Abstract
Ethnopharmacological relevance
The herbal formula Huo Luo Xiao Ling Dan (HLXL) and its modifications have been used in traditional Chinese medicine for about one hundred years to alleviate pain and inflammation.
Aim
To investigate the effects of HLXL on complete Freund’s adjuvant (CFA)-induced multiple-joint arthritis in rats.
Materials and Methods
Male Lewis rats, 190–210g, were immunized subcutaneously at the base of the tail with 200 µl of heat-killed M. tuberculosis in mineral oil (5 mg/ml). HLXL (2.30g/kg and 4.60g/kg) or vehicle control (n=8 per group) was administered orally (i.g.) once a day between days 16–25 post-CFA injection. The rats were observed for signs of arthritis with arthritic changes (erythema, edema, induration) being scored on a scale of 0 to 4 of increasing severity using a standard scoring system. The maximum arthritis score per rat was 16. A plethysmometer was used to measure edema volume in each paw. Adverse effects of HLXL were monitored by closely observing the animals for unusual behavioral changes. Levels of tumor necrosis factor alpha (TNF-α) and interleukin 1 beta (IL-1β) in local tissue were measured by enzyme-linked immunosorbent assay on day 25 post-CFA.
Results
HLXL significantly decreased arthritis scores between days 23–25 in the 2.30g/kg group and 21–25 in the 4.60g/kg group (p<0.05). It reduced paw edema on days 22 and 24 in the 2.30g/kg group and on days 20, 22 and 24 in the 4.60g/kg group compared to control (p<0.05). Local tissue TNF-α and IL-1β levels on day 25 post-CFA injection were significantly (p<0.05) lower in rats treated with HLXL than in control rats. No observable adverse effects were found.
Conclusion
The data suggest that HLXL produces significant anti-arthritic effects that may be mediated by suppressing pro-inflammatory cytokines, and it appears to be safe.
Keywords: Arthritis, Chinese Herbs, IL-1β, TNF-α, Rats
1. Introduction
Arthritis and related disorders, including rheumatoid arthritis (RA), are common diseases affecting millions (Kvien, 2004). Conventional medicine, including treatment with steroids, non-steroidal anti-inflammatory drugs (NSAIDs) and such biological agents as tumor necrosis factor alpha (TNF-α) and interleukin-1 beta (IL-1β) antagonists (Fleischmann et al., 2004), has shown only limited success against all forms of arthritis (Chandrashekara et al., 2002). Furthermore, these drugs are associated with unpleasant side effects such as gastrointestinal disturbances (Scheiman, 2001) and cardiovascular risks (Davies and Jamali, 2004). Considering the potential adverse activities of these drugs, as well as their limited ability to provide long-term remission (Davies and Jamali, 2004; Fleischmann et al., 2004), newer conventional drugs and complementary and alternative medicine (CAM) products, including herbs, are continuously being sought (Barnes et al., 2004). Herbal products are receiving increasing public interest, and herbal treatment is now the most popular CAM therapy.
Chinese herbal medicine, a major modality in traditional Chinese medicine (TCM) and practiced for thousands of years in China and other Asian countries, is used for treating arthritis and related disorders, or “Bi syndrome”(Bensky and Gamble, 1993; Ho and Lai, 2004). Herbal formulations are the common form of administration in Chinese herbal practice, and herbal formulas are well documented in ancient and modern literature (Peng, 1998; Sun, 682). According to Chinese herbal theory, interactions among the different herbs in a formula exert a synergistic effect and neutralize potential toxicity and side effects of the individual constituents (Bensky and Barolet, 1990; Bensky and Gamble, 1993). However, there is as yet a lack of rigorous scientific evaluation of such formulas.
The classical formula Huo Luo Xiao Ling Dan (HLXL, “Effective Pill to Invigorate the Collaterals”), first documented in the book Yi Xue Zhong Zhong Can Xi Lu--Records of Heart-felt Experiences in Medicine with the Reference to the West (Zhang, 1974), consists of four component herbs, Ru Xiang (Boswellia carterii Birdw.), Mo Yao (Commiphora myrrha Engl.), Dang Gui (Angelica sinensis [Oliv.] Diels), and Dan Shen (Salvia miltiorrhiza Bge.). The formula and its modifications have been used to treat arthritis and various conditions (Bensky and Gamble, 1993; Liang, 1997; Wang et al., 2002).
In order to strengthen the effects in arthritis-related diseases (Bi syndrome) and minimize potential toxicity and adverse effects according to TCM herbal theory, a modified Huo Luo Xiao Ling Dan (HLXL) containing 11 herbs was developed, the main actions of which are to promote circulation, alleviate pain and reduce swelling (Bensky and Barolet, 1990; Peng, 1998). The herbs, with their Chinese names, botanical names, family names, and clinical dosages, are: Ruxiang [Boswellia carterii Birdw. (Burseraceae )] 15g, Qianghuo [Notopterygium incisum Ting ex H.T. Chang (Apiaceae)] 12g, Danggui [Angelica sinensis (Oliv.) Diels. (Apiaceae)] 12g, Chishao [Paeonia lactiflora Pall. (Paeoniaceae)] 12g, Gancao [Glycyrrhiza uralensis Fisch. (Fabaceae)] 12g, Yanhusuo [Corydalis yanhusuo W.T. Wang. (Papaveraceae )] 12g, Danshen [Salvia miltiorrhiza Bge. (Lamiaceae )] 12g, Chuanxiong [Ligusticum chuanxiong S.H. Qiu. (Apiaceae)] 12g, Qinjiao [Gentiana macrophylla Pall.(Gentianaceae)] 12g, Guizhi [Cinnamomum cassia Presl. (Lauraceae)] 15g and Duhuo [Angelica pubescens Maxim. (Apiaceae)] 12g. In a previous study, we demonstrated that this formula produces anti-hyperalgesia and anti-edema in a rat model of persistent inflammation (Lao et al., 2006).
Adjuvant arthritis (AA), induced in susceptible strains of Lewis rats by subcutaneous injection of heat-killed Mycobacterium tuberculosis, is a widely used model (Durai et al., 2004). The AA model shares some features with human RA (Taurog et al., 1988). During the developmental course of AA, there is initially an acute periarticular inflammation characterized by synovial mononuclear cell infiltration (Taurog et al., 1988). This is followed by chronic arthritis involving synovial hyperplasia and destruction of periarticular bone and cartilage similar to that seen in human arthritis, particularly RA. Severe arthritis eventually leads to bony ankylosis and deformities of the paws.
The purpose of the present study was to evaluate the efficacy, safety and mechanism of action of an extract of HLXL using the rat AA model. Specific aims were to: (1) determine the anti-arthritic effects of HLXL by systematically scoring arthritis symptoms and by measuring paw edema, (2) evaluate potential adverse effects of the formula, and (3) investigate the formula’s effects on pro-inflammatory cytokines by examining local tissue levels of TNF-α and IL-1β.
2. Material and Methods
2.1 Animal preparation
Male Lewis rats (Harlan, Indianapolis, IN), 5–6 weeks old (190–210g), were kept under controlled environmental conditions (22°C ± 0.5°C relative humidity 40–60%, 7am to 7pm alternate light-dark cycles, food and water ad libitum). The animals were purchased one week before the experiment and allowed to acclimate. The floors of their cages were covered with paper pellets to minimize the possibility of painful contact with a hard surface. The animal protocol was approved by the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine.
AA was induced by injecting 200 µl of complete Freund’s adjuvant (CFA) solution, prepared by suspending heat-killed M. tuberculosis (strain H37Ra, Difco Laboratories, Detroit, MI, USA) in mineral oil (Sigma, St. Louis, MO, USA; 5 mg/ml), subcutaneously at the base of the tail (Durai et al., 2004). Rats developed signs of polyarthritis 8–10 days following the injection. After injection, the clinical features of AA, erythema, induration, and edema, presented in multiple joints as follows: (1) onset occurred between days 8–10, (2) progressive severity developed over the next 7–10 days (early phase), and (3) spontaneous regression followed over the next 10–14 days (late phase).
2.2 Herbal preparation and extraction
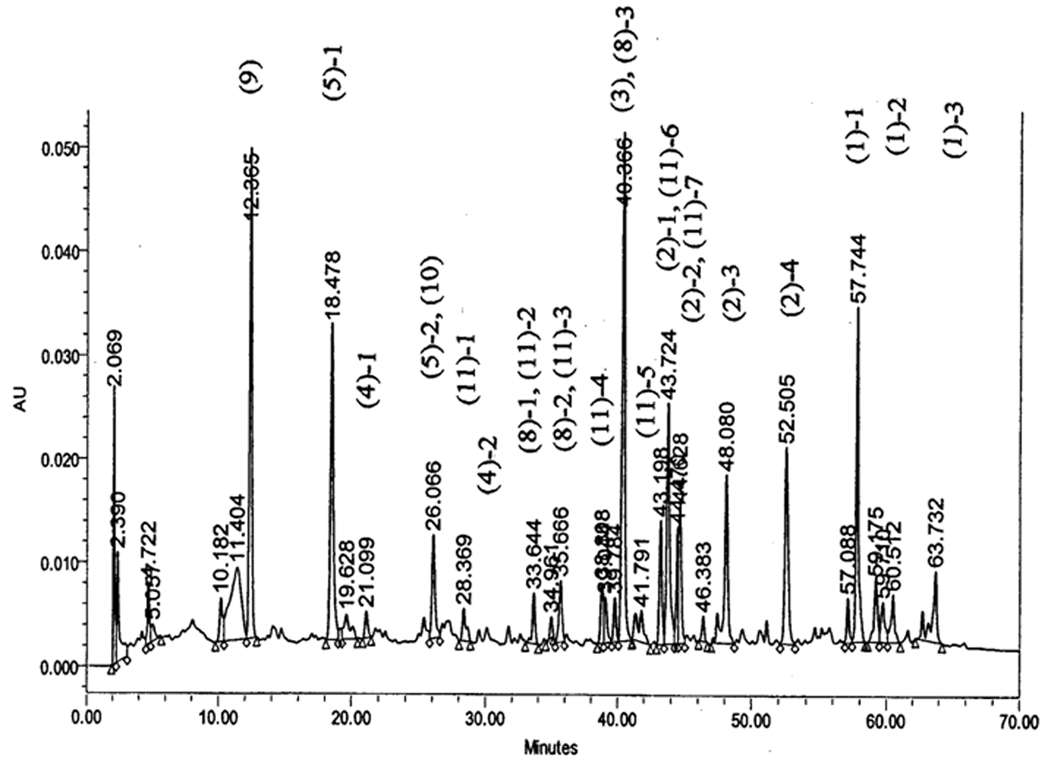
The method of herbal preparation has been previously reported (Lao et al., 2006). In brief, the 11 herbs used in HLXL were prepared with traditional methods after harvest (Bensky and Gamble, 1993) and purchased from the Tong Ren Tang pharmaceutical company (Beijing, China). Unprocessed component herbs of HLXL were acquired through the Institute of Medicinal Plant Development (IMPLAD), Chinese Academy of Medical Sciences, Beijing, China, and the identities authenticated based on the pharmacopoeia (The Pharmacopoeia Commission of PRC, 2000). The herbs were processed and extracted with aqueous acetone as previously described (Lao et al., 2006). Each extract was monitored for the absence of contaminants (heavy metals, pesticides, and mycotoxins) prior to formulation. The final extract was 25% the weight of the raw herbs. The formulated HLXL was subjected to high performance liquid chromatography (HPLC) finger printing analysis in which major peaks were identified as the marker compounds to their originating individual herbs (unpublished data).The HPLC trace of the initial extract is showed in Figure 1.
Fig. 1.
HPLC trace of the HLXL extract. The major representative peaks of herbs in the formula are marked. The numbers in parentheses are the numbers assigned to the herbs as listed in Table One. The second number indicates the marker number for the respective herb. For example, (1)-3 represents the third marker of the first herb (Boswellia carterii Birdw.) listed in the HLXL formula. The HPLC conditions were YMC C-18 analytical column (4.6 mm × 15 cm); 100% Methanol at 1.0 ml/min; UV monitor at 210 nm. Retention times of purified HLXL are delineated directly on this tracing.
A complete set of voucher samples of the authenticated herbs has been retained at IMPLAD.
2.3 Intervention
Intervention commenced on day 16 post-CFA injection and continued for 10 consecutive days. The CFA-injected rats were randomly divided into HLXL treatment and vehicle control groups. A single dose of 2.30g/kg or 4.60g/kg HLXL extract or vehicle was administered once a day to the respective groups. Each dose was dissolved in a 2 ml mixture of distilled water and sesame oil (2:1) and administered intragastrally (i.g.) using a 5 ml syringe with a 4 cm long gavage needle through the mouth to the stomach. The initial dosage of 2.30g/kg was based on body surface area and calculated from the daily human HLXL clinical dosage (FDA, 2002). Control rats received 2 ml of vehicle. In all cases, the administration was performed gently and swiftly and no analgesics were required for the procedure.
2.4 Arthritis assessments
The rats were assessed daily for signs of arthritis between days 7 and 25 post-CFA using a well-established, widely-used scoring system developed to evaluate the severity of AA (Durai et al., 2004). Paws were examined and graded for severity and loci of erythema, swelling and induration using a 5-point scale: 0 = no signs of disease, 1 = signs involving the ankle/wrist, 2 = signs involving the ankle plus tarsal of the hind paw and/or wrist plus carpals of the forepaw, 3 = signs extending to the metatarsals or metacarpals, and 4 = severe disease involving the entire hind or fore paw (Durai et al., 2004). The maximum arthritic score per rat was set at 16 (4 points × 4 paws).
During treatment, paw volume was measured every other day with a plethysmometer (IITC Inc, Woodland Hills, CA), a device that consists of two vertical, interconnected, water-filled Perspex cells. The paw to be measured was dipped into the larger cell to the ankle line, causing the water level in the smaller cell, which contains a transducer, to rise. The transducer converts detected paw volume into milliliters, then registers the exact volume electronically on a monitor. Total paw volume, the sum of the 4 paws, was calculated to monitor the paw edema. To avoid bias, investigators performing all assessments and measurements were blinded to treatment group assignments.
2.5 Local tissue collection and ELISA
After arthritis assessment on day 25 post-injection, the rats were deeply anesthetized with sodium pentobarbital (80 mg/kg, i.p.). The ankle tissue was collected, frozen, weighed and immediately placed in 0.50 ml of ice-cold homogenization buffer (50 mmol/l NaCl, 10 mmol/l Tris, 2.5 mmol/l MgCl2, pH 7.4), to 50 ml of which one complete protease inhibitor tablet (Boehringer Mannheim, Germany) was added. The tissues were homogenized using an ultrasonic tissue homogenizer (Ultrasonic Processors, Vernon Hills, IL). They were spun at 20,000 g for 30 minutes at 4°C, and the supernatant was aliquoted and stored at −80°C. The animals were euthanized following the tissue collection.
TNF-α and IL-1β levels of the local tissue homogenate supernatant were measured using enzyme-linked immunosorbent assay (ELISA) kits according to the procedures recommended by the manufacturer (BioSource International Inc., Camarillo, CA). In brief, IL-1β levels were measured by pipetting 50 µl of sample and 50 µl of standard diluent buffer into the wells of a microtiter plate coated with an antibody specific to rat IL-1β, then incubated for 3 hours at 37°C. After two 10-minute washings with PBS, biotinylated anti-rat IL-1β antibody was added and incubated at ambient temperature for 1 hour. Streptavidin-peroxidase HRP was then added and incubated for 30 minutes to bind to the biotinylated antibody. After two more 10-minute washings with PBS to remove unbound enzymes, color was developed by adding stabilized chromogen tetramethylbenzidine and a stop solution was added. Finally, the optical density was calculated with an automated Coulter microplate reader (Coulter Electronics, Kendall, FL) at 450 nm, and the IL-1β protein was quantified by comparing the sample to the standard curve generated from the kit. The results were expressed as cytokine concentrations (i.e. pg/mg protein). The same procedure was used to assay TNF-α, with the substitution of antibodies specific to that cytokine. The assay was performed by an investigator blinded to the treatment group assignments.
2.6 Toxicity/adverse effects assessment
2.6.1 Unusual behavioral changes
In the second experiment, thirty naive rats were randomly divided into two groups. For 42 consecutive days, an HLXL group (20 rats, half male and half female) received 2.3g/kg i.g. HLXL, while a control group (10 rats, half male and half female) received only the same amount of vehicle, 2:1 distilled water and sesame oil.
Assessment for toxicity/adverse effects was carried out in compliance with Chinese (China State Administration of Traditional Chinese Medicine, 1999) and American (FDA, 2004) guidelines for developing new Chinese herbal medicines and botanical drugs. Standard pharmacological categories of toxic and adverse behavioral reactions were used, following Dr. Gad’s methods (Gad, 2002). All animals, including CFA-injected animals, were closely monitored for unusual behavioral changes and such symptoms as obvious temperature change, diarrhea, weight loss, fur discoloration, lethargy, irritation, and convulsion during treatment. Rectal temperature was taken with a digital thermometer before each HLXL or vehicle administration, and rats were weighed every other day. All observations were performed by investigators blinded to treatment group assignments.
On day 42, after the observation period, all animals in both groups were anesthetized (sodium pentobarbital, 50 mg/kg, i.p.), blood samples were collected, and gross necropsy and histopathological studies were performed.
2.6.2 Blood biochemistry tests
Blood biochemistry tests were conducted by an independent laboratory, the research animal diagnostic laboratory of the University of Missouri. Sera alanine aminotransferae (ALT), aspartate aminotransferae (AST), blood urea nitrogen (BUN), creatinine (Cr), lactate dehydrogenase (LDH), creatine kinase (CK), total protein, albumin and globulin were tested using commercial kits to detect any HLXL effects on liver, kidney and heart function.
2.6.3 Gross necropsy and histopathology
Each animal was subjected to full necropsy, including examination of the external surface of the body, all orifices, and the thoracic, abdominal and cranial cavities and their contents. The brain, lungs, heart, liver, kidneys, spleen, stomach, adrenals, and testes or ovaries were checked immediately after dissection. Tissues from the liver, kidneys, heart and spleen of each animal were preserved in 10% neutral buffered formalin for histopathological examination. The preserved tissues were embedded in paraffin, and 5-micron-thick sections were cut and stained using the standard Haematoxylin and Eosin (H&E) method. Histopathological observation was conducted under a light microscope (Nikon E600), and the responsible investigator was blinded to animal treatment assignment.
2.7 Statistical analysis
The results are presented as mean ± standard error. For arthritic score and edema assessments, one-way repeated measure ANOVA was used for statistical comparison among three groups; for cytokine testing, one-way ANOVA was used. All post hoc comparisons were conducted using the Dunnett test. P<0.05 was considered significant in all cases.
3. Results
3.1 Effects of HLXL on arthritic score and paw edema
Beginning on day 8 after CFA injection, the rats gradually began to develop multiple-joint AA; manifestations peaked between days 18 and 20 (Fig. 2 and Fig. 3). There were no significant differences in arthritic score or multiple-paw arthritic edema between the groups before HLXL or vehicle administration (p>0.05).
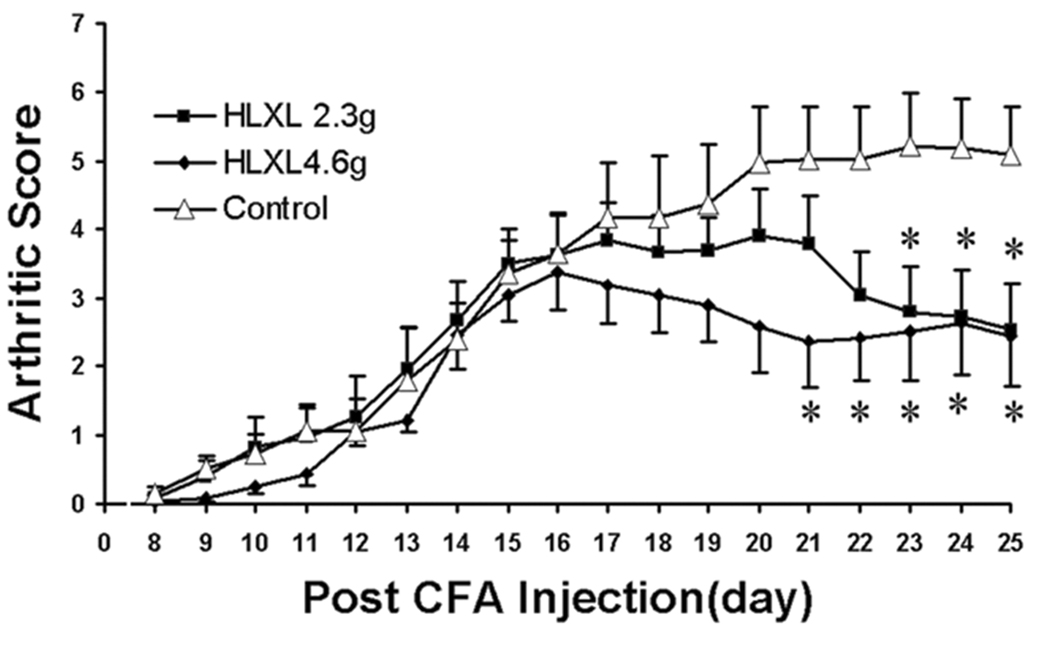
Fig. 2.
Effect of HLXL on arthritis as assessed with arthritic scores (Mean ± S.E., n = 8/group) in rats. Note that both 2.30g/kg and 4.60g/kg (daily, i.g.) of HLXL significantly decreased the arthritic score and suppressed peak arthritic severity compared to vehicle control, *p<0.05 vs. vehicle control, at the same time point.
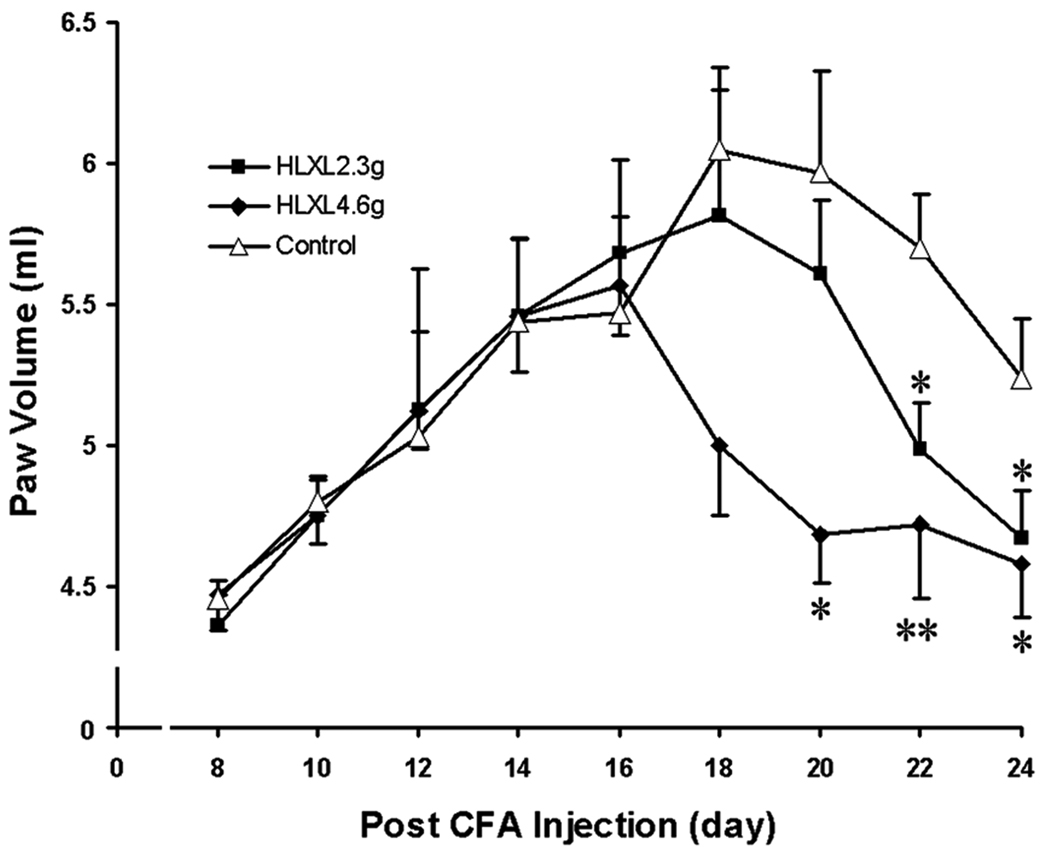
Fig. 3.
Effect of HLXL on arthritis assessed with paw volume (Mean ± S.E., n = 8/group) in rats. Data showed that paw volume increased remarkably following arthritis formation and peaked around day 18 in the control group. HLXL (daily, i.g.) significantly decreased arthritic paw edema volume and suppressed peak arthritic edema in both 2.30 g/kg and 4.60g/kg groups, *p<0.05 and **p<0.01 vs. vehicle control, at the same time point.
HLXL at both 2.30 and 4.60/kg significantly decreased arthritic scores compared to the vehicle control (p<0.001). At 2.30g/kg, scores significantly (p<0.05) decreased between days 23 to 25. At 4.60/kg, scores significantly (p<0.05) decreased between days 21 to 25. See Fig. 2.
Concomitantly, HLXL at both 2.30 and 4.60g/kg attenuated multiple-paw edema (p<0.001). At 2.30g/kg, edema was significantly (p<0.05) attenuated compared to control on days 22 and 24. At 4.60g/kg, edema was significantly (p<0.05) attenuated compared to control on days 20, 22 and 24. See Fig. 3.
3.2 HLXL effects on local arthritic tissue cytokines
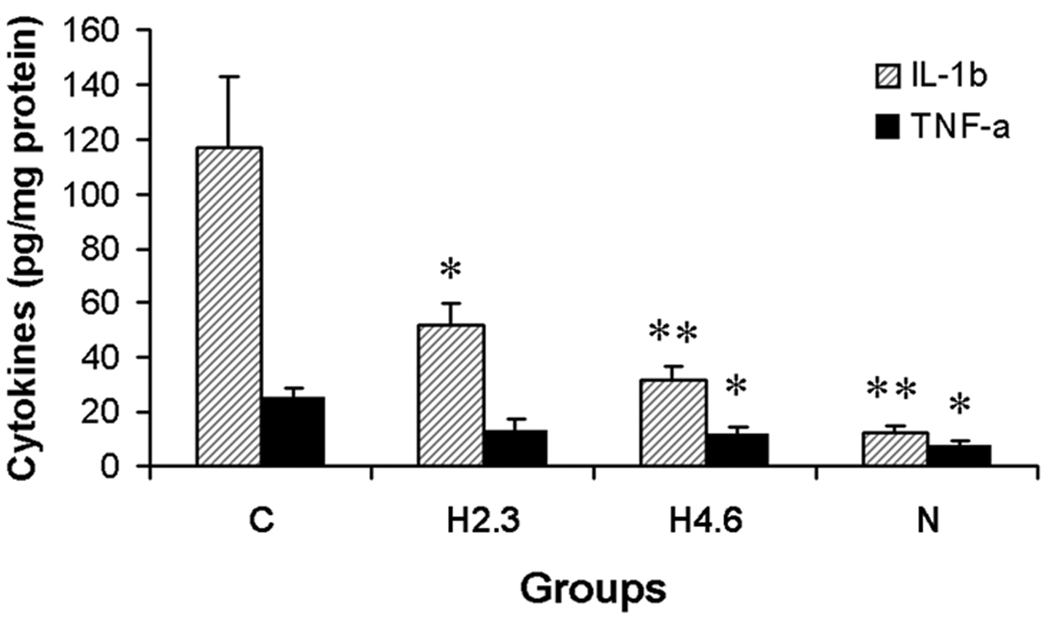
On day 25 post-CFA injection, IL-1β levels in local tissue of the vehicle control arthritic rats were about 10-fold that of the non-arthritis vehicle group (117.29±25.58 pg/mg protein vs. 12.44±2.90 pg/mg protein, p<0.01). HLXL dosage-dependently suppressed IL-1β in local arthritic tissue by half in the 2.3g/kg group (51.41±8.36 pg/mg protein, p<0.05) and by two-thirds in the 4.6g/kg group (31.63±5.01 pg/mg protein, p<0.01) compared to the vehicle control (117.29±25.58 pg/mg protein). See Fig.4.
Fig. 4.
Effect of HLXL on IL-1β and TNF-α levels (pg/mg protein, Mean ± S.E.) 25 days post-CFA injection. Tissue was obtained from four groups of rats: group N (no arthritis + vehicle treatment, n=4), group H4.6 (arthritis + HLXL treatment at 4.60g/kg/day, n=7), group H2.3 (arthritis + HLXL treatment at 2.30g/kg/day n=6), and group C (arthritis + vehicle treatment n= 6). Data showed that both IL-1β and TNF-α increased significantly in local tissue following development of arthritis. However, after HLXL treatment, local tissue IL-1β and TNF-α decreased significantly, *p<0.05 and **p<0.01, compared to the vehicle control (Group C).
On day 25 post-CFA injection, TNF-α levels in local tissue of vehicle control arthritic rats were about three times that of the non-arthritis vehicle group (24.78±4.18 pg/mg protein vs. 7.27±1.80 pg/mg protein, p<0.05). HLXL at 4.60g/kg significantly decreased TNF-α levels (11.14±3.52 pg/mg protein, q=2.64, p<0.05) compared to those in the vehicle control arthritis rats (24.78±4.18 pg/mg protein. Fig.4). HLXL at 2.30g/kg also lowered TNF-α (12.96±4.28 pg/mg protein) although statistical significance was not reached (p>0.05).
3.3 Adverse effects and toxicity
No abnormal animal behavioral changes or clinical signs were observed at either 2.30 or 4.60g/kg in AA rats or in naive rats. No abnormalities were found in the sera biochemistry assay: all ALT, AST, BUN, Cr, LDH, CK, total protein, albumin and globulin data were in the normal range for rats (Loeb WF and FW, 1999), and there were no significant differences between the HLXL (2.30g/kg per day) group and vehicle group. Gross necropsy revealed no damage to organs or tissues and no stomach ulcers or bleeding foci in any animal. Microscopic examination of the tissue sections from liver, heart, kidney and spleen revealed no abnormal changes in either HLXL or control groups.
4. Discussion
The present study demonstrated that an aqueous acetone extract of the Chinese herbal formula HLXL dosage-dependently attenuated adjuvant-induced multiple-joint arthritis and facilitated recovery as measured by decreasing arthritic scores and paw edema volume. These findings are consistent with the results of our previous studies on the effects of HLXL on a CFA-induced persistent inflammation model (Lao et al., 2006) and suggest that the formula may be effective, especially in the recovery stage, and safe against arthritis and its related disorders.
In TCM practice, the early acute stage of arthritis is usually considered an excess-heat syndrome (Hot Bi), and heat-clearing herbs are most suitable for this stage of treatment (Bensky and Barolet, 1990; Bensky and Gamble, 1993). In contrast, the manifestations in the recovery or the chronic stage are usually categorized as Wind syndrome, Dampness syndrome, Deficiency-Cold, or a combination of two or more of these syndromes (mixed syndrome) (Bensky and Barolet, 1990), and signs of Qi and Blood stasis are commonly seen (Bensky and Barolet, 1990; Liang, 1997).
HLXL consists of herbs known in TCM theory to activate Qi and Blood circulation, warm the channels, relieve Wind-Dampness, alleviate pain and reduce swelling (Bensky and Gamble, 1993; The Pharmacopoeia Commission of PRC, 2000). The herb Gui Zhi (Cinnamomum cassia Presl.) has a particularly warm nature that is contraindicated in Hot Bi syndrome such as that seen in acute inflammation (Bensky and Gamble, 1993; The Pharmacopoeia Commission of PRC, 2000). This might explain our observation that the formula showed no effect when used in the early stage of symptoms in this AA animal model (unpublished data) but showed significant anti-arthritic effects in the later stage. The findings of our study seem to point up the importance of syndrome differentiation stressed in TCM theory and suggest that this formula may be useful in treating the chronic stage of inflammatory arthritis.
Further, we found that the HLXL extract significantly decreased the concentration of the pro-inflammatory cytokines TNF-α and IL-1β at the local inflammation site in the AA model. IL-1β and TNF-α originate from activated macrophages, and TNF-α is also produced by antigen-primed helper T cells (Harris, 1990). These cytokines have been documented as critically important both in RA in humans (Fleischmann et al., 2004; Jacques et al., 2006) and AA in rats (Smith-Oliver et al., 1993; Wei et al., 2004). They contribute to many features of arthritic inflammation, including synovial tissue inflammation, synovial proliferation, and cartilage and bone damage (Harris, 1990).
Several ingredients of HLXL have been reported to possess immunomodulatory properties. For example, it has been reported that boswellic acids, chemical components of the herb Boswellia carterii, selectively decrease the formation of leukotriene LTB4, a potent chemoattractant and activator of both granulocytes and macrophages (Safayhi et al., 1992), and reduce the infiltration of leucocytes into an inflammation site (Sharma et al., 1989). Acetyl-boswellic acids inhibit IkappaB kinases, resulting in both inhibition of NF-kappaB and down-regulation of TNF-α expression in activated human monocytes (Syrovets et al., 2005). Angelica sinensis polysaccharide root extract has immuno-inhibitory effects on TNF-α (Liu et al., 2003). Paeonia lactiflora root extract decreases human fibroblasts secretion of monocyte chemotactic proteins (MCP)-1 and MCP-3, which are known as the most potent chemokines to mediate allergic inflammation (Leem et al., 2004). Thus our data suggest that the potent therapeutic effect of HLXL in the AA model is due in part to the inhibition of the activity of pro-inflammatory cytokines.
Furthermore, except for Corydalis yanhusuo, the herbs in HLXL have anti-inflammatory effects on inflammation models (Grand Pharmacopeia of Chinese Materia Medica Editing Committee, 1999). Among the constituents of HLXL, several are reported to have significant inhibitory effects on fibrosis and granuloma formation, proliferative activities that occur in chronic inflammation. For example, the ferulic acid in Angelica sinensis significantly inhibits the granuloma formation induced by cotton pellets (Ozaki, 1992). Isoliquiritin, which occurs in Glycyrrhiza uralensis, also has significantly inhibited granuloma formation (Kobayashi et al., 1995), and Salvia miltiorrhiza significantly inhibits liver and lung fibrosis in animal models (Grand Pharmacopeia of Chinese Materia Medica Editing Committee, 1999). These studies suggest that HLXL may alleviate arthritis in part by inhibiting the formation of granulomas.
In recent years the medical community and public have been concerned about herbal product safety (White House Commission on Complementary and Alternative Medicine, 2002). During the 10 consecutive days of repeat-dose study, 4.60g/kg per day of HLXL showed no observable adverse effects on the AA model, and sera biochemical examination and histology observation showed no toxicities or adverse effects on the naive rats during the 42 day repeat-dose study. These findings suggest that the HLXL used in the present study will show no toxicity or adverse effects in clinic.
In conclusion, the present study indicates that HLXL appears to be safe and that it produces significant anti-arthritic effects in the AA model by suppressing pro-inflammatory cytokines. This suggests that HLXL may be beneficial as an adjuvant to conventional drugs in the treatment of arthritis and related inflammatory disorders.
Acknowledgments
This study was supported by NIH/NCCAM grant PO1 AT002605-01A1 from the National Center for Complementary and Alternative Medicine (NCCAM) at the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCCAM. We thank Prof. Chen Shi-lin, (IMPLAD, Chinese Academy of Medical Sciences, Beijing, China) for assistance in the acquisition and identities authentication of herbal source materials and Dr. Lyn Lowry for her editorial support.
Reference
- Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and Alternative Medicine Use Among Adults: United States, 2002. Advance Data From Vital and Health Statistics. 2004 [PubMed] [Google Scholar]
- Bensky D, Barolet R. Chinese herbal medicine: Formulas & Strategies. Seattle: Eastland Press; 1990. [Google Scholar]
- Bensky D, Gamble A. Chinese Herbal Medicine: Materia Medica. revised edition. Seattle, Washington: Eastland Press; 1993. [Google Scholar]
- Chandrashekara S, Anilkumar T, Jamuna S. Complementary and alternative drug therapy in arthritis. Journal of the Association of Physicians of India. 2002;50:225–227. [PubMed] [Google Scholar]
- China State Administration of Traditional Chinese Medicine. Testing Guidelines for Safety Evaluation of New Drug. Beijing: The Guidelines for Developing New Chinese Herbal Medicine. 1999
- Davies NM, Jamali F. COX-2 selective inhibitors cardiac toxicity: getting to the heart of the matter. Journal of Pharmacy & Pharmaceutical Sciences. 2004;7:332–336. [PubMed] [Google Scholar]
- Durai M, Kim HR, Moudgil KD. The regulatory C-terminal determinants within mycobacterial heat shock protein 65 are cryptic and cross-reactive with the dominant self homologs: implications for the pathogenesis of autoimmune arthritis. Journal of Immunology. 2004;173:181–188. doi: 10.4049/jimmunol.173.1.181. [DOI] [PubMed] [Google Scholar]
- FDA. Guidance for Industry and Reviewers: Estimate the Safe Starting Dose in Clinical Trials for Therapeutics in Adult Healthy Volunteers, Appendix A (Draft Guidance) 2002 http://www.fda.gov/cber/gdlns/dose.htm.
- FDA; Center for Drug Evaluation and Research (CDER) Guidance for Industry: Botanic drug products. 2004 www.fda.gov/cder/guidance/4592fnl.htm.
- Fleischmann R, Stern R, Iqbal I. Anakinra: an inhibitor of IL-1 for the treatment of rheumatoid arthritis. Expert Opinion on Biological Therapy. 2004;4:1333–1344. doi: 10.1517/14712598.4.8.1333. [DOI] [PubMed] [Google Scholar]
- Gad SC. Drug Safety Evaluation. Wiley-Interscience New York; 2002. [Google Scholar]
- Grand Pharmacopeia of Chinese Materia Medica Editing Committee. Grand Pharmacopeia of Chinese Materia Medica (Zhong Hua Ben Cao) Shanghai: Shanghai Scientific Press; 1999. [Google Scholar]
- Harris ED., Jr Rheumatoid arthritis. Pathophysiology and implications for therapy. New England Journal of Medicine. 1990;322:1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- Ho LJ, Lai JH. Chinese herbs as immunomodulators and potential disease-modifying antirheumatic drugs in autoimmune disorders. Current Drug Metabolism. 2004;5:181–192. doi: 10.2174/1389200043489081. [DOI] [PubMed] [Google Scholar]
- Jacques C, Gosset M, Berenbaum F, Gabay C. The role of IL-1 and IL-1Ra in joint inflammation and cartilage degradation. Vitamins & Hormones. 2006;74:371–403. doi: 10.1016/S0083-6729(06)74016-X. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Miyamoto T, Kimura I, Kimura M. Inhibitory effect of isoliquiritin, a compound in licorice root, on angiogenesis in vivo and tube formation in vitro. Biological & Pharmaceutical Bulletin. 1995;18:1382–1386. doi: 10.1248/bpb.18.1382. [DOI] [PubMed] [Google Scholar]
- Kvien TK. Epidemiology and burden of illness of rheumatoid arthritis. Pharmacoeconomics. 2004;22:1–12. doi: 10.2165/00019053-200422001-00002. [DOI] [PubMed] [Google Scholar]
- Lao L, Fan AY, Zhang R-X, Zhou AN, Ma ZZ, Lee DY-W, K R, Berman B. Anti-hyperalgesic and anti-inflammatory effects of the modified Chinese herbal formula Huo Luo Xiao Ling Dan (HLXL) in Rats. American Journal of Chinese Medicine. 2006;34:833–844. doi: 10.1142/S0192415X06004326. [DOI] [PubMed] [Google Scholar]
- Leem K, Kim H, Boo Y, Lee HS, Kim JS, Yoo YC, Ahn HJ, Park HJ, Seo JC, Kim HK, Jin SY, Park HK, Chung JH, Cho JJ. Effects of Paeonia lactiflora root extracts on the secretions of monocyte chemotactic protein-1 and -3 in human nasal fibroblasts. Phytotherapy Research. 2004;18:241–243. doi: 10.1002/ptr.1392. [DOI] [PubMed] [Google Scholar]
- Liang YY. The relation between rheumatoid arthritis and TCM blood stagnation syndrome. Tradit Chin Med Res. 1997;10:1–3. [Google Scholar]
- Liu SP, Dong WG, Wu DF, Luo HS, Yu JP. Protective effect of angelica sinensis polysaccharide on experimental immunological colon injury in rats. World Journal of Gastroenterology. 2003;9:2786–2790. doi: 10.3748/wjg.v9.i12.2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb WF, FW Q, editors. The Clinical Chemistry of Laboratory Animals. 2nd ed. Taylor & Francis Philadelphia; 1999. [Google Scholar]
- Ozaki Y. Antiinflammatory effect of tetramethylpyrazine and ferulic acid. Chemical & Pharmaceutical Bulletin. 1992;40:954–956. doi: 10.1248/cpb.40.954. [DOI] [PubMed] [Google Scholar]
- Peng HR. Grand Dictionary of Chinese medicinal formula (Zhong Yi Fang Ji Da Ci Dian) Beijing: People's Health Press; 1998. [Google Scholar]
- Safayhi H, Mack T, Sabieraj J, Anazodo MI, Subramanian LR, Ammon HP. Boswellic acids: novel, specific, nonredox inhibitors of 5-lipoxygenase. Journal of Pharmacology & Experimental Therapeutics. 1992;261:1143–1146. [PubMed] [Google Scholar]
- Scheiman JM. The impact of nonsteroidal anti-inflammatory drug-induced gastropathy. American Journal of Managed Care. 2001;7:S10–S14. [PubMed] [Google Scholar]
- Sharma ML, Bani S, Singh GB. Anti-arthritic activity of boswellic acids in bovine serum albumin (BSA)-induced arthritis. International Journal of Immunopharmacology. 1989;11:647–652. doi: 10.1016/0192-0561(89)90150-1. [DOI] [PubMed] [Google Scholar]
- Smith-Oliver T, Noel LS, Stimpson SS, Yarnall DP, Connolly KM. Elevated levels of TNF in the joints of adjuvant arthritic rats. Cytokine. 1993;5:298–304. doi: 10.1016/1043-4666(93)90060-i. [DOI] [PubMed] [Google Scholar]
- Sun SM. 682. Thousand Ducat Prescriptions (Qian Jing Fan) [Google Scholar]
- Syrovets T, Buchele B, Krauss C, Laumonnier Y, Simmet T. Acetyl-boswellic acids inhibit lipopolysaccharide-mediated TNF-alpha induction in monocytes by direct interaction with IkappaB kinases. Journal of Immunology. 2005;174:498–506. doi: 10.4049/jimmunol.174.1.498. [DOI] [PubMed] [Google Scholar]
- Taurog JD, Argentieri DC, McReynolds RA. Adjuvant arthritis. Methods in Enzymology. 1988;162:339–355. doi: 10.1016/0076-6879(88)62089-1. [DOI] [PubMed] [Google Scholar]
- The Pharmacopoeia Commission of PRC. Beijing: Chemical Industry Press; Pharmacopoeia of the People's Republic of China. 2000
- Wang YC, Ji SY, Wang R. The clinical application of Huo Luo Xiao Ling Dan. Acta Tradit Chin Med. 2002;30:53. [Google Scholar]
- Wei YH, Li Y, Qiang CJ. Effects and mechanisms of FR167653, a dual inhibitor of interleukin-1 and tumor necrosis factor, on adjuvant arthritis in rats. International Immunopharmacology. 2004;4:1625–1632. doi: 10.1016/j.intimp.2004.07.022. [DOI] [PubMed] [Google Scholar]
- White House Commission on Complementary and Alternative Medicine. White House Commission on Complementary and Alternative Medicine Policy. 2002 http://www.whccamp.hhs.gov/finalreport.html.
- Zhang XC. Yi Xue Zhong Zhong Can Xi Lu/Records of Heart-felt Experiences in Medicine with the Reference to the West. Shijiazhuang: Hebei people's Publisher; 1974