to be present in carboxyl terminated dendrimers due to defects formed during their synthesis [28]. Dendrimers and dendrimer-PEG conjugates were dissolved in methanol and reacted with three equivalents of [3H]-acetic anhydride in the presence of excess triethylamine overnight. The methanol was dried under a stream of nitrogen and the product was redissolved in distilled water. Triethylamine and unreacted acetic anhydride were removed by Sephadex G-25 protein desalting (PD-10) columns. Specific activities of the radiolabeled compounds are reported in Table 1.
Caco-2 Cell Culture
Caco-2 cells (passages 20-40) were grown at 37°C in an atmosphere of 95% relative humidity and 5% CO2. Cells were maintained in T-75 flasks using Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% non-essential amino acids, 10,000 units/ml penicillin, 10,000 μg/ml streptomycin and 25 μg/ml amphotericin. Media was changed every other day and cells were passaged at 80-90% confluence using a 0.25% trypsin/ ethylenendiamine tetraacetic acid (EDTA) solution. Incubation buffer used in assays consisted of Hank's balanced salt solution (HBSS), supplemented with 1 mM N-(2-hydroxyelthyl)piperazine-N'-(2 ethanesulfonic acid) hemisodium salt (HEPES) buffer (pH 7.4).
Cytotoxicity Assay
Cytotoxicity of unmodified and differentially PEGylated PAMAM dendrimers was assessed by the water soluble tetrazolium salt (WST-1) assay. Caco-2 cells were seeded at 50,000 cells per well in 96 well cell culture plates (Corning, Corning NY) and maintained at 37°C, 95% relative humidity and 5% CO2 for forty eight hours. Cells were washed with warm HBSS buffer and incubated for 2 hours with 100 μL of varying concentrations of dendrimer and dendrimer-PEG conjugates in HBSS. After two hours, the dendrimer solutions were removed and the cells were washed with warm HBSS buffer. 10 μL of WST-1 cell proliferation reagent in 100 μL of HBSS buffer was added to each well and incubated for four hours at 37°C. Absorbance at 460 nm and background at 600 nm were measured using a SpectraMax 384 plate reader (Molecular Devices, Sunnyvale, CA). HBSS was used as a negative control for 100% cell viability. Cytotoxicity of each dendrimer at each concentration was assessed in six replicates and a two-way analysis of variance was performed to determine if concentration or dendrimer type significantly impacted cytotoxicity.
Cellular Uptake Studies
The effect of PEGylation on cellular uptake of radiolabeled dendrimers was investigated. Caco-2 cells were seeded at 40,000 cells/ well in 24-well cell culture plates (Corning, Corning NY) and maintained at 37°C, 95% relative humidity and 5% CO2 for forty eight hours. Cells were washed with warm HBSS buffer and 300 μL of 0.02 mM dendrimer solution in HBSS was added for 30 or 60 minutes. After the given incubation period, the cells were washed twice with ice cold HBSS to halt the uptake process. The cells were then lysed with NaOH and neutralized with HCl. Uptake was measured by quantifying the cell-associated radioactivity using a liquid scintillation counter (Beckman Coulter, Fullerton, CA) with Econosafe scintillation cocktail (Research Products International, Mount Prospect, IL). Uptake was normalized to total protein content using the BCA protein assay kit (Pierce, Evanston, IL). Statistical significance was determined by a two-way analysis of variance and Bonferroni post-hoc correction.
Transepithelial Permeability Assessment
The effect of differential PEGylation on transport of radiolabeled dendrimers across Caco-2 cell monolayers was assessed in the BD Biocoat HTS Caco-2 Assay System (BD Biosciences, San Hose, CA). The three day assay protocol defined by the manufacturer was used to prepare the monolayers. Briefly, Caco-2 cells were grown past confluency to a density of >250,000 cells/ cm2 in T25 flasks. Cells were seeded at 200,000 cells per well, in 24 well transwell plates with fibrillar collagen coated cell culture inserts in basal cell seeding medium supplemented with MITO+ (mitogenic stimulating) serum extender and 10% FBS. After 24 hours, the medium was switched to entero-STIM, a fully defined media containing butyric acid, which induces differentiation of intestinal epithelial cells and form a competent monolayer [29]. After an additional forty-eight hours, the monolayers were used for experiments. Cells were washed twice with warm HBSS buffer. To test dendrimer permeability, 100 μL of dendrimer solution (0.1 mM) was added to the apical compartment and 600 μL of HBSS added to the basal compartment. After two hours, 400 μL from the basal compartment was taken for scintillation counting. Apparent permeability (Papp) was calculated by:
| (Eq.1) |
where dQ/dt is the change in the amount of solute over time (permeability rate), A is the surface area of the insert, and C0 is the donor concentration. [14C]-Mannitol was used to monitor monolayer integrity. The transepithelial flux of the paracellular marker [14C]-mannitol was 2.6 × 10−6 cm/s, which demonstrates monolayer integrity as shown previously [30, 31]. Statistical significance was determined by Tukey's Multiple Comparison test.
Occludin Staining
Caco-2 cells were seeded at 20,000 cells/cm2 on collagen-coated four chamber culture slides (BD Biosciences, Bedford MA), maintained under normal cell culture conditions for 5 days and used for experiments when the cells reached confluence. The cells were equilibrated in HBSS for two hours prior to the experiment. The cells were incubated with 300 μl of 0.1 mM dendrimer solutions for 2 hours at 37°C and then washed three times with ice cold PBS to remove the dendrimers. G3.5, G3.5-P1, G4.5 and G4.5-P.85 were used with HBSS as a control. The cells were then fixed with 300 μl of 4% paraformaldehyde solution for 20 minutes at room temperature, washed twice with 25 mM glycine and once with PBS and then permeabilized with 300 μl of 0.2% Triton X-100 in blocking solution made of 1% bovine serum albumin (BSA) in PBS for 20 minutes at room temperature. Cells were washed three times with PBS and then incubated with 1% BSA/PBS for 30 minutes. The blocking solution was removed and the cells were incubated with 300 μl of 2 μM mouse anti-occludin (Invitrogen (Zymed), Carlsbad, CA) and kept at 4°C overnight. Cells were washed three times with 1% BSA/PBS and then incubated with 1% BSA/PBS as before. After the blocking solution was removed, the cells were incubated with 300 μl of 10 μg/mL Alexa Fluor 568 goat anti-mouse IgG (H+L) (Invitrogen, Molecular Probes, Carlsbad, CA) for 1 hour. The cells were then washed three times with PBS and the chambers were removed. Gel mount was added to each region and allowed to dry for 2 hours at room temperature. The slides were then sealed with clear nail polish and kept at 4°C prior to visualization.
Slides were visualized using a Zeiss LSM510 META confocal laser scanning microscope (Carl Zeiss, Jena, Germany). Alexa Fluor 568 was visualized with excitation and emission wavelengths of 543 and 603 nm respectively. Z-stacks were obtained with the following microscope settings: 63x oil objective, 1 airy unit pinhole, 543 nm laser set to 20% power with 1000 gain, 0.1 amplifier offset and 1 amplifier gain. The scan speed was set to 8 in line mode with mean averaging set to 4. Z-stack sections were taken 0.5 μm apart with 15 sections per region. Three z-stacks were acquired per treatment and analyzed using Volocity 3D imaging software. (Version 4.3.2; Improvision, Inc., Lexington, MA). Red voxels, corresponding to occludin staining, were quantified by thresholding the intensity between 25% and 100%. The number of red voxels was quantified for each region and normalized to the number of cells. Results are reported as mean ± standard deviation and statistical significance was determined using a one-tailed Student's t-test.
Results and Discussion
Synthesis and Characterization of PEGylated Anionic PAMAM Dendrimers
1H NMR studies confirmed the formation of the conjugates with new peaks corresponding to the protons from PEG. 1H NMR quantification showed a concomitant increase in the number of PEG moieties conjugated with the number of equivalents of PEG, but this increase was less than the stoichiometric amount used (Table 1). This may be attributed to steric hindrance, which was more evident in lower generation dendrimers likely due to their lower number of available acid groups and smaller surface area.
Characterization by SEC showed the absence of free PEG and other small molecular weight impurities (data not shown). Elution volumes of native and PEGylated dendrimers are shown in Table 1. While there is a decrease in elution volume from G3.5 dendrimers to G4.5 dendrimers due to the larger size, native and modified dendrimers of the same generation do not show any significant differences in elution volume. This illustrates that PEGylation does not increase the hydrodynamic volume of the dendrimers, suggesting that PEG is preferentially wrapped around the dendrimer rather than protruding from the surface. Hydrodynamic radii of the conjugates measured by dynamic light scattering support this hypothesis (Table 1). Increasing the degree of PEGylation does not increase the hydrodynamic radii. In fact, the hydrodynamic radii are slightly decreased, indicating that PEG may shield the surface charge and decrease charge-charge repulsion of functional groups and therefore the size of the polymers in solution.
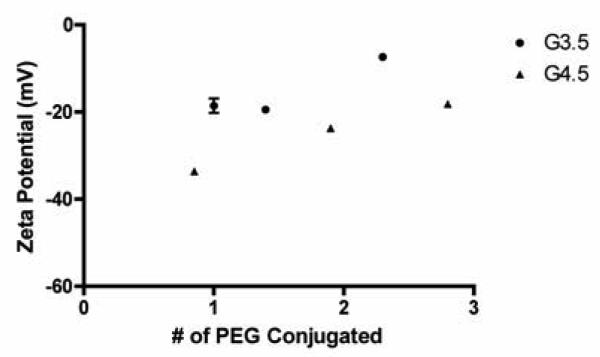
Zeta potential measurements shed light on the impact of PEGylation on dendrimer surface charge. Figure 1 illustrates that zeta potential becomes less negative upon further PEGylation with G3.5 and G4.5 dendrimers showing similar behavior, confirming that PEG shields surface charge. Shcharbin et al. [32] reported a zeta potential for G4.5 PAMAM dendrimers in deionized water as −56 ± 0.5 mV. Comparing this with our data shows that even one PEG is able to shield a significant amount of charge on the dendrimer surface.
Figure 1.
Zeta potential of PEGylated G3.5 and G4.5 PAMAM Dendrimers. n=3, Mean ± standard deviation
PEGylated Dendrimers Maintain Cell Viability
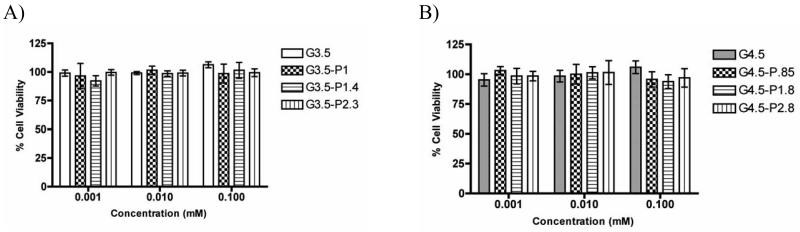
While amine terminated PAMAM dendrimers are known to be highly cytotoxic at higher generations, anionic dendrimers have been found to be much less toxic [33]. In this study we examined whether PEGylation of anionic dendrimers would influence their toxicity profile. G3.5 and G4.5 dendrimers and dendrimer-PEG conjugates were tested at concentrations up to 0.1 mM and did not show appreciable cytotoxicity (cell viability > 90%) over the entire range of concentrations tested (Figure 2A, B). This toxicity profile confirmed that treatment of Caco-2 cells with up to 0.1 mM of the dendrimer-PEG conjugates would not affect cell viability during uptake or transport experiments.
Figure 2.
Caco-2 cell viability in the presence of G3.5 (A) and G4.5 (B) native and PEGylated dendrimers after a 2 hour incubation time. n=6, Mean ± standard deviation.
PEGylation Affects Uptake of Anionic Dendrimers in Caco-2 Cells
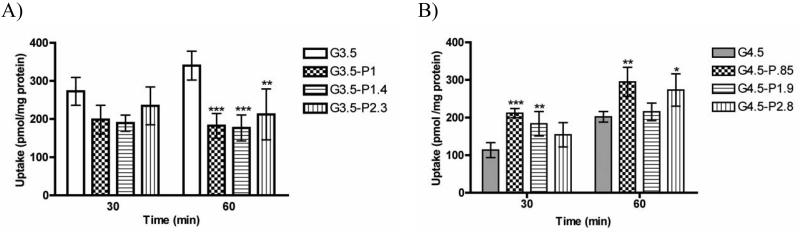
The impact of PEGylation on time-dependent cellular uptake of dendrimers was assessed in Caco-2 cells (Figure 3). Uptake studies shed light on the degree to which the dendrimers are transported through transcellular pathways (passive diffusion and vesicular mechanisms) in the gastrointestinal epithelium and their eventual uptake in tumor cells at the site of action. In general, PEGylation decreased uptake of G3.5 dendrimers, but this effect is statistically significant only after 60 minutes. Notably, there is no significant impact of the degree of PEGylation on uptake, i.e. a single PEG chain can reduce dendrimer uptake by almost 50% after a 60 minute incubation period. In contrast, for G4.5 dendrimers, conjugation of 1 PEG chain appears to increase uptake, with further addition of PEG decreasing uptake from this point.
Figure 3.
Uptake of G3.5 (A) and G4.5 (B) native and differentially PEGylated dendrimers at 0.02 mM for 30 and 60 minutes in Caco-2 cells. n=3, Mean ± standard deviation. (*),(**) and (***) denote significant differences from unmodified dendrimers with p<0.05, p<0.01 and p<0.001 respectively.
The flexible chain of PEG molecules can wrap around the rigid, spherical dendrimer and shield some of the negative charge on the dendrimer surface. Methoxy-terminated 750 Da PEG contains 16 subunits, giving it an overall extended chain length of 5.7 nm based on calculated bond lengths and angles. In comparison, G3.5 dendrimers have a reported diameter of approximately 4 nm and G4.5 dendrimers approximately 5 nm [34]. This suggests that the PEG chain, due to its flexible random coil conformation, is of comparable size to the dendrimers. For the smaller G3.5 dendrimers, one PEG chain is enough to reduce the interactions with the cells, leading to decreased cellular uptake for all PEGylation ratios. This may be due to a stealth-like effect imposed by PEG, creating fewer interactions between the dendrimers and the cells. In contrast, for G4.5 dendrimers, addition of 1 PEG chain actually increases uptake as compared to unmodified G4.5. This may be explained by the charge density of the dendrimers, which can be calculated by dividing the number of charges (64, 128) by the surface area of the dendrimer, assuming a spherical conformation [35]. The higher charge density of G4.5 dendrimers (1.6 charges/nm2) compared to G3.5 dendrimers (1.3 charges/nm2), can presumably cause repulsion with the negatively charged cell membrane. Addition of 1 PEG chain reduces the charge on the surface, increasing the uptake to a point comparable to G3.5 dendrimers. However, addition of more PEG shields more of the negative charge, again, creating a stealth system and decreasing cellular uptake. Correlating the uptake of the dendrimers with the zeta potential data shows that there may be an ideal zeta potential around −30 mV that promotes cellular uptake. In general, dendrimers with zeta potentials more negative than −30 mV had reduced cellular uptake (ie. G4.5) and dendrimers with zeta potentials greater than −30 mV (ie PEGylated G3.5) also show lower uptake. Interestingly, incubation time-dependent behavior was observed between dendrimer generations. While there is no statistical difference between uptake of unmodified or PEGylated G3.5 at 30 and 60 minutes, there is a significant increase (p<0.01) in uptake for the G4.5 dendrimers, suggesting that steady-state uptake of these larger dendrimers has not yet been achieved.
Dendrimers are known to be transported across Caco-2 cell monolayers by both transcellular and paracellular pathways [13, 14, 33, 36]. Uptake studies in Caco-2 cells help to elucidate the relative contributions of these processes. This is significant for oral drug delivery as premature drug release within intestinal cells can cause undesirable side effects. Therefore, using PEGylated G3.5 dendrimers to decrease uptake in intestinal cells may be of use in cases where it is desirable to transport the cargo across the cells while avoiding entrapment within the cells.
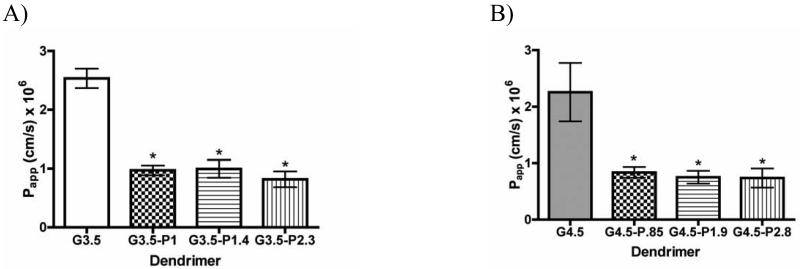
Dendrimer PEGylation Significantly Decreases Transepithelial Transport
In order to assess the suitability of PEGylated dendrimers for oral drug delivery, their transport across- Caco-2 cell monolayers was studied and compared to unmodified dendrimers. Transport experiments complement the uptake studies, giving an overall picture of transepithelial transport including both transcellular and paracellular pathways. PEGylation significantly decreased transport of both G3.5 and G4.5 dendrimers (Figure 4 A, B), yielding apparent permeabilities approximately 60-70% lower than unmodified dendrimers. Despite this decrease in overall transport flux, PEGylated dendrimers still show appreciable transport as compared to traditional linear polymers [37], indicating that the PEGylated dendrimers continue to enhance their own transport to some degree. Addition of more than one PEG per dendrimer does not further decrease transport. This can be explained by PEG shielding the negative charge on the dendrimer, thereby potentially reducing previously described [12-14] tight junctional opening and enhanced epithelial permeation. By attaching a linear, neutral molecule with a flexible chain structure to the spherical semi-rigid dendrimer, the dendrimer surface charge is partially shielded, leading to fewer interactions between the dendrimers and the cells, and therefore, a potential reduction in transepithelial transport.
Figure 4.
Apparent permeability of G3.5 (A) and G4.5 (B) native and differentially PEGylated dendrimers at 0.1 mM after a 2 hour incubation time with Caco-2 cell monolayers. n=3, Mean ± standard deviation. (*) denotes a significant difference from unmodified dendrimers with p<0.001.
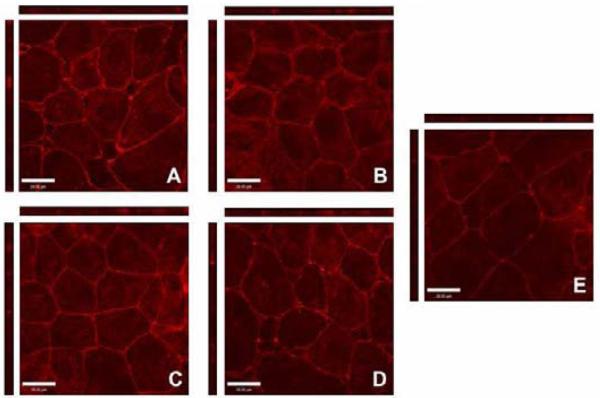
Tight Junction Opening is Reduced by PEGylation of Anionic Dendrimers
To further assess the influence of dendrimer PEGylation on tight junctional modulation, Caco-2 cells were pretreated with native and PEGylated dendrimers and stained for occludin, a marker protein for tight junction integrity [38]. Previously, it was shown that Caco-2 cells treated with 1 mM G1.5, G2.5 and G3.5 dendrimers showed disrupted occludin staining patterns and increased occludin accessibility as compared to cells with no polymer treatment [12]. In this study, we used a ten-fold lower concentration (0.1 mM) in order to mimic the conditions used in the transport assay. Control cells treated with HBSS alone (Figure 5E) show thin lines of red staining corresponding to occludin proteins linking cell membranes. In contrast, dendrimer-treated cells (Figure 5 A-D) show brighter, wider bands of staining with increased intracellular staining, indicating accessibility of occludin protein to its antibody due to the opening of the tight junctions. In the case of G3.5 (Figure 5A) confocal images show some amount of cell detachment, indicating tight junction opening.
Figure 5.
Staining of the tight junction protein occludin in the presence and absence of dendrimers visualized by confocal microscopy. Caco-2 cells incubated for 2 hours with 0.1 mM (A) G3.5, (B) G3.5-P1, (C) G4.5, (D) G4.5-P.85 and (E) with no polymer treatment. Main panels illustrate the xy plane; horizontal bars illustrate the xz plane; vertical bars illustrate the yz plane. Scale bars equal 28 μm.
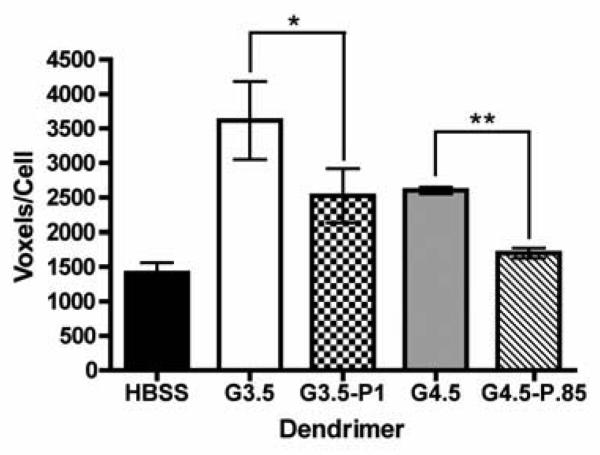
All dendrimers studied showed a statistically significant increase in occludin staining compared to the HBSS control (p<0.05) (Figure 6), illustrating that native and PEGylated dendrimers open tight junctions to some degree, even at relatively low concentrations (0.1 mM).
Figure 6.
Quantification of occludin staining by Volocity 3D imaging software in the presence and absence of dendrimers. Results reported are number of “red” voxels per region normalized to the number of cells in each region (n=3). One-tailed Student's t-tests were used to determine statistical significance. (*) and (**) denote significant differences from unmodified dendrimers with p<0.05 and p<0.01 respectively.
PEGylated dendrimers (Figure 5 B, D) show much lower occludin staining than native G3.5 and G4.5 dendrimers (Figure 5 A, C), suggesting that PEGylated dendrimers interact with and open the tight junctions to a lesser degree. This is consistent with the transport results as PEGylated dendrimers had lower transport rates than both G3.5 and G4.5 dendrimers. This decrease in tight junction modulation can be attributed to the charge shielding properties of PEG. Previous studies have shown that neutral dendrimers have the lowest transport rates and lowest disruption of tight junctional occludin as compared to charged dendrimers of the same generation [12]. Therefore, PEGylation can potentially shield the anionic charge and cause anionic dendrimers to behave more like hydroxyl-terminated dendrimers. Comparing G3.5 and G4.5 dendrimers, we see that G3.5 dendrimers show higher occludin staining than G4.5 dendrimers. Since G4.5 dendrimers have a higher charge density and the negative charge is known to enhance interaction with the tight junctions, this may seem counterintuitive; however, it is likely that the increased size of G4.5 dendrimers may inhibit their ability to open the tight junctions.
Combining the uptake results with the occludin staining results allows us to understand the relative contributions of paracellular and transcellular transport of PEGylated dendrimers compared to unmodified dendrimers. In the case of G3.5 dendrimers, PEGylation causes a significant decrease in uptake and a moderate decrease in tight junction modulation, indicating that these dendrimers have less paracellular and less transcellular transport compared to unmodified dendrimers. By contrast, PEGylation of G4.5 dendrimers causes a modest increase in uptake and has only slightly more occludin accessibility than the control, suggesting that PEGylated G4.5 dendrimers have more transcellular transport and much less paracellular transport than unmodified G4.5 dendrimers. This sheds light on how PEGylation can impact transport mechanisms as well as overall transport rates. The strong correlation between the transport studies and the occludin staining studies suggest that overall, anionic dendrimers are transported primarily through the paracellular route.
PAMAM dendrimers can be used for oral drug delivery to release drugs in the gastrointestinal tract and transport drugs into or across the intestinal barrier. PEGylation of PAMAM dendrimers can modulate toxicity, functionalize the surface for drug conjugation and facilitate drug release. While PEGylation decreases the transepithelial transport of anionic dendrimers, the conjugates still show appreciable transport, compared to traditional linear polymers [37], and have the potential for increased functionality. This work demonstrates that degree of PEGylation and dendrimer generation can modulate the mechanisms of transport, and can be custom tailored for different oral drug delivery applications.
Conclusion
In this work we report the effect of PEGylation on cytotoxicity, uptake and transport of G3.5 and G4.5 anionic PAMAM dendrimers across Caco-2 cells. At the concentration range studied, PEGylation of these dendrimers maintained cell viability. PEGylation decreased uptake and transport for G3.5 dendrimers, whereas PEGylated G4.5 dendrimers demonstrated increased uptake with a concomitant decrease in overall dendrimer transport. Occludin staining of Caco-2 cell monolayers in the presence of conjugates showed that PEGylated dendrimers opened the tight junctions to a lesser extent than native dendrimers, indicating a reduction in paracellular transport. While PEGylated dendrimers showed decreased transport rates, they were still transported to an appreciable extent compared to traditional linear macromolecules [37] and offer advantages of facilitated drug conjugation and release as well as potential for improved biodistribution. Together these studies demonstrate that in the design of PAMAM-PEG conjugates for oral drug delivery, the extent of transepithelial transport, uptake as well as the mechanism of transport can be controlled by a judicious choice of dendrimer generation and degree of PEGylation. Taken together, these parameters can be custom- tailored to the specific needs of a desired drug delivery application.
Acknowledgments
Financial support was provided by an NSF Graduate Research Fellowship to Deborah Sweet, a Department of Defense Multidisciplinary Postdoctoral Fellowship (W81XWH-06-1-0698) to Rohit Kolhatkar, a University System of Maryland Integrated Nanobio seed grant from the Maryland Department of Business and Economic Development and NIH R01EB07470.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vicent MJ, Duncan R. Polymer conjugates: nanosized medicines for treating cancer. Trends Biotechnol. 2006;24(1):39–47. doi: 10.1016/j.tibtech.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Lu Z, Ye F, Vaidya A. Polymer platforms for drug delivery and biomedical imaging. J. Controlled Release. 2007;122(3):269–277. doi: 10.1016/j.jconrel.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomalia DA, Reyna LA, Svenson S. Dendrimers as multi-purpose nanodevices for oncology drug delivery and diagnostic imaging. Biochem. Soc. Trans. 2007;35(Pt 1):61–67. doi: 10.1042/BST0350061. [DOI] [PubMed] [Google Scholar]
- 4.Patri AK, Kukowska-Latallo JF, Baker JR. Targeted drug delivery with dendrimers: Comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complex. Adv. Drug Delivery Rev. 2005;57:2203–2214. doi: 10.1016/j.addr.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Lai P, Lou P, Peng C, Pai C, Yen W, Huang M, Young T, Shieh M. Doxorubicin delivery by polyamidoamine dendrimer conjugation and photochemical internalization for cancer therapy. J. Controlled Release. 2007;122(1):39–46. doi: 10.1016/j.jconrel.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Majoros IJ, Myc A, Thomas T, Mehta CB, Baker JR. PAMAM dendrimer-based multifunctional conjugate for cancer therapy: synthesis, characterization, and functionality. Biomacromolecules. 2006;7(2):572–579. doi: 10.1021/bm0506142. [DOI] [PubMed] [Google Scholar]
- 7.Koyama Y, Talanov VS, Bernardo M, Hama Y, Regino CA, Brechbiel MW, Choyke PL, Kobayashi H. A dendrimer-based nanosized contrast agent dual-labeled for magnetic resonance and optical fluorescence imaging to localize the sentinel lymph node in mice. J. Magn. Reson. Imaging. 2007;25(4):866–871. doi: 10.1002/jmri.20852. [DOI] [PubMed] [Google Scholar]
- 8.Lee CC, MacKay JA, Fréchet JM, Szoka FC. Designing dendrimers for biological applications. Nat. Biotechnol. 2005;23(12):1517–1526. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]
- 9.Jevprasesphant R, Penny J, Jalal R, Attwood D, McKeown NB, D'Emanuele A. The influence of surface modification on the cytotoxicity of PAMAM dendrimers. Int. J. Pharm. 2003;252(12):263–266. doi: 10.1016/s0378-5173(02)00623-3. [DOI] [PubMed] [Google Scholar]
- 10.Jevprasesphant R, Penny J, Attwood D, McKeown NB, D'Emanuele A. Engineering of dendrimer surfaces to enhance transepithelial transport and reduce cytotoxicity. Pharm. Res. 2003;20(10):1543–1550. doi: 10.1023/a:1026166729873. [DOI] [PubMed] [Google Scholar]
- 11.Kolhatkar RB, Kitchens KM, Swaan PW, Ghandehari H. Surface acetylation of polyamidoamine (PAMAM) dendrimers decreases cytotoxicity while maintaining membrane permeability. Bioconjugate Chem. 2007;18(6):2054–2060. doi: 10.1021/bc0603889. [DOI] [PubMed] [Google Scholar]
- 12.Kitchens KM, Kolhatkar RB, Swaan PW, Eddington ND, Ghandehari H. Transport of poly(amidoamine) dendrimers across Caco-2 cell monolayers: Influence of size, charge and fluorescent labeling. Pharm. Res. 2006;23(12):2818–2826. doi: 10.1007/s11095-006-9122-2. [DOI] [PubMed] [Google Scholar]
- 13.Kitchens KM, Kolhatkar RB, Swaan PW, Ghandehari H. Endocytosis inhibitors prevent poly(amidoamine) dendrimer internalization and permeability across Caco-2 cells. Mol. Pharmaceutics. 2008;5(2):364–369. doi: 10.1021/mp700089s. [DOI] [PubMed] [Google Scholar]
- 14.Kitchens K, Foraker A, Kolhatkar R, Swaan P, Ghandehari H. Endocytosis and interaction of poly (amidoamine) dendrimers with Caco-2 Cells. Pharm. Res. 2007;24(11):2138–2145. doi: 10.1007/s11095-007-9415-0. [DOI] [PubMed] [Google Scholar]
- 15.Kolhatkar R, Sweet D, Ghandehari H. Torchilin V, editor. Multifunctional Pharmaceutical Nanocarriers. Springer. 2008:201–232. [Google Scholar]
- 16.Wiwattanapatapee R, Carreño-Gómez B, Malik N, Duncan R. Anionic PAMAM dendrimers rapidly cross adult rat intestine in vitro: a potential oral delivery system? Pharm. Res. 2000;17(8):991–998. doi: 10.1023/a:1007587523543. [DOI] [PubMed] [Google Scholar]
- 17.Sakthivel T, Toth I, Florence AT. Distribution of a lipidic 2.5 nm diameter dendrimer carrier after oral administration. Int. J. Pharm. 1999;183(1):51–55. doi: 10.1016/s0378-5173(99)00043-5. [DOI] [PubMed] [Google Scholar]
- 18.Jevprasesphant R, Penny J, Attwood D, D'Emanuele A. Transport of dendrimer nanocarriers through epithelial cells via the transcellular route. J. Controlled Release. 2004;97:259–267. doi: 10.1016/j.jconrel.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 19.Pisal D, Yellepeddi V, Kumar A, Kaushik R, Hildreth M, Guan X, Palakurthi S. Permeability of surface-modified polyamidoamine (PAMAM) dendrimers across Caco-2 cell monolayers. Int. J. Pharm. 2008;350(12):113–121. doi: 10.1016/j.ijpharm.2007.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D'Emanuele A, Jevprasesphant R, Penny J, Attwood D. The use of a dendrimer-propranolol prodrug to bypass efflux transporters and enhance oral bioavailability. J. Controlled Release. 2004;95(3):447–453. doi: 10.1016/j.jconrel.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Ke W, Zhao Y, Huang R, Jiang C, Pei Y. Enhanced oral bioavailability of doxorubicin in a dendrimer drug delivery system. J. Pharm. Sci. 2008;97(6):2208–2216. doi: 10.1002/jps.21155. [DOI] [PubMed] [Google Scholar]
- 22.Kolhatkar RB, Swaan PW, Ghandehari H. Potential oral delivery of 7-Ethyl-10-hydroxy-camptothecin (SN-38) using poly(amidoamine) dendrimers. Pharm. Res. 2008;25(7):1723–1729. doi: 10.1007/s11095-008-9572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Najlah M, Freeman S, Attwood D, D'Emanuele A. In vitro evaluation of dendrimer prodrugs for oral drug delivery. Int. J. Pharm. 2007;336(1):183–190. doi: 10.1016/j.ijpharm.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 24.El-Sayed M, Ginski M, Rhodes C, Ghandehari H. Influence of surface chemistry of poly (amidoamine) dendrimers on Caco-2 cell monolayers. J. Bioact. Compat. Polym. 2003;18(1):7–22. [Google Scholar]
- 25.Parveen S, Sahoo SK. Nanomedicine: clinical applications of polyethylene glycol conjugated proteins and drugs. Clin. Pharmacokinet. 2006;45(10):965–988. doi: 10.2165/00003088-200645100-00002. [DOI] [PubMed] [Google Scholar]
- 26.Okuda T, Kawakami S, Maeie T, Niidome T, Yamashita F, Hashida M. Biodistribution characteristics of amino acid dendrimers and their PEGylated derivatives after intravenous administration. J. Controlled Release. 2006;114:69–77. doi: 10.1016/j.jconrel.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Okuda T, Kawakami S, Akimoto N, Niidome T, Yamashita F, Hashida M. PEGylated lysine dendrimers for tumor-selective targeting after intravenous injection in tumor-bearing mice. J. Controlled Release. 2006;116:320–336. doi: 10.1016/j.jconrel.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 28.Yang H, Kao WJ. Dendrimers for pharmaceutical and biomedical applications. J. Biomater. Sci., Polym. Ed. 2006;17(12):3–19. doi: 10.1163/156856206774879171. [DOI] [PubMed] [Google Scholar]
- 29.Chong S, Dando SA, Morrison RA. Evaluation of biocoat intestinal epithelium differentiation environment (3-day cultured Caco-2 cells) as an absorption screening model with improved productivity. Pharm. Res. 1997;14(12):1835–1837. doi: 10.1023/a:1012112820371. [DOI] [PubMed] [Google Scholar]
- 30.Foraker AB, Walczak RJ, Cohen MH, Boiarski TA, Grove CF, Swaan PW. Microfabricated porous silicon particles enhance paracellular delivery of insulin across intestinal Caco-2 cell monolayers. Pharm. Res. 2003;20(1):110–116. doi: 10.1023/a:1022211127890. [DOI] [PubMed] [Google Scholar]
- 31.Swaan PW, Hillgren KM, Szoka FC, Jr., Oie S. Enhanced transepithelial transport of peptides by conjugation to cholic acid. Bioconjugate Chem. 1997;8(4):520–525. doi: 10.1021/bc970076t. [DOI] [PubMed] [Google Scholar]
- 32.Shcharbin D, Mazur J, Szwedzka M, Wasiak M, Palecz B, Przybyszewska M, Zaborski M, Bryszewska M. Interaction between PAMAM 4.5 dendrimer, cadmium and bovine serum albumin: a study using equilibrium dialysis, isothermal titration calorimetry, zeta-potential and fluorescence. Colloids Surf. B Biointerfaces. 2007;58(2):286–289. doi: 10.1016/j.colsurfb.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Kitchens KM, El-Sayed ME, Ghandehari H. Transepithelial and endothelial transport of poly (amidoamine) dendrimers. Adv. Drug Delivery Rev. 2005;57(15):2163–2176. doi: 10.1016/j.addr.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Esfand R, Tomalia DA. Poly (amidoamine)(PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. Drug Discovery Today. 2001;6(8):427–426. doi: 10.1016/s1359-6446(01)01757-3. [DOI] [PubMed] [Google Scholar]
- 35.Paulo PM, Lopes JN, Costa SM. Molecular dynamics simulations of charged dendrimers: low-to-intermediate half-generation PAMAMs. J. Phys. Chem. B. 2007;111(36):10651–10664. doi: 10.1021/jp072211x. [DOI] [PubMed] [Google Scholar]
- 36.El-Sayed M, Rhodes CA, Ginski M, Ghandehari H. Transport mechanism(s) of poly (amidoamine) dendrimers across Caco-2 cell monolayers. Int. J. Pharm. 2003;265(12):151–157. doi: 10.1016/s0378-5173(03)00391-0. [DOI] [PubMed] [Google Scholar]
- 37.Ghandehari H, Smith PL, Ellens H, Yeh PY, Kopecek J. Size-dependent permeability of hydrophilic probes across rabbit colonic epithelium. J. Pharmacol. Exp. Ther. 1997;280(2):747–753. [PubMed] [Google Scholar]
- 38.Kitchens KM, Kolhatkar RB, Swaan PW, Eddington ND, Ghandehari H. Transport of poly(amidoamine) dendrimers across Caco-2 cell monolayers: Influence of size, charge and fluorescent labeling. Pharm Res. 2006;23(12):2818–2826. doi: 10.1007/s11095-006-9122-2. [DOI] [PubMed] [Google Scholar]