Abstract
We report the isolation and reactivity of a series of “ligandless,” anionic arylpalladium complexes of the general structure [Pd(Ar)Br2]22- by the reaction of (tBu3P)Pd(Ar)(Br) and bromide. These anionic complexes insert olefins at room temperature, and these fast insertions indicate that the anionic complexes are kinetically competent as intermediates in Heck-Mizoroki reactions conducted under “ligandless” conditions (lacking added dative ligand). Kinetic studies showed that the anionic complexes insert olefins much faster than the corresponding neutral, P(t-Bu)3-ligated complexes. Addition of halide to the reaction of the neutral complex (tBu3P)Pd(Ar)(Br) and styrene led to a significant rate acceleration, suggesting that the anionic complex forms rapidly and reversibly in situ from the neutral species prior to migratory insertion. These data, along with studies on the regioselectivity for reaction of aryl halides with butyl vinyl ether in the presence of the different starting catalysts, are consistent with the intermediacy of the same anionic, arylpalladium intermediates in Heck reactions catalyzed by palladium complexes containing bulky trialkylphosphine ligands as in reactions conducted under ligandless conditions.
Palladium-catalyzed couplings conducted without added dative ligand are run on both small and industrial scale, despite the development of modern phosphine and carbene ligands. Studies to reveal the mechanism of reactions under these “ligandless” conditions are more challenging than those of systems known to react through discrete complexes containing supporting ligands. Studies of “ligandless” systems have identified the presence of colloidal palladium in such reactions1-3 and molecular species generated from these nanoparticles are proposed as the active catalyst in solution,3 but conclusions about the structures of such species have been largely speculative. The anionic species [(Ar)PdX2]-and [PdX3]- lacking dative ligands have been detected by mass spectrometry and EXAFS,2,3 and complexes of the formula [(Ar)PdX2]22- containing perhalogenated aryl groups have been isolated.4 However, ligandless arylpalladium halide complexes lacking the stability imparted by a perhaloaryl group have never been isolated, and the reactions of discrete, “ligandless” anionic arylpalladium halide complexes have not been described.5
 |
(1) |
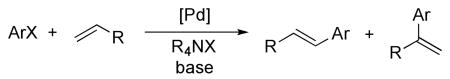
“Jeffery conditions” involving simple palladium salts, inorganic base, and tetraalkylammonium salts,6 are classic conditions for the Heck reaction (eq 1).7,8 Depending on the identity of the halogen and the alkene, the oxidative addition or the olefin insertions steps of the catalytic cycle can be turnover limiting.9 In either case, migratory insertion of the alkene into an arylpalladium complex forms the carbon-carbon bond in the product and controls the selectivity for formation of one isomer over another. Thus, to understand and affect the yields of the Heck reaction, the arylpalladium intermediate and the pathway by which it reacts with alkenes under conditions commonly used for the Heck reaction must be identified.
Here, we report the synthesis, isolation, structural characterization, and reactivity of anionic arylpalladium halide complexes [(Ar)PdBr2]22- lacking a dative ligand. Mechanistic data imply that these complexes are intermediates in Heck reactions catalyzed by ligandless palladium. However, we also provide data that imply that that such “ligandless” species are also intermediates in reactions catalyzed by complexes of some of the most commonly used phosphines, including those catalyzed by the combination of palladium and a bulky trialkylphosphine.
 |
(2) |
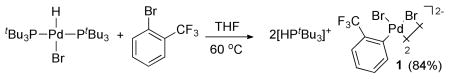
We first observed the formation of anionic arylpalladium complexes lacking dative ligands from the combination of 2-bromobenzotrifluoride and the Pd(II) complex (tBu3P)2Pd(H)(Br) (eq 2).10,11 This reaction formed the anionic 2-trifluoromethyl-phenyl complex 1 lacking any neutral, L-type, ligands in high yield. The 1H NMR spectrum of 1 contained a doublet at 5.49 ppm for the proton of the phosphonium salt, in addition to the resonances for the palladium-bound aryl group and the tert-butyl groups on phosphorus. The 31P{1H} NMR spectrum consisted of a signal with a chemical shift similar to that of [HPtBu3]Br.
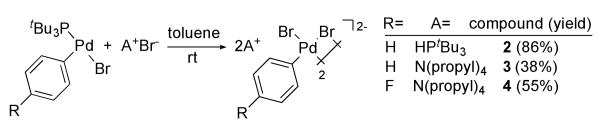
A more general synthesis of dimeric, dianionic arylpalladium halide complexes is shown in Scheme 1. Phenyl and 4-fluorophenyl complexes 2-4 were prepared from the reaction of trialkylphosphonium and tetraalkylammonium halides with (tBu3P)Pd(Ar)(Br).12 The products were obtained directly from a nonpolar reaction solvent in 38-86% yield. Complexes 1-4 are air-stable, crystalline solids. Single-crystal X-ray diffraction indicates that they are dimeric complexes containing two μ-halide ligands in the solid state (see Supporting Information for X-ray data). Conductivity measurements indicate that the complexes remain dimeric in solution. The conductivity of a 1 mM solution of 3 in nitrobenzene was 49 ohm-1 cm2 mol-1. This value falls in the range of conductivities of 2:1 electrolytes, and a 2:1 electrolyte is consistent with a dianionic, dimeric structure.13
Scheme 1.
 |
(3) |
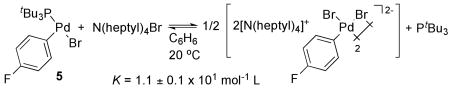
The combination of halide and neutral, phosphine-ligated aryl-palladium halide complexes equilibrates with the combination of the anionic complexes and free phosphine. Exchange of halide for phosphine occurred immediately upon mixing the neutral complex 5 with NR4Br (eq 3). The equilibrium constant determined by 19F and 31P NMR spectroscopy at 20 °C was 1.1 ± 0.1 × 101 mol-1 L.14
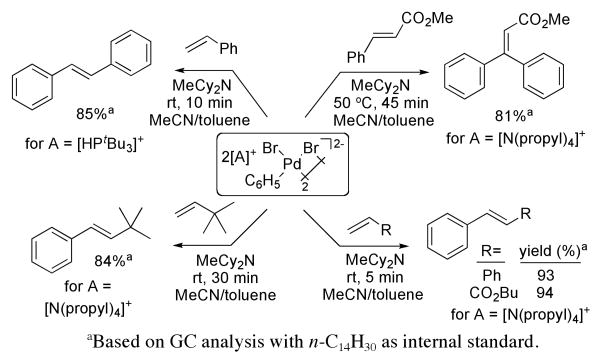
Anionic complexes 2 and 3 react with alkenes and vinylarenes under mild conditions (Scheme 2). Complex 2 reacted with styrene in a mixture of acetonitrile and toluene with added MeCy2N to form trans-stilbene in high yield within 10 min at 25 °C. Reactions occurred in the absence of base; however higher yields of the vinylarenes (ca 10%) were obtained in the presence of added amine, presumably because the consumption of the H-Pd-X product prevents unproductive consumption of the starting complex. Reactions of the anionic complexes with olefins typically result in fast precipitation of a black solid (presumably Pd0) during the reaction. In the case of the reaction of 2 with styrene (Scheme 2), the soluble palladium products were quantified by 31P NMR spectroscopy. The only species observed in solution was Pd(PtBu3)2 (25%). The remaining palladium product is likely the precipitated species.
Scheme 2.
To assess whether the phosphonium cation generates a phosphine ligand and the reaction occurs through this phosphine complex, we studied the reactions of alkenes and vinylarenes with complex 3 containing an ammonium, rather than a phosphonium, ion. Acrylates and styrene reacted with 3 to give the cinnamate and stilbene products in high yield. Even 3,3-dimethyl-1-butene reacted to form a vinylarene product at room temperature. 1,2-Disubstituted alkenes react more slowly than less substituted alkenes in the Heck arylation, but the anionic complex 3 reacted with trans-methyl cinnamate to afford the tri-substituted olefin product in good yield at only 50 °C.
These results show that the anionic complex [(Ar)PdX2]22- is competent to be an intermediate in Heck reactions catalyzed by ligandless palladium systems that typically occur over several hours at elevated temperature.15 Moreover, these results show that the anionic arylpalladium complexes are more reactive than neutral analogs ligated by PPh3.16-17 The faster reactions of alkenes with these anionic complexes than with related neutral complexes runs counter to the typically faster insertions of alkenes into the M-C bonds of more electrophilic complexes.
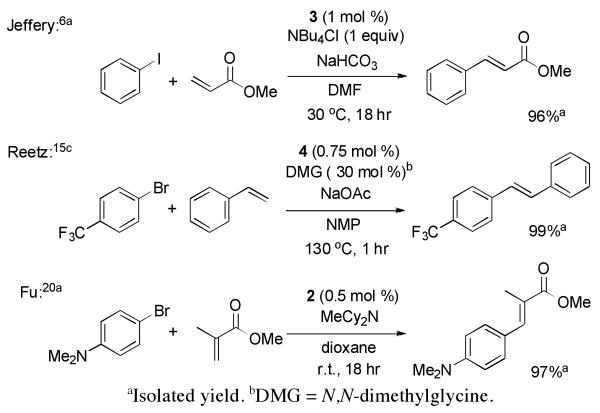
To assess the competence of 2-4 to be intermediates in the Heck reaction, we conducted reactions of aryl halides with olefins catalyzed by 2-4 under three common sets of reaction conditions (Scheme 3). The reaction of iodobenzene with methyl acrylate catalyzed by the parent phenyl complex 3 at 30 °C gave trans-methyl cinnamate in 96% yield. Similarly, the reaction of 4-bromo-benzotrifluoride with styrene catalyzed by p-fluorophenyl complex 4 at 130 °C gave the substituted stilbene in high yield. Finally, the reaction of the deactivated aryl bromide 1-bromo-4-N,N-dimethyl-aniline with methyl methacrylate occurred in the presence of 2 to give the substituted cinnamate product in high yield at room temperature. Presumably the phosphonium cation in 2 releases free P(t-Bu)3 in the presence of amine base18 and forms a highly active P(t-Bu)3-ligated Pd(0) species for oxidative addition of this aryl bromide.19 Thus, these reactions of the anionic species with alkenes imply that the isolated complexes are competent to be intermediates in the Heck reactions of iodo- and bromoarenes with terminal and disubstituted olefins.
Scheme 3.
Further studies implied that these ligandless complexes are also intermediates in Heck reactions conducted under some of the mildest conditions developed recently.20 Because “ligandless” complexes 2-4 were prepared from arylpalladium complexes ligated by P(t-Bu)3, ligandless, anionic complexes would be formed reversibly in a catalytic system involving P(t-Bu)3-ligated palladium complexes. If the anionic complexes were more reactive than the neutral species, then the products would actually result from reaction of the alkene with the species lacking any dative ligand. Thus, we conducted experiments to compare the rates of reactions of the anionic arylpalladium complexes to those of neutral P(t-Bu)3-ligated analogs. These experiments revealed the unexpected result that the anionic complexes react faster than the neutral analogs ligated by P(t-Bu)3.
Comparisons of the rates of reactions of styrene with ligandless and P(t-Bu)3-ligated arylpalladium halide complexes 4 and 5 are shown in Figure 1. Reaction of anionic 4 with styrene and MeCy2N in a mixture of acetonitrile and toluene (4:1) at 20 °C occurred with a kobs value of 2.9 × 10-3 s-1. This value was much faster than that of the analogous reaction of the neutral, three-coordinate 5 with the same quantity of styrene in the presence of MeCy2N21 (0.05 M) and PtBu3 (0.17 M).
Figure 1.
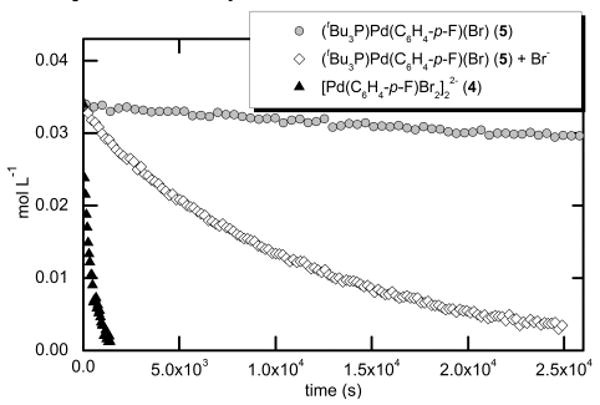
Decay of 5 (0.034 M) in the presence of styrene (0.17 M), PtBu3 (0.17 M), N(heptyl)4Br (0-0.68 M) and MeCy2N (0.05 M)21 in acetonitrile and toluene (4:1) at 20 °C, and decay of 4 (0.017 M) in the presence of styrene (0.17 M) and MeCy2N (0.05 M) in acetonitrile and toluene (4:1) at 20 °C as monitored by 19F NMR spectroscopy.
The rate of decay of the neutral, three-coordinate species in the presence of added halide (Figure 1) was measured to test the potential that the anionic species are intermediates in reactions of phosphine-ligated complexes. Reaction of (tBu3P)Pd(C6H4-p-F)(Br) (5) with styrene in the presence of N(heptyl)4Br (0.68 M) occurred in good yield (92% yield as determined by 19F NMR spectroscopy) and to 90% conversion after 7 h (kobs = 8.9 × 10-5 s-1), while the same reaction conducted in the absence of added bromide proceeded to only 13% conversion over the same time.22
To test further the potential that the ligandless and P(t-Bu)3-ligated complexes react with olefins through a common intermediate, we evaluated the ratios of products formed from the isolated anionic complexes and from reactions conducted with common catalysts for the Heck reaction. Because prior studies23 have indicated that the distribution of isomers formed from the Heck reaction varies with the steric and electronic properties of the electrophile, olefin, and catalyst, we considered that this selectivity could be used to probe for an intermediate that is common to reactions initiated with different catalyst precursors.
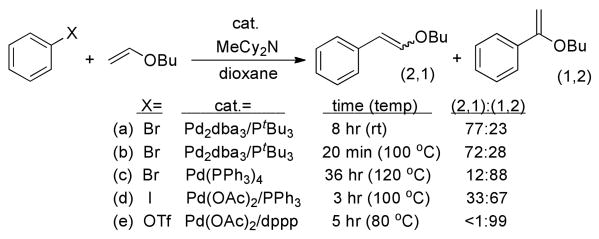
In particular, Heck reactions of vinyl ethers form different ratios of isomeric products with different catalysts,23 and we measured the isomeric ratios of products of the reactions catalyzed by palladium complexes of triphenylphosphine, 1,3-bis-(diphenyl-phosphino)propane (dppp), and P(t-Bu)3 under the same conditions. The distribution of products from the reaction of bromobenzene or iodobenzene with butyl vinyl ether catalyzed by Pd(PPh3)4 or the combination of Pd(OAc)2 and PPh3, as well as that from the reaction of phenyl triflate and butyl vinyl ether catalyzed by Pd(OAc)2 and dppp (Scheme 4) were much different from that obtained from reaction of Pd2dba3 and P(t-Bu)3. Thus, we concluded this selectivity would be a valid probe for a common intermediate.
Scheme 4.
The reaction of butyl vinyl ether with the isolated anionic 3 and with the combination of (tBu3P)Pd(Ph)(Br) and a tetraalkylammonium bromide salt are shown in eq 4 and 5. These two stoichiometric reactions occurred with similar regioselectivity. Moreover, this regioselectivity was indistinguishable from that of the catalytic reaction conducted with Pd2dba3 and P(t-Bu)3. The similarity of these regioselectivities is consistent with the presence of a common intermediate that reacts with the olefin. The selectivity from the stoichiometric reaction of the vinyl ether with complex 3 indicates that this common intermediate is the “ligandless,” anionic arylpalladium halide complex we isolated.
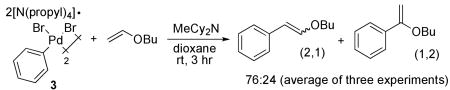 |
(4) |
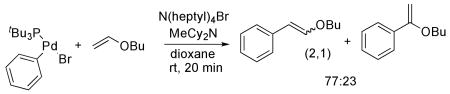 |
(5) |
The studies reported here reveal some basic principles about the reactivity of alkenes with transition-metal complexes and the design of catalysts for reactions occurring by alkene insertion. First, these studies show that alkene insertion can be fast, even for complexes lacking a positive charge or electron-poor dative ligands. The faster reactions of the anionic complex versus that of the neutral species contrasts the typical trend of faster insertions of alkenes into the metal-carbon bonds of complexes that are more electrophilic,24 including cationic organopalladium complexes.25 The anionic complexes are more electron-rich, as probed by computed CO stretching frequencies,26 and therefore, we suggest that the anionic complexes in this work are more reactive than neutral, phosphine-ligated analogs because they are less hindered.
Second, these studies show that the fast rates of the catalyst containing the strongly donating P(t-Bu)3 ligand relies on the ability of this ligand to fully dissociate to generate a less hindered “ligandless” species. At the same time, these studies show that efforts to use ancillary ligands to control selectivity should focus on dative ligands more tightly bound than P(t-Bu)3 or on anionic additives. Future studies will test this hypothesis and explore the role of these species in other palladium-catalyzed processes.
Supplementary Material
Acknowledgments
We thank the NIH (NIGMS, GM 58108) for support of this work and Johnson-Matthey for a gift of PdCl2. We thank Dr. Per Ryberg for helpful discussions on the insertions of vinyl ethers and Dale Pahls for DFT calculations of the νCO values.
Footnotes
Supporting Information Available. Crystallographic data for 1 and 2, experimental procedures and characterization of palladium complexes. This information is available free of charge via the Internet at www.pubs.acs.org.
References
- 1.(a) Reetz MT, Westermann E. Angew Chem Int Ed. 2000;39:165. doi: 10.1002/(sici)1521-3773(20000103)39:1<165::aid-anie165>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]; (b) de Vries JG. J Chem Soc Dalton Trans. 2006:421. doi: 10.1039/b506276b. [DOI] [PubMed] [Google Scholar]
- 2.Evans J, O'Neill L, Kambhampati VL, Rayner G, Turin S, Genge A, Dent AJ, Neisius T. J Chem Soc Dalton Trans. 2002:2207. [Google Scholar]
- 3.de Vries AHM, Parlevliet FJ, Schmieder-van de Vondervoort L, Mommers JHM, Henderickx HJW, Walet MAM, de Vries JG. Adv Synth Catal. 2002;344:996. [Google Scholar]
- 4.(a) Albeniz AC, Espinet P, Martin-Ruiz B, Milstein D. J Am Chem Soc. 2001;123:11504. doi: 10.1021/ja016315b. [DOI] [PubMed] [Google Scholar]; (b) Usón R, Forniés J, Nalda JA, Lozano MJ, Espinet P, Albéniz AC. Inorg Chim Acta. 1989;156:251. [Google Scholar]
- 5.For seminal studies on oxidative addition to phosphine-ligated anionic Pd(0) complexes see: (a) ref 17a. Amatore C, Carré E, Jutand A, M'Barki M. Organometallics. 1995;14:1818.
- 6.(a) Jeffery T. J Chem Soc Chem Commun. 1984:1287. [Google Scholar]; (b) Jeffery T. Tetrahedron Lett. 1985;26:2667. [Google Scholar]
- 7.(a) Heck RF, Nolley JP. J Org Chem. 1972;37:2320. [Google Scholar]; (b) Mizoroki T, Mori K, Ozaki A. Bull Chem Soc Jpn. 1971;44:581. [Google Scholar]
- 8.(a) Reetz MT, Breinbauer R, Wanninger K. Tetrahedron Lett. 1996;37:4499. [Google Scholar]; (b) Beller M, Fischer H, Kühlein K, Reisinger CP, Herrmann WA. J Organomet Chem. 1996;520:257. [Google Scholar]; (c) Reetz MT, Lohmer G, Schwickardi R. Angew Chem Int Ed. 1998;37:481. doi: 10.1002/(SICI)1521-3773(19980302)37:4<481::AID-ANIE481>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt AF, Halaiqa AA, Smirnov VV. Synlett. 2006;18:2861. [Google Scholar]
- 10.Barrios-Landeros F, Carrow BP, Hartwig JF. J Am Chem Soc. 2008;130:5842. doi: 10.1021/ja711159y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amatore C, Jutand A. Acc Chem Res. 2000;33:314. doi: 10.1021/ar980063a. [DOI] [PubMed] [Google Scholar]
- 12.(a) Stambuli JP, Bühl M, Hartwig JF. J Am Chem Soc. 2002;124:9346. doi: 10.1021/ja0264394. [DOI] [PubMed] [Google Scholar]; (b) Stambuli JP, Incarvito CD, Bühl M, Hartwig JF. J Am Chem Soc. 2004;126:1184. doi: 10.1021/ja037928m. [DOI] [PubMed] [Google Scholar]
- 13.Geary WJ. Coord Chem Rev. 1971;7:81. [Google Scholar]
- 14.The equilibrium constant was found to be sensitive to the polarity of the medium.
- 15.(a) Reetz MT, de Vries JG. Chem Commun. 2004:1559. doi: 10.1039/b406719n. [DOI] [PubMed] [Google Scholar]; (b) Guertler C, Buchwald SL. Chem Eur J. 1999;5:3107. [Google Scholar]; (c) Reetz MT, Westermann E, Lohmer R, Lohmer G. Tetrahedron Lett. 1998;39:8449. [Google Scholar]
- 16.The reactions of PPh3 complexes with styrene or acrylates require elevated temperature in the absence of additives and give modest to poor yields of the stilbene or cinnamate products.
- 17.(a) Amatore C, Carré E, Jutand A, M'Barki MA, Meyer G. Organometallics. 1995;14:5605. [Google Scholar]; (b) Dieck HA, Heck RF. J Am Chem Soc. 1974;96:1133. [Google Scholar]; (c) Lee Kyoung H, Youngim N, Junseong L, Youngkyu D, Sukbok C. Angew Chem Int Ed. 2005;44:6166. [Google Scholar]
- 18.Netherton MR, Fu GC. Org Lett. 2001;3:4295. doi: 10.1021/ol016971g. [DOI] [PubMed] [Google Scholar]
- 19.The reaction of 1-bromo-4-N,N-dimethyl-aniline with methyl methacrylate catalyzed by the parent complex 3 containing an ammonium, rather than a phosphonium, cation did not form any cinnamate product at room temperature, and proceeded to only 17% conversion at 100 °C.
- 20.(a) Littke AF, Fu GC. J Am Chem Soc. 2001;123:6989. doi: 10.1021/ja010988c. [DOI] [PubMed] [Google Scholar]; (b) Shaughnessy KH, Kim P, Hartwig JF. J Am Chem Soc. 1999;121:2123. [Google Scholar]; (c) Ehrentraut A, Zapf A, Beller M. Synlett. 2000;120:1589. [Google Scholar]; (d) Littke AF, Fu GC. J Org Chem. 1999;64:10. doi: 10.1021/jo9820059. [DOI] [PubMed] [Google Scholar]; (e) Stambuli JP, Stauffer SR, Shaughnessy KH, Hartwig JF. J Am Chem Soc. 2001;123:2677. doi: 10.1021/ja0058435. [DOI] [PubMed] [Google Scholar]
- 21.In the absence of added halide, rate acceleration by the palladium product (tBu3P)2Pd(H)(Br) was observed and addition of MeCy2N was necessary to scavenge HBr. The resulting salt (MeCy2N · HBr) subsequently crystallized from solution during the course of the reaction.
- 22.No intermediates were observed and the olefin product was formed with a rate constant that was similar to that of the decay of starting complex 5 (kobs = 7.4 × 10-5 s-1).
- 23.(a) Cabri W, Candiani I, Bedeschi A, Santi R. J Org Chem. 1992;57:3558. [Google Scholar]; (b) Cabri W, Candiani I, Bedeschi A, Penco S, Santi R. J Org Chem. 1992;57:1481. [Google Scholar]; (c) Andersson CM, Hallberg A, Daves GD., Jr J Org Chem. 1987;52:3529. [Google Scholar]
- 24.Svejda SA, Brookhart M. Organometallics. 1998;18:65. [Google Scholar]
- 25.Szabo MJ, Jordan RF, Michalak A, Piers WE, Weiss T, Yang SY, Ziegler T. Organometallics. 2004;23:5565. [Google Scholar]
- 26.DFT calculations of CO stretching frequencies for neutral and anionic Pd complexes suggest that the anionic complex contains a more electron rich metal center (see Supporting Information).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.