Abstract
Background
Solid organ transplant recipients must take immune suppressive medications that have side effects, cause complications and lead to distressing symptoms that reduce health-related quality of life. Mindfulness meditation has been shown to reduce these symptoms in other patient populations, and it is unlikely to interfere with the immune suppressive medication regimen.
Purpose
This paper describes the design and rationale of a clinical trial to determine whether training in mindfulness meditation can reduce depression, anxiety and insomnia after transplantation, and summarizes baseline characteristics of the participants.
Methods
Transplant recipients were randomized in equal numbers to one of three arms: a Mindfulness-Based Stress Reduction (MBSR) program consisting of 8 weeks of group instruction, home practice and telephone monitoring; a time and attention control Health Education program; or a waitlist arm. After serving 6 months as waitlist controls, these participants were re-randomized to MBSR or Health Education. Evaluations were obtained at baseline (prior to the active interventions), 8 weeks, 6 months and 1 year (after randomization to MBSR or Health Education only). The primary analysis will compare composite symptom scores between MBSR and Health Education, initially or after serving in the waitlist. Subsequent analyses will compare these two groups on depression, anxiety and insomnia symptom scales and secondary outcomes of health-related quality of life, actigraphy and health care utilization. A separate analysis, using only data collected before re-randomization, will compare short-term outcomes between the waitlist and active treatment arms.
Results
One hundred fifty recipients were randomized and 72% of waitlist participants (31/43) were recycled to an active intervention after 6 months. Patient characteristics were balanced across trial arms after initial and secondary randomizations.
Limitations
Transplant recipients are a very select population. Their adherence to the intervention and willingness to serve as waitlist controls prior to re-randomization may be atypical. Participants were not blinded to treatment and primary outcomes are self-reports.
Conclusion
The innovative design used in the trial enabled the waitlist group to directly contribute to the number in the primary analysis of active arms, and to also serve as an internal validation test. The trial may be a useful model for trials involving very small target populations.
Keywords: waitlist, mindfulness meditation, solid organ transplant
Introduction
Solid organ transplantation improves health-related quality of life, but does not restore patients to normal health.[1-3] Transplant recipients must take immune suppression medications daily, and these drugs are costly, have serious side effects and may cause major complications.[4-6] Psychosocial stress is one of the most prominent negative sequelae of organ transplantation, and symptoms of depression, anxiety and insomnia are common.[7-10] It is thus important to develop non-pharmacologic interventions that provide transplant recipients relief from these distressful symptoms.
The Mindfulness-Based Stress Reduction (MBSR) program has been shown to improve mood and health-related quality of life for patients with cancer,[11, 12] anxiety,[13, 14] and depression,[15, 16] and to improve stress management among non-clinical populations.[17, 18] While MBSR is one of the most solidly researched meditation programs,[19, 20] a 2007 Agency for Healthcare Research and Quality evidence report on MBSR and other forms of meditation termed the field “beset with uncertainty.”[21] The report rated clinical trials of meditation as poorly designed, and, in particular, faulted the selection of controls. The report concluded that current literature does not provide clear support for the therapeutic application of any meditation program.
This paper presents the design, rationale and methods of a randomized trial of MBSR to reduce symptom distress in solid organ transplant recipients, and summarizes baseline characteristics of participants. The trial faced two major challenges: recruiting sufficient participants from a small target population and evaluating a holistic intervention with an undetermined mechanism of action.
The target population, organ transplant recipients, is extremely small. We estimated that only 2,200 adults living in the study area had received organ transplants over the past 10 years. Small trials are warranted to study unique, distinctive populations like transplant recipients, but investigators are expected to ensure that all trials are adequately powered.[22] Several designs are optimal for small samples, but these options - crossover, adaptive and sequential designs - are not feasible with mindfulness meditation training, because mindfulness training does not washout and does not have an immediately detectable and reversible impact on health.
Like most forms of meditation training, MBSR is complex and multi-faceted, a mixture of specific therapeutic elements and incidental elements[23] that interact in a synergistic manner to affect health outcomes. If MBSR were dismantled and individual elements studied in isolation, the results could be misleading, and would not serve as a valid test of MBSR.[21] Key features that enabled this trial to meet its recruiting target and control for incidental elements in evaluating the impact of MBSR on outcomes are described below.
Trial Design and Methods
Overview
The study was a randomized controlled clinical trial to test the hypothesis that MBSR would significantly reduce depression, anxiety and insomnia in transplant recipients. Participants were recruited through advertisements in transplant clinics, by direct mail and by provider referrals. After screening and consent, they were randomized to one of three arms: 1) the MBSR program; 2) a Health Education program; or 3) a waitlist control. After 6 months of data collection, waitlisted participants were re-randomized to either MBSR or Health Education. (Figure 1) Health Education was an active time and attention control condition consisting of peer-led chronic disease self-management classes. The primary outcomes were self-reported symptoms and health-related quality of life, collected by mailed surveys at baseline before randomization; at 8 weeks, coinciding with the end of the active intervention period; and at 6 month follow-up. Twelve month follow-up data were collected from all participants randomized to the active arms (MBSR or Health Education). All participants completed diaries throughout the study period, recording use of health services, medications, sick days, time exercising and time meditating (MBSR group only). Seven sets of MBSR and Health Education classes were conducted between 2003 and 2007. Follow-up measurements will be completed in 2008.
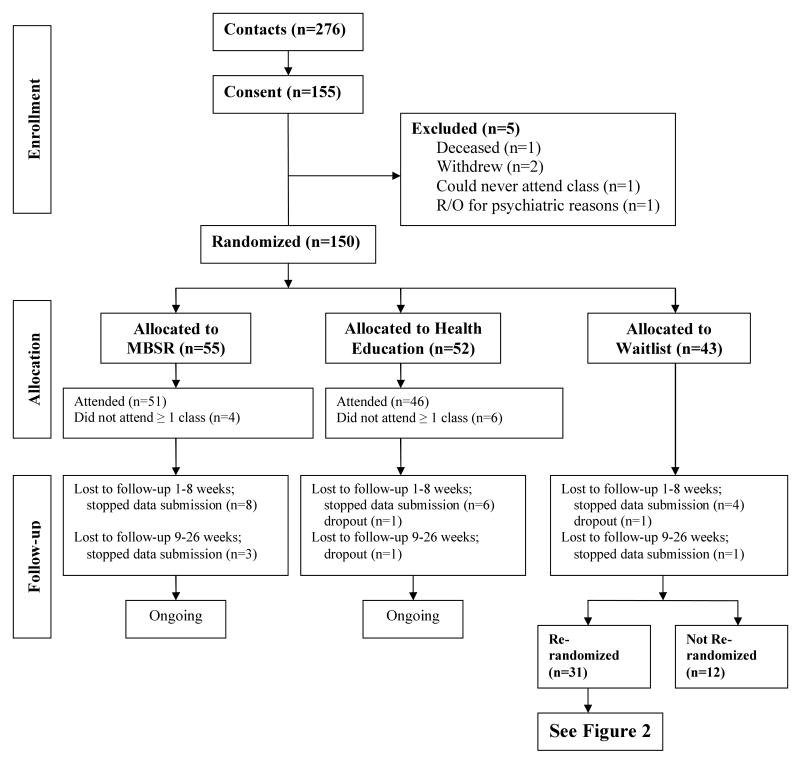
Figure 1.
Stage 1 Randomization. Flow diagram of all participants by first randomization assignments. One year follow-up is ongoing. Figure includes counts as of 1/15/08.
Inclusion and Exclusion Criteria
This study is being conducted in the Minneapolis-St. Paul metropolitan area, which is served by two major transplant programs. Study participants were adult solid organ transplant recipients (kidney, kidney/pancreas, pancreas, lung, liver, heart or heart-lung) whose transplant surgery was at least 6 months prior to enrollment. Participants were 18 years old or older; English-speaking, literate and mentally intact; reachable by telephone; on immune suppressive medication and receiving regular medical follow-up care; and able to attend weekly classes in Minneapolis. To avoid the confounding effects of other health conditions, persons were excluded if they had serious preexisting mental health issues (e.g., psychosis), were medically unstable (non-elective hospital admission in the last 3 months or major surgery planned) or on kidney dialysis. Persons regularly practicing mindfulness meditation were also excluded. All participants completed the informed consent process and signed informed consent and HIPAA documents.
The sample was diverse with respect to the underlying disease cured or treated by transplantation. However, living with an organ transplant and a complex medication regimen generates shared experiences, common health concerns and side effects. Since both the MBSR and Health Education interventions were developed for use with diverse groups of chronically ill patients, it was reasonable to expect treatment impact in this mixed transplant group. Further, by including all types of organ transplants, we substantially increased our pool of potential participants, and our results will therefore be more generalizable.
Recruiting and Screening
Numerous strategies were used to inform transplant recipients about the study. In an earlier pilot [24] we found that using the mass media (e.g., newspapers, radio) was not cost effective for this small, select population. As an alternative, a registry of interested persons was established and these individuals were notified when active recruiting for the trial began. This enabled us to easily fill our initial set of MBSR and Health Education classes. Other recruiting strategies included a physician co-investigator and recruiting liaisons who were staff at the transplant clinics and informed patients about the trial and conducted initial screening. These strategies were augmented by direct mailings from the transplant centers to recipients and by clinical champions who spoke with potential participants individually. We held both evening and afternoon classes and publicized the availability of transportation assistance for those unable to drive to class. In our final year, updated recruiting flyers, posters and color brochures were widely distributed at transplant centers and sent to local transplant-related organizations. Recruiting efforts resulted in 276 patients contacting study staff, and over half (54%) were subsequently enrolled and randomized. Overall, we distributed more than 3000 study brochures and mailed over 1500 letters to providers and patients to obtain our target sample of 150 participants.
Randomization
A stratified two-stage randomization scheme[25] was used. In the first stage, all participants were randomized to one of the three study arms (MBSR, Health Education or waitlist). (Figure 1) In the second stage, the waitlist participants were randomly allocated to MBSR or Health Education. (Figure 2) The purpose of the two-stage scheme was to enhance power for testing the primary hypothesis that MBSR would have significantly more impact than Health Education, and to provide an internal validation test. To ensure balance across the groups, participants were stratified by: (1) current use of medications or past treatment for depression, anxiety or sleep (yes/no); (2) type of transplant (3 groups - kidney/liver/heart or lung); and (3) Type 1 diabetes (yes/no). Using blocks of 6, patients were randomized within strata in equal numbers to three groups: the MBSR program; Health Education; or the waitlist. (Figure 1) The waitlist participants contributed data at points comparable to pre- and post-intervention and 6 month follow-up, and then had the opportunity to be randomized to MBSR or Health Education. (Figure 2)
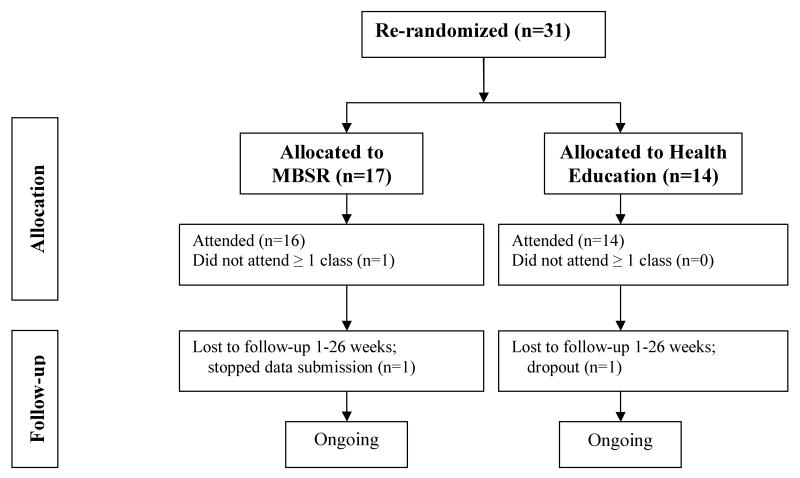
Figure 2.
Stage 2 Randomization. Flow diagram of waitlist participants who were re-randomized. One year follow-up is ongoing, and a fifth dropout has occurred post-26 weeks in the MBSR group. Figure includes counts as of 1/15/08.
Use of the Recycled Waitlist Design
During the informed consent process, a diagram (similar to Figure 1) was used to explain the study design, and potential participants appeared to welcome the information that, regardless of initial randomization, they would have an opportunity for an active treatment. During the waiting period dropouts from the waitlist were few in number, and less than the number of dropouts in either active intervention arm. (See Figure 1) Re-randomizations were occasionally postponed beyond the planned 6 month period, due to time conflicts with the next series of study classes (e.g., vacations, work, health changes) that could not be predicted at the time of initial study enrollment. Some waitlist participants were never re-randomized, and this attrition should be addressed in estimating sample size for this design. Trials with shorter intervention cycles or briefer follow-up intervals might retain a higher proportion of their waitlist arm for randomization to active treatment.
After 6 months, 72% of waitlist participants (31 of 43) were re-randomized to attend MBSR or Health Education classes and 29 completed the 6 month post-intervention follow-up. Re-randomizing increased the sample size for the primary outcome comparison by 29%, a notable gain, given the size of the target population. Equally important, comparability of baseline characteristics between MBSR and Health Education groups was maintained after the second randomization.
As shown in Table 1, the randomization process achieved a reasonable balance across the three arms at baseline. The only variable found to differ in the three arms at baseline was education, and these differences were modest and could be addressed in multivariate analyses. The distribution of baseline characteristics across the two active arms after waitlist participants were re-randomized and combined with the original MBSR and Health Education groups is shown in Table 2. Variables known to be associated with the outcomes, such as gender (46% vs. 44%), medication use (44%, both) and type of transplant (68% vs. 65% kidney or kidney/pancreas recipients) were very similar between the active arms after the second randomization.
Table 1. Baseline characteristics of all randomized participants: Stage 1 randomization.
| 3 groups randomized (N=150) | ||||
|---|---|---|---|---|
| MBSR | Health Education | Waitlist | P value1 | |
| n (%) | 55 (37) | 52 (35) | 43 (29) | |
| Age (SD) | 55 (12) | 51 (10) | 56 (11) | .11 |
| Female n (%) | 21 (38) | 24 (46) | 22 (51) | .42 |
| Race n (%) | .52 | |||
| White | 48 (87) | 49 (94) | 39 (91) | |
| Black/African American | 3 (5) | 0 (0) | 1 (2) | |
| Asian or American Indian | 4 (8) | 3 (6) | 3 (7) | |
| Hispanic/Latino n (%) | 1 (2) | 2 (4) | 4 (9) | .21 |
| Marital status n (%) | .06 | |||
| Never married | 8 (15) | 4 (8) | 4 (9) | |
| Married | 38 (69) | 26 (50) | 26 (61) | |
| Other | 9 (16) | 22 (42) | 13 (10) | |
| Employment n (%) | .36 | |||
| Full-time | 19 (35) | 27 (52) | 15 (35) | |
| Part-time | 8 (15) | 5 (10) | 7 (16) | |
| Other | 28 (50) | 20 (38) | 21 (49) | |
| Education n (%) | .05 | |||
| High-school or partial | 2 (4) | 10 (19) | 1 (2) | |
| Some college | 21 (38) | 16 (31) | 13 (31) | |
| College graduate | 22 (40) | 17 (33) | 16 (38) | |
| Post-graduate | 10 (18) | 9 (17) | 12 (29) | |
| Treated and/or takes medication for sleep, depression and/or anxiety in past year | 24 (44) | 24 (46) | 17 (40) | .81 |
| Transplant type n (%) | .99 | |||
| Lung or heart | 10 (18) | 9 (17) | 7 (16) | |
| Liver | 10 (18) | 9 (17) | 7 (16) | |
| Kidney and/or pancreas | 35 (64) | 34 (65) | 29 (67) | |
| Years since original transplant (SD) | 5.6 (5.8) | 7.6 (7.4) | 5.8 (5.2) | .21 |
P values for the continuous variables are derived from ANOVAs and from chi square tests for categorical variables.
Table 2. Baseline characteristics of participants in the active arms: Stage 1 and stage 2 randomizations, combined.
| 2 groups randomized (N=138) | |||
|---|---|---|---|
| MBSR | Health Education | P value1 | |
| n (%) | 72 (52) | 66 (48) | |
| Age (SD) | 55 (12) | 52 (10) | .17 |
| Female n (%) | 33 (46) | 29 (44) | .82 |
| Race n (%) | .25 | ||
| White | 65 (91) | 62 (94) | |
| Black/African American | 3 (4) | 0 (0) | |
| Asian or American Indian | 4 (6) | 4 (6) | |
| Hispanic/Latino Ethnicity n (%) | 3 (4) | 2 (3) | .72 |
| Marital Status n (%) | .12 | ||
| Never married | 9 (13) | 5 (8) | |
| Married | 47 (65) | 36 (55) | |
| Others | 16 (22) | 25 (38) | |
| Employment n (%) | .26 | ||
| Full-time | 24 (33) | 31 (47) | |
| Part-time | 11 (15) | 8 (12) | |
| Others | 37 (52) | 27 (41) | |
| Education n (%) | .17 | ||
| High-school or partial | 3 (4) | 10 (15) | |
| Some college | 24 (33) | 21 (32) | |
| College graduate | 30 (42) | 24 (36) | |
| Post-graduate | 15 (21) | 11 (17) | |
| Treated and/or takes medication for sleep, depression and/or anxiety in past year | 32 (44) | 29 (44) | .95 |
| Transplant Type n (%) | .90 | ||
| Lung or heart | 12 (17) | 11 (17) | |
| Liver | 11 (15) | 12 (18) | |
| Kidney and/or pancreas | 49 (68) | 43 (65) | |
| Years since original transplant (SD) | 5.7 (6.0) | 7.1 (6.9) | .20 |
P values for the continuous variables are derived from ANOVAs and from chi square tests for categorical variables.
Logistically, implementing the two-stage randomization was not difficult or excessively time consuming. The implementation process for this trial required mounting the two active intervention arms simultaneously, and in tandem, monitoring the data collection from the newly randomized participants in the waitlist arm. Tracking and maintaining contact with individuals who completed their waitlist period and then deferred entering an active intervention was the most time consuming aspect of this design.
Re-Randomization
Re-randomization was undertaken in order to make optimal use of efficacy data in a trial in which the treatments did not wash out and enrollment continued over several years. Others have used re-randomization to refine optimal dosing schedules, to “enrich” clinical trials by adding stages in which some patients continued, added or withdrew from therapies, or to evaluate salvage therapies among patients randomized to placebo or failing a first therapy.[26] In these trials, the second randomization data were generally analyzed and reported as a separate, ancillary trial. Re-randomization in clinical trials is relatively rare, and there are no guidelines for reporting re-randomized trials.[26]
Waitlists
Waitlists have been found to be effective in recruiting and retaining control group participants in trials of meditation and yoga.[27, 28] Our design was an extension and modification of the standard waitlist. In a standard waitlist, there is no second randomization and investigators fail to take full advantage of data from treatment of the waitlist participants. Often the period of treatment for the waitlist is not part of the formal trial, and waitlist treatment results are not reported. In a recent paper, Brown et al.[29] described a dynamic waitlist design to achieve both internal control and efficiency. Brown's dynamic waitlist required that all enrollees be known at baseline so that intervention times could be randomly assigned. Therefore, this approach was not feasible in our trial. While our approach shared the objectives of the dynamic waitlist, our design was possible with a rolling enrollment.
Waitlist designs can be controversial, particularly when a standard treatment is being temporarily withheld from trial participants.[30] In this study, there was not restriction on the use of standard treatment (i.e., symptom medications) in any study arm. Symptom medication use was collected throughout the study, and exploratory analyses will evaluate how patterns of medication use changed during the trial. A potential limitation of this design is that spontaneous symptom reductions, before re-randomization, could reduce the responsiveness of the waitlist participants to study interventions. Participants may enter a trial when their symptoms are particularly burdensome, and improve on their own during the waiting period. [31] This bias can be directly evaluated with this design, and if the waitlist responds differently to therapy, analyses can be restricted to the stage 1 randomization.
Interventions
Mindfulness-Based Stress Reduction
Mindfulness-Based Stress Reduction originated in the Stress Reduction Clinic at the University of Massachusetts (UMass) Medical Center and is currently used in over 250 clinics, hospitals, and HMOs in the US and abroad.[32] MBSR was developed to facilitate adaptation to the stressors of medical illness. It is an 8-week, generic, skills-based program led by an instructor, in a class format. MBSR has a well delineated manual, structured class activities, a repertoire of yoga postures, homework assignments and recordings to guide home practice. There are also training programs, certification and continuing education for instructors to ensure a consistent, standardized program. Our MBSR instructors, both trained at the UMass program, follow this standard model. The MBSR program is described in the text Full Catastrophe Living by Kabat-Zinn.[33]
The eight MBSR classes are each 2 ½ hours long. Sessions include information about stress, cognition and health, but primarily concentrate on learning to focus attention through a variety of meditative techniques, such as focusing on the breath, body-scan, sitting and walking meditations and gentle yoga. A day of mindfulness retreat is held on the weekend between Weeks 6 and 7. This 6-hour retreat consists of 5 hours of silent mindful meditation practice with the instructor leading meditations and yoga, a mindful meal, and an hour of discussion of the experience.
MBSR participants are trained to recognize their immediate emotional and physical state, including pain or discomfort, and to let thoughts come and go in awareness with no attempt to change, suppress or elaborate on thoughts. By incorporating mindfulness techniques into their daily life, practitioners learn to “find breathing space” in order to skillfully respond to stressors with appropriate action, rather than reacting “on automatic pilot” with conditioned responses that can be emotionally arousing or unhelpful. Mindfulness is hypothesized to facilitate adaptation to the stressors of chronic illness,[34] and the goal of MBSR is to promote lifelong self-management.[33]
Benefits of MBSR are posited to depend on regular, personal meditation practice. Participants are encouraged to practice meditation for 45 minutes at least once a day, 6 days a week, throughout the course. In this trial, home practice expectations during the follow-up period were 20 minutes per day, 6 days a week, plus informal use of mindfulness, with the intention of maintaining MBSR practice for life. A unique feature of our trial was the inclusion of a system of telephone contacts to promote adoption of meditation practice during follow-up. During the first 4 months of follow-up, the study coordinator called each participant to identify obstacles to practice, and encouraged use of techniques to resume practice when lapses occurred. Individualized feedback was based on the Transtheoretical Model of Behavior Change.[35] Participants were called on a tapering schedule, weekly during Month 3, twice in Month 4 and only once in Months 5 and 6.
Health Education
The peer-led chronic disease self-management program developed at Stanford University by Lorig and associates was selected as the core of our active control condition.[36] The Stanford program is highly structured and follows a program manual for class content and “action planning,” a process in which participants identify a specific task that they can confidently accomplish in the coming week and share outcomes with the group. The program is typically delivered in six health education classes with 5 to 15 participants, led by two trained peer leaders. Each class session includes a generic chronic disease self-management topic, a problem-solving or communication activity and action planning. Our study interventionist and one transplant recipient attended the Stanford training program for program leaders. The study interventionist trained three additional leaders and supervised all Health Education classes. The Stanford program classes meet weekly for 2 ½ hours, similar to a MBSR class. To roughly equate time and attention for this trial, the Stanford program was followed by two classes with transplant-specific content (e.g., traveling after transplant, risks of complications), and Health Education participants received phone calls from study staff on the same schedule as the MBSR group. During these calls Health Education participants reported on their action plans.
Based on results from the Stanford program, we expected Health Education to improve self-efficacy for managing health and to reduce health care utilization.[37, 38] Health Education was not expected to have an impact on the primary symptoms (depression, anxiety and insomnia) studied in our trial. Contrasting the active arms (MBSR and Health Education) with the waitlist group was expected to elucidate the differential impacts of the arms.
Rationale for Control Selection
Non-specific yet potentially beneficial elements of MBSR include instructor attention, group support and expectancy, factors that may bias comparisons to a no treatment control arm.[39] We decided that it was critical to equate these non-specific factors because the content and delivery of the MBSR program made masking instructors and study participants unreasonable. The study team chose a known active control (Health Education) over a contrived sham time and attention arm, because the extensive time requirements of MBSR class and practice convinced us that only a potentially beneficial control could ethically justify a comparable level of participant burden.[30] An active control condition with a track record of providing meaningful benefits for persons with chronic illness could also serve to attract participants and overcome resistance to being randomized. It has been found that randomization to no treatment can be a barrier to recruiting, generate resentful demoralization, and increase dropouts in unblinded trials.[40, 41] However, comparing MBSR only to this active control would result in reduced power and fail to control for maturation or spontaneous improvement in both arms.[42] A standard three-armed trial with both active and passive controls overcomes these limitations, but at a cost of diminished statistical power for the primary comparison of active treatment arms.[43] Our two-stage randomization approach resolved this dilemma, and offered the advantages of the three-group design while mitigating the loss of power from multiple arms. In this trial, Health Education ensured that individuals in the active arms had similar study-related time demands, group, instructor and study staff contact, and justifiable expectations of personal benefit.
Primary Outcomes
The primary study outcome was a composite, the sum of ranked changes from baseline in symptom distress based on widely used and well-validated measures of anxiety, depression and sleep. The State-Trait Anxiety Inventory (STAI) (Y-1) state version measures anxiety at the present time with 20 brief questions such as “I am tense”, rated on a scale from 1 = not at all to 4 = very much so.[44] The Center for Epidemiological Studies Depression Scale (CES-D) measures depression symptom intensity in the past week. It consists of 20 questions such as “I felt sad,” with responses from 1 = rarely or none at all to 4 = most or all of the time.[45] The Pittsburgh Sleep Quality Index (PSQI) measures sleep quality, based on recall of sleep behaviors in the past month. The PSQI has 18 questions addressing 7 sub-domains of sleep: quality, latency, duration, efficiency (quotient of hours asleep divided by hours in bed), disturbances (like feeling too hot or too cold), use of medications, and daytime dysfunction.[46] The STAI and CES-D have strong psychometric properties, and norm-based cutoffs for determining clinically meaningful levels of symptoms. The PSQI is the most widely used standardized measure of sleep quality;[47] its psychometric properties are good, and it too has cutoffs for clinically meaningful impact. Higher scores on the STAI, CES-D and PSQI represent increased symptom distress. The original time frames for the STAI, CES-D and PSQI were preserved in this study to maintain the validity of the instruments and consistency with norms and other literature. The measures were found to have high reliability and validity with transplant recipients in our pilot study.[24]
Composite change scores were calculated following the approach of O'Brien [48] by computing the changes from baseline in the primary outcomes - STAI, CES-D and PSQI - at 8 weeks, 6 months and one year for each participant; next, within each outcome separately at each time, changes were ranked across the sample from best (=1) to worst in terms of improvement; finally, the three outcome ranks assigned to each participant at one time were summed. The participant with the smallest rank-sum at a time point reported the most improvement from baseline in these symptoms, consistent with the direction of low symptom burden on the original scales. Basing the composite on ranks ensures that the three components will be equally weighed in the global test of the composite outcome, despite differences in scale means and variances. As noted by Pocock [49], global tests are useful when it is desirable to judge treatment success by a consistent pattern of benefit across a specified set of correlated outcomes. As noted by Tilley et al.[50], following the global test one analyses the individual component outcomes for clinical interpretability. Our components, the STAI, CES-D and PSQI, have norms and accepted benchmarks to assess clinical benefit, while the rank-based composite does not.
In this trial, benefits to anxiety, depression and sleep were considered equally important and equally likely; i.e., a common intervention effect was expected. The composite score in the O'Brien approach is used to test for a consistent pattern of improvement or worsening across these indicators of symptom burden. The non-parametric approach weighs the indicators equally, and the resulting sum can be analyzed using normal theory statistics. Because the conceptual domains of insomnia, anxiety and depression overlap (e.g., sleep dysfunction is part of DSM-IV criteria for depression), these symptoms are positively correlated, and therefore, a Bonferroni correction would be too conservative and have low power. The O'Brien method is more appropriate than Hotellings' T2 or MANOVA, which do not test for a consistent trend. The major virtue of the composite is its parsimony, enabling a powerful omnibus test that can be followed with symptom-specific analyses based on three widely used, reliable measures. Because it was hypothesized that the size of the intervention's effects on these outcomes would be similar, the single test of a composite outcome would be efficient and more powerful than separate tests adjusted for multiple comparisons.[51]
Secondary Outcomes
Actigraphy, an objective technique to measure sleep / wake patterns, was used to complement self-reported PSQI results. An actigraph is a wristwatch-like device that records movement, and the data generated are summarized by validated software programs to measure key sleep/wake parameters. Actigraphy has been found to be highly reliable for the measurement of group changes, and it can replace the more expensive and burdensome polysomnography for many research purposes.[52] Participants in the active arms completed 2 weeks of actigraphy at baseline and at 6 month follow-up. Actigraphy readings, masked to treatment assignment, were the only blinded study outcome.
Health-related quality of life, quality of life, perceived transplant-related stress, serenity, use of health care resources, and costs were also secondary outcomes in the trial. Quality of life was defined as a sense of well-being, satisfaction and happiness with all personally important aspects of life. Health-related quality of life was defined as subjective health status, and referred to the physical, mental and social domains of function and well-being that can be influenced by illness and treatment.[53] The Short Form-12, version 2 (SF-12v2), a brief version of the widely used SF-36 generic health profile, was the main health-related quality of life measure.[54] The first SF-12 item, self-rated health, can be compared to national surveys and has been shown to predict mortality.[55] The SF-12 physical (PCS) and mental (MCS) component scores are unbiased and close approximations to the SF-36 MCS and PCS scores, which have extensive norms.[56]
Quality of life was measured with a visual analogue scale similar to that included in the widely used EQ-5D.[57] The simplicity of the one item quality of life scale permits individuals to rate their quality of life without framing effects from investigator-selected domains of importance. Treatment-specific quality of life, defined as distress from transplant-related factors (e.g., fear of organ rejection, susceptibility to infection), persists for years after transplantation surgery.[58, 59] A 10-item version of Frazier's Transplant-Related Stressors scale was therefore included in the trial.[59] Serenity has been defined as a non-religious dimension of spirituality that decreases stress and promotes optimal health. To test the hypothesis that MBSR increased serenity, a 22-item version of Roberts and Aspy's Serenity Scale was included in the trial.[60]
Data on use of health care resources, including hospital, urgent care, emergency department or health provider visits, use of medications for depression, anxiety or insomnia, and sick days were collected using daily diaries distributed to participants and returned by mail each month. Participants who ceased to complete monthly calendars were given the option of receiving one call a month to report hospitalizations.
Mediators and Moderators
To evaluate the hypothesis that MBSR participation would lead to increased mindfulness, home meditation practice time was recorded on diaries throughout the study period. A scale measuring attitudinal mindful awareness[61] was collected at baseline and at every follow-up. Social support, self-efficacy to manage chronic health conditions, and coping strategies were three additional mediators posited to influence outcomes. Brief self-report scales were used to measure these putative mediators longitudinally, concurrently with our outcomes, so that they could be examined as either intermediate outcomes or mediators of impact.
Factors that might moderate outcomes, including demographics, medical history, positive and negative affect[62] and type of transplant(s), were collected at baseline. Adherence to immune suppressive medications was collected by self-report on the Morisky Medication-Taking Scale.[63]
Retention and Dropouts
Class attendance, home practice and study form completion were closely monitored. Participants were encouraged to continue contributing data regardless of class attendance or meditation practice. Birthday cards and seasonal study newsletters were sent to maintain contact with all active participants. The newsletters were also sent to transplant organizations to increase members' awareness of the study. Department store gift cards in small denominations ($5 and $10) were mailed with follow-up surveys as thank-you incentives. An offer of a small gift (e.g., pedometer, Transplant Olympics recipe book) accompanied the one year survey. Individuals who did not return surveys in a timely manner after repeated mailings were offered an abbreviated “core outcomes” short form to complete by mail or phone interview. Only 5 randomized participants have dropped out of the study to date; however, 18 were “pocket refusals” who, despite a re-mailing and reminder calls, never returned post-intervention 8-week surveys.
Sample Size and Power
Sample size was calculated to provide 80% power to detect at least a moderate difference between MBSR and Health Education, the active control, in 6 month change from baseline on the composite score (defined above), and in the primary outcomes of depression, anxiety and insomnia. A meta-analysis [64] of 10 randomized trials using standardized mindfulness training (using almost exclusively MBSR programs) with outcomes of anxiety, depression, sleep, and/or psychological well-being gave an effect size of d/σ = 0.54; Baer's meta-analysis of MBSR found similar results, with effect size .59.[65] These represent medium effects in Cohen's taxonomy.[43] To have 80% power to detect an effect size of at least d/σ = 0.5 at the 0.05 level using a two-sample two-sided t-test, 64 participants are required in each group. This effect size corresponds to a difference of 1.2, 3.0, and 3.6 points on the PSQI, CES-D, and STAI, respectively. The estimated group size of 64 was increased by 20% to 75 in each group to allow for dropouts based on a MBSR trial with cancer patients which reported 17% dropouts over a year[12] and on our pilot study which had 5% dropouts over 6 months.[24] The 75 per group includes both those randomized in stage 1 and stage 2.
Statistical Analyses
For internal validation of the interventions, we will compare the active interventions, MBSR and Health Education, to the waitlist (passive control) using change from baseline to 8 weeks, immediately post-course for the interventions. This will be an intent-to-treat analysis using assignments from the first-stage randomization to three groups, and baseline-carried-forward imputation of missing values. A global test of the composite outcome between the waitlist and active arms (MBSR and Health Education combined) will be followed by separate analyses of the STAI, CES-D and PSQI between each active arm and the waitlist. Demographic and baseline clinical characteristics of the waitlist participants who were and were not re-randomized at stage 2 will be compared to detect selection effects that might impact the primary comparison.
The primary comparison is between MBSR and Health Education in 6 month change from intervention baseline on the composite score. This intent-to-treat comparison will be based on all participants as randomized to MBSR or Health Education either at the first or second stage of randomization, with baseline-carried-forward imputation: those missing 6 month outcomes are assumed to have zero change, i.e., no improvement from baseline. This comparison will be performed using a fixed-effects general linear model that adjusts the main comparison for the design stratification and for waitlist status. Inclusion of stratifying variables in the analysis will adjust for residual imbalances between groups on these factors, a particular concern for small trials with blocked designs.[66] Inclusion of a waitlist variable will enable this analysis to detect a possible impact of waitlist service on treatment response (i.e., differential responses between stage 1 (not waitlisted) and stage 2 (waitlisted) randomized participants). However, there were no important differences in demographic characteristics between waitlist participants and those assigned to MSBR or Health Education at stage 1 (Table 1).
This primary comparison will be supplemented by a comparison between MBSR and Health Education on longitudinal changes to 8 weeks, 6 months and one year, using a mixed-effect linear model with a random effect for participant, to model correlation within participants, and fixed-effects adjustors for the design stratification and for waitlist status. In addition, using counts of participants in clinically symptomatic and normal categories for anxiety, depression, and insomnia, a parallel comparison of MBSR and Health Education longitudinally will use generalized estimating equations to account for the correlated binary data within participants.[67]
Clinical significance and determination of treatment success will be based on established cutpoints. For depression, the proportions of the sample at or above 16 (proposed cut point for clinically meaningful depression symptoms) on the CES-D at baseline, post-course and follow-up will be determined. This cut point corresponds to the most symptomatic 15-19% of the general population.[45] For anxiety, proportions in the moderate (STAI of 40 to 59) and severe (STAI of 60 or more) anxiety ranges will be determined.[44] For self-reported sleep scores, the proportions of the sample above the score of 5, the cut point for ‘poor sleep’ on the PSQI, at baseline, post-course and follow-up will be determined. This corresponds to the worst 11.5% in a group of healthy adult volunteers.[46] An overall determination of treatment success will be based on meeting criteria for benefit on at least one symptom scale. For actigraphy, standard cutpoints for poor sleep are sleep efficiency <85%, sleep latency > 30 minutes, wake time after sleep onset > 30 minutes or less than 6 hours of total sleep time.[68] We will also count the number of patients whose scores move from the symptomatic to the normal range from pre-to-post-treatment. If efficacy is shown, cost-effectiveness analyses will form incremental cost effectiveness ratios using an algorithm to convert SF-12 results to quality-adjusted life years[69] and standard charges used to convert health utilization data to dollars.
Discussion
This trial design may be a useful model for other trials with very small target populations. Recommendations for small clinical trials include tailoring the design and performing corroborative statistical analyses. Tailoring involves selecting outcomes that make the best possible use of available participants and customizing the statistical design and analytic approaches to the study population and research question.[22] To obtain data for corroborative analyses, investigators are encouraged to collect multiple outcome measures and measures of factors that may mediate or moderate treatment effects. To obtain the most information from available participants, investigators may employ repeated measures or other longitudinal reports. All of these recommendations have been followed in this trial of MBSR.
Limitations
Every trial, regardless of its size, is expected to be adequately powered.[22] This trial employed a composite outcome and innovative recycled waitlist design to increase the power of the primary outcome analysis, in order to fully utilize a sample of participants from a very small target population. These strategies are most effective when the intervention (i.e., MBSR) generates similarly sized effects on the specified outcomes (depression, anxiety and insomnia, in this case), and these outcomes are inter-correlated.[70] The analysis will be unbiased if serving in the waitlist has no influence on subsequent response to the active treatments. Should these conditions not hold, the alternative is to analyze outcomes separately and to limit the primary analysis to data collected following the stage 1 randomization.
Risks analogous to these are inherent in many clinical trial designs, particularly those most powerful for small trials, such as crossovers, where incomplete washout or sequence effects may require limiting the analysis to the first period of study. A limitation of this study is that the power calculation was adjusted only for dropouts from MBSR or Health Education, but not for the rate of re-randomization (72%). This oversight was partly a consequence of the relative rarity of re-randomization designs; others interested in using a similar approach need to address this in their designs.
The time demands of the “full dose” MBSR intervention could be viewed as a study limitation. This intervention requires engaged, motivated participants who are willing to make a large time investment in their health. If the MBSR program is highly efficacious, it may be appropriate to investigate alternative formats that may be less time consuming but equally effective. Other limitations include the fact that meditation is individually experienced and self-reported; it cannot be confirmed or its quality objectively rated. Self-report measures of mindfulness and recorded practice time are only surrogates. Other limitations of this study include the very select population. Nevertheless, while transplant recipients are a relatively small population, they can serve as a model for other chronic disease populations whose medication regimen is complex and demanding.
Conclusions
Overall, our experience with the two-staged randomization design was very positive. Comments from prospective participants indicated that the waitlist was an important factor in their decision to accept randomization. Logistically, implementing the two-stage randomization was not difficult for the study staff. The requirement to collect longitudinal data from re-randomized participants was a modest increment in staff effort to achieve the considerable benefit of increased sample size for the primary analysis. The design also supports a range of analytic options. Investigation of three-group differences, using data from only the first randomization will be used to calibrate the clinical importance of outcome differences between MBSR and Health Education. This design may be particularly appropriate and useful in clinical trials with shorter intervention cycles and briefer follow-up intervals, where re-randomization rates from the waitlist might be higher. Researchers studying unique, small populations may also find this design particularly valuable. Other situations where a recycled waitlist approach may be valuable are trials that need internal validation control groups. These include trials to compare dissimilar active interventions, such as comparisons of medication to behavioral treatment, and trials designed to “dismantle” effective but costly or complex interventions to identify the most active components in order to develop more accessible and less costly treatments.
Acknowledgments
The authors wish to recognize Carol Skay for her assistance with data management. We also wish to thank our MBSR instructors and peer leaders, clinical co-investigators and all the transplant recipients who agreed to participate.
FUNDING: Funding for this study was provided by grants from the National Institutes of Health, National Institute of Nursing Research grant R01 NR008585 and National Center for Research Resources grant M01 RR00400. ClinTrials.gov identifier NCT00367809.
List of abbreviations
- MBSR
Mindfulness-Based Stress Reduction
- STAI
State-Trait Anxiety Inventory
- CES-D
Center for Epidemiologic Studies Depression Scale
- PSQI
Pittsburgh Sleep Quality Index
- QOL
quality of life
- SF-12
Short Form-12 Health Survey
- SF-36
Short Form-36 Health Survey
- MCS
Mental Component Summary
- PCS
Physical Component Summary
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, Version IV
- d
difference between treatment means
Contributor Information
Cynthia R. Gross, College of Pharmacy and School of Nursing, University of Minnesota, Minneapolis, Minnesota, United States.
Mary Jo Kreitzer, School of Nursing and Center for Spirituality and Healing, University of Minnesota, Minneapolis, Minnesota, United States.
Maryanne Reilly-Spong, College of Pharmacy, University of Minnesota, Minneapolis, Minnesota, United States.
Nicole Y. Winbush, Family Medicine and Community Practice, University of Minnesota, Minneapolis, Minnesota, United States.
E. Katherine Schomaker, Department of Biostatistics, School of Public Health, University of Minnesota, Minneapolis, Minnesota, United States.
William Thomas, Department of Biostatistics, School of Public Health, University of Minnesota, Minneapolis, Minnesota, United States.
References
- 1.Dew MA, Switzer GE, Goycoolea JM, Allen AS, DiMartini A, Karmos RL, et al. Does transplantation produce quality of life benefits? Transplantation. 1997;64(9):1261. doi: 10.1097/00007890-199711150-00006. [DOI] [PubMed] [Google Scholar]
- 2.Gross CR, Malinchoc M, Kim RW, Evans RW, Wiesner RH, Petz JL, et al. Quality of life before and after liver transplantation for cholestatic liver disease. Hepatology. 1999;29:356. doi: 10.1002/hep.510290229. [DOI] [PubMed] [Google Scholar]
- 3.Lanuza DM, Lefaiver CA, Farcas GA. Research on the quality of life of lung transplant candidates and recipients: an integrative review. Heart & Lung. 2000;29(3):180. doi: 10.1067/mhl.2000.105691. [DOI] [PubMed] [Google Scholar]
- 4.Kasiske BL, Cohen D, Lucey MR, Neylan JF. Payment for immunosuppression after organ transplantation. American Society of Transplantation. JAMA. 2000;283(18):2445. doi: 10.1001/jama.283.18.2445. [DOI] [PubMed] [Google Scholar]
- 5.Silkensen JR. Long-term complications in renal transplantation. Journal of the American Society of Nephrology. 2000;11(3):582. doi: 10.1681/ASN.V113582. [DOI] [PubMed] [Google Scholar]
- 6.Vasquez EM. Sirolimus: a new agent for prevention of renal allograft rejection. American Journal of Health-System Pharmacy. 2000;57(5):437. doi: 10.1093/ajhp/57.5.437. [DOI] [PubMed] [Google Scholar]
- 7.Akman B, Ozdemir FN, Sezer S, Micozkadioglu H, Haberal M. Depression levels before and after renal transplantation. Transplantation Proceedings. 2004;36:111. doi: 10.1016/j.transproceed.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 8.Dew MA, Kormos RL, DiMartini AF, Switzer GE, Schulberg HC, Roth LH, et al. Prevalence and risk of depression and anxiety-related disorders during the first three years after heart transplantation. Psychosomatics. 2001;42(4):300. doi: 10.1176/appi.psy.42.4.300. [DOI] [PubMed] [Google Scholar]
- 9.Matas AJ, McHugh L, Payne WD, Wrenshall LE, Dunn DL, Gruessner RW, et al. Long-term quality of life after kidney and simultaneous pancreas-kidney transplantation. Clinical Transplantation. 1998;12(3):233. [PubMed] [Google Scholar]
- 10.Sigmon HD, Grady PA. Quality of life for transplantation patients: National Institute of Nursing Research Spring Science Work Group. Heart & Lung. 2001;30(1):5. doi: 10.1067/mhl.2001.111728. [DOI] [PubMed] [Google Scholar]
- 11.Carlson LE, Ursuliak Z, Goodey E, Angen M, Speca M. The effects of a mindfulness meditation-based stress reduction program on mood and symptoms of stress in cancer outpatients: 6-month follow-up. Supportive Care in Cancer. 2001;9(2):112. doi: 10.1007/s005200000206. [DOI] [PubMed] [Google Scholar]
- 12.Speca M, Carlson LE, Goodey E, Angen M. A randomized, wait-list controlled clinical trial: the effect of a mindfulness meditation-based stress reduction program on mood and symptoms of stress in cancer outpatients. Psychosomatic Medicine. 2000;62(5):613. doi: 10.1097/00006842-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Kabat-Zinn J, Massion AO, Kristeller J, Peterson LG, Fletcher KE, Pbert L, et al. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. American Journal of Psychiatry. 1992;149:936. doi: 10.1176/ajp.149.7.936. [DOI] [PubMed] [Google Scholar]
- 14.Miller J, Fletcher K, Kabat-Zinn J. Three year follow-up and clinical implications of a mindfulness-based stress reduction intervention in the treatment of anxiety disorders. Gen Hosp Psychiatry. 1995;17:192. doi: 10.1016/0163-8343(95)00025-m. [DOI] [PubMed] [Google Scholar]
- 15.Ma SH, Teasdale JD. Mindfulness-based cognitive therapy for depression: Replication and exploration of differential relapse prevention effects. Journal of Consulting and Clinical Psychology. 2004;72(1):31. doi: 10.1037/0022-006X.72.1.31. [DOI] [PubMed] [Google Scholar]
- 16.Teasdale JD, Segal ZV, Williams JM, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol. 2000;68(4):615. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]
- 17.Rosenzweig S, Reibel D, Greeson J, Brainard G, Hojat M. Mindfulness-based stress reduction lowers psychological distress in medical students. Teaching & Learning in Medicine. 2003;15(2):88. doi: 10.1207/S15328015TLM1502_03. [DOI] [PubMed] [Google Scholar]
- 18.Williams KA, Kolar MM, Reger BE, Pearson JC. Evaluation of a wellness-based mindfulness stress reduction intervention: A controlled trial. American Journal of Health Promotion. 2001;15(6):422. doi: 10.4278/0890-1171-15.6.422. [DOI] [PubMed] [Google Scholar]
- 19.Baer R, editor. Mindfulness-Based Treatment Approaches: A clinician's guide to evidence-base and applications. Elsevier Academic Press; Amsterdam: 2006. [Google Scholar]
- 20.Lazar SW. Mindfulness Research. In: Germer CK, Siegel RD, Fulton PR, editors. Mindfulness and Psychotherapy. New York: The Guilford Press; 2005. [Google Scholar]
- 21.Ospina M, Bond T, Karkhaneh M, Tjosvold L, Vandermeer B, Liang Y, et al. Meditation Practices for Health: State of the Research. Rockville, MD: Agency for Healthcare Research and Quality; Jun, 2007. [PMC free article] [PubMed] [Google Scholar]
- 22.Evans C, Ildstad S, editors. Small Clinical Trials: Issues and Challenges. Washington, DC: National Academy Press; 2001. p. 11.p. 15.p. 21. [PubMed] [Google Scholar]
- 23.Caspi O, Burleson K. Methodological challenges in meditation research. Advances in Mind-Body Medicine. 2005;21(1):4. [PubMed] [Google Scholar]
- 24.Gross CR, Kreitzer MJ, Russas V, Treesak C, Frazier PA, Hertz MI. Mindfulness meditation to reduce symptoms after organ transplant: A pilot study. Alternative Therapies in Health and Medicine. 2004;10(3):58. [PubMed] [Google Scholar]
- 25.Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE. Cognitive behavioral therapy for treatment of chronic primary insomnia: a randomized controlled trial. Jama. 2001;285(14):1856. doi: 10.1001/jama.285.14.1856. [DOI] [PubMed] [Google Scholar]
- 26.Mills E, Kelly S, Wu P, Guyatt G. Epidemiology and reporting of randomized trials employing re-randomization of patient groups: A systematic survey. Contemporary Clinical Trials. 2007;28:268. doi: 10.1016/j.cct.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Cohen L, Warneke C, Fouladi RT, Rodriguez MA, Chaoul-Reich A. Psychological adjustment and sleep quality in a randomized trial of the effects of a Tibetan yoga intervention in patients with lymphoma. Cancer. 2004;100(10):2253. doi: 10.1002/cncr.20236. [DOI] [PubMed] [Google Scholar]
- 28.Moritz S, Quan H, Richki B, Liu M, Angen M, Vintila R, et al. Home study-based spirituality education program decreases emotional stress and increases quality of life - a randomized, controlled trial. Alternative Therapies in Health and Medicine. 2006;12(6):26. [PubMed] [Google Scholar]
- 29.Brown C, Wyman P, Guo J, Pena J. Dynamic wait-listed designs for randomized trials: New designs for prevention of youth suicide. Clinical Trials. 2006;3:259. doi: 10.1191/1740774506cn152oa. [DOI] [PubMed] [Google Scholar]
- 30.Street LL, Luoma JB. Control groups in psychosocial intervention research: Ethical and methodological issues. Ethics Behav. 2002;12(1):1. doi: 10.1207/S15327019EB1201_1. [DOI] [PubMed] [Google Scholar]
- 31.Elliott SA, Brown JSL. What are we doing to waitlist controls? Behavior Research and Therapy. 2002;40:1047. doi: 10.1016/s0005-7967(01)00082-1. [DOI] [PubMed] [Google Scholar]
- 32.Stress Reduction Program - Center for Mindfulness - UMass Medical School. 2006 [cited 2006 March 27]; Available from: http://www.umassmed.edu/cfm/srp/
- 33.Kabat-Zinn J. Full Catastrophe Living: Using the wisdom of your body and mind to face stress, pain, and illness. New York: Dell Publishing; 1990. [Google Scholar]
- 34.Shapiro SL, Carlson LE, Astin JA, Freedman B. Mechanisms of mindfulness. Journal of Clinical Psychology. 2006;82(3):373. doi: 10.1002/jclp.20237. [DOI] [PubMed] [Google Scholar]
- 35.Prochaska J, Velicer W. The transtheoretical model of health behavior change. American Journal of Health Promotion. 1997;12(1):38. doi: 10.4278/0890-1171-12.1.38. [DOI] [PubMed] [Google Scholar]
- 36.Lorig K, Holman H, Sobel D, Laurent D, Gonzalez V, Minor M. Living a Healthy Life with Chronic Conditions: self-management of heart disease, arthritis, diabetes, asthma, bronchitis, emphysema and others. 2nd. Palo Alto, CA: Bull Publishing; 2000. [Google Scholar]
- 37.Lorig KR, Ritter P, Stewart AL, Sobel DS, Brown BW, Jr, Bandura A, et al. Chronic disease self-management program: 2-year health status and health care utilization outcomes. Medical Care. 2001;39(11):1217. doi: 10.1097/00005650-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Lorig KR, Sobel DS, Stewart AL, Brown BW, Jr, Bandura A, Ritter P, et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: a randomized trial. Medical Care. 1999;37(1):5. doi: 10.1097/00005650-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Bishop S. What do we really know about mindfulness-based stress reduction? Psychosomatic Medicine. 2002;64:71. doi: 10.1097/00006842-200201000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Barkauskas V, Lusk S, Eakin B. Selecting control interventions for clinical outcome studies. Western Journal of Nursing Research. 2005;27(3):346. doi: 10.1177/0193945904271446. [DOI] [PubMed] [Google Scholar]
- 41.Given BA, Keilman LJ, Collins C, Given WC. Strategies to minimize attrition in longitudinal studies. Nursing Research. 1990;39:184. [PubMed] [Google Scholar]
- 42.Lindquist R, Wyman J, Talley K, Findorff M, Gross C. Design of control group conditions in clinical trials of behavioral interventions. Journal of Nursing Scholarship. 2007;39(3):214. doi: 10.1111/j.1547-5069.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- 43.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd. Hillsdale, New Jersey: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 44.Spielberger C. Manual for the State-Trait Anxiety Inventory (Form Y) Redwood City, CA: Mind Garden, Inc.; 1983. [Google Scholar]
- 45.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;3:385. [Google Scholar]
- 46.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989;28(2):193. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 47.Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. Journal of Psychosomatic Research. 1998;45(1):5. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- 48.O'Brien PC, Shampo MA. Statistical considerations for performing multiple tests in a single experiment. 5. Comparing two therapies with respect to several endpoints. Mayo Clin Proc. 1988;63:1140. doi: 10.1016/s0025-6196(12)65511-6. [DOI] [PubMed] [Google Scholar]
- 49.Pocock SJ. Clinical trials with multiple outcomes: A statistical perspective on their design, analysis and interpretation. Controlled Clinical Trials. 1997;18:530. doi: 10.1016/s0197-2456(97)00008-1. [DOI] [PubMed] [Google Scholar]
- 50.Tilley BC, Marler J, Geller NL, Lu M, Legler J, Brott T, et al. Use of a global test for multiple outcomes in stroke trials with application to the National Institute of Neurological Disorders and Stroke t-PA Stroke Trial. 1996. [DOI] [PubMed] [Google Scholar]
- 51.Huang P, Woolson RF, O'Brien PC. A rank-based sample size method for multiple outcomes in clinical trials. Statistics in Medicine. 2008;27:3084–3104. doi: 10.1002/sim.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 53.Schipper H, Clinch JJ, Olweny CLM. Quality of life studies: Definitions and conceptual issues. In: Spilker B, editor. Quality of Life and Pharmacoeconomics. 2. Philadelphia: Lippincott-Raven Publishers; 1996. p. 11. [Google Scholar]
- 54.Ware JE, Jr, Kosinski MA, Keller SD. A 12-Item short-form health survey. Construction of scales and preliminary tests of reliability and validity. Medical Care. 1996;34(3):220. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Mossey J, Shapiro E. Self-rated health: A predictor of mortality among the elderly. Am J Public Health. 1982;72(8):800. doi: 10.2105/ajph.72.8.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ware J, Kosinski M, Dewey J. How to score version 2 of the SF-36(R) Health Survey. Lincoln, RI: QualityMetric Incorporated; 2000. [Google Scholar]
- 57.Kind P. The EuroQoL Instrument: an index of health-related quality of life. In: Spilker B, editor. Quality of Life and Pharmacoeconomics in Clinical Trials. 2nd. Philadelphia, PA: Lippincott-Raven Publishers; 1996. p. 191. [Google Scholar]
- 58.Frazier PA, Davis-Ali SH, Dahl KE. Correlates of noncompliance among renal transplant recipients. Clinical Transplant. 1994;8(6):550. [PubMed] [Google Scholar]
- 59.Frazier PA, Davis-Ali SH, Dahl KE. Stressors, social support, and adjustment in kidney transplant patients and their spouses. Social Work in Health Care. 1995;21(2):93. doi: 10.1300/J010v21n02_07. [DOI] [PubMed] [Google Scholar]
- 60.Roberts K, Aspy C. Development of the serenity scale. Journal of Nursing Measurement. 1993;1(2):145. [PubMed] [Google Scholar]
- 61.Brown KW, Ryan RM. The benefits of being present: Mindfulness and its role in psychological well-being. J Pers Soc Psychol. 2003;84(4):822. doi: 10.1037/0022-3514.84.4.822. [DOI] [PubMed] [Google Scholar]
- 62.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality & Social Psychology. 1988;54(6):1063. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 63.Morisky D, Green L, Levine D. Concurrent and predictive validity of a self-reported measure of medication adherence. Medical Care. 1986;24(1):67. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 64.Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits. A meta-analysis. J Psychosom Res. 2004;57(1):35. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- 65.Baer RA. Mindfulness training as a clinical intervention: A conceptual and empirical review. Clinical Psychology Science and Practice. 2003;10(2):125. [Google Scholar]
- 66.Kernan WN, Viscoli CM, Makuch RW, Brass LM, Horwitz RI. Stratified randomization for clinical trials. J Clin Epidemiol. 1999;52(1):19. doi: 10.1016/s0895-4356(98)00138-3. [DOI] [PubMed] [Google Scholar]
- 67.Diggle PJ, Heagerty P, Liang KY, Zeger SL. Analysis of Longitudinal Data. 2nd. Oxford: Oxford University Press; 2002. [Google Scholar]
- 68.Edinger JD, Sampson WS. A primary care “Friendly” cognitive behavioral insomnia therapy. Sleep. 2003;26(2):177. doi: 10.1093/sleep/26.2.177. [DOI] [PubMed] [Google Scholar]
- 69.Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Medical Care. 2004;42(9):851. doi: 10.1097/01.mlr.0000135827.18610.0d. [DOI] [PubMed] [Google Scholar]
- 70.Vickers A. Statistical considerations for use of composite health-related quality-of-life scores in randomized trials. Quality of Life Research. 2004;13:717. doi: 10.1023/b:qure.0000021686.47079.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]