Abstract
Theories of Attention-Deficit/Hyperactivity Disorder (ADHD) implicate dysfunctional regulation mechanisms that have been conceptually grouped into “top-down” control and “bottom-up” affective/reactive processes. This dual-process account can be invoked in relation to temperament or personality traits and may clarify how traits relate to ADHD. Two samples were examined to illuminate developmental effects. The younger sample was 179 youngsters aged 7 to 12 years (113 boys; 107 with ADHD). The older sample was 184 adolescents (109 boys; 87 with ADHD) aged 13 to 18 years. Structural equation models included parent-rated traits, teacher-rated ADHD symptoms, and laboratory-obtained executive functions. A control or “top-down” factor included cognitive control and conscientiousness/effortful control. A second factor labeled affective or “bottom-up” included neuroticism/negative emotionality, agreeableness, and reactive control. In the younger sample, these two factors were differentially and specifically related to inattention and hyperactivity, respectively. However, in the older sample, the first factor was related to inattention and hyperactivity, whereas the second factor was related to hyperactivity. Personality traits appear to map onto ADHD symptoms in a meaningful manner consistent with a dual-process model of temperament and ADHD.
Keywords: Temperament, Personality, Executive function, Attention-deficit/hyperactivity disorder
Temperament provides an important avenue for understanding ADHD, but attention to this matter has been more in the realm of theory than empirical data, with some exceptions we note later. ADHD’s trait-like features make it particularly well-suited to examination via a temperament or personality perspective (White 1999). ADHD as a syndrome is characterized by two domains of behavioral problems used to create subtypes. The first reflects six or more behavioral symptoms of inattention-disorganization (e.g., often fails to give close attention to details or makes careless mistakes in schoolwork, work, or other activities; ADHD, predominantly inattentive type [ADHD-PI]). The second reflects six or more symptoms of hyperactivity-impulsivity (e.g., often fidgets with hands or feet or squirms in seat; often has difficulty awaiting turn; ADHD, predominantly hyperactive-impulsive type or combined type [ADHD-PHI; ADHD-C]; APA 2000). These features often persist into adulthood (Biederman et al. 1996; Kessler et al. 2005), although many individuals do not meet full criteria as adults and developmental changes in symptoms have been noted (Hart et al. 1995). A further examination of psychological mechanisms associated with these two symptom domains from the perspective of temperament is of interest for several reasons.
Prior, correlational work in children and adults with ADHD suggests that they are often characterized by low conscientiousness or low effortful control, as well as low reactive control, high extraversion, low agreeableness, high neuroticism, and high negative emotionality (Martel and Nigg 2006; Nigg et al. 2002; White 1999). Examination of the correlation tables in prior studies suggests that these traits may be somewhat differentially related to the ADHD symptom domains of inattention versus hyperactivity-impulsivity. This possibility warrants more formal examination in light of theoretical developments. For many years, theories about mechanisms involved in ADHD emphasized regulation broadly-defined, with a focus on laboratory measures of executive functioning, cognitive control, and their neural underpinnings (e.g., Barkley 1997; Pennington and Ozonoff 1996). More recently, theorists have emphasized the non-unitary nature of ADHD and of regulatory control (Nigg et al. 2004; Nigg 2006; Sonuga-Barke 2005). Following up on his earlier theory papers, Sonuga-Barke (2005) suggested that inattentive symptoms are related to a meso-cortical dopamine system and deficits in effortful control such as executive function, whereas hyperactivity-impulsivity is related to a meso-limbic dopamine system and deficits in motivational or incentive-based control. At the level of temperament traits, Nigg (2006) and Martel and Nigg (2006) suggested that inattention was related to the trait of effortful control and hyperactivity-impulsivity to reactive traits such as reactive control. These two control processes, or dual pathways, may shape ADHD, tending to influence one aspect of the disorder more than the other (Sonuga-Barke 2005).
It is increasingly clear that regulation of behavior entails the confluence of two fundamental kinds of processes. We term these, for heuristic purposes, “top-down” and “bottom-up” (Holroyd and Coles 2002; Nigg and Casey 2005). For this discussion, top-down processes are related to more effortful forms of control. Behaviorally, this kind of control refers to behavior that is goal-directed, resource-demanding, and planful, or when there is a need to overcome immediate stimuli in order to maintain progress toward a goal held in working memory. Neurally, top-down processes are thought to rely primarily on prefrontal circuitry, particularly in its role of suppressing task irrelevant activation elsewhere in the brain (Casey 2005; Nigg and Casey 2005). Bottom-up processes are thought to be related to behavior that does not demand conscious mental resources and which are more heavily influenced by immediate incentive or affective response. This kind of processing is believed to rely on stimulus-driven activation either in parietal cortex (in the case of attentional capture; Corbetta and Shulman 2002) or subcortically (e.g., in striatum or limbic regions; Eisenberg et al. 1996), particularly in its role of interrupting motor or cognitive processing being implemented in frontal cortex, redirecting attention to an immediate salient event (Casey 2005; Holroyd and Coles 2002; Nigg and Casey 2005).
We suggest that these two basic kinds of processes may be used to provide a meta-conceptual framework for temperament and personality (for the moment we do not distinguish those two trait domains) that in turn can be related to a dual process model of ADHD, and thus to provide part of a nomological network validating such theoretical proposals. To constrain the discussion with regard to temperament and personality, we draw upon two trait models.
Of these two models, the first, associated with work of Eisenberg et al (1996), suggests four major temperament traits: reactive control (i.e., reflexive, automatic forms of control), effortful control, resiliency (i.e., flexibility of control in response to context demands), and negative emotionality. The second model is the well-known Big Five (McCrae and Costa 1999), which suggests that the major personality traits are neuroticism, extraversion, openness, agreeableness, and conscientiousness. In line with most recent reviewers (e.g., Rothbart and Posner 2006) we suggest that these models are likely tapping overlapping trait domains in somewhat different fashions. It has often been suggested in the literature that, at the behavioral level of analysis, two or three super-ordinate trait factors can be broken out, at different levels of abstraction, from the four or five major traits (e.g., Block and Block 2006; Eysenck 1981; Markon et al. 2005; Watson et al. 1994; Watson et al. 2006). Thus, seeking a two-factor organization of these four and five trait models is conceptually feasible.
A viable two-factor organization of these four and five traits models might consist of a distinction between top-down and bottom-up processes. Traits that reflect top-down or resource-demanding processing include effortful control, conscientiousness, and, perhaps, resiliency. Executive function and cognitive control laboratory tasks are also related to top-down control processes in our conception (Eisenberg et al. 1996; John 1990; Martel and Nigg 2006; Nigg 2000; Rothbart and Posner 2006). The remaining traits are viewed as reflecting rather more bottom-up processing that does not require mental resources (although it can interrupt current thoughts). These include reactive control, negative emotionality, neuroticism, agreeableness, and extraversion. Each of these can be viewed as related to immediate incentive response (albeit of distinct valences, such as approach or avoidance) and propensity to related affective states (such as excitement, positive mood, anxiety, negative mood).
Consistent with this perspective, neuroimaging research suggests that neuroticism is related to amygdala activation and extraversion to nucleus accumbens activation (Depue and Lenzenweger 2006; Horvitz 2002; Omura et al. 2005), both reflecting variants of subcortical response presumed to activate cortical response. Furthermore, children with ADHD exhibit group abnormalities in the frontal-striatal neural circuitry believed to underpin top-down control (Durston 2003; Pliszka et al. 2006; Seidman et al. 2005) and in the basal ganglia regions believed to underpin bottom-up processing (Dickstein et al. 2006; Krain and Castellanos 2006; Wiersema et al. 2006).
Top-down and bottom-up processes may be differentially related to the ADHD symptom domains of inattention and hyperactivity-impulsivity. It might be argued that top-down control, including traits such as conscientiousness and effortful control, as well as experimental measures of executive function or top-down cognitive control, would be more strongly related to symptoms of inattention-disorganization. In turn, traits hypothesized to reflect relatively more bottom-up processing, including negative and positive emotionality and hostility, would be more strongly related to hyperactivity-impulsivity. However, note that based on the measures available in the current study (i.e., measures largely related to negative affect), we created a two-factor model that omitted what is usually seen as the third superfactor in three-factor conceptions of personality, namely approach/extraversion. Instead, we simply conjectured that affective traits like neuroticism and agreeableness would load together in a two-factor structure (the bottom-up factor), whereas executive function and more effortful traits would load together on the other factor (the top-down factor). Further, we expected that the top-down factor would be specifically related to inattentive ADHD symptoms, whereas the bottom-up factor would be specifically related to hyperactive-impulsive ADHD symptoms.
This top-down and bottom-up framework has many appealing features, but it faces at least one significant problem: none of the behavioral or neural systems under discussion here are developmentally static during childhood and adolescence. Just as ADHD has some stability but also some characteristic patterns of change in development, so the underlying top-down and bottom-up processes may be susceptible to developmental change. This is particularly, but not exclusively, important for top-down control. The prefrontal neural circuitry underpinning top-down control exhibits dramatic development during adolescence (Giedd et al. 2006), potentially moderating the impact of other cognitive and motivational systems (Halperin and Schultz 2006). Limbic circuitry also undergoes rapid developmental change during adolescence (Casey et al. in press). We therefore considered the possibility of developmental change via the examination of these models separately in younger and older groups of children.
Method
Participants
Overview
The current study examined two samples: a young sample (referred to as the “child” sample) and a slightly older group (referred to as the “adolescent” sample) for purposes of replication and to enable some comment on developmental continuity of effects. The samples were not combined into a single sample because there were some differences in how they were assessed and in the measures available.
The child sample participants were 179 children (113 boys, 66 girls) age six through 12 years. Twenty-eight percent identified themselves as ethnic minorities. Children were initially included in one of two groups: those diagnosed with ADHD (n=107) and controls (n=72). The ADHD group included 24 ADHD-PI (i.e., met criteria for 6 or more inattentive symptoms) and 68 ADHD-C (i.e., met criteria for 6 or more inattentive symptoms and 6 or more hyperactive-impulsive symptoms). Fifteen children had five symptoms of ADHD, falling just slightly below diagnostic threshold, and were included in regression models in view of evidence that ADHD represents an arbitrary cut point on a continuous dimension of behavior (a trait), which favors the statistically more powerful dimensional approach over the categorical approach (Levy et al. 1997; Sherman et al. 1997). Fifty-six children met criteria for Oppositional-Defiant Disorder (ODD); 19 for Conduct Disorder (CD).
Adolescent sample participants were 184 youth (109 boys, 75 girls) age 13 to 18 years. Twenty-two percent identified themselves as ethnic minorities. Adolescents were initially included in one of two groups: those diagnosed with ADHD, any type (n=87), and controls (n= 97). The ADHD group included 48 ADHD-PI and 39 ADHD-C. Twenty-two adolescents met criteria for ODD; four for CD.
Recruitment and Identification
A broad community recruitment strategy was used, with mass mailings to parents in local school districts and public advertisements, in order to obtain as broadly representative of a sample as possible. Families initially recruited then passed through a standard multi-gate screening process to establish diagnostic groupings. At Stage 1, all families (N=969 for children and N= 672 for adolescents) were screened by phone to rule out youth prescribed long-acting psychotropic medication (e.g. antidepressants), neurological impairments, seizure history, head injury with loss of consciousness, other major medical conditions, or a prior diagnosis of mental retardation or autistic disorder, as reported by the parent.
At Stage 2, parents and teachers or remaining eligible youth (N=301 for children and N=469 for adolescents) completed rating scales (Child Behavior Checklist [CBCL; Achenbach 1991], Conners Parent and Teacher Rating Scales [Conners 1997], ADHD Rating Scale [DuPaul et al. 1998]). Parents completed a structured clinical interview, and children completed IQ and achievement testing. Families were screened out here if they failed to attend the diagnostic visit or if teacher ratings could not be obtained.
In the child sample, eligible primary caretakers (N=218) completed the Diagnostic Interview Schedule for Children (DISC-IV; Shaffer et al. 2000) by telephone or during on-campus visits. The DISC-IV, a computer-guided, structured interview designed for use by non-clinicians, assesses symptoms as well as onset, duration, and impairment criterion for disorders in the DSM-IV. A trained interviewer (a graduate student or advanced undergraduate with at least 10 hours of training) administered the DISC-IV. The quality of the interviews was calibrated by having the interview recorded with five percent reviewed by a certified trainer. A four-subtest short form of the WISC-III (data collection was begun prior to publication of the WISC-IV) was administered; estimated full scale IQ over 75 was required for inclusion.
For the adolescent sample, youth and their primary caregiver completed the KSADS-E (Puig-Antich and Ryan 1986), rather than the DISC-IV, due to changes in study procedures and funding when the adolescent sample was recruited. The data from the interviews and parent and teacher rating scales were then presented to a clinical diagnostic team consisting of a board certified child psychiatrist and licensed clinical child psychologist. Their agreement rates were acceptable for ADHD diagnosis, subtypes, and current ODD and CD (all kappas≥0.89).
All youth came back for a second laboratory visit a few weeks later during which time parents completed temperament measures, and youth completed neuropsychological measures.
Final ADHD Classification and Data Combination
Final ADHD classification procedures were similar for children and adolescents. For children, the DISC-IV supplement with an “or” algorithm was used to confirm the diagnosis. If children met criteria regarding age of onset, duration, impairment, and cross-situational manifestation, the diagnostic assignment was determined by adding the endorsed symptoms of the DISC-IV with the teacher-reported symptoms on the ADHD-RI (that is, items rated as a “2” or “3” on the 0–3 scale) to get the total number of symptoms (Lahey et al. 1994). Children failing to meet cutoffs for all parent and teacher ADHD rating scales at the 80th percentile and having 4 or fewer symptoms of ADHD with the “or” algorithm were considered controls (to avoid a “supernormal” comparison group). For adolescents, a “best estimate” diagnostic process was implemented, in which the psychiatrist and psychologist independently arrive at a clinical decision regarding ADHD diagnosis, subtype, and presence of comorbid disorders. To do so, they employed the “or” algorithm across parent structured interview and teacher ratings to count symptoms and reviewed evidence of onset, duration, impairment, and cross-situational manifestation in the interview and rating data.
Comorbid Child Diagnoses
The DISC-IV or KSADS-E interview, respectively, was used for establishing the presence of child and adolescent ODD, CD, anxiety disorders, and depressive disorders using DSM-IV criteria in the two samples.
Measures
Temperament and Personality Traits—California Q-Sort
To assess traits from the two models using the same methods, as many traits as possible were assessed with a parent-completed California Child Q-Sort (CCQ), specifically the common language version (Caspi et al. 1992). The CCQ is a typical Q-Sort consisting of 100 cards which must be placed in a forced-choice, nine-category, rectangular distribution. The rater (in this case, the mother) describes the child by placing descriptive cards in one of the categories, ranging from one (least descriptive) to nine (most descriptive). Thus, items at the extreme ends of this range are most descriptive. Instructions were derived from the standard instruction set provided by Jack Block (personal communication, 1996). To measure reactive control, resiliency, and negative emotionality, scales developed by Eisenberg et al (1996; 2003; personal communication, 2006) were used (e.g., reactive control, “is shy and reserved;” resiliency, “is resourceful in initiating activities;” negative emotionality, “cries easily”). To measure the Big Five, scales developed by John and colleagues (1994) were used (e.g., neuroticism, “tends to brood and ruminate;” agreeableness, “is eager to please;” conscientiousness, “is competent, skillful”). Items from these scales were averaged after reverse-scoring selected items.
Handling of Item Overlap
Because scale scores for the personality and temperament dimensions were constructed by independent groups from the same broad pool of items, there was potential for considerable item overlap across scales. In order to avoid artificially inflated estimates of construct covariance influencing latent factor loadings, item overlap was eliminated using a procedure in which the item with the highest alpha-of-item-deleted index was removed first. This procedure was continued in a sequential fashion, starting with the longest scale first. After this procedure, alpha reliability for all scales remained acceptable (lowest α≥0.67). Correlations between non-overlapping and overlapping trait scales were all 0.93 or above (p<0.01), supporting the validity of this procedure. However, due to the fact that the Big Five trait of openness has been poorly validated in models of child temperament and personality (Caspi et al. 2005; John et al. 1994), it was excluded from analyses. Finally, a decision was made to exclude extraversion from analyses because it was hypothesized to be related to an approach factor that had no other measurable indicators in the current study.
Laboratory Measures of Top-Down Control
Two variables were selected for this study because they were available on all of the youth in both samples. Response inhibition, that is, the suppression of a prepotent motor response, was measured with the Stopping Task (Logan 1994; Nigg 2000). This task assesses the ability to rapidly interrupt a planned response. This function entails activation of areas in the prefrontal cortex, particularly the right inferior frontal gyrus (Aron et al. 2003) and associated regions in the striatum, particularly the caudate (Casey et al. 1997). During this two-alternative choice reaction time task with four blocks of 64 trials, participants see an X or an O on a computer screen and respond rapidly with one of two keys. On 25 percent of trials, a tone sounds shortly after the X or O is displayed, indicating that participants are to withhold their response. A stochastic tracking methodology provides reliable estimates of stop signal reaction time (Band et al. 2003). A quantitative model of RT processes enables calculation of each participant’s speed of stopping or inhibiting a response (the stop signal RT or SSRT) by subtracting average stop signal delay from average RT (Logan 1994).
In addition, participants completed the Trail-making B task which served as an additional index of cognitive control. It taps set-shifting ability (Spreen and Strauss 1991). In the Trail-making B task, participants are asked to draw a line between alternating numbers and letters in numerical and alphabetical order as quickly as possible (e.g., 1-A-2-B-3-C…25). Time to completion was the outcome variable used.
Dependent variables—Clinical Symptom Counts
For SEM analyses testing the main dual-path hypothesis, it was necessary to have a quantitative measure of the two ADHD symptom domains. To this end, teacher ratings on the cognitive problems (i.e., inattention) and hyperactivity subscales of the Conners Rating Forms (1997) were used to avoid source variance confounding with parent-reported personality traits. Note that the Conners scale called “cognitive problems” is identical to the DSM-IV symptoms of “inattention-disorganization.” For simplicity, we refer to this scale as “inattention” from here on.
Data Reduction
No data were missing in the adolescent sample. However, in the child sample, two percent of Q-Sort data were missing. Missingness on each variable was uncorrelated with scores on the available data for all the other variables (average r=−0.02, only 1 out of 48 significant at p<0.05, consistent with what would be expected by chance). Therefore, missing data were imputed using the expectation maximization (EM) algorithm, which is one form of maximum likelihood estimation (Shafer and Graham 2002). The mean change in correlations between the trait variables and ADHD symptoms due to imputation was less than r=0.01.
Data Analysis
Within the child and adolescent samples, the ADHD and non-ADHD comparison groups were combined in order to assess a full range of traits, cognitive control, and ADHD symptoms. With regard to testing the primary hypotheses, two structural equation models (SEMs) were conducted in LISREL: one for children and one for adolescents. These SEMs were conducted in order to examine indicators of the two hypothesized latent factors described earlier. These latent factors were then used to predict manifest variables of teacher-rated inattention problems and hyperactivity. Following the dual process model, it was hypothesized that the “top-down” factor would be specifically related to inattention problems, while the “bottom-up” factor would be specifically related to hyperactivity-impulsivity.
The two latent factors were allowed to correlate because the conceptual neural systems at issue are closely interrelated and act in concert to influence behavior. Correlated error terms were allowed among measures obtained from the same instrument. Thus, the error terms of trait scales obtained from the Q-Sort were allowed to correlate, as were ratings from the Conners’ Teacher Rating Form. Models were estimated separately for children and adolescents in order to evaluate replication of the findings (a common concern with SEMs), and to provide a view of developmental change in effects.
In evaluating adequacy of model fit, several indices were examined. The chi-square statistic is reported, with smaller chi-square values indicating better fit. In addition, the root mean square error of approximation (RMSEA) is reported with values at or below 0.05 indicating close approximate fit, and an RMSEA of 0.08 or below indicating reasonable fit (Kline 2005). The comparative fit index (CFI), normed fit index (NFI), and goodness-of-fit index (GFI) are also reported. Values closer to 1.0 indicate better fit for all of these indices (Kline 2005), with values above 0.90 generally considered to represent satisfactory fit. In order to compare fit between non-nested models while controlling for differences in the number of parameters specified (one with more parameters otherwise would artifactually fit “better” in that it explained more variance than one with fewer parameters), the Akaike information criterion (AIC) was used, with lower values indicating better model fit (Kline 2005).
Results
Preliminary Examination of the Data
Descriptive statistics on the two samples can be seen in Tables 1 and 2 respectively. As shown, both samples showed differences between ADHD and non-ADHD youth in all personality traits, on stop signal reaction time (but not Trail-making time) and, as expected, on Conners symptom scales (p<0.01). These data confirm and extend prior reports that ADHD youth are characterized by differences on common measures of temperament and/or personality versus non-ADHD youth. Most between-group temperament/personality effects still held when ODD and CD were used as covariates. The only exception was that, in the adolescent sample, there was no significant between-group difference in Agreeableness (p>0.05) when ODD and CD were used as covariates.
Table 1.
Descriptive Statistics for ADHD, Control, and Total Groups of Children
| ADHD (n=92) | Control (n=87) | Total (n=179) | |
|---|---|---|---|
| M(SD) | M(SD) | M(SD) | |
| N (%) Males | 78 (65.5) | 35 (58.3) | 113 (63.1) |
| N (%) Ethnic Minority | 34 (28.5) | 17 (28.3) | 51 (28.4) |
| Child age in years | 9.50 (1.14) | 9.44 (1.23) | 9.47 (1.18) |
| SES | 35.57 (14.41) | 37.90 (15.46) | 36.73 (14.94) |
| Neuroticism | 4.55 (1.09) | 3.68 (0.93) | 4.26 (1.11)** |
| Agreeableness | 5.65 (1.24) | 6.60 (1.06) | 6.11 (1.25)** |
| Conscientiousness | 3.95 (0.95) | 5.51 (1.52) | 4.71 (1.48)** |
| Reactive Control | 3.94 (1.03) | 4.77 (0.97) | 4.34 (1.08)** |
| Resiliency | 5.62 (0.89) | 6.36 (0.83) | 5.73 (1.02)** |
| Negative Emotionality | 4.73 (1.23) | 3.43 (0.90) | 4.30 (1.28)** |
| Response Inhibition (SSRT) | 426 (160.98) | 358.18 (134.24) | 392.29 (151.71)** |
| Set-Shifting (Trails B Time) | 68.59 (63.51) | 58.02 (54.30) | 63.52 (59.33) |
| Teacher-rated Cognitive Problems (T-score) | 59.42 (10.33) | 49.92 (8.9) | 54.89 (10.76)** |
| Teacher-rated Hyperactivity (T-score) | 63.14 (11.5) | 51.07 (9.52) | 57.32 (12.17)** |
Significant differences between ADHD and control groups, measured by t-tests and chi-squares, indicated under total column. “Cognitive problems” is the label on the Conners Scale for DSM-IV ADHD symptoms of inattention.
p<0.05;
p<0.01.
Table 2.
Descriptive Statistics for ADHD, Control, and Total Groups of Adolescents
| ADHD (n=87) | Control (n=97) | Total (n=184) | |
|---|---|---|---|
| M (SD) | M (SD) | M (SD) | |
| N (%) Males | 57 (65.5) | 52 (53.6) | 109 (59.2) |
| N (%) Ethnic Minority | 18 (20.5) | 22 (22.8) | 40 (21.6) |
| Child age in years | 15.28 (1.13) | 15.58 (1.07) | 15.44 (1.11) |
| Neuroticism | 4.47 (1.13) | 3.53 (0.87) | 3.97 (1.11)* |
| Agreeableness | 6.13 (1.27) | 6.64 (1.01) | 6.40 (1.16)* |
| Conscientiousness | 4.18 (1.25) | 6.54 (1.11) | 5.43 (1.67)* |
| Reactive Control | 4.54 (1.16) | 5.30 (0.96) | 4.95 (1.13)* |
| Resiliency | 5.31 (0.97) | 6.37 (0.69) | 5.87 (0.99)* |
| Negative Emotionality | 4.12 (1.17) | 3.22 (0.81) | 3.65 (1.09)* |
| Response Inhibition (SSRT) | 319.63 (120.7) | 252.94 (71.23) | 283.81 (102.58)* |
| Set-Shifting (Trails B Time) | 61.69 (17.6) | 55.6 (27.32) | 58.47 (23.38) |
| Teacher-rated Cognitive Problems (T-score) | 60.99 (11.75) | 48.59 (7.62) | 54.43 (11.56)* |
| Teacher-rated Hyperactivity (T-score) | 63.46 (16.64) | 49.53 (9.28) | 56.1 (14.95)* |
Significant differences between ADHD and control groups, measured by t-tests and chi-squares, indicated under total column. “Cognitive problems” is the label on the Conners Scale for DSM-IV ADHD symptoms of inattention-disorganization.
p<0.01.
In preparation for the SEM analyses, correlation matrices for traits, cognitive control measures, and problem behavior, calculated separately for children and adolescents, were examined; these are shown in Tables 3 and 4 respectively. Note that, although correlation matrices are shown for ease of interpretation, SEM computations were based on the covariance matrices, not shown.
Table 3.
Correlation Matrix of Traits, Cognitive Control, Inattention, and Hyperactivity in Children
| Inatt | Hyper | Cons | Resl | SSRT | TrailsB | RxCont | Neur | NegEm | Agree | |
|---|---|---|---|---|---|---|---|---|---|---|
| Inatt | 1 | |||||||||
| Hyper | 0.42 | 1 | ||||||||
| Cons | −0.45 | −0.47 | 1 | |||||||
| Resl | −0.42 | −0.23 | 0.61 | 1 | ||||||
| SSRT | 0.24 | 0.22 | −0.28 | −0.14 | 1 | |||||
| TrailsB | 0.22 | −0.00 | −0.11 | −0.12 | 0.06 | 1 | ||||
| RxCont | −0.17 | −0.44 | 0.54 | 0.24 | −0.18 | −0.06 | 1 | |||
| Neur | 0.29 | 0.23 | −0.47 | −0.58 | 0.04 | 0.11 | −0.07 | 1 | ||
| NegEm | 0.28 | 0.38 | −0.62 | −0.56 | 0.17 | 0.02 | −0.41 | 0.47 | 1 | |
| Agree | −0.26 | −0.39 | 0.51 | 0.41 | −0.06 | −0.12 | 0.51 | −0.25 | −0.73 | 1 |
Inatt teacher-rated symptoms of inattention, Hyper teacher-rated hyperactivity, Cons conscientiousness, Resl resiliency, SSRT Stop Signal reaction time; response inhibition, TrailB time on Trails B; set-shifting, RxCont reactive control, Neur neuroticism, NegEm negative emotionality, Agree agreeableness.
Table 4.
Correlation Matrix of Traits, Cognitive Control, Inattention, and Hyperactivity in Adolescents
| Inatt | Hyper | Cons | Resl | SSRT | TrailsB | RxCont | Neur | NegEm | Agree | |
|---|---|---|---|---|---|---|---|---|---|---|
| Inatt | 1 | |||||||||
| Hyper | 0.53 | 1 | ||||||||
| Cons | −0.49 | −0.45 | 1 | |||||||
| Resl | −0.42 | −0.34 | 0.61 | 1 | ||||||
| SSRT | 0.20 | 0.15 | −0.41 | −0.21 | 1 | |||||
| TrailB | 0.25 | 0.09 | −0.16 | −0.13 | 0.29 | 1 | ||||
| RxCont | −0.18 | −0.29 | 0.48 | 0.17 | −0.13 | 0.04 | 1 | |||
| Neur | 0.31 | 0.31 | −0.35 | −0.54 | 0.11 | 0.06 | 0.14 | 1 | ||
| NegEm | 0.33 | 0.41 | −0.49 | −0.48 | 0.22 | 0.04 | −0.33 | 0.45 | 1 | |
| Agree | −0.17 | −0.26 | 0.44 | 0.35 | −0.29 | −0.08 | 0.40 | −0.13 | −0.56 | 1 |
Inatt teacher-rated inattention, Hyper teacher-rated hyperactivity, Cons conscientiousness, Resl resiliency, SSRT Stop Signal reaction time; response inhibition, TrailB time on Trails B; set-shifting, RxCont reactive control, Neur neuroticism, NegEm negative emotionality, Agree agreeableness.
Measurement Models
In order to test the validity of a distinction of a two-factor versus a one-factor model (necessary for our basic two-process story to be supported), preliminary comparisons were conducted. A one-factor model was tested and its fit index compared to the hypothesized two-factor model within each sample. Within the child sample, a one-factor model (comprised of all traits and task measures) had worse fit (AIC=124.63) than the hypothesized two-factor model (AIC=106.51). All other fit indices also indicated marginal fit, although this model was not nested (χ2[27]=68.26, p< 0.01; RSMEA=0.09; NFI=0.93; CFI=0.96; GFI=0.93; these indices can be compared to the two-factor model shown later). Within the adolescent sample, results were similar: the one-factor model (AIC=158.59) did not fit as well as the two-factor model (AIC=99.63). All other fit indices were also rather marginal (χ2[28]=104.59, p<0.01; RSMEA=0.12; NFI=0.89; CFI=0.92; GFI=0.90; compare to two-factor solution below). Thus, it was concluded that two-factor models fit better than one-factor models within both samples, supporting further scrutiny of the two-factor hypothesis.
Results of Structural Models
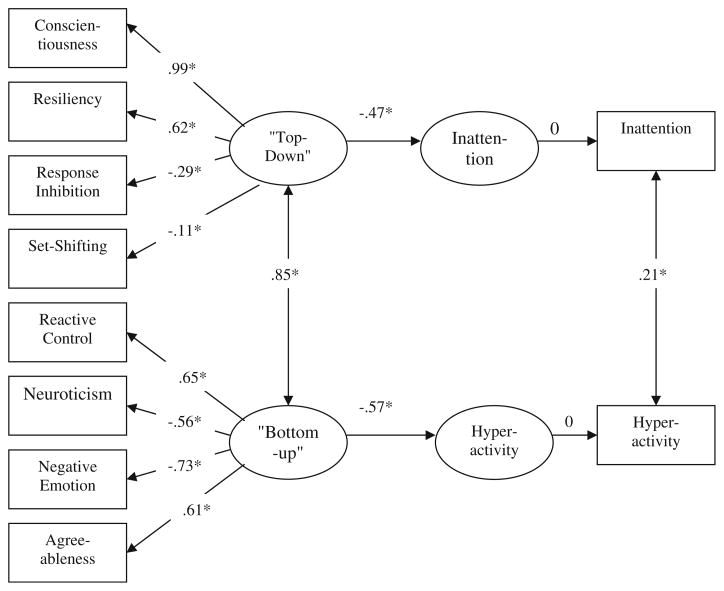
With regard to the two-factor model, then, the child model, shown in Fig. 1, obtained a reasonable fit to the data, according to our criteria (χ2[26]=48.51, p<0.01; RSMEA= 0.07; NFI=0.95; CFI=0.98; GFI=0.95; AIC=106.51). Two latent factors were fit and were labeled “top-down” and “bottom-up.” High levels of conscientiousness, resiliency, better response inhibition, and more efficient set-shifting were indicators of the first, or top-down control, factor. High levels of reactive control and agreeableness and low levels of neuroticism and negative emotionality were indicators of the second, or bottom-up affect, factor. Note that these factors were significantly correlated (r=0.85, p< 0.05), indicating that they shared about 72% of their reliable variance (reliability of the latent variable is perfect so, despite the 0.85 correlation, the factors are still partially distinct). However, as noted above, a one-factor model yielded a markedly worse fit than this two-factor model after adjusting for number of parameters allowed.
Fig. 1.
“Top-down” control and “Bottom-up” affect as predictors of inattention and hyperactivity in Children with ADHD and controls Note. χ2[26]=48.51, p<0.05. RMSEA=0.07. NFI=0.95. CFI=0.98. GFI=0.95. *p<0.05; **p<0.01
Also as shown in Fig. 1, the first factor was significantly, inversely related to teacher-rated inattention and the second factor was significantly, inversely related to hyperactivity-impulsivity in the expected direction. The first or “top-down” factor explained 22% of the variance in inattention, whereas the second or “bottom-up” factor explained 23% percent of the variance in hyperactivity. The crucial test of the dual process model concerned what would happen when the cross paths were freed. Doing so did not significantly improve the model (Δχ2[2]=1.77, p>0.05), supporting the specificity of relations shown in Fig. 1.
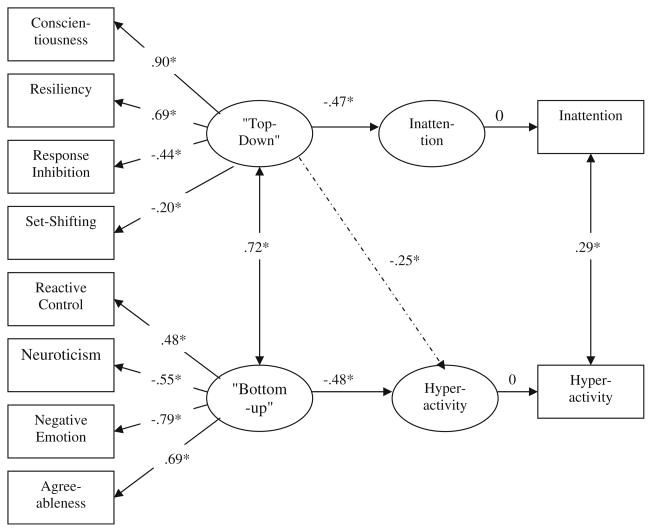
The same models were tested in the adolescent sample, with results shown in Fig. 2. As before, this model resulted in a reasonable fit to the data by our criteria (χ2[27]=43.63, p<0.05; RSMEA=0.058; NFI=0.95; CFI=0.98; GFI=0.95; AIC=99.63). The two latent factors labeled top-down control and bottom-up affect were replicated. Once again, these factors were substantially correlated (r=0.72, p< 0.05), indicating that in this second and older sample they shared about half of their reliable variance. The adolescent sample also replicated the finding that the first factor, which we called top-down control, was significantly, inversely related to teacher-rated inattention and the second, or bottom-up, factor was significantly, inversely related to hyperactivity-impulsivity.
Fig. 2.
“Top-down” control and “Bottom-up” affect as predictors of inattention and hyperactivity in Adolescents with ADHD and controls Note. χ2[27]=43.63, p<0.05. RMSEA=0.058. NFI= 0.95. CFI=0.98. GFI=0.95. *p<0.05; **p<0.01
With regard to the critical test using the cross paths, however, the adolescent sample failed to replicate the child sample with regard to the complete dissociation of these paths. In the adolescent sample, adding two paths, one between top-down control and hyperactivity and another between bottom-up affect and inattention, significantly improved the model (Δχ2[2]=8.89, p<0.05). This was due to the path between the first factor (top-down control) and hyperactivity, which was significant (standardized estimate=−0.25 based on correlation matrix). Thus, in adolescents, unlike in children, there was weaker specificity of latent traits to symptom dimensions.
Discussion
The current study examined temperament and personality traits drawn from two popular and widely cited models of traits, and fit them into a dual-process framework in order to elucidate their relations to ADHD symptom dimensions. Measures of executive control were used as external validators to distinguish the two trait constructs. In both children and adolescents, a two-factor model that we characterized as consisting of a superordinate latent factor for “top-down” control (i.e., high conscientiousness, resiliency, better response inhibition, and more efficient set-shifting) and another for “bottom-up” affect (i.e., high reactive control and agreeableness and low neuroticism and negative emotionality) fit the data well and markedly better than a one-factor model. Replication of that finding across two independent samples added some confidence in the higher-order two-process concept of how the traits might fit together in relation to ADHD.
Note that the idea of a two-factor higher-order structure to these major traits follows a long tradition in the literature. Several personality theories and the data they build upon have suggested that, at the highest order of abstraction, personality traits break into two or three factors (Block and Block 2006; Eysenck 1981; Markon et al. 2005; Watson et al. 1994, 2006). John (1990) suggested that the Block and Block traits of ego-resilience and ego-control were more abstract than the Big Five. Gramzow et al (2004) found that ego-control and ego-resilience were independent predictors of the Big Five. Of course, three factor models have also been suggested at a super-ordinate level (e.g., Eysenck 1981; Rothbart and Bates 1998; Watson et al. 1994). The present findings do not contradict such a supposition. That is because in the present instance, we did not attempt to model a positive affect or approach factor (extraversion), so the two-factor solution was viewed as conceptually satisfying, but was not assumed to cover the entire domain of higher order temperament or personality traits in this age range.
What was new in the current study was that we anchored the top-down factor with laboratory measures, and in turn linked these to a temperament model related to effortful and reactive processes. These findings confirm that temperament/personality traits of effortful processes and laboratory measures of cognitive control are tapping similar processes (see Nigg, 2000 for more discussion of this expectation) and may be able to be used in a bootstrapping manner to enable incremental prediction of developmental psychopathology. Secondly, these data help link temperament traits to ADHD with new clarity. Looking at the findings in the child sample (Fig. 1), they indicate specificity of relations between the first factor (top-down control) and inattention on the one hand, and between the second factor (bottom-up affect) and hyperactivity-impulsivity on the other hand. This finding supported the dual-process concept of ADHD that guided this work, at least in children. However, developmental modulation of the theory of ADHD may be warranted. Although the basic two-factor model fit the traits well in children and adolescents, their relations to ADHD were not as specific in adolescents as in children. In the adolescents, the first or top-down control factor predicted both less inattention and less hyperactivity, while bottom-up affect also predicted less hyperactivity. This suggests that a distinct two process model of ADHD is not so clear for adolescents.
Aside from the possibility of sampling differences or other artifact, this failure to replicate the specificity of associations in the adolescent sample is theoretically interesting from a developmental perspective. It may be that this finding reflects developmental change in the mechanisms related to ADHD symptoms from childhood to adolescence. That is, top-down neural circuitry (i.e., efficiency of prefrontal cortical projections to posterior and subcortical regions) dramatically develops during adolescence via continued pruning and myelination of those networks (Lenroot and Giedd 2006). As a result, top-down circuitry might have an increasingly important influence on descending neural circuitry during late adolescence (Halperin and Schultz 2006), allowing individuals to more efficiently control affect and affectively-driven behavior such as hyperactivity (Sowell et al. 2001).
These findings provide some support for recent work on dual pathway models of ADHD, at least in childhood. It also is consistent with the general model of different temperament routes to ADHD suggested by Nigg et al. (2004) in the earlier special section. Also in line with recent theory (e.g., Nigg 2006; Nigg and Casey 2005; Sonuga-Barke 2005), top-down control processes, believed to be underpinned by frontal-striatal circuits, appear to be more specifically related to inattention in children. In contrast, bottom-up affective processes, believed to be underpinned by fronto-amygdala circuits, appear to be more specifically related to hyperactivity in children. However, the support for this model was only partial. Top-down control and bottom-up affective processes appear to show some dissociation in childhood, even though they were highly correlated, and their influences on ADHD symptom dimensions were distinct. However, in adolescence, the two latent traits dissociated better, but their influences on specific symptom dimensions were non-specific.
Thus, the dual process model may require some revision. It is recognized that top-down and bottom-up processes are mutually influencing in early childhood (Rothbart and Bates 1998) and no doubt they are later as well. What remains to be worked out is how the evolution of cognitive control in adolescence intersects with changes in ADHD presentation (Halperin and Schultz 2006). Why does hyperactivity normatively decrease, but inattention become more salient as a problem, during adolescence? The present data of course cannot directly answer that question, but they suggest that the trait influences on ADHD symptoms are not entirely the same in adolescence as they are in childhood.
These data help to integrate temperament trait models (developed for children although, in this case, partially reliant on an earlier personality theory by Block and Block [1980]) and personality traits (derived from studies of adults) with one another and with cognitive control as assessed in the laboratory. Despite modest correlations between laboratory and trait measures (as is common), reliable latent factors were identified and replicated. These factors incorporated traits and laboratory measures of top-down control in the manner expected. As recent work on hierarchical models of personality suggest (DeYoung 2006; Markon et al. 2005), there appear to be underlying similarities between temperament and personality models, especially at higher levels of abstraction. In addition, cognitive control appears to share some similarities with these higher-order traits. In turn, those relations can be mapped onto relevant neural circuits (Rothbart and Bates 1998). These more precise, latent factors, captured by a rich array of trait and cognitive control indicators, may allow the more accurate depiction and prediction of child and adolescent outcomes than manifest single-factor trait measures or laboratory tasks.
Limitations of the current study should be noted. Although a child and adolescent sample were available, they were cross-sectional; thus, intra-individual development over time cannot be assessed. In addition, maternal report was used for all temperament and personality traits to enable a large sample to be obtained (although note that ADHD symptoms were based on teacher ratings to eliminate source variance as a confound). Future research should examine whether current results can be replicated using observational measures of temperament and personality. We also did not have laboratory probes of affective response, which might be an interesting direction for future work. Oppositional-defiant disorder symptoms and their relation to top-down control and bottom-up affective processes might also be further assessed in a sample in which these kinds of problems are more prevalent. However, their presence did not alter the results reported here. Overlapping items were removed from the trait scales, but this may have slightly altered their content.
In conclusion, the current study supported two major processes linking temperament and laboratory measures of regulation. In turn, these mapped onto a two-factor model of ADHD. Top-down control processes reflected in the temperament measures were related to inattentive ADHD symptoms, whereas bottom-up reactive/affective processes were related to hyperactive-impulsive ADHD symptoms in childhood but only partially so in adolescence. Thus, developmental changes in the temperament processes related to ADHD may be important to consider in future work.
Acknowledgments
This research was supported by NIH National Institute of Mental Health Grant R01-MH63146, MH59105, and MH70542 to Joel Nigg. Martel was supported by NIH F31 MH075533. We are indebted to the families and staff who made this study possible.
References
- Achenbach TM. Manual for the child behavior checklist/4–18 and 1991 profile. Burlington, VT: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, D. C: American Psychiatric Association; 2000. [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Band GP, van der Molen MW, Logan GD. Horse-race model simulations of the stop signal procedure. Acta Psychologica. 2003;112(2):105–142. doi: 10.1016/S0001-6918(02) 00079-3. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone S, Milberger S, Curtis S, Chen L, Ouellette C, et al. Predictors of persistence and remission of ADHD into adolescence: results from a four-year prospective follow-up study. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35(3):343–351. doi: 10.1097/00004583-199603000-00016. [DOI] [PubMed] [Google Scholar]
- Block JH, Block J. The role of ego-control and ego-resiliency in the organization of behavior. In: Collins WA, editor. Development of cognition, affect, and social relations: The Minnesota Symposia on Child Psychology. Vol. 13. Hillsdale, NJ: Lawrence Erlbaum; 1980. pp. 39–101. [Google Scholar]
- Block J, Block JH. Venturing a 30-year longitudinal study. The American Psychologist. 2006;61(4):315–327. doi: 10.1037/0003-066X.61.4.315. [DOI] [PubMed] [Google Scholar]
- Casey BJ. Frontostriatal and frontocerebellar circuitry underlying cognitive control. In: Mayr V, Awh E, Keele SW, editors. Developing individuality in the human brain: A tribute to Michael I. Posner. Washington, D.C: APA; 2005. [Google Scholar]
- Casey BJ, Jones R, Hare T. The adolescent brain. The year in cognitive neuroscience. Annals of the New York Academy of Sciences. 2008;11:84–94. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom Le, Gieddd JN, et al. A developmental functional MRI study of prefrontal activation during performance of the Go-No-Go task. Journal of Cognitive Neuroscience. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Caspi A, Block J, Block JH, Klopp B, Lynam D, Moffitt TE, et al. A “common-language” version of the California child Q-Set for personality assessment. Psychological Assessment. 1992;4(4):512–523. doi: 10.1037/1040-3590.4.4.512. [DOI] [Google Scholar]
- Caspi A, Roberts BW, Shiner RL. Personality development: stability and change. Annual Review of Psychology. 2005;56:453–584. doi: 10.1146/annurev.psych.55.090902.141913. [DOI] [PubMed] [Google Scholar]
- Conners CK. Conners rating scales—revised. Toronto: Multi-Health Systems, Inc; 1997. [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Depue RA, Lanzenweger MF. Toward a developmental psychopathology of personality disturbance: A neurobehavioral dimensional model. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology, vol. 2: Developmental neuroscience. Hoboken, New Jersey: Wiley; 2006. pp. 762–796. [Google Scholar]
- DeYoung CG. Higher-order factors of the big five in a multi-informant sample. Journal of Personality and Social Psychology. 2006;91(6):1138–1151. doi: 10.1037/0022-3514.91.6.1138. [DOI] [PubMed] [Google Scholar]
- Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2006;47(10):1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopolous AD, Reid R. ADHD Rating scale—IV: Checklists, norms, & clinical interpretation. New York: Guilford; 1998. [Google Scholar]
- Durston S. A review of the biological bases of ADHD: what have we learned from imaging studies? Mental Retardation and Developmental Disabilities Research Reviews. 2003;9:184–195. doi: 10.1002/mrdd.10079. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Guthrie IK, Murphy BC, Maszk P, Holmgren R, et al. The relations of regulation and emotionality to problem behavior in elementary school children. Development and Psychopathology. 1996;8:141–162. [PubMed] [Google Scholar]
- Eisenberg N, Qhou Q, Losoya SH, Fabes RA, Shepard SA, Murphy BC, et al. The relations of parenting, effortful control, and ego control to children’s emotional expressivity. Child Development. 2003;74(3):875–895. doi: 10.1111/1467-8624.00573. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ. General features of the model. In: Eysenck HJ, editor. A model for personality. New York: Springer-Verlag; 1981. pp. 1–37. [Google Scholar]
- Giedd JN, Shaw P, Wallace G, Gogtay N, Lenroot RK. Anatomic brain imaging studies of normal and abnormal brain development in children and adolescents. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology, vol. 2: Developmental neuroscience. Hoboken, NJ: Wiley; 2006. pp. 127–196. [Google Scholar]
- Gramzow RH, Sedikides C, Panter AT, Sathy V, Harris J, Insko CA. Patterns of self-regulation and the big five. European Journal of Personality. 2004;18:367–385. doi: 10.1002/per.513. [DOI] [Google Scholar]
- Halperin JM, Schultz KP. Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychological Bulletin. 2006;132(4):560–581. doi: 10.1037/0033-2909.132.4.560. [DOI] [PubMed] [Google Scholar]
- Hart EL, Lahey BB, Loeber R, Applegate B, Frick PJ. Developmental change in attention-deficit hyperactivity disorder in boys: a four-year longitudinal study. Journal of Abnormal Child Psychology. 1995;23(6):729–749. doi: 10.1007/BF01447474. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH. The neural basis of human error processing: Reinforcement learning, dopamine, and error-related negativity. Psychological Review. 2002;109(4):679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. Dopamine gating of glutamatergic sensorimotor and incentive motivational input signals to the striatum. Behavioural Brain Research. 2002;137(1–2):65–74. doi: 10.1016/S0166-4328(02)00285-1.. [DOI] [PubMed] [Google Scholar]
- John OP. The “Big Five” factor taxonomy: Dimensions of personality in the natural language and in questionnaires. In: Pervin LA, editor. Handbook of personality: Theory and research. New York: Guilford; 1990. pp. 66–100. [Google Scholar]
- John OP, Caspi A, Robins RW, Moffitt TE, Stouthamer-Loeber M. The little-five: exploring the nomological network of the five-factor model of personality in adolescent boys. Child Development. 1994;65:160–178. doi: 10.2307/1131373. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Adler LA, Barkley R, Biederman J, Conners CK, Faraone SV, et al. Patterns and predictors of attention-deficit/hyperactivity disorder persistence into adulthood: results from the National comorbidity survey replication. Biological Psychiatry. 2005;57:1442–1451. doi: 10.1016/j.biopsych.2005.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. 2. New York: Guilford; 2005. [Google Scholar]
- Krain AL, Castellanos FX. Brain development and ADHD. Clinical Psychology Review. 2006;26:433–444. doi: 10.1016/j.cpr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Applegate B, McBurnett K, Biederman J, Greenhill L, Hynd GW, et al. DSM-IV field trials for attention deficit hyperactivity disorder in children and adolescents. The American Journal of Psychiatry. 1994;151:1673–1685. doi: 10.1176/ajp.151.11.1673. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neuroscience and Biobehavioral Reviews. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Levy F, Hay DA, McStephen M, Wood CH, Waldman I. Attention-deficit/hyperactivity disorder: a category or a continuum? Genetic analysis of a large-scale twin study. American Academy of Child & Adolescent Psychiatry. 1997;36(6):737–744. doi: 10.1097/00004583-199706000-00009. [DOI] [PubMed] [Google Scholar]
- Logan GD. A users guide to the stop signal paradigm. In: Dagenbach D, Carr TH, editors. Inhibitory processes in attention, memory, and language. San Diego: Academic; 1994. pp. 189–239. [Google Scholar]
- Markon KE, Krueger RF, Watson D. Delineating the structure of normal nad abnormal personality: an integrative hierarchical approach. Journal of Personality and Social Psychology. 2005;88(1):139–157. doi: 10.1037/0022-3514.88.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MM, Nigg JT. Child ADHD and personality/temperament traits of reactive and effortful control, resiliency, and emotionality. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2006;47(11):1175–1183. doi: 10.1111/j.1469-7610.2006.01629.x. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PT. A five-factor model of personality. In: Pervin LA, John OP, editors. Handbook of personality: Theory and research. New York: Guilford; 1999. pp. 139–153. [Google Scholar]
- Nigg JT. On inhibition/disinhibition in developmental psychopathology: view from cognitive and personality psychology and a working inhibition taxonomy. Psychological Bulletin. 2000;126:220–246. doi: 10.1037/0033-2909.126.2.220. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Temperament and developmental psychopathology. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2006;47:395–422. doi: 10.1111/j.1469-7610.2006.01612.x. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Casey BJ. An integrative theory of attention-deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Development and Psychopathology. 2005;17(3):785–806. doi: 10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Goldsmith HH, Sachek J. Temperament and attention deficit hyperactivity disorder: the development of a multiple pathway model. Journal of Clinical Child and Adolescent Psychology. 2004;33:42–53. doi: 10.1207/S15374424JCCP3301_5. [DOI] [PubMed] [Google Scholar]
- Nigg JT, John OP, Blaskey LG, Huang-Pollock CL, Willcutt EG, Hinshaw SP, et al. Big five dimensions and ADHD symptoms: links between personality traits and clinical symptoms. Journal of Personality and Social Psychology. 2002;83(2):451–469. doi: 10.1037/0022-3514.83.2.451. [DOI] [PubMed] [Google Scholar]
- Omura K, Todd CR, Canli T. Amygdala gray matter concentration is associated with extraversion and neuroticism. Neuroreport. 2005;16(17):1905–1908. doi: 10.1097/01.wnr.0000186596.64458.76. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1996;37:51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Pliszka SR, Glahn DC, Semrud-Clikeman M, Franklin C, Perez R, Xiong J, et al. Neuroimaging of inhibitory control areas in children with attention deficit hyperactivity disorder who were treatment naïve or in long-term treatment. The American Journal of Psychiatry. 2006;163(6):1052–1060. doi: 10.1176/appi.ajp.163.6.1052. [DOI] [PubMed] [Google Scholar]
- Puig-Antich J, Ryan N. Kiddie schedule for affective disorders and schizophrenia. Pittsburgh, PA: Western Psychiatric Institute; 1986. [Google Scholar]
- Rothbart MK, Bates JE. Temperament. In: Damon W, Eisenberg N, editors. Handbook of child psychology: Social, emotional, and personality development. Vol. 3. New York: Wiley; 1998. pp. 105–176. [Google Scholar]
- Rothbart MK, Posner MI. Temperament, attention, and developmental psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology, vol. 2: Developmental neuroscience. Hoboken, New Jersey: Wiley; 2006. pp. 465–501. [Google Scholar]
- Seidman LJ, Valera EM, Makris N. Structural brain imaging of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57(11):1263–1272. doi: 10.1016/j.biopsych.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Shafer JL, Graham JW. Missing data: our view of the state of the art. Psychological Methods. 2002;7:147–177. doi: 10.1037/1082-989X.7.2.147. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas C, Dulcan MK, Schwab-Stone M. NIMH Diagnostic Interview Schedule for Children, Version IV (NIMH DISC-IV): description, differences from previous versions and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Sherman DK, Iancono WG, McGue MK. Attention-deficit hyperactivity disorder dimensions: a twin study of inattention and impulsivity-hyperactivity. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(6):745–753. doi: 10.1097/00004583-199706000-00010. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biological Psychiatry. 2005;57(11):1231–1238. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Delis D, Stiles J, Jernigan TL. Improved memory functioning and frontal lobe maturation between childhood and adolescence: a structural MRI study. Journal of the International Neuropsychological Society. 2001;7(3):312–322. doi: 10.1017/S135561770173305X. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. Compendium of neuropsychological tests: Administration, norms, and commentary. New York: US Oxford University Press; 1991. [Google Scholar]
- Watson D, Clark LA, Harkness AR. Structures of personality and their relevance to psychopathology. Journal of Abnormal Psychology. 1994;103(1):18–31. doi: 10.1037/0021-843X.103.1.18. [DOI] [PubMed] [Google Scholar]
- Watson D, Kotov R, Gamez W. Basic dimensions of temperament in relation to personality and psychopathology. In: Krueger RF, Tackett JL, editors. Personality and psychopathology. New York: Guildford; 2006. pp. 7–38. [Google Scholar]
- White JD. Personality, temperament, and ADHD: a review of the literature. Personality and individual differences. 1999;27:589–598. doi: 10.1016/S0191-8869(98)00273-6. [DOI] [Google Scholar]
- Wiersema R, van der Meere J, Roeyers H, Van Coster R, Baeyens D. Event rate and event-related potentials in ADHD. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2006;47(6):560–567. doi: 10.1111/j.1469-7610.2005.01592.x. [DOI] [PubMed] [Google Scholar]