Abstract
Background
Attention-deficit/hyperactivity disorder (ADHD) and low-level lead exposure are high-prevalence conditions among children, and studies of large populations have suggested that these conditions are related. We examine this relationship in children from a community sample exposed to average background levels of lead who have a diagnosis of ADHD that is established by clinical criteria.
Methods
One hundred fifty children ages 8–17 years participated (mean age = 14 years; 53 control subjects, 47 ADHD Predominantly Inattentive type, 50 ADHD-Combined type). Diagnosis was formally established with a semi-structured clinical interview and parent and teacher ratings. Children completed intelligence quotient (IQ) measures and the stop task (a neuropsychological measure). Lead was assayed from whole blood with inductively coupled plasma mass spectrometry.
Results
Blood lead levels in this sample closely matched US population exposure averages, with a maximum level of 3.4 μg/dL. Blood lead levels were statistically significantly higher in ADHD-combined type than in non-ADHD control (p < .05) children. Blood lead was associated with symptoms of hyperactivity-impulsivity but not inattention-disorganization, after control of covariates. Blood lead levels were linked with a lower IQ (p < .05), but IQ did not account for effects on hyperactivity. Instead, hyperactivity mediated effects of lead on IQ. Effects of blood lead on hyperactivity-impulsivity were mediated by poor performance on the stop task. This mediation effect was independent of effects of lead on IQ.
Conclusions
Low-level lead exposure might be an important contributor to ADHD. Its effects seem to be mediated by less effective cognitive control, consistent with a route of influence via striatal-frontal neural circuits.
Keywords: ADHD, blood lead, cognitive control, hyperactivity
Attention-deficit/hyperactivity disorder (ADHD) occurs in 3% to 7% of children, causing impairment due to extreme hyperactivity, impulsivity, and/or inattention (1,2). The syndrome frequently co-occurs with other disruptive behaviors, particularly conduct and oppositional defiant disorders (3), so their effects must be considered. The DSM-IV specifies three clinical subtypes: predominantly hyperactive (ADHD-PH), predominantly inattentive (ADHD-PI), and combined (ADHD-C). The degree of shared etiology between subtypes remains an important question (4). These subtypes are arrived at through combinations of two primary symptom dimensions: inattention-disorganization, and hyperactivity/impulsivity. Theorists suggest that symptoms of inattention-disorganization are related to breakdown in top-down cognitive control processes, such as executive functioning, effortful attention, and selection (5,6). These processes depend on integrity of prefrontal circuitry and its projections both subcortically to basal ganglia and cortically to posterior cortex (7). Symptoms of hyperactivity-impulsivity might be related to breakdowns in bottom-up aspects of cognitive control, including anticipation of rewards, interruption or suppression of response, and maintenance of response consistency (8). Bottom-up signaling is of particular interest because of its dependence on striatum and projections from striatum to prefrontal cortex (7) and because lead is thought to target the striatum (9–11).
Lead exposure via water, soil, and other sources remains a worldwide pediatric health concern (12). In the United States, regulation has reduced the incidence of lead poisoning to < 4%, but low-level exposure early in development remains common even in the United States (12). Blood lead levels from 1 to 10 μg/dL are associated with lower child intelligence quotient (IQ; 13), weaker executive cognitive abilities (14,15), behavioral symptoms of ADHD (16–18), and diagnosis of ADHD (19,20) in community surveys. Early studies (18–20) confirmed that blood lead was associated with clinical ADHD even after control for numerous confounders although generally at lead levels higher than typical today (i.e., 10–20 μg/dL vs. the current population average in US children of 1–2 μg/dL) (21). Lacking has been study of ADHD by independently and reliably verified DSM-IV criteria at these lower blood lead levels.
Lead might influence symptoms of ADHD via disruption of striatal functioning and associated striatal-frontal circuitry. Among the psychological functions related to these circuits are response suppression (the ability to withhold a prepared response on a sudden signal; 22) and response variability (the ability to maintain a consistent high speed of response across multiple experimental trials; 8). Although top-down effects probably contribute also, striatal signaling seems to be involved in both abilities (7,8).
Methods and Materials
Recruitment and Multi-Stage Case-Finding Procedure
One hundred fifty children ages 8–17 completed the study. They were recruited via mailings to parents in regional school districts, public advertisements, and outreach to local clinics in an effort to obtain the broadest and most representative sampling possible. Parents provided written informed consent and children provided written informed assent. Families entered a multistage screening process to establish diagnostic groupings. To obtain equal numbers of ADHD and non-ADHD children, we advertised both for “healthy” children and for “children with suspected or diagnosed attention problems, ADD, or ADHD” and over-selected for ADHD when screening.
Stage 1 entailed a phone screen for eligibility. Eight hundred forty families contacted the project to express interest in the study. Rule-outs were long-acting psychotropic medication (e.g., antidepressant drugs), history of seizure, neurological impairments, a prior diagnosis of mental retardation or autistic disorder, head injury with loss of consciousness, sensorimotor handicap, or other major medical conditions in the child, as reported by the parent. Four hundred one children passed the initial (Stage 1) phone screen and were scheduled for the clinical screen.
Stage 2 included the following three elements: First, teachers and parents completed the respective versions of the Child Behavior Checklist (CBCL; 23), Conners Rating Scale-Revised (24), and the ADHD Rating Scale (25), all reliable and well-validated for assessing ADHD and other child disorders. Second, parents visited campus to complete the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS-E) during an interview with a trained, masters-level clinician. Sample interviews were videotaped and checked for fidelity as well as for inter-interviewer consistency in KSADs diagnosis (all k > .80 in this report). Third, children accompanied parents and completed a screen for IQ with a three-subtest short form of the Wechsler Intelligence Scales for Children-4th Edition (WISC-IV; 26) to estimate full scale IQ and word reading and spelling subtests of the Wechsler Individual Achievement Test-2nd Edition (WIAT-II; 27) to estimate academic achievement and learning disability. At this (Stage 2) clinical screen, 82 families dropped out, owing to lack of interest or inability to schedule an available appointment. Of the remainder, children were excluded after the screen if they had current major depressive episode (n = 20), situational symptoms of ADHD (only noticeable at home or only at school (n = 41) or ADHD-PH (n = 2), other major psychopathology (substance addiction, bipolar disorder, history of psychosis, sleep disorder, medical or neurological condition discovered at the clinical screen, or IQ < 75 (n = 92). Potential control children were excluded for ADHD, learning disability, or conduct disorder. Their psychiatric status was otherwise free to vary, so as to approximate population averages for psychopathology. Four eligible children declined the blood draw, yielding 150 children.
Establishment of Final ADHD and Other Diagnoses
With all available data, a best estimate diagnosis was arrived at independently by two licensed clinicians (a board-certified child psychiatrist and a fully licensed child clinical psychologist) who were blind to study hypotheses, blood lead levels, and cognitive control measures. Their agreement rates for ADHD, conduct disorder, and oppositional defiant disorder were acceptable (all k > .80). Disagreements were discussed to arrive at consensus, which was readily achieved in all cases. Before diagnosing ADHD, the clinicians required that, consistent with DSM-IV criteria, another disorder did not better account for symptoms, evidence of impairment, and evidence of cross-situational symptoms. If these conditions were met, then symptoms were counted as present if endorsed by parent on the KSADS or teacher on the ADHD rating scale (a symptom was counted as present on the ADHD rating scale if rated as a “2” or a “3” on the 0–3 scale). This procedure, referred to as the “or” algorithm, was validated in the DSM-IV field trials (28). The DSM-IV symptom counts were used in regression analyses for inattention, hyperactivity, or total ADHD symptoms. To assure that oppositional and conduct disorders did not explain ADHD findings, a disruptive behavior disorder diagnosis was assigned to 25 children who had either full or subthreshold (one symptom shy, to avoid false negatives) criteria for oppositional or conduct disorder by DSM-IV on the KSADS-E.
Cognitive Control
Cognitive control was assessed by having children complete a tracking version of the Stop task, a widely used and well validated measure to assess the ability to interrupt or suppress a prepared response (29). On 75% of trials (called here “go trials”) they were to decide as quickly as possible whether a letter on the computer screen was an “x” or an “o” and press the appropriate key. On 25% of trials (called “stop trials”) they heard a tone at the last moment and were to interrupt their response. Timing of the tone was varied in a stochastic tracking procedure to enable estimate of time needed to interrupt a response (stop signal reaction time). Variability of reaction time on the “go” trials of this task (when no stop tone sounded and children simply made a rapid response) was recorded as an index of response variability, readiness, and control. Stop signal reaction time scores < 125 msec were judged physiologically implausible and eliminated from analyses (n = 8). Due to a computer failure early in the project, an additional 23 were missing stop task data, so the total n for the stop task was 119 children (49 Control, 33 ADHD-PI, and 37 ADHD-C). Children with ADHD whose stop task data were lost or excluded did not differ from the other children with ADHD on level of blood lead or on symptoms of inattention or hyperactivity-impulsivity (all F < 1.0, p > .50). The control children without stop data also did not differ on these measures from the other control subjects (F < 1.0, p > .50). If missing data were imputed by maximum likelihood, results were unchanged.
Measurement of Blood Lead
The child and parent had 2 mL whole blood drawn through venipuncture in the arm. The blood was drawn into a 2-mL purple-top Vacutainer tube (vacutainer tubes were lot checked for lead by lab before use). Blood samples were frozen and stored at −20°C. Samples were assayed with the process of inductively coupled plasma mass spectrometry (ICP-MS). This method had a detection limit for lead of .3 μg/dL; inter-run precision was 5.8% (coefficient of variation) at a lead value of 2.9 μg/dL. The process began with whole blood samples brought to room temperature and vortexed, so no particulate matter remained at the bottom of the sample. Samples were diluted 1:50 with a diluent composed of 1.0% tetramethylammonium hydroxide, internal standard (iridium), 10% ethyl alcohol, 100 ppb gold, and .05% wetting solution (Triton X). Samples were then mixed by inverting 3–4 times. The analysis then entailed quantitating the sum of masses 206, 207, and 208 on the basis of three replicates/sample on a Perkin Elmer Elan DRC Plus ICP-MS (Waltham, Massachusetts). Because blood levels were low, lead assays were repeated a second time on the samples with the same results (r = .97, intraclass correlation absolute agreement coefficient = .94; all findings for ADHD unchanged).
Data Reduction and Data Analysis
For analysis of variance, we report the effect size partial η2. Mediation was evaluated by estimating confidence intervals around the indirect effect, with regression-based path analysis (30) and non-parametric resampling (bootstrapping with bias correction; 30,31). This procedure yields a path model that directly estimates the significance of the indirect effect appropriately for relatively small samples (30,31). It therefore is similar to but more powerful than the older procedure recommended by Baron and Kenny (32). All variables were standardized before analysis; path coefficients are labeled as B.
Results
Descriptive Overview
The sample comprised three groups: non-ADHD, ADHD-PI, and ADHD-C (the few children identified with ADHD-PH were excluded). Table 1 provides a descriptive and clinical overview of the sample, including blood lead levels; Table 2 compares blood lead levels in the current sample with the national average; and Table 3 shows correlations of blood lead with other variables in the current sample. With regard to Table 1, although ADHD and control groups did not differ in proportion of boys (61% vs. 65%, p = .58), the proportion of boys differed with respect to the ADHD subtypes, as shown in Table 1. Gender of child did not interact with lead in predicting ADHD (F < 1.0; p > .9); we therefore treated gender of child as a covariate rather than a factor in all analyses. Child estimated Full Scale IQ ranged from 78 to 139 with a mean of 109. Control children tended to have higher IQ than children with ADHD, as shown in Table 1; the two ADHD subtype groups had essentially identical IQ scores. The ADHD groups came from families with lower incomes than the non-ADHD children, and income was associated inversely with blood lead (Table 3). Income was therefore covaried in all analyses.
Table 1.
Sample Summary Statistics (mean + SD) for Three Groups
| Control | ADHD-PI | ADHD-C | p | |
|---|---|---|---|---|
| n | 53 | 47 | 50 | |
| % male | 60% | 49% | 80%a | <.05 |
| % White | 71% | 81% | 80% | ns |
| Child Age (yrs) | 14.7 (2.5)a | 13.0 (2.4)b | 12.1 (2.9)b | <.01 |
| Annual Home Income ($) | 86.0 (46)a | 71.4 (41)ab | 52.7 (32)b | <.05 |
| Child Full Scale IQ | 116.2 (11.9)a | 105.6 (14.5)b | 104.1 (10.8)b | <.01 |
| # DSM-IV Inattention | 1.9 (2.0)a | 8.3 (.9)b | 8.7 (.48)b | <.01 |
| # DSM-IV Hyperactivity | 1.0 (1.1)a | 3.4 (2.3)b | 8.2 (.9)c | <.01 |
| CBCL Externalizing T | 47.1 (11.5)a | 52.9 (10.4)b | 61.2 (9.5)c | <.01 |
| CBCL Internalizing T | 52.2 (12.6)a | 58.1 (9.4)b | 57.8 (9.3)b | <.01 |
| Conners Cognitive T | 47.6 (8.4)a | 73.6 (9.4)b | 69.2 (9.5)b | <.01 |
| Conners Hyperactivity T | 48.8 (4.9)a | 58.8 (12.9)b | 74.3 (14.2)c | <.01 |
| Conners Oppositional T | 47.6 (7.8)a | 55.6 (11.8)b | 65.2 (12.0)b | <.01 |
| Conners ADHD Index T | 48.3 (8.7)a | 72.0 (7.9)b | 71.8 (9.0)b | <.01 |
| Child Unadjusted Blood Lead | .89 (.39)a | .95 (.46)a | 1.26 (.67)b | <.01 |
The DSM-IV scores represent number of symptoms obtained by the “or” algorithm on the basis of parent Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS) and teacher attention-deficit/hyperactivity disorder (ADHD) Rating Scale. For dimensional scores, post hoc Tukey tests were conducted in the presence of 3-group effects; 2-way χ2 was conducted for categorical variables. Income is in thousands of dollars per year; blood lead is in μg/dL. CBCL, Child Behavior Checklist; Conners, parent rated T-scores.
Different superscripts indicate pair-wise differences on post hoc tests at p < .05. For example: “a” under Control Conners’ Cognitive indicates a significant difference from “b” for predominantly inattentive (ADHD-PI) for the same variable; because combined (ADHD-C) also has a “b” it differs from control subjects also but not from ADHD-PI. “ab” indicates does not differ from the group with the “a” or “b” superscript. Another example: Under CBCL Externalizing, group 1 is “a,” group 2 is “b,” and group 3 is “c.” This indicates that all three groups differ significantly from each other.
Table 2.
Mean Blood Lead Level with Comparison to National Sample, 1999–2002
| Sample | Age (yrs) | Mean Blood Lead μg/dL |
|---|---|---|
| National (CDC) | 12–19 | .94–1.10 |
| Current (n = 115) | 12–17 | 1.03 (SE = .05) |
| National (CDC) | 6–11 | 1.25–1.51 |
| Current (n = 35) | 8–11 | 1.04 (SE = .09) |
The National Centers for Disease Control (CDC) data (from the CDC National Health and Nutrition Examination Survey [NHANES] sample) reflect surveys at two points in time, one in 1999 and one in 2002. The lower value represents the 2002 value, and the higher value represents the 1999 value, indicating slight decrease over time in average population blood lead level.
Table 3.
Pearson Correlations of Blood Lead with Key Demographic and Behavioral Measures
| Child Lead | Maternal Lead | |
|---|---|---|
| Gender of the Child | −.28a | −.06 |
| Age of the Child | −.03 | .20b |
| Child Estimated FSIQ | −.18b | .10 |
| Household Income | −.27a | −.01 |
| DSM-IV Inattention | .18b | −.10 |
| DSM-IV Hyperactivity | .31a | .03 |
| DSM-IV ADHD Diagnosis | .24a | −.10 |
| DSM-IV Conduct Disorder | .02 | .21b |
| Conners Rating Scale Cognitive Problems | .14 | −.10 |
| Conners Rating Scale Hyperactivity | .25a | −.10 |
| Conners Rating Scale Oppositional | .18b | −.06 |
| Conners Rating Scale ADHD Index | .17b | −.06 |
| CBCL Attention Problems | .21b | −.09 |
The DSM-IV scores represent symptom counts derived from the “or” algorithm counting symptoms from parent KSADS and teacher ADHD Rating Scale. FSIQ, Estimated Full Scale Intelligence Quotient; Conners Rating Scale, parent rated Conners Revised Short Form; Cognitive Problems, inattention symptoms on that scale; CBCL, maternal rated Child Behavior Checklist; other abbreviations as in Table 1.
p < .01.
p < .05.
As shown in Table 1, the ADHD groups tended to be younger than the control group. Blood lead was not correlated with age in either the control (r =−.08, p > .50) or ADHD (r = −.01, p > .80) group. However, age was associated with cognitive control; so in mediation models involving cognitive control, age was covaried. Non-white race was unassociated with blood lead (White, m = 1.01; non-White, m = 1.09, F < 1.0; p > .40), and covarying child race did not affect any results. Race therefore was omitted from results. As expected by the fact that they could not have ADHD or conduct disorder, the control group had slightly lower than average externalizing behavior problems (national average T = 50). However, their internalizing symptoms were consistent with population norms. Thus, this was a relatively population-representative control group with regard to levels of psychopathology.
Pediatric blood lead levels ranged from .40 to 3.47 μg/dL with a mean of 1.03 (SE = .04). The levels were generally similar to or lower than recent national averages. Table 2 illustrates this by presenting national average levels reported by the Centers for Disease Control (22) in relation to our sample. These data suggest that the current sample was reasonably representative with regard to lead exposure and, in particular, was not exposed more than is typical in the United States. Table 3 shows correlations of blood lead with a range of other measures. Maternal blood lead level ranged from 0 to 5.3 μg/dL and was unrelated to child ADHD diagnosis or symptoms (maternal and child blood lead were weakly correlated; r = .16, p = .053). This finding suggests that lead effects are more likely related to postnatal than prenatal exposure; maternal blood lead was not further examined. The relation of child blood lead to lower IQ, also shown in Table 3, remained significant after covarying gender and income (β = −.19, p < .05).
Association of ADHD Diagnosis with Blood Lead Level
The three-group analysis of covariance (gender and income covaried) yielded a medium effect size [F(2,145) = 6.08, η2 = .04, p = .015; with age also covaried, p = .001]. The ADHD-C type had higher lead levels than the non-ADHD group [F(1,99) = 4.2, p = .04]. The ADHD-PI group did not differ from the non-ADHD group (F < 1.0, p > .50) or from the ADHD-C group (p = .15). To evaluate whether the ADHD-C effect could be explained by the overlap of externalizing behavior with ADHD-C, analyses were rechecked after adding oppositional defiant and conduct disorders as covariates; the difference between ADHD-C and non-ADHD remained significant (p = .03).
Figure 1 provides an overview scatterplot of blood lead and total ADHD symptoms, showing the significant association [r = .29, r2 = .07, p < .05]. With regard to ADHD symptom domains, blood lead was associated with both DSM-IV symptom counts for both inattention-disorganization and hyperactivity-impulsivity, as shown in Table 3. However, the effect on inattention was nonsignificant after covarying income and gender, whereas the association of blood lead with hyperactivity-impulsivity remained significant (p < .05). The relationship of lead with hyperactivity-impulsivity remained significant after statistically removing the relationship of inattention with hyperactivity (β = .19, p = .001). The reverse was not true; with hyperactivity controlled, inattention was unrelated to blood lead (p > .40). Therefore, mediation effects were examined only for the symptom domain of hyperactivity-impulsivity.
Figure 1.
Scatterplot of total attention-deficit/hyperactivity disorder (ADHD) symptoms (parent + teacher “or” algorithm) and child blood lead (r = .29, r2 = .07, p < .05).
Mediation Effects of Cognitive Functioning and ADHD
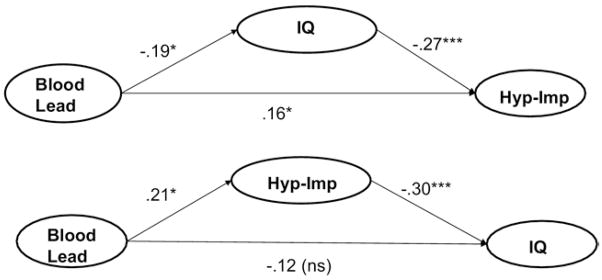
As shown in Figure 2 (top portion), the effect of lead on hyperactivity-impulsivity was not fully mediated by IQ (remaining path from lead to ADHD, β = .16, p = .046), although IQ served as a partial mediator (see figure footnote; age is not covaried, because IQ is already age adjusted). Figure 2, second part, shows that the relation of lead to IQ was mediated by the effect of lead on hyperactivity-impulsivity (remaining path from lead to IQ, β = −.12, p = .13; indirect path through ADHD, p < .05; 95% confidence interval = −.13 to −.02). Thus, effects of lead on IQ did not explain effects on hyperactivity-impulsivity.
Figure 2. Path diagram showing hyperactivity mediates effect of blood lead on child IQ but not the reverse (income and gender covaried).
Total indirect effects: Model 1, B = .05 (SE = .02), p<.05; 95; CI = .01–.10. Model 2, B=−.06 (SE=.03), p<.05; 95%; CI = −.14 to −.02. Hyp-imp = ADHD hypereactive symptoms as a composite of parent interview and teacher rating. Numbers on the figures are path coefficients (standardized regression coefficients). Lead=child’s blood lead.
Two cognitive control measures were hypothesized to mediate lead effects. We checked whether Baron and Kenny’s (33) conditions for mediation were present (income, gender, and age covaried). Blood lead level statistically predicted inefficient response inhibition as indexed by slower stop signal reaction time (β = .38, p < .001) and excess response variability (β = .39, p < .001). Hyperactivity-impulsivity was statistically predicted by slower stop signal reaction time (β = .20, p = .012) and greater response variability (β = .27, p < .001). Mediation was therefore examined formally in a path model. The two cognitive control variables were entered simultaneously in the path model because they were correlated.
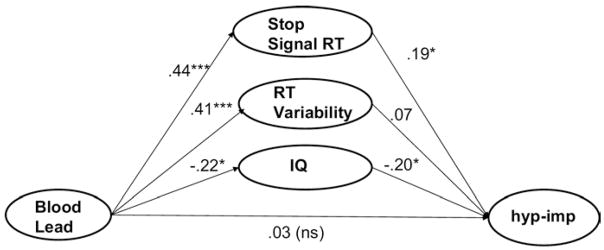
Results are depicted in Figure 3. The overall indirect path was significant (p < .05; see figure footnote) and completely mediated the effect of blood lead on hyperactivity. The effect of response inhibition rendered the unique effect of response variability nonsignificant. As a final check on simultaneous effects, we added IQ as a third mediator. As shown in Figure 4, these three variables together fully mediated the effect of lead on hyperactivity (total indirect path, p < .05); note that response inhibition continued to exert a significant mediation effect over and above the effect of IQ. All results held when results were checked for individual variables with the Baron and Kenny methods. Last, moderated mediation (differential mediation effects by diagnostic group) were examined, with no significant effects for either ADHD symptoms or IQ.
Figure 3. Cognitive control mediates effect of blood lead on attention-deficit/hyperactivity disorder (ADHD) hyperactive-impulsive symptoms (n= 119) with income, gender, and age covaried.
Total indirect effect B=.13 (SE .056), p<.05 (95% CI=.03–.26). Hyp-imp=ADHD hyperactive symptoms as a composite of parent interview and teacher rating. Numbers on the figure are path coefficients (standardized regression coefficients). Lead=child’s blood lead.
Figure 4. Cognitive control and IQ mediate effect of blood lead on attention-deficit/hyperactivity disorder (ADHD) hyperactive symptoms (n = 119) with income, gender, and age covaried.
Total indirect effect B=.16 (SE .06), p<.05 (95% CI=.05–.30). ADHD hyperactive-implsive symptoms are a composite of parent interview and teacher rating. Numbers on the figure are path coefficients (standardized regression coefficients). Lead=child’s blood lead.
Discussion
Whereas ADHD carries genetic influences on susceptibility, environmental risk factors might activate that susceptibility (33,34). Prior studies have shown that blood lead is related to symptoms of ADHD in children in a linear dose-dependent fashion (20) but typically had samples with lead levels higher than current population averages. Low-level lead exposure remains an important candidate for etiological influences on ADHD, because it remains widespread in children in the United States and throughout the world, it disrupts midbrain dopamine circuitry (35), and this is the same circuitry that is implicated in ADHD (7,36). To our knowledge, the present study is the first to examine low levels of lead (< 5 μg/dL) and its relationship to children diagnosed with ADHD by formal clinical criteria with DSM-IV as well as the first to examine low-level blood lead in relation to response inhibition and variability. Children’s lead exposure in this sample was similar to the national average. Within the limits of our sample, results suggest several conclusions.
First, background low-level lead exposure was associated with ADHD. This finding can be taken with additional confidence in that it converges well with conclusions from a recent large-sample population survey that used a nationally representative sample but lacked verification of ADHD diagnosis by clinical criteria (37). The present result lends greater confidence to both those findings and those reported here, each study balancing the other’s limitations. The finding also converges with earlier studies that used other definitions of ADHD and addressed extensive covariates (18–20).
Second, the effect of blood lead was specific to ADHD-C and was not observed with ADHD-PI (cases of ADHD-PH were too rare to study). Third, consistent with that subtype finding, effects on symptoms were most reliable for symptoms of hyperactivity-impulsivity rather than inattention-disorganization. These effects were not explained by group differences in household income or gender of the child. They also remained after accounting for potential mediation effects of IQ. Finally, they remained after adjusting for the presence of externalizing disorders that are often comorbid with ADHD.
Fourth, a secondary but noteworthy finding was that we replicated prior studies showing that lead exposure at very low levels is associated with lower IQ (13). This suggests that lead effects in our selected, clinically defined sample were operating similarly to what is observed in unselected population samples. It was also notable that effects of lead on IQ were mediated by the effects of lead on hyperactivity. Although these were cross-sectional data, it is possible that lead disrupts early cognitive control mechanisms, leading to hyperactive-impulsivity and secondarily to lower IQ.
Fifth, and of importance to theories of ADHD causal mechanism, we offer the first data on blood lead and specific measures of cognitive control thought to be associated with ADHD. Neurotoxic mechanisms of lead in the developing brain might vary (35). Of most relevance to ADHD is the extensive evidence that lead alters midbrain/striatal dopamine functioning (9,10) as well as gene expression in the striatum (11), with differential effects at low versus high exposure (38,39). Thus, the low-level lead associations observed here might be part of one pathway to ADHD. Low-level lead exposure might contribute specifically to ADHD by disrupting cognitive control operations instantiated in striatum and in frontal-striatal neural loops, including apparent effects on neurotransmission involving dopamine, acetylcholine, glutamate, and γ-amino butyric acid (35). It might thereby disrupt bottom-up signaling needed for cognitive control and thus influence hyperactive and impulsive behaviors. Indeed, lead exposure in animals creates cognitive deficits similar to the mechanisms suggested to underlie ADHD (38). Missing until now were data on these operations in children who actually were diagnosed with ADHD with DSM-IV criteria.
We therefore expanded prior findings in a clinical sample with two measures widely theorized to be relevant to ADHD and widely studied in relation to it—response suppression and response variability. These measures are important because they are central to theories of ADHD mechanism and are related to striatal-frontal neural circuitry yet have not been previously studied in an ADHD sample in relation to low-level lead exposure. As expected, both mechanisms were related to ADHD symptoms. Both cognitive mechanisms were disrupted by low levels of lead exposure, consistent with lead effects on striatum. Furthermore, weakness in these cognitive control operations partially accounted for the effect of lead on ADHD symptoms, even with IQ in the model, consistent with a model of lead effects on ADHD via midbrain dopamine systems. This finding should stimulate further efforts to evaluate performance and direct neural measures of striatum decrements in relation to lead effects on ADHD.
Limitations of this study should be noted. It is unclear how well concurrent lead levels reflect risks that probably occurred earlier in development. However, lead has a long half-life: years to decades in bones (40), and years in blood (41). Thus, current blood levels also reflect past exposures. Some data suggest that concurrent blood lead levels are superior to lead sampled earlier in life in predicting IQ (13), but as those authors point out, such data are lacking for behavioral measures. Effects of lead on the brain might depend on age of exposure. We do not know the ages of exposure of the children in this study. Second, it is possible that hyperactive children ingest more lead rather than that lead causes hyperactivity. However, the only study we are aware of to test that question (42) found that lead levels were not elevated in hyperactive children with a known organic etiology (e.g., head injury) but were elevated in other hyperactive children. With regard to subtypes, ADHD-PH, rare in this age range, was rarely identified by our sampling procedure. Further examination of children with ADHD-PH will be of interest.
Last, this was not a random population sample, so sampling biases cannot be fully ruled out (characteristics of refusers were unknown). However, our recruitment was community-based, and participants were selected via pre-established criteria in a multi-gate selection process, as is standard in studies of relatively rare clinical conditions sampled from the general population. The control group had lead exposure and behavior profiles consistent with national averages (Table 2). Although the control group had higher-than-average IQ and higher income than the ADHD group, associations of blood lead with ADHD were not accounted for by group differences in IQ or income. Furthermore, the ADHD effects here replicate past findings using less well defined ADHD measures as cited earlier as well as recent findings from a national population survey (37) that did not have formal ADHD diagnoses. In short, this study is important because it is the first to verify in a clinically validated sample that DSM-IV diagnostic criteria for ADHD are associated with blood lead at low exposure levels.
In conclusion, we found that average, background-levels of lead exposure were associated with ADHD in a clinically characterized sample. We extended prior findings by demonstrating this effect in a sample with diagnosis confirmed by full clinical criteria, showing the effect was independent of effects of lead on IQ or other disruptive behavior problems and illustrating potential mediation via lead effects on cognitive control. The implications of these findings for understanding attributable risk to ADHD are substantial (37). Notably, the lead exposure in the current sample was low; the control and ADHD sample overlapped in lead exposure level. As noted in Figure 1, some children with mildly elevated blood lead did not have elevated ADHD symptoms, and some with ADHD did not have elevated blood lead. Low-level lead exposure is not the only route to ADHD, and it is most likely that some children are more vulnerable to lead effects. One reason might be specific genetic vulnerability to low-level lead effects. For example, it might be that genes expressed particularly in striatum (e.g., dopamine transporter gene) are especially important in susceptibility to the hyperactive-impulsive symptoms of ADHD and interact with low levels of blood lead to exacerbate risk. Therefore, studies that examine interplay between low-level lead exposure and genotype are now needed.
Acknowledgments
This work was supported by R21 MH 070542 (PI: JTN) and by an MSU HBRI grant for data collection and laboratory analyses and by funding from CDC via Public Health Preparedness and Response for Bioterrorism to Michigan Department of Community Health that partially supported the laboratory analysis.
We thank Dr. B.J. Casey, Dr. Sandra Jacobson, and Dr. Joseph Jacobson for helpful comments regarding this study.
Footnotes
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. Text Rev. [Google Scholar]
- 2.Rappley MD. Clinical practice. Attention deficit-hyperactivity disorder. New Engl J Med. 2005;352:165–173. doi: 10.1056/NEJMcp032387. [DOI] [PubMed] [Google Scholar]
- 3.Jensen PS, Hinshaw SP, Kraemer HC, Lenora N, Newcorn JH, Abikoff HB, et al. ADHD comorbidity findings from the MTA study: Comparing comorbid subgroups. J Am Acad Child Adolesc Psychiatry. 2001;40:147–158. doi: 10.1097/00004583-200102000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Milich R, Balentine AC, Lynam DR. ADHD combined type and ADHD predominantly inattentive type are distinct and unrelated disorders. Clin Psychol: Science Pract. 2001;8:463–468. [Google Scholar]
- 5.Nigg JT. Understanding What Goes Wrong and Why. New York: The Guilford Press; 2006. What Causes ADHD? [Google Scholar]
- 6.Sonuga-Barke EJ. Causal models of attention-deficit/hyperactivity disorder: From common simple deficits to multiple developmental pathways. Biol Psychiatry. 2005;57:1231–1238. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Nigg JT, Casey BJ. An integrative theory of attention-deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Dev Psychopathol. 2005;17:785–806. doi: 10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- 8.Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R. Characterizing cognition in ADHD: Beyond executive dysfunction. Trends Cogn Sci. 2006;10:117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Kala SV, Jadhav AL. Low level lead exposure decreases in vivo release of dopamine in the rat nucleus accumbens: A microdialysis study. J Neurochem. 1995;65:1631–1655. doi: 10.1046/j.1471-4159.1995.65041631.x. [DOI] [PubMed] [Google Scholar]
- 10.Jadhav AL, Ramesh GT. Pb-induced alterations in tyrosine hydroxylase activity in rat brain. Mol Cell Biochem. 1997;175:137–141. doi: 10.1023/a:1006891830182. [DOI] [PubMed] [Google Scholar]
- 11.Lewis MW, Pitts DK. Inorganic lead exposure in the rat activates striatal cFOS expression at lower blood levels and inhibits amphetamine-induced cFOS expression at higher blood levels. J Pharmacol Exp Ther. 2004;310:815–820. doi: 10.1124/jpet.103.063941. [DOI] [PubMed] [Google Scholar]
- 12.Fewtrell LJ, Pruss-Ustun A, Landrigan P, Ayuso-Mateos JL. Estimating the global burden of disease of mild mental retardation and cardiovascular diseases from environmental lead exposure. Environ Res. 2004;94:120–133. doi: 10.1016/s0013-9351(03)00132-4. [DOI] [PubMed] [Google Scholar]
- 13.Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, et al. Low-level environmental lead exposure and children’s intellectual function: An international pooled analysis. Environ Health Perspect. 2005;113:894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canfield RL, Kreher DA, Cornwell C, Henderson CR. Low-level lead exposure, executive functioning, and learning in early childhood. Child Neuropsychol. 2003;9:35–53. doi: 10.1076/chin.9.1.35.14496. [DOI] [PubMed] [Google Scholar]
- 15.Canfield RL, Gendle MH, Cory-Slechta DA. Impaired neuropsychological functioning in lead-exposed children. Dev Neuropsychol. 2004;26:513–540. doi: 10.1207/s15326942dn2601_8. [DOI] [PubMed] [Google Scholar]
- 16.Tuthill RW. Hair lead levels related to children’s classroom attention-deficit behavior. Arch Environ Health. 1996;51:214–220. doi: 10.1080/00039896.1996.9936018. [DOI] [PubMed] [Google Scholar]
- 17.Minder B, Das-Smaal EA, Brand EF, Orlebeke JF. Exposure to lead and specific attentional problems in schoolchildren. J Learn Disabil. 1994;27:393–399. doi: 10.1177/002221949402700606. [DOI] [PubMed] [Google Scholar]
- 18.Gittelman R, Eskenazi B. Lead and hyperactivity revisited. An investigation of nondisadvantaged children. Arch Gen Psychiatry. 1983;40:827–833. doi: 10.1001/archpsyc.1983.01790070017002. [DOI] [PubMed] [Google Scholar]
- 19.Silva PA, Hughes P, Williams S, Faed JM. Blood lead, intelligence, reading attainment, and behaviour in eleven year old children in Dunedin, New Zealand. J Child Psychol Psychiatry. 1988;29:43–52. doi: 10.1111/j.1469-7610.1988.tb00687.x. [DOI] [PubMed] [Google Scholar]
- 20.Thomson GO, Raab GM, Hepburn WS, Hunter R, Fulton M, Laxen DP. Blood-lead levels and childrens behavior results from the Edinburg Lead Study. J Child Psychol Psychiatry. 1989;30:515–528. doi: 10.1111/j.1469-7610.1989.tb00265.x. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control. Third National Report on Human Exposure to Environmental Chemicals. Atlanta, Georgia: National Center for Environmental Health; 2005. Publication No. 05–0570. [Google Scholar]
- 22.Barkley RA. Attention-deficit/hyperactivity disorder, self-regulation, and time: Toward a more comprehensive theory. J Dev Behav Pediatr. 1997;18:271–279. [PubMed] [Google Scholar]
- 23.Achenbach TM. Manual for the Child Behavior Checklist/4-18 and 1991 Profile. Burlington, Vermont: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- 24.Conners CK. Conners Rating Scales-Revised. Toronto: Multi-Health Systems; 1997. [Google Scholar]
- 25.DuPaul GJ, Power TJ, Anastopolous AD, Reid R. ADHD Rating Scale—IV: Checklists, Norms, & Clinical Interpretation. New York: Guilford Press; 1998. [Google Scholar]
- 26.Wechsler D. Technical and Interpretive Manual. 4. San Antonio: The Psychological Corporation; 2003. Wechsler Intelligence Scale for Children. [Google Scholar]
- 27.Wechsler D. Examiner’s Manual. 2. San Antonio: Psychological Corporation; 2005. Weschler Individual Achievement Test. [Google Scholar]
- 28.Lahey BB, Applegate B, McBurnett K, Biederman J, Greenhill L, Hynd GW, et al. DSM-IV field trials for attention deficit hyperactivity disorder in children and adolescents. Am J Psychiatry. 1994;151:1673–1685. doi: 10.1176/ajp.151.11.1673. [DOI] [PubMed] [Google Scholar]
- 29.Logan GD, Schachar RJ, Tannock R. Impulsivity and inhibitory control. Psych Sci. 1997;8:60–64. [Google Scholar]
- 30.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 31.MacKinnon DP. Analysis of mediating variables in prevention and intervention research. In: Cázares A, Beatty L, editors. Scientific Methods for Prevention Intervention Research (NIDA Monograph No. 139) Rockville, Maryland: National Institute on Drug Abuse; 1994. pp. 127–153. [PubMed] [Google Scholar]
- 32.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Person Social Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 33.Waldman ID, Gizer IR. The genetics of attention deficit hyperactivity disorder. Clin Psychol Rev. 2006;26:396–432. doi: 10.1016/j.cpr.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Purcell S. Variance components models for gene-environment interactions in twin analysis. Twin Research. 2002;5:554–571. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- 35.Cory-Slechta DA. Relationships between lead-induced learning impairments and changes in dopaminergic, cholinergic and glutamatergic neurotransmitter system functions. Annu Rev Pharmacol Toxicol. 1995;35:391–415. doi: 10.1146/annurev.pa.35.040195.002135. [DOI] [PubMed] [Google Scholar]
- 36.Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav Brain Sci. 2005;28:397–419. doi: 10.1017/S0140525X05000075. [DOI] [PubMed] [Google Scholar]
- 37.Braun J, Kahn RS, Froehlich T, Auinger P, Lanphear BP. Exposures to environmental toxicants and attention deficit hyperactivity disorder in U.S. children. Environ Health Perspect. 2006;114:1904–1909. doi: 10.1289/ehp.9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cory-Slechta DA. Lead-induced impairments in complex cognitive function: Offerings from experimental studies. Child Neuropsychol. 2003;9:54–75. doi: 10.1076/chin.9.1.54.14499. [DOI] [PubMed] [Google Scholar]
- 39.Johnston MV, Goldstein GW. Selective vulnerability of the developing brain to lead. Curr Opin Neurol. 1998;11:689–693. doi: 10.1097/00019052-199812000-00013. [DOI] [PubMed] [Google Scholar]
- 40.Hu H, Rabinowitz M, Smith D. Bone lead as a biological marker in epidemiologic studies of chronic toxicity: Conceptual paradigms. Environ Health Perspect. 1998;106:1–8. doi: 10.1289/ehp.981061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manton WI, Angle CR, Stanek KL, Reese YR, Kuehnemann TJ. Acquisition and retention of lead by young children. Environ Res. 2000;82:60–80. doi: 10.1006/enrs.1999.4003. [DOI] [PubMed] [Google Scholar]
- 42.David OJ, Hoffman SP, Sverd J, Clark J. Lead and hyperactivity: Lead levels among hyperactive children. J Abnorm Child Psychol. 1977;5:405–416. doi: 10.1007/BF00915088. [DOI] [PubMed] [Google Scholar]