Abstract
Hair follicle stem cells in the epithelial bulge are responsible for the continual regeneration of the hair follicle during cycling. The bulge cells reside in a niche composed of dermal cells. The dermal compartment of the hair follicle consists of the dermal papilla and dermal sheath. Interactions between hair follicle epithelial and dermal cells are necessary for hair follicle morphogenesis during development and in hair reconstitution assays. Dermal papilla and dermal sheath cells express specific markers and possess distinctive morphology and behavior in culture. These cells can induce hair follicle differentiation in epithelial cells and are required in hair reconstitution assays either in the form of intact tissue, dissociated freshly-prepared cells or cultured cells. This review will focus on hair follicle dermal cells since most therapeutic efforts to date have concentrated on this aspect of the hair follicle, with the idea that enriching hair-inductive dermal cell populations and expanding their number by culture while maintaining their properties, will establish an efficient hair reconstitution assay that could eventually have therapeutic implications.
Keywords: Hair follicle, Dermal papilla, Dermal sheath, Reconstitution assay
1. Dermal components of the hair follicle
1.1. Introduction
The hair follicle is composed of epidermal (epithelial) and dermal (mesenchymal) compartments and their interaction plays an important role in the morphogenesis and growth of the hair follicle [1,2]. Effective cross-talk between these two compartments is also thought to be key for successful reconstitution of hair follicles for research or therapeutic purposes. Generally dermal cells are considered as inducers and epithelial cells as responders in the process of hair formation although the signaling in between the two cells type is reciprocal and complicated.
Several models have been established for the study of dermal-epidermal interactions, as well as the reconstitution of hair follicles [3]. Most hair reconstitution assays take place in vivo in immunodeficient host mice. Although these assays work well with mouse cells, regenerating human hair follicles is still a challenge requiring breakthroughs in several aspects, including enriching cells with trichogenic capacity, maintaining their trichogenic capacity during processing, and providing them with an adequate host environment.
This article focuses on the dermal components of the hair follicle and provides an overview on the role of dermal cells in hair reconstitution assays, which hold promise for dissecting out factors and cellular subpopulations necessary for hair follicle regeneration.
1.2. Dermal papilla (DP) and dermal sheath (DS)
The dermal portion of the hair follicle can be divided into two compartments, the dermal papilla (DP) and dermal sheath (DS) [4] (Fig. 1). The DP is located at the base of the hair follicle. The DS, or connective tissue sheath, lines the epithelium of the hair follicle from the bulge level downward and is contiguous with the base of the DP through a stalk. DP and DS are separated from the epithelial portion of the hair follicle by a basement membrane. The DS consists of three layers of collagen fibers running in different directions, with fibroblasts mostly residing in the thickened middle collagen layer [5]. Cells within DP and DS are specialized fibroblasts of mesenchymal origin. However, expression of neuronal markers by DP cells and their transcription profiles suggest a neural crest origin [6–8]. By tracing the progeny of neural-crest stem cells, investigators found that a portion of the DP cells are derived from the neural crest [6].
Fig. 1.
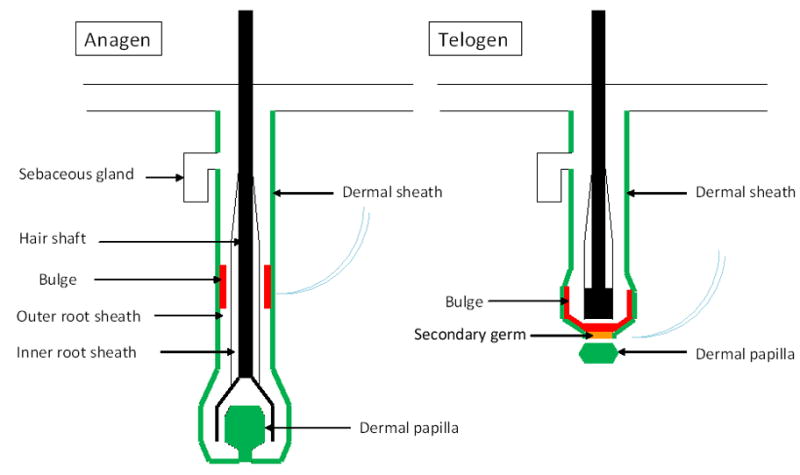
The structure of a hair follicle. The hair follicle stem cells (red) are located in a niche created by the dermal sheath and dermal papilla cells (green). In anagen, the dermal papilla leads the down-growth from the secondary germ and bulge cells to the formation of the bulb. In telogen, the dermal papilla resides adjacent to the secondary germ (yellow), which is derived from bulge stem cells during late catagen phase.
DS has been considered as a cellular reservoir of DP cells during the hair follicle cycle[9], and it was hypothesized that stem cells might reside in the DS similar to its epithelial counterpart, the outer root sheath [10]. DS cells share similar characteristics with the DP and can regenerate a new DP after loss of the DP [11–13]. In classic transection studies of rat vibrissae, Oliver and colleagues demonstrated that removal of the lower follicle resulted in regeneration of the DP, apparently from the DS, while removal of the follicle at the level of the bulge did not allow for DP regeneration [14]. There may be two-way cellular traffic between the DP and DS during normal hair follicle cycling [15]. The DP reaches its maximal size in anagen IV when cell number is double that in telogen. This increase in cell number is thought to be due to recruitment of cells from the DS. DP cells may migrate out into the DS again prior to the next resting phase [9]. One recent study suggests that thrombin signaling through PI3K-Akt pathway regulates the transformation between DS and DP cells [16]. The exact relationship between the DP and DS awaits specific promoters that target each cell population. This approach has been useful for understanding heterogeneity of epidermal stem cells [10].
2. Dermal cells and dermal signals in hair morphogenesis and cycling
2.1. Dermal cells in hair morphogenesis and cycling
During embryonic hair follicle development, mesenchymal cells aggregate immediately below the epidermis in a patterned manner. These aggregates or “condensates” mark the location of the new hair follicle. The mesenchymal condensates maintain close contact with the base of down-growing epithelial columns and are eventually ensheathed by the hair bulb during the peg stage [17]. In a fully developed anagen hair follicle, the DP resides deep in the subcutaneous fat and is surrounded by hair matrix cells. In catagen, the DP moves up to the dermis as the epithelial strand regresses. As the secondary hair germ forms from the bottom of the bulge at the end of catagen, the DP comes to rest immediately adjacent to these cells that will form the next lower hair follicle. [18].
2.2. Selected dermal signals during hair follicle morphogenesis
This topic has been reviewed extensively [2,8,19] and here we mention only some major players. Wnt signaling in the dermis may be the “first dermal message” responsible for inducing formation of hair placodes from the overlying epidermis [2]. Dickkopf-1 (Dkk-1), a potent inhibitor of Wnt signaling is expressed by the interfollicular dermis surrounding new hair follicles at this stage [20]. Other dermal factors required for epithelial down-growth during hair morphogenesis include fibroblast growth factors and the bone morphogenetic protein (BMP) inhibitor, noggin [21,22].
Shh signaling is important for early DP development and maturation. It is expressed by the hair follicle epithelium and influences both mesenchyme and epithelium. In the Shh knockout mouse, hair follicle development is severely compromised; either arrested in the germ stage or exhibiting tiny hair follicle structures expressing hair shaft differentiation keratins [23–25]. Recent studies show primary cilia in DP cells are required for Shh signaling, and laminin-511 is critical for the formation of primary cilia in DP, as well as maintaining the expression of noggin [26,27]. PDGF-A is another epidermal factor which cooperates with Shh signaling and contributes to the formation of DP and DS [25].
3. Isolation and culture of dermal cells
3.1. Techniques for isolating and culturing dermal cells
The most widely used method to isolate DP is surgical micro-dissection, which has been well established in rat and murine vibrissae follicles as well as human hair follicles [28,29]. Surgical micro-dissection has also been used to isolate DS adjacent to DP [11,12]. However, the technique is laborious and time-consuming. Outgrowths of cells from DP explants can be expected within 1 week in culture medium. Cultured DP cells show flattened and polygonal morphology and tend to grow into multi-layered aggregates in the first few passages. The behavior of aggregate formation in DP cell culture seems to be correlated with their hair inductivity [30]. DP cells gradually lost their proliferative capacity after being passaged. Adding FGF-2 into the culture medium promotes long-term culture of mouse vibrissa and human DP cells to more than 30 passages [30].
Human DP can be harvested more efficiently by applying dispase and subsequent collagenase treatment to the lower anagen hair follicles which reside in the subcutaneous fat [31]. Taking advantage of transgenic expression of fluorescent protein in targeted cells and fluorescence activated cell sorting (FACS), dissociated DP cells can be obtained in a larger scale in mice using versican or Lef1 promotors which are preferentially up-regulated in DP [8,32].
3.2. Dermal-epidermal interaction in vitro
Although DP cells and keratinocytes change their properties in culture, co-culturing these two cells in vivo still provides useful information about their interactions. Keratinocytes co-cultured with DP cells have increased proliferation rate and show significant migration toward DP cells as well as conditioned medium prepared with cultured DP cells [33]. When matrix cells from the hair bulb grow on the top of DP cells, occasionally keratinocytic spheres form and are surrounded by DP cells with the formation of basement membrane-like structure. This phenomenon, which showed the attempt to form hair follicles in vitro, was only observed when combining matrix cells with DP cells.
4. Biochemical and molecular signatures of dermal cells
Cells within DP and DS are specialized mesenchymal cells and express specific enzymes and molecules. Although the functions of most marker proteins are unknown, they have been widely used to identify DP and DS. The expression of some markers, e.g. alkaline phosphatase and versican correlates with hair inductive properties.
4.1. Alkaline phosphatase (AP)
The activity of AP has been used as a marker to detect the presence of DP and regarded as an indicator for hair inductivity [13,34]. Handjiski et al. show that pelage DP of mice expressed strong and persistent AP activity throughout the entire hair cycle [35]. However, a recent study by Iida et al. shows dynamic change of AP activity in DP and bulbar dermal sheath. AP activity in DP reach its maximal level in early anagen, and decreased at the proximal half (below Auber’s line) of DP after mid-anagen growing phase [34]. In DS, AP activity is shown in proximal DS adjacent to DP, with the highest level detected in early anagen [13,34]. The hair-inductivity of cultured DP cells is known to decrease after passage, as is the expression of AP [36]. The temporal and spatial changes of AP activity coincide with the hair-inductive property of DP and DS.
4.2. α-Smooth muscle actin (αSMA)
αSMA was present in the mid- to lower DS in rat and human hair follicles but not in DP [37]. However, DP cells become αSMA-positive in culture [37]. Therefore, αSMA is a marker for DS in vivo, and a marker for both DP and DS in vitro.
4.3. Versican
In human hair follicles, versican is reported specifically expressed in DP during anagen. Weak versican immunoreactivity has also been shown at the dermal sheath outside K15-positive bulge epithelial cells. Versican expression in DP is lost in miniaturized hair follicles of androgenetic alopecia [38]. In mouse, versican is expressed in anagen hair follicles but absent in telogen hair follicles. Therefore, versican may play an important role in anagen induction and maintenance of anagen. Ascorbic acid 2-phosphate induces expression of versican in human dermal cells which may in turn enhance the initiation and growth of hair follicles [39]. Kishimoto et al. use GFP driven by versican promoter as a way to enrich DP cells by FACS [32]. These GFP-positive cells show behavior and morphology consistent with DP cells. They induce hair neogenesis in engraft assay when combined with epidermal cells, while GFP-negative cells did not induce the formation of hair follicles [32]. Subsequent studies showed that while Versican-GFP cells are enriched for DP, more specific DP markers were needed.
4.4. Corin
Corin encodes a transmembrane protease that is expressed in the heart and participates in the processing of natriuretic peptides. In mouse pelage skin, Corin is expressed specifically in the DP from the earliest stage [40]. Corin also plays a role in the coat color specification. However, it not required for hair morphogenesis based on lack of phenotype by mutation of Corin gene in mice [40].
4.5. CD133
CD133, or Prominin-1, is a known hematopoietic stem cell marker that is strongly expressed in DP of stage 3–4 developing hair follicles and early anagen in mouse skin. CD133-positive cells isolated from mouse skin by FACS resemble DP cells in morphology and behavior when plated in culture and they have similar molecular and transcript profiles [41]. CD133-positive dermal cells, but not CD133-negative dermal cells, induce hair follicle neogenesis 10 times more efficiently than unsorted dermal cells in terms of required cell numbers [41]. The different result by CD133-positive and negative cells demonstrates the power of surface markers in isolating subpopulations from hair follicles and the importance of choosing the right subpopulation for hair regeneration assay.
5. Dermal cells in hair reconstitution assays and maintenance of hair inductivity
The hair inductive property of dermal cells, especially DP cells, has been clearly demonstrated. The epithelial part of a pre-existing hair follicle needs a DP to maintain its growth, and dissociated epidermal cells require the guidance of dermal cells to be organized into complicated hair structures, otherwise, a simple epithelial cyst forms. Therefore, dermal cells are critical in hair regeneration assay and it is important to keep dermal cells inductive during preparation.
5.1. Hair inductivity in dermal cells
The inductive property in dermal cells was elegantly shown by Oliver when he transposed DP beneath the upper half of amputated vibrissa hair follicles [42]. He and his colleagues then transplanted DP into afollicular skin and induced hair growth [43]. Cultured DP and DS cells, as well as intact DS tissue are able to reconstitute a new functioning DP in vivo [11–13,44]. Reynolds et al. introduced DS from male human scalp into the forearm skin of a female donor and induced growth of a terminal hair follicle with a DP containing a Y-chromosome indicating the donor origin [12]. Although we cannot be sure of the target epithelial population in this experiment, a subsequent study also showed trans-species induction of hair follicles by human DP [45]. If validated, these studies are significant for their demonstration of DP inductive abilities as well as the possible immune privilege state of the hair follicle championed by Paus and coworkers [46].
The inductive property of dermal cells is affected by donor status including hair cycle stage and age. Both the hair-forming ability and the hair-inductivity of DP change during hair cycle and affect the frequency of hair formation in transplantation study [47]. Dermal condensates from embryonic tissue and DP from adult skin induce hair formation through different mechanism [48]. When combining glabrous skin from adult mice onto embryonic dermis with dermal condensates, hair neogenesis follows the process of embryonic hair development. When using adult DP from vibrissa to induce hair neogenesis from the same epidermis, hair placode-like structures are not found.
The inductive property seems to be uniformly distributed in cells in a single DP although cells in different area express different markers. Osada et al. showed 80% clones from single DP cells have hair-inducing ability [49]. Each individual DP is designated to induce different type of hair follicles. Sox2-positive DP cells only induce guard/awl/auchen follicles while the DP of a zigzag hair follicle is Sox2-negative [7].
5.2. Hair reconstitution assays: in vivo model
Hair reconstitution assays are useful for assessing the hair inductive properties of isolated cell populations. Early reconstitution assays consisted of transposing dermal tissue or cells beneath or in close contact to the upper half of an amputated hair follicle in vivo [42] (Fig. 2). The inserted dermal tissue regenerates a new DP and helps to restore the truncated epithelial structure with new hair shaft growth. This type of model restores a pre-existing injured hair follicle rather than regenerating a hair follicle de novo. It is also laborious to dissect and amputate hair follicles and insert dermal population. Only large follicles can be used in this model so there are many limitations in its application. In the subsequent studies, dermal tissues or cells are implanted into the dermis of the skin in order to induce the formation of hair follicles from the overlying epidermis [43,50]. The origin of the dermal tissue determines the type of new hair follicles [51]. Questions still exist in some studies whether these implanted dermal tissues or cells induce de novo hair follicles, or transform pre-existing hair follicles into another type. Hair formation by implantation of DP cells into glabrous skin favors de novo hair neogenesis [48,50].
Fig. 2.
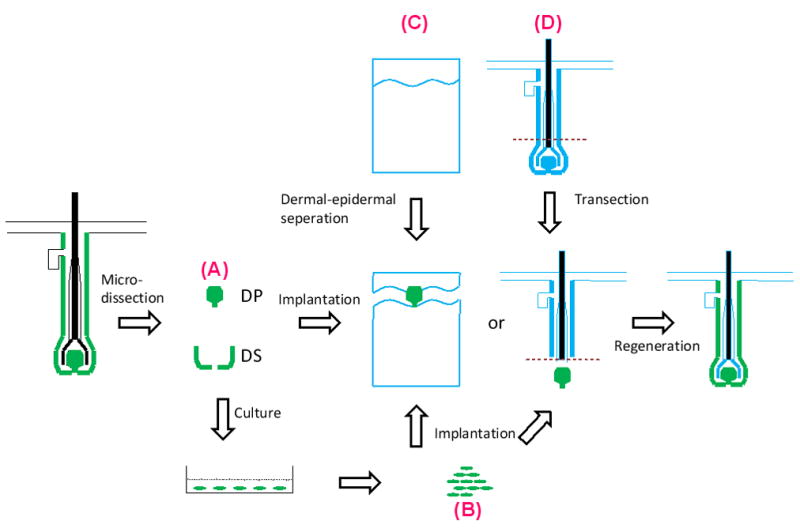
Transposition of intact dermal tissue (A) or cultured dermal cells (B) into a dermal-epidermal junctional pocket created by dispase (C) or under amputated hair follicles (D) as a model for hair regeneration.
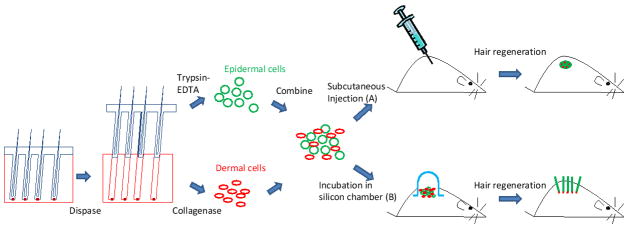
More recently, two hair regeneration models are established using dissociated cell aggregates or single cells from hair follicles as the epidermal and dermal components (Fig. 3). This type of model gives us more flexibility to select and manipulate epidermal and dermal populations. The first model involves the use of a silicon graft chamber [52]. The mixture of epidermal and dermal cells is placed onto a full thickness wound on the back of immunodeficient mice. The wound is covered by a bell-shaped silicon chamber which confines and protects the cells on the wound bed. The chamber is usually taken off after 1 week and hair neogenesis can be seen from the surface of back skin in 3 weeks by using cells from mouse neonates [53].
Fig. 3.
Hair regeneration models using dissociated epidermal and dermal cells. The cells are either injected subcutaneously or seeded on a full-thickness wound protected by a chamber on the back of the immunodeficient host mouse.
In the second method, the mixture of epidermal and dermal cells is injected subcutaneously into the back skin of immunodeficient mice [54]. The injected epidermal cells aggregate into large cystic spheres seen as nodules in the host skin. Stenn calls this the “patch assay” [55]. Epidermal aggregates undergo central apoptosis and result in cyst formation. Dermal condensates outside the epidermal spheres start to induce hair follicles growing outward from the cyst wall with hair shafts projecting inward to the cyst cavity [55]. For epidermal and dermal cells from mouse neonates, this process of hair neogenesis takes less than 2 weeks. The back skin is cut off and the patch with regenerated hair follicles can be seen at the undersurface of the skin (Fig. 4).
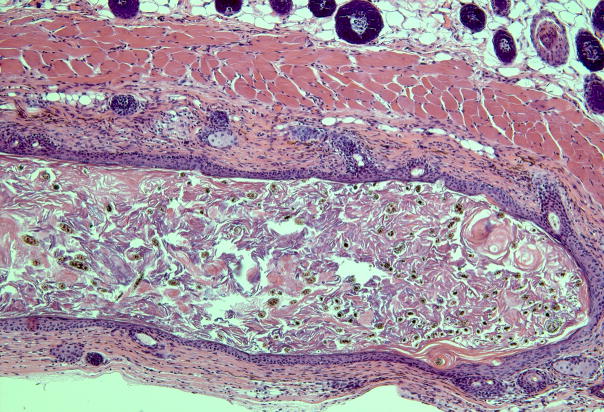
Fig. 4.
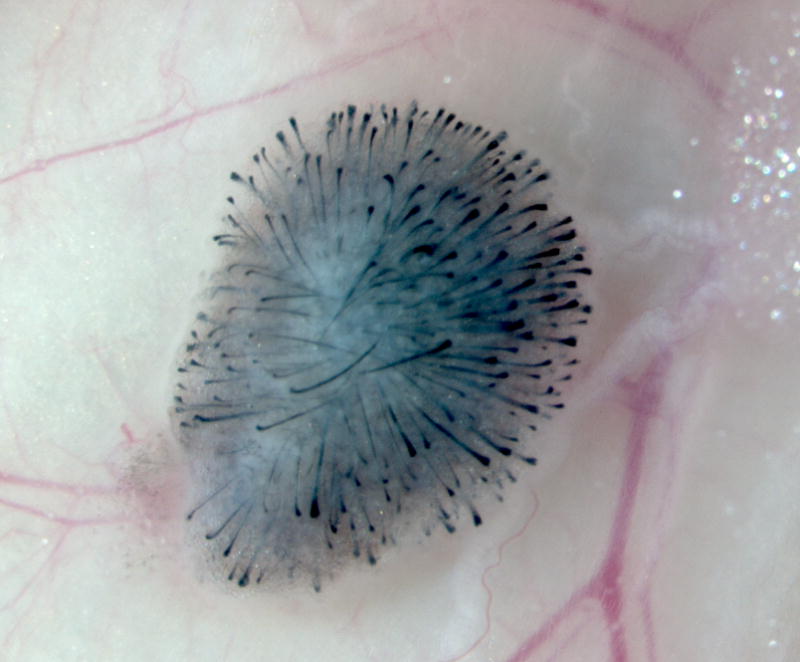
Subcutaneous injection of dissociated murine epidermal and dermal cells into the back skin of a nude mouse. (a) A nodule or “patch” with regenerated hair follicles can be observed from the undersurface of the skin in 2 weeks. (b) The histology shows formation of a cyst with hair follicles coming out from the cyst wall.
The two models have been used widely. We injected a mixture of K15-positive epithelial cells from adult mice and dermal cells from mouse neonates into immunodeficient mice. Isolated adult epithelial stem cells from the hair follicle were multipotent and capable of regenerating the cutaneous epithelial lineages of the hair follicle, sebaceous gland and interfollicular epidermis. This study highlighted the importance of choosing the epithelial cell subpopulation with stem cell characteristics to reconstitute hair follicles [54]. Similarly, Ito et al. use CD133 as a marker to enrich hair-inductive dermal cells for hair regeneration assay.
In another in vivo model, glabrous skin was treated by dispase to create a small pocket between epidermis and dermis. Dermal cells are then inserted into the pocket before the whole skin grafted into nude mice [48,49].
However, the use of immunodeficient host mice in these in vivo models possesses drawbacks especially when applying these systems to regenerate human hair follicles. Very little is known about the influence of host factors on hair follicle neogenesis. Yet host immunity is not totally ablated and may confound the result. The placement of human cells into mouse hosts further aggravates this issue. These host factors may explain the low efficiency of these in vivo hair regeneration models in which several thousands to millions of cells are required to regenerate a single hair follicle. It may also explain why regeneration of pure human hair follicles has never been achieved so far.
5.3. In vitro hair regeneration model
In vitro environment is more controllable with fewer unknown factors. An in vitro hair reconstitution assay can provide high throughput analysis and production. However, it is not easy to simulate the complicated niche where hair follicles reside. Usually collagen is used as the matrix for dermal-epidermal interaction to take place. Epidermal and dermal cells can either be incorporated into the gel or sit on the top of the gel [56]. Dermal-epidermal interaction can also take place in a hanging-drop system [57]. However, all the structures that initially formed in the gel or liquid phase in vitro have to be eventually grafted into immunodeficient host mice in order to achieve complete hair morphogenesis [57,58]. Otherwise, only cell aggregates can be seen which are still far from the structure of a real hair follicle. This suggests that current in vitro systems are still over-simplified for complete hair morphogenesis.
5.4. Regeneration of human hair follicles
Current success in de novo hair regeneration has been dependent on the use of mouse cells, especially cells from newborn or embryonic mice. Ehama et al. used a graft chamber to generate hair follicle-like structure from the combination of human epidermal cells and mouse dermal cells [53]. No pure human hair follicles can be made so far, suggesting that factors or microenvironment are missing.
6. Maintaining hair inductivity in dermal cell culture
It is important to keep cells competent in hair regeneration assays while processing and expanding them in culture. However, it is known that cultured DP cells gradually lose their hair inductivity and proliferative capacity after being passaged [30,59].
6.1. Sphere formation
Only early passaged DP cells manifesting aggregative behavior are able to form DP after implantation into transected upper hair follicles [59]. Cultured DP cells grown as spheres showed higher hair-inductivity than 2-D cells and these spheres retain their hair inductivity even in later passage (passage 26) [30].
In order to enhance self-aggregating and spheroid-forming behavior, DP cells can be cultured on a biomaterial surface with lower adhesivity [60]. However, the growth rate of DP cells becomes compromised on this surface. In addition, fibronectin coating on culture dish enhanced the number of spheroids by 67% [61].
6.2. BMP signaling
Hair bulb and DP are rich for BMPs and BMP receptor 1a. When DP cells are cultured in medium containing BMP 2, 4, 5 or 6, their expression of AP is preserved relative to cells cultured without BMPs [36]. BMP6-treated mouse DP cells have improved hair inductivity compared to control DP cells and the inductive capacity is retained up until passage 8. By ablating BMP receptor 1a in cultured DP cells, DP cells fail to reconstitute hair follicles [36].
6.3. Wnt Signaling
Activation of Wnt signaling is important for the initiation and maintenance of hair morphogenesis [2]. Kishimoto et al. demonstrate that Wnt-3a treated DP cells had a higher capacity to induce hair formation compared to non-treated or Shh-treated DP cells in engraft study [62]. Similar result from Ouji et al. showed that co-transplantation of Wnt-10b-secreting cells promoted hair neogenesis [63].
Glycogen synthase kinase-3 (GSK-3) inhibits Wnt signaling by phosphorylating β-catenin. GSK-3 inhibitor-treated human DP cells showed increased activity and expression of AP and IGF-1, two indicators of hair-inductivity. However, the hair regeneration assay using GSK-3-treated human DP cells with mouse epidermal cells fails to result in hair neogenesis [64].
7. Dermal cells in hair diseases
The role of the DP in diseases of skin and hair is not known, but some have speculated that androgenetic alopecia, which is characterized by miniaturized hair follicles and shortening of anagen phase in a defined pattern, may be due to the effect of testosterone and dihydrotestosterone acting on androgen receptors in the DP and causing changes in transcription of genes such as TGF-β and IGF-1 [65–67]. Androgens may also drive the DP to secret inhibitory autocrine factors [68]. Bahta et al. cultured DP cell from balding and non-balding scalp and found that balding DP cells showed characteristics of senescence including loss of proliferation capacity, change of morphology and expression of senescence-related markers and oxidative stress markers such as p16INK4a/pRb, heat shock protein-27 [69]. One recent study showed androgen regulated Wnt signaling in DP cells again suggesting their involvement in the disease process of AGA [66].
8. Conclusion
Dermal cells play a pivotal role in the regulation of hair growth. For hair follicle regeneration purposes, important issues include isolation of inductive dermal population, expanding their number by culture, maintenance of their hair inductive property and providing them an adequate niche and exogenous signals to enhance their interaction with epidermal cells toward the fate of hair neogenesis. An ideal hair regeneration model for human hair follicles has yet to be developed. The efficiency has to be improved with fewer starting cell number and higher number of regenerated hair follicles. The variables should be easily controllable for studying hair biology. The multipotent cells reside in dermal compartment of the hair follicle warrant further studies for their potential clinical application in regenerative medicine.
Supplementary Material
Table 1.
Summary of tissue and cellular recombination assays
| Author Year | Epidermal component | Dermal component | Brief methods | Results and significance |
|---|---|---|---|---|
| Oliver [14] 1966 | Rat, transected vibrissa follicles, upper half | Rat, vibrissa DP | Implantation of DP underneath the transected follicles | Regrowth of whisker follicles after DP implantation, not without DP implantation |
| Oliver [43] 1970 | Rat, 1–3 mm epidermal sheets from ear and scrotal skin, and lip mucosa | Rat, vibrissa DP | Implantation into rat ear skin | Vibrissa DP induce hair follicle neogenesis from epidermal sheet, even afollicular epidermis |
| Jahoda et al. [44] 1984 | Rat, transected vibrissa follicles, upper half | Rat, cultured vibrissa DP cells (passage 1–3) | Implantation of DP underneath the transected follicles | Hair follicle neogenesis induced by cultured DP cells |
| Horne et al. [59] 1986 | Rat, transected vibrissa follicles, upper half | Rat,
|
Implanted dermal cells beneath the transected HF |
|
| Horne and Jahoda [11] 1992 | Rat, transected vibrissa follicles, upper half | Rat, lower DS from the same follicle | Implantation of DP underneath the transected follicles | Lower DS is able to regenerate a new DP |
| Reynolds and Jahoda [50] 1992 | Rat, adult, foot pad epidermis | Rat, cultured vibrissa DP cells | Implantation of DP cells under foot pad epidermis and transplantation into a chamber on rat skin | Hair induction in glabrous epidermis by cultured DP cells |
| Jahoda [51] 1992 | Rat, adult, ear epidermis | Rat, vibrissa DP | Implantation of DP into small incisional wound on rat ear | Growth of vibrissa-type hair follicles supports DP’s role in determining hair follicle type |
| Jahoda et al. [70] 1993 | Rat, adult, ear epidermis | Rat,
|
Implantation of cultured dermal cells into a wound create on rat ear |
|
| Weinberg et al. [71] 1993 | Mouse, neonatal, epidermal cell aggregates (hair buds) | Rat and mouse, dissociated cells from the whole dermis | Chamber method | Regeneration of hair follicles from dissociated cells |
| Lichti et al. [72] 1993 | Mouse, neonatal, hair buds |
|
Chamber method |
|
| Jahoda et al. [73] 1996 | Human, transected hair follicles, upper half | Subcutaneous implantation into nude mice | DS is able to replace DP | |
| Reynolds et al. [12] 1999 | Human, forearm epidermis | Human, DS from scalp | Implantation of DS into a wound on forearm |
|
| Kishimoto et al. [62] 2000 | Mouse, neonatal, dissociated cells | Mouse, neonatal, cultured dermal cells, treated with Shh, Wnt3a | Chamber method |
|
| Jahoda et al. [45] 2001 | Rat, transected vibrissa follicle, upper part | Human, intact DP from scalp | Recombination and implantation into nude mice | Trans-species induction of hair by human DP |
| McElwee et al. [13] 2003 | Mouse, ear epidermis | Mouse, cultured DP, DSC, DS cells | Injection of the cellular mixture into mouse ear | Hair induction by DP and DSC cells |
| Morris et al. [54] 2004 | Mouse, adult K15-positive cells (K15-EGFP) | Mouse, neonatal, dissociated dermal cells | Subcutaneous injection | Hair follicle can be generated from multipotent adult stem cells but not from the whole epidermal population |
| Halvickova et al. [56] 2004 | Human, cultured outer root sheath cells (ORSK) | Human, cultured DP cells | Fibroblasts- incorporated collagen matrix with ORSK and DP cells in Matrigel on the top | An epithelial-mesenchy mal interaction assay system yields hair follicle-like structures. |
| Reynolds and Jahoda [74] 2004 | Rat, transected vibrissa follicle, upper half |
|
Implantation into kidney capsule of nude mice | Cross-appendage (hair/teeth) and interspecies (mouse/human) interaction |
| Zheng et al. [55] 2005 | Mouse, neonatal, epidermal aggregates | Mouse, neonatal, dissociated cells | Subcutaneous injection | Mechanism of cyst formation with hair follicle neogenesis |
| Ouji et al. [63] 2006 | Mouse, neonatal, cultured cells | Mouse, neonatal, freshly prepared cells | Chamber method, graft with Wnt-secreting cells | The importance of Wnt-10b in hair development. |
| Inamatsu et al. [48] 2006 | Rat, small pieces of sole epidermis | Rat,
|
Epidermal and dermal portions combined and grafted onto nude mouse |
|
| Osada et al. [30] 2007 | Mouse, neonatal, dissociated cells | Mouse, DP cell culture | Implantation into nude mouse skin | DP cells grown in spheres induce more hair follicles |
| Nakao et al. [58] 2007 | Mouse, embryonic dissociated epidermal cells from vibrissa | Mouse, neonatal, dissociated dermal cells from vibrissa | Organ culture in collagen gel for 2 days then transplant into subrenal capsule of mice | Regeneration of structurally corrected vibrissa |
| Ehama et al. [53] 2007 | Human, neonatal and adult, foreskin epidermal cells | Mouse, dissociated dermal cells (frozen and thawed) | Chamber method | Chimeric hair follicle-like structure |
| Ito et al. [41] 2007 | Mouse, embryonic, dissociated epidermal cells | Mouse, CD133+, CD133− and whole dermal cells | Subcutaneous injection | CD133 as a marker for the isolation of hair-inducing cells |
| Qiao et al. [75] 2008 | Mouse, embryonic, epidermal sheets | Cultured mouse embryonic dermal cells and human neonatal foreskin dermal cells | Grafted epidermal sheet plus dermal cells into nude mice under a protective skin flap | A graft model that can be used to test the hair-inductivity of dermal cells |
| Qiao et al. [57] 2008 | Mouse, embryonic, mixture of single follicular epidermal cells and dermal cells | Suspension culture to form aggregates followed by transfer to 96-well plate to form proto-hair and transplantation to nude mice ear | In vitro epidermal-dermal aggregation and proto-hair formation with in vivo hair formation | |
| Osada et al. [49] 2009 | Mouse, foot pad epidermis | Mouse, DP cells, clones from single DP cell | Insertion of DP sphere into epidermal-derma l separation and implantation into nude mice | DP cells have intrinsic hair follicle-inducing ability |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cotsarelis G. Epithelial stem cells: a folliculocentric view. J Invest Dermatol. 2006;126:1459–68. doi: 10.1038/sj.jid.5700376. [DOI] [PubMed] [Google Scholar]
- 2.Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol. 2002;118:216–25. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- 3.Chuong CM, Cotsarelis G, Stenn K. Defining hair follicles in the age of stem cell bioengineering. J Invest Dermatol. 2007;127:2098–100. doi: 10.1038/sj.jid.5700947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341:491–7. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- 5.Ito M, Sato Y. Dynamic ultrastructural changes of the connective tissue sheath of human hair follicles during hair cycle. Arch Dermatol Res. 1990;282:434–41. doi: 10.1007/BF00402618. [DOI] [PubMed] [Google Scholar]
- 6.Fernandes KJ, McKenzie IA, Mill P, Smith KM, Akhavan M, Barnabe-Heider F, et al. A dermal niche for multipotent adult skin-derived precursor cells. Nat Cell Biol. 2004;6:1082–93. doi: 10.1038/ncb1181. [DOI] [PubMed] [Google Scholar]
- 7.Driskell RR, Giangreco A, Jensen KB, Mulder KW, Watt FM. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development. 2009;136:2815–23. doi: 10.1242/dev.038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rendl M, Lewis L, Fuchs E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 2005;3:e331. doi: 10.1371/journal.pbio.0030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tobin DJ, Gunin A, Magerl M, Handijski B, Paus R. Plasticity and cytokinetic dynamics of the hair follicle mesenchyme: implications for hair growth control. J Invest Dermatol. 2003;120:895–904. doi: 10.1046/j.1523-1747.2003.12237.x. [DOI] [PubMed] [Google Scholar]
- 10.Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–4. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 11.Horne KA, Jahoda CA. Restoration of hair growth by surgical implantation of follicular dermal sheath. Development. 1992;116:563–71. doi: 10.1242/dev.116.3.563. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds AJ, Lawrence C, Cserhalmi-Friedman PB, Christiano AM, Jahoda CA. Trans-gender induction of hair follicles. Nature. 1999;402:33–4. doi: 10.1038/46938. [DOI] [PubMed] [Google Scholar]
- 13.McElwee KJ, Kissling S, Wenzel E, Huth A, Hoffmann R. Cultured peribulbar dermal sheath cells can induce hair follicle development and contribute to the dermal sheath and dermal papilla. J Invest Dermatol. 2003;121:1267–75. doi: 10.1111/j.1523-1747.2003.12568.x. [DOI] [PubMed] [Google Scholar]
- 14.Oliver RF. Whisker growth after removal of the dermal papilla and lengths of follicle in the hooded rat. J Embryol Exp Morphol. 1966;15:331–47. [PubMed] [Google Scholar]
- 15.Jahoda CA. Cell movement in the hair follicle dermis - more than a two-way street? J Invest Dermatol. 2003;121:ix–xi. doi: 10.1111/j.1523-1747.2003.12585.x. [DOI] [PubMed] [Google Scholar]
- 16.Feutz AC, Barrandon Y, Monard D. Control of thrombin signaling through PI3K is a mechanism underlying plasticity between hair follicle dermal sheath and papilla cells. J Cell Sci. 2008;121:1435–43. doi: 10.1242/jcs.018689. [DOI] [PubMed] [Google Scholar]
- 17.Paus R, Muller-Rover S, Van Der Veen C, Maurer M, Eichmuller S, Ling G, et al. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol. 1999;113:523–32. doi: 10.1046/j.1523-1747.1999.00740.x. [DOI] [PubMed] [Google Scholar]
- 18.Ito M, Kizawa K, Hamada K, Cotsarelis G. Hair follicle stem cells in the lower bulge form the secondary germ, a biochemically distinct but functionally equivalent progenitor cell population, at the termination of catagen. Differentiation. 2004;72:548–57. doi: 10.1111/j.1432-0436.2004.07209008.x. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt-Ullrich R, Paus R. Molecular principles of hair follicle induction and morphogenesis. Bioessays. 2005;27:247–61. doi: 10.1002/bies.20184. [DOI] [PubMed] [Google Scholar]
- 20.Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643–53. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- 21.Petiot A, Conti FJ, Grose R, Revest JM, Hodivala-Dilke KM, Dickson C. A crucial role for Fgfr2-IIIb signalling in epidermal development and hair follicle patterning. Development. 2003;130:5493–501. doi: 10.1242/dev.00788. [DOI] [PubMed] [Google Scholar]
- 22.Botchkarev VA, Botchkareva NV, Roth W, Nakamura M, Chen LH, Herzog W, et al. Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nat Cell Biol. 1999;1:158–64. doi: 10.1038/11078. [DOI] [PubMed] [Google Scholar]
- 23.Chiang C, Swan RZ, Grachtchouk M, Bolinger M, Litingtung Y, Robertson EK, et al. Essential role for Sonic hedgehog during hair follicle morphogenesis. Dev Biol. 1999;205:1–9. doi: 10.1006/dbio.1998.9103. [DOI] [PubMed] [Google Scholar]
- 24.St-Jacques B, Dassule HR, Karavanova I, Botchkarev VA, Li J, Danielian PS, et al. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058–68. doi: 10.1016/s0960-9822(98)70443-9. [DOI] [PubMed] [Google Scholar]
- 25.Karlsson L, Bondjers C, Betsholtz C. Roles for PDGF-A and sonic hedgehog in development of mesenchymal components of the hair follicle. Development. 1999;126:2611–21. doi: 10.1242/dev.126.12.2611. [DOI] [PubMed] [Google Scholar]
- 26.Lehman JM, Laag E, Michaud EJ, Yoder BK. An Essential Role for Dermal Primary Cilia in Hair Follicle Morphogenesis. J Invest Dermatol. 2008 doi: 10.1038/jid.2008.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao J, DeRouen MC, Chen CH, Nguyen M, Nguyen NT, Ido H, et al. Laminin-511 is an epithelial message promoting dermal papilla development and function during early hair morphogenesis. Genes Dev. 2008;22:2111–24. doi: 10.1101/gad.1689908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jahoda C, Oliver RF. The growth of vibrissa dermal papilla cells in vitro. Br J Dermatol. 1981;105:623–7. doi: 10.1111/j.1365-2133.1981.tb00971.x. [DOI] [PubMed] [Google Scholar]
- 29.Messenger AG. The culture of dermal papilla cells from human hair follicles. Br J Dermatol. 1984;110:685–9. doi: 10.1111/j.1365-2133.1984.tb04705.x. [DOI] [PubMed] [Google Scholar]
- 30.Osada A, Iwabuchi T, Kishimoto J, Hamazaki TS, Okochi H. Long-term culture of mouse vibrissa dermal papilla cells and de novo hair follicle induction. Tissue Eng. 2007;13:975–82. doi: 10.1089/ten.2006.0304. [DOI] [PubMed] [Google Scholar]
- 31.Wu JJ, Liu RQ, Lu YG, Zhu TY, Cheng B, Men X. Enzyme digestion to isolate and culture human scalp dermal papilla cells: a more efficient method. Arch Dermatol Res. 2005;297:60–7. doi: 10.1007/s00403-005-0554-z. [DOI] [PubMed] [Google Scholar]
- 32.Kishimoto J, Ehama R, Wu L, Jiang S, Jiang N, Burgeson RE. Selective activation of the versican promoter by epithelial- mesenchymal interactions during hair follicle development. Proc Natl Acad Sci U S A. 1999;96:7336–41. doi: 10.1073/pnas.96.13.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujie T, Katoh S, Oura H, Urano Y, Arase S. The chemotactic effect of a dermal papilla cell-derived factor on outer root sheath cells. J Dermatol Sci. 2001;25:206–12. doi: 10.1016/s0923-1811(00)00130-4. [DOI] [PubMed] [Google Scholar]
- 34.Iida M, Ihara S, Matsuzaki T. Hair cycle-dependent changes of alkaline phosphatase activity in the mesenchyme and epithelium in mouse vibrissa follicles. Dev Growth Differ. 2007;49:185–95. doi: 10.1111/j.1440-169X.2007.00907.x. [DOI] [PubMed] [Google Scholar]
- 35.Handjiski BK, Eichmuller S, Hofmann U, Czarnetzki BM, Paus R. Alkaline phosphatase activity and localization during the murine hair cycle. Br J Dermatol. 1994;131:303–10. doi: 10.1111/j.1365-2133.1994.tb08515.x. [DOI] [PubMed] [Google Scholar]
- 36.Rendl M, Polak L, Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008;22:543–57. doi: 10.1101/gad.1614408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jahoda CA, Reynolds AJ, Chaponnier C, Forester JC, Gabbiani G. Smooth muscle alpha-actin is a marker for hair follicle dermis in vivo and in vitro. J Cell Sci. 1991;99 (Pt 3):627–36. doi: 10.1242/jcs.99.3.627. [DOI] [PubMed] [Google Scholar]
- 38.Soma T, Tajima M, Kishimoto J. Hair cycle-specific expression of versican in human hair follicles. J Dermatol Sci. 2005;39:147–54. doi: 10.1016/j.jdermsci.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 39.Kim SR, Cha SY, Kim MK, Kim JC, Sung YK. Induction of versican by ascorbic acid 2-phosphate in dermal papilla cells. J Dermatol Sci. 2006;43:60–2. doi: 10.1016/j.jdermsci.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Enshell-Seijffers D, Lindon C, Morgan BA. The serine protease Corin is a novel modifier of the Agouti pathway. Development. 2008;135:217–25. doi: 10.1242/dev.011031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ito Y, Hamazaki TS, Ohnuma K, Tamaki K, Asashima M, Okochi H. Isolation of murine hair-inducing cells using the cell surface marker prominin-1/CD133. J Invest Dermatol. 2007;127:1052–60. doi: 10.1038/sj.jid.5700665. [DOI] [PubMed] [Google Scholar]
- 42.Oliver RF. The experimental induction of whisker growth in the hooded rat by implantation of dermal papillae. J Embryol Exp Morphol. 1967;18:43–51. [PubMed] [Google Scholar]
- 43.Oliver RF. The induction of hair follicle formation in the adult hooded rat by vibrissa dermal papillae. J Embryol Exp Morphol. 1970;23:219–36. [PubMed] [Google Scholar]
- 44.Jahoda CA, Horne KA, Oliver RF. Induction of hair growth by implantation of cultured dermal papilla cells. Nature. 1984;311:560–2. doi: 10.1038/311560a0. [DOI] [PubMed] [Google Scholar]
- 45.Jahoda CA, Oliver RF, Reynolds AJ, Forrester JC, Gillespie JW, Cserhalmi-Friedman PB, et al. Trans-species hair growth induction by human hair follicle dermal papillae. Exp Dermatol. 2001;10:229–37. doi: 10.1034/j.1600-0625.2001.100402.x. [DOI] [PubMed] [Google Scholar]
- 46.Paus R, Ito N, Takigawa M, Ito T. The hair follicle and immune privilege. J Investig Dermatol Symp Proc. 2003;8:188–94. doi: 10.1046/j.1087-0024.2003.00807.x. [DOI] [PubMed] [Google Scholar]
- 47.Iida M, Ihara S, Matsuzaki T. Follicular epithelia and dermal papillae of mouse vibrissa follicles qualitatively change their hair-forming ability during anagen. Differentiation. 2007;75:371–81. doi: 10.1111/j.1432-0436.2006.00152.x. [DOI] [PubMed] [Google Scholar]
- 48.Inamatsu M, Tochio T, Makabe A, Endo T, Oomizu S, Kobayashi E, et al. Embryonic dermal condensation and adult dermal papilla induce hair follicles in adult glabrous epidermis through different mechanisms. Dev Growth Differ. 2006;48:73–86. doi: 10.1111/j.1440-169X.2006.00848.x. [DOI] [PubMed] [Google Scholar]
- 49.Osada A, Kobayashi K, Masui S, Hamazaki TS, Yasuda K, Okochi H. Cloned cells from the murine dermal papilla have hair-inducing ability. J Dermatol Sci. 2009;54:129–31. doi: 10.1016/j.jdermsci.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 50.Reynolds AJ, Jahoda CA. Cultured dermal papilla cells induce follicle formation and hair growth by transdifferentiation of an adult epidermis. Development. 1992;115:587–93. doi: 10.1242/dev.115.2.587. [DOI] [PubMed] [Google Scholar]
- 51.Jahoda CA. Induction of follicle formation and hair growth by vibrissa dermal papillae implanted into rat ear wounds: vibrissa-type fibres are specified. Development. 1992;115:1103–9. doi: 10.1242/dev.115.4.1103. [DOI] [PubMed] [Google Scholar]
- 52.Lichti U, Anders J, Yuspa SH. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat Protoc. 2008;3:799–810. doi: 10.1038/nprot.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ehama R, Ishimatsu-Tsuji Y, Iriyama S, Ideta R, Soma T, Yano K, et al. Hair follicle regeneration using grafted rodent and human cells. J Invest Dermatol. 2007;127:2106–15. doi: 10.1038/sj.jid.5700823. [DOI] [PubMed] [Google Scholar]
- 54.Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, et al. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–7. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 55.Zheng Y, Du X, Wang W, Boucher M, Parimoo S, Stenn K. Organogenesis from dissociated cells: generation of mature cycling hair follicles from skin-derived cells. J Invest Dermatol. 2005;124:867–76. doi: 10.1111/j.0022-202X.2005.23716.x. [DOI] [PubMed] [Google Scholar]
- 56.Havlickova B, Biro T, Mescalchin A, Arenberger P, Paus R. Towards optimization of an organotypic assay system that imitates human hair follicle-like epithelial-mesenchymal interactions. Br J Dermatol. 2004;151:753–65. doi: 10.1111/j.1365-2133.2004.06184.x. [DOI] [PubMed] [Google Scholar]
- 57.Qiao J, Turetsky A, Kemp P, Teumer J. Hair morphogenesis in vitro: formation of hair structures suitable for implantation. Regen Med. 2008;3:683–92. doi: 10.2217/17460751.3.5.683. [DOI] [PubMed] [Google Scholar]
- 58.Nakao K, Morita R, Saji Y, Ishida K, Tomita Y, Ogawa M, et al. The development of a bioengineered organ germ method. Nat Methods. 2007;4:227–30. doi: 10.1038/nmeth1012. [DOI] [PubMed] [Google Scholar]
- 59.Horne KA, Jahoda CA, Oliver RF. Whisker growth induced by implantation of cultured vibrissa dermal papilla cells in the adult rat. J Embryol Exp Morphol. 1986;97:111–24. [PubMed] [Google Scholar]
- 60.Young TH, Lee CY, Chiu HC, Hsu CJ, Lin SJ. Self-assembly of dermal papilla cells into inductive spheroidal microtissues on poly(ethylene-co-vinyl alcohol) membranes for hair follicle regeneration. Biomaterials. 2008;29:3521–30. doi: 10.1016/j.biomaterials.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 61.Young TH, Tu HR, Chan CC, Huang YC, Yen MH, Cheng NC, et al. The enhancement of dermal papilla cell aggregation by extracellular matrix proteins through effects on cell-substratum adhesivity and cell motility. Biomaterials. 2009 doi: 10.1016/j.biomaterials.2009.05.065. [DOI] [PubMed] [Google Scholar]
- 62.Kishimoto J, Burgeson RE, Morgan BA. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 2000;14:1181–5. [PMC free article] [PubMed] [Google Scholar]
- 63.Ouji Y, Yoshikawa M, Shiroi A, Ishizaka S. Promotion of hair follicle development and trichogenesis by Wnt-10b in cultured embryonic skin and in reconstituted skin. Biochem Biophys Res Commun. 2006;345:581–7. doi: 10.1016/j.bbrc.2006.04.142. [DOI] [PubMed] [Google Scholar]
- 64.Yamauchi K, Kurosaka A. Inhibition of glycogen synthase kinase-3 enhances the expression of alkaline phosphatase and insulin-like growth factor-1 in human primary dermal papilla cell culture and maintains mouse hair bulbs in organ culture. Arch Dermatol Res. 2009;301:357–65. doi: 10.1007/s00403-009-0929-7. [DOI] [PubMed] [Google Scholar]
- 65.Kwack MH, Sung YK, Chung EJ, Im SU, Ahn JS, Kim MK, et al. Dihydrotestosterone-inducible dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J Invest Dermatol. 2008;128:262–9. doi: 10.1038/sj.jid.5700999. [DOI] [PubMed] [Google Scholar]
- 66.Kitagawa T, Matsuda K, Inui S, Takenaka H, Katoh N, Itami S, et al. Keratinocyte growth inhibition through the modification of Wnt signaling by androgen in balding dermal papilla cells. J Clin Endocrinol Metab. 2009;94:1288–94. doi: 10.1210/jc.2008-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Inui S, Fukuzato Y, Nakajima T, Yoshikawa K, Itami S. Androgen-inducible TGF-beta1 from balding dermal papilla cells inhibits epithelial cell growth: a clue to understand paradoxical effects of androgen on human hair growth. FASEB J. 2002;16:1967–9. doi: 10.1096/fj.02-0043fje. [DOI] [PubMed] [Google Scholar]
- 68.Hamada K, Randall VA. Inhibitory autocrine factors produced by the mesenchyme-derived hair follicle dermal papilla may be a key to male pattern baldness. Br J Dermatol. 2006;154:609–18. doi: 10.1111/j.1365-2133.2006.07144.x. [DOI] [PubMed] [Google Scholar]
- 69.Bahta AW, Farjo N, Farjo B, Philpott MP. Premature senescence of balding dermal papilla cells in vitro is associated with p16(INK4a) expression. J Invest Dermatol. 2008;128:1088–94. doi: 10.1038/sj.jid.5701147. [DOI] [PubMed] [Google Scholar]
- 70.Jahoda CA, Reynolds AJ, Oliver RF. Induction of hair growth in ear wounds by cultured dermal papilla cells. J Invest Dermatol. 1993;101:584–90. doi: 10.1111/1523-1747.ep12366039. [DOI] [PubMed] [Google Scholar]
- 71.Weinberg WC, Goodman LV, George C, Morgan DL, Ledbetter S, Yuspa SH, et al. Reconstitution of hair follicle development in vivo: determination of follicle formation, hair growth, and hair quality by dermal cells. J Invest Dermatol. 1993;100:229–36. doi: 10.1111/1523-1747.ep12468971. [DOI] [PubMed] [Google Scholar]
- 72.Lichti U, Weinberg WC, Goodman L, Ledbetter S, Dooley T, Morgan D, et al. In vivo regulation of murine hair growth: insights from grafting defined cell populations onto nude mice. J Invest Dermatol. 1993;101:124S–9S. doi: 10.1111/1523-1747.ep12363165. [DOI] [PubMed] [Google Scholar]
- 73.Jahoda CA, Oliver RF, Reynolds AJ, Forrester JC, Horne KA. Human hair follicle regeneration following amputation and grafting into the nude mouse. J Invest Dermatol. 1996;107:804–7. doi: 10.1111/1523-1747.ep12330565. [DOI] [PubMed] [Google Scholar]
- 74.Reynolds AJ, Jahoda CA. Cultured human and rat tooth papilla cells induce hair follicle regeneration and fiber growth. Differentiation. 2004;72:566–75. doi: 10.1111/j.1432-0436.2004.07209010.x. [DOI] [PubMed] [Google Scholar]
- 75.Qiao J, Philips E, Teumer J. A graft model for hair development. Exp Dermatol. 2008;17:512–8. doi: 10.1111/j.1600-0625.2007.00661.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.