Abstract
Rationale
Angiotensin converting enzyme 2 (ACE2) is a new member of the brain renin-angiotensin system, that might be activated by an overactive renin-angiotensin system.
Objective
To clarify the role of central ACE2 using a new transgenic mouse model with hACE2 under the control of a synapsin promoter, allowing neuron-targeted expression in the central nervous system.
Methods and Results
Syn-hACE2 (SA) transgenic mice exhibit high hACE2 protein expression and activity throughout the brain. Baseline hemodynamic parameters (telemetry), autonomic function and spontaneous baroreflex sensitivity (SBRS) were not significantly different between SA mice and non-transgenic littermates (NT). Brain-targeted ACE2 over-expression attenuated the development of neurogenic hypertension (Ang II infusion: 600 ng/kg.min/14 days) and the associated reduction of both SBRS and parasympathetic tone. This prevention of hypertension by ACE2 over-expression was reversed by blockade of the Ang-(1–7) receptor (D-ala7-Ang-(1–7); 600 ng/kg.min). Brain AT2/AT1 and Mas/AT1 receptor ratios were significantly increased in SA mice. They remained higher following Ang-II infusion but were dramatically reduced after Ang-(1–7) receptor blockade. ACE2 over-expression resulted in increased NOS and NO levels in the brain, and prevented the Ang-II-mediated decrease in NOS expression in regions modulating BP regulation.
Conclusions
ACE2 over-expression attenuates the development of neurogenic hypertension partially by preventing the decrease in both SBRS and parasympathetic tone. These protective effects might be mediated by enhanced NO release in the brain resulting from Mas and AT2 receptor up-regulation. Taken together; our data highlight the compensatory role of central ACE2 and its potential benefits as a therapeutic target for neurogenic hypertension.
Keywords: carboxypeptidase, blood pressure, nitric oxide, baroreflex, autonomic function
Introduction
The renin angiotensin system (RAS) is well known for its physiological and pathophysiological roles in the regulation of blood pressure (BP) and cardiovascular function.1,2 A new component of the RAS, Angiotensin (Ang) converting enzyme (ACE) 2 has been identified, from human heart failure ventricle and lymphoma cDNA libraries [for reviews see3,4]. Although the ACE2 transcript was first described in heart, kidney and testis, additional studies reported ACE2 mRNA in rat medulla oblongata4 and ACE2 activity in mouse brain.5 Recently, we showed the presence of both ACE2 protein and mRNA widespread throughout the murine brain, in regions involved in the central regulation of cardiovascular function as well as non-cardiovascular regions.6 ACE2 converts Ang II into the vasodilatory peptide Ang-(1–7) with an affinity 400-fold higher than for Ang I.7 In the central nervous system (CNS), Ang-(1–7) has been shown to enhance sensitivity of the bradycardic component of the cardiac baroreceptor reflex8 and to promote vasodilation in hypertensive animals.9,10 As a key enzyme in generating Ang-(1–7), ACE2 is thought to be a pivotal player in central BP regulation.3,5
Several evidences from various laboratories have shown the beneficial effects of peripheral ACE2 in the regulation of cardiovascular hypertrophy and BP control.10–12 In the CNS, using a lentivirus coding for ACE2, Yamazato et al. previously showed that ACE2 over-expression in the rostral ventrolateral medulla, could reverse hypertension in spontaneously hypertensive rats (SHR).13 More recently, we reported that brain-targeted ACE2 over-expression in the subfornical organ (SFO) prevents the acute Ang-II-mediated pressor and drinking responses.5 However, due to the short term expression and the low efficiency of the virus vectors, these acute studies could not address the long-term effects of ACE2 expression. This is particularly important as previous studies using transgenic mice over-expressing ACE2 selectively in the heart, resulted in lethal alterations of cardiac rhythm.14 Accordingly, the role of ACE2 in the central regulation of BP and the effects of chronic ACE2 over-expression on the development of hypertension need further investigation.
To achieve this goal, we developed a new transgenic mouse model with neuron-targeted over-expression of human ACE2 (hACE2) in the CNS. Our data suggest that chronic ACE2 over-expression in syn-hACE2 (SA) mice impairs the development of neurogenic hypertension partially by preventing the decreases in baroreflex sensitivity and parasympathetic tone. Importantly, these protective effects may involve AT2 and Mas receptors up-regulation thus leading to increased nitric oxide (NO) signaling pathways and NO levels in the cerebrospinal fluid (CSF).
Material and Methods
A detailed description of methods andexperimental protocols can be found in the online data supplement available at http://www.circresaha.org.
Generation of transgenic mice
The SA fusion transgene (Fig. 1A) was constructed and microinjected into fertilized C57BL/6J×SJL/J (B6SJLF2) mouse embryos at the University of Iowa Transgenic Animal Facilities. All mice were fed standard mouse chow and water ad libitum. All procedures were approved by Institutional Animal Care andUse Committees at the University of Iowa and Louisiana State University Health Science Center.
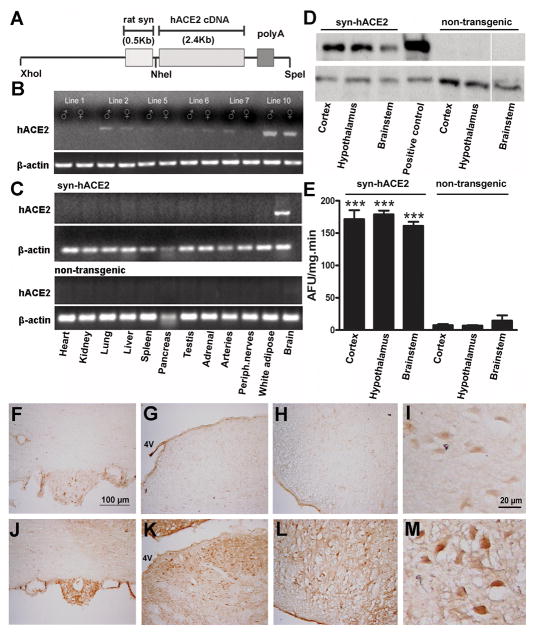
Figure 1.
Generation and characterization of SA transgenic mice. (A) Schematic of the fusion transgene containing the full length hACE2 cDNA, driven by a rat synapsin promoter. (B) Representative RT-PCR showing transgene expression in males and females from various founders. The highest level of hACE2 transgene expression was detected in line 10. (C) hACE2 mRNA expression was identified only in the brain of SA mice. (D) Representative Western blotting showing hACE2 protein expression in cortex, hypothalamus and brainstem of SA but not NT mice. Neuro2A cells transfected with hACE2 are used as positive control. (E) ACE2 activity assay showing the enzyme functionality and enhanced activity in SA compared to NT mice. Representative immunohistochemistry showing high widespread hACE2 expression throughout the brain of SA, as illustrated in the SFO (J), NTS (K) and RVLM (L) compared to NT mice (F, G, H, I). Higher magnification shows neuron-targeted expression of hACE2 in SA (M), while only background was detected in NT mice (I). Statistical significance: ***P<0.001 vs. NT mice. (AFU: arbitrary fluorescent units).
RNA isolation and reverse transcription PCR
Total RNA was isolated from various tissues from both transgenic and NT mice. Specific primers for hACE2 and β-actin were designed using PrimerQuest Software (IDT, Coralville, IA).
Western blot
Tissue lysates (10 μg) from brain cortex, hypothalamus and brainstem were collected separately and processed against hACE2 antibodies. Specific bands were detected by chemiluminescence according and quantitated by laser densitometry.
ACE2 activity
ACE2 activity was measured in tissue lysates from brain cortex, hypothalamus and brainstem. Data are presented as amounts of substrate FPSVI converted to product per minute and are normalized for total protein.
Immunohistochemistry
Brains sections were processed for hACE2, AT1 and AT2 receptors, Mas protooncogene, endothelial nitric oxide synthase (eNOS), Ser1177-phosphorylated-eNOS, Thr495-phosphorylated-eNOS and neuronal NOS (nNOS) detection.
Nitric Oxide measurement
Mice were anesthetized and NO measured using a Free Radical Analyzer. The NO probe was positioned into the mouse lateral ventricle and the current measured following stabilization.
Angiotensin II and Ang 1–7 levels
Brain regions and blood samples were collected and processed for Ang II and Ang-(1–7) measurement by the Wake Forest University Hypertension Core Laboratory.
Physiological recordings
Male SA and control littermates (n=12), 8–10 weeks old, were instrumented with radiotelemetry probes. Following recovery, baseline BP was recorded for 4 days. Mice were then infused subcutaneously for 14 days using osmotic minipumps (Alzet) containing either 1) saline; 2) Ang-II (600 ng/kg.min), a model for neurogenic hypertension; 3) D-Ala7-Ang-(1–7) (600 ng/kg.min), an Ang-(1–7) antagonist or 4) Ang-II + D-Ala7-Ang-(1–7). Water intake was recorded daily. Spontaneous baroreflex sensitivity (SBRS) and autonomic function were also assessed.
Statistical Analysis
Data are expressed as mean ±SEM. Data were analyzed by Student’s t test or two-way ANOVA (Bonferroni post hoc tests to compare replicate means) when appropriate. Statistical comparisons were performed using Prism5 (GraphPad Software, San Diego, CA). Differences were considered statistically significant at P<0.05.
Results
Characterization of transgenic mice
Using standard transgenic technology, we generated 11 SA founders expressing the hACE2 transgene (Figure 1A). All founders were successfully bred to establish transgenic lines. Out of these founders, line 10 exhibited the highest level of transgene expression, while others exhibited moderate or low levels of hACE2 mRNA in the brain (Figure 1B). Using RT-PCR, we detected high level expression of the hACE2 transgene in the brains from line 10, but not in other tissues, confirming the brain-specificity of the synapsin promoter (Figure 1C). To determine whether SA transgene expression was also reflected at the protein level, Western blotting was performed on brains from SA (line 10) and NT mice using a specific hACE2 antibody (Figure 1D). Expression of hACE2 protein (120 kDa) was detected in different brain regions in SA (i.e. cortex, hypothalamus, and brainstem), but not in NT mice. To verify whether transgenic expression of the hACE2 protein is functional, ACE2 activity was assessed in both genotypes. As shown in Figure 1E, SA have significantly higher (10 to 15-fold, P<0.001) brain ACE2 activity compared to NT mice harboring only the endogenous ACE2 gene.
Using immunohistochemistry, we examined the distribution of transgene expression in the brain of SA mice. Widespread hACE2 immunostaining was detected throughout the CNS in cardiovascular regions like the SFO, nucleus of tractus solitarius (NTS) and rostral ventrolateral medulla (RVLM) (Figure 1J, K, L), as well as in non-cardiovascular regions (Online Figure I). Very low levels were detected in NT mice (Figure 1F, G, H), suggesting weak mouse ACE2 cross-reactivity for this hACE2 antibody. Higher magnification revealed neuron-targeted hACE2 immunostaining (Figure 1M), consistent with the synapsin promoter specificity. Sections incubated without primary antibody showed a complete lack of immunostaining from both transgenic and NT mice (data not shown) confirming the specificity of staining.
Finally, to address the consequences of ACE2 overexpression on Ang peptides, Ang II and Ang-(1–7) were measured in the cortex, hypothalamus and brainstem of control and transgenic mice (Table 1). Ang II was significantly reduced in the brainstem of SA (7.0 ±1.1 pg/mg protein, n=12, P<0.05) compared to NT (10.1 ±1.0 pg/mg protein, n=12) mice. Moreover, the balance between Ang II and Ang-(1–7) peptides was significantly altered in both hypothalamus and brainstem of SA mice, in favor of Ang-(1–7). Plasma peptides were not significantly different (Table 1).
Table 1.
Baseline Angiotensin peptides levels in the brain and plasma.
| NT | SA | |
|---|---|---|
| Cortex (pg/mg protein) | ||
| Ang-II | 8.1 ±0.4 | 7.1 ±0.7 |
| Ang-(1–7) | 7.1 ±1.1 | 6.6 ±0.9 |
| Ang-II/Ang-(1–7) | 1.7 ±0.2 | 2.0 ±0.2 |
| Ang-(1–7)/Ang-II | 0.6 ±0.1 | 0.5 ±0.1 |
| Hypothalamus (pg/mg protein) | ||
| Ang-II | 11.0 ±1.8 | 8.6 ±1.6 |
| Ang-(1–7) | 7.7 ±1.3 | 10.0 ±1.2 |
| Ang-II/Ang-(1–7) | 1.4 ±0.2 | 0.9 ±0.2* |
| Ang-(1–7)/Ang-II | 0.7 ±0.1 | 1.2 ±0.1* |
| Brainstem (pg/mg protein) | ||
| Ang-II | 10.1 ±1.0 | 7.0 ±1.1* |
| Ang-(1–7) | 13.8 ±1.7 | 14.9 ±1.5 |
| Ang-II/Ang-(1–7) | 0.7± 0.1 | 0.5 ±0.1* |
| Ang-(1–7)/Ang-II | 1.4± 0.2 | 2.1 ±0.2* |
| Plasma (pg/ml) | ||
| Ang-II | 195 ±55 | 154 ±56 |
| Ang-(1–7) | 264 ±51 | 191 ±45 |
| Ang-II/Ang-(1–7) | 0.7 ±0.2 | 0.8 ±0.3 |
| Ang-(1–7)/Ang-II | 1.4 ±0.3 | 1.2 ±0.3 |
Data represent the Ang II, Ang-(1–7) levels and their ratios. Values are expressed as mean ±SEM.
P<0.05 vs. NT mice.
ACE2 over-expression reverses the development of neurogenic hypertension
To determine the functional consequences of hACE2 expression in SA mice, we first assessed the integrity of the BP response following acute intracerebroventricular (ICV) Ang II (200 ng/200 nL) administration in conscious freely moving mice. Expression of exogenous hACE2 blunted the Ang II-mediated pressor response in SA mice (3 ±2 mmHg, n=5) compared to their NT littermates (15 ±3 mmHg, n=5, P<0.05), consistent with our previous report showing that adenovirus-mediated ACE2 over-expression prevents the pressor response to acute ICV Ang II.5 To examine whether this functional expression of hACE2 in the brain can prevent the development of hypertension, we chronically infused mice, via the osmotic minipump, with a slow-pressor dose of Ang II (600 ng/kg.min/14 days) and monitored BP changes in conscious freely moving animals using telemetry. The 24-hour baseline hemodynamic parameters and activity data are summarized in Online Table I. BP was not significantly different between genotypes (SA: 101.8±2.0, NT: 97.2±2.2 mmHg, P>0.05), indicating that mice with brain-targeted ACE2 over-expression remain normotensive. Saline infusion did not alter BP level in any of the genotypes (Figure 2A). Chronic Ang II infusion significantly increased BP in NT mice, reaching a hypertensive level after 14 days (132 ±5 mmHg, n=12) compared to saline-infused controls (95 ±1 mmHg, n=6, P<0.05) (Figure 2A). Interestingly, SA mice exhibited a biphasic pattern. In the first week BP increased, reaching the same level (121 ±4 mmHg, n=12) as in NT mice (122 ±4 mmHg, n=12). However, in the second week, BP decreased, achieving non-hypertensive levels (109.7 ±4.1 mmHg, n=12) by the end of the Ang II infusion, although still higher than in saline-infused SA mice (93.3 ±3.7 mmHg, n=5). To confirm the ability of hACE2 expression to reduce Ang II-mediated responses, we assessed the drinking behavior during the 2-week Ang II infusion. Baseline water intake was not significantly different (P>0.05) between genotypes (Figure 2B). Ang II infusion resulted in a dramatic increase in daily water intake in NT mice by the end of the infusion protocol. Importantly, ACE2 over-expression prevented the enhanced water intake in SA compared to NT mice (4.9 ±0.4, n=9 vs. 9.5 ±1.5 mL/day, n=11, P<0.05) (Figure 2B). However, it remained higher in Ang II-infused SA compared to saline-infused mice (4.9 ±0.4, n=9 vs. 2.6 ±0.2 mL/day, n=4, P<0.01). Taken together, these data suggest that Ang II pressor and drinking responses are dramatically reduced but not totally prevented by neuronal ACE2 over-expression.
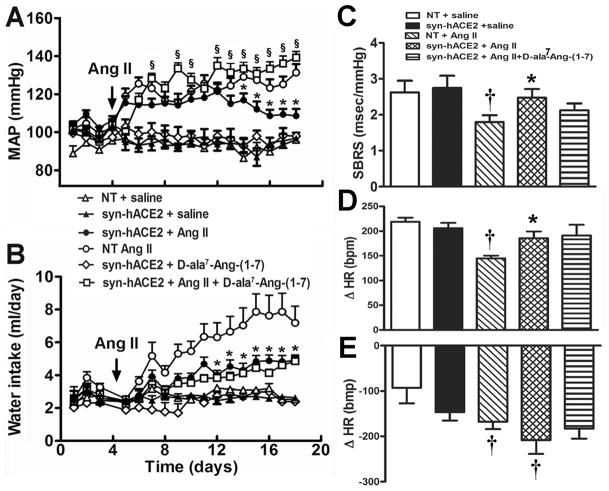
Figure 2.
ACE2 over-expression prevents the development of neurogenic hypertension. (A) Chronic Ang II infusion produced a progressive pressor response that was reversed in SA mice (n=12) in the second week of treatment. Chronic infusion of D-ala7-Ang-(1–7) totally restored the hypertensive response when concomitantly infused with Ang II in SA mice (n=11). (B) Ang II-mediated water intake was blunted in SA mice (n=12) and could not be restored by the Ang-(1–7) receptor blocker (n=11). Baseline SBRS (C), vagal tone (D) and sympathetic drive (E) were not different between groups. ACE2 over-expression prevented the Ang II-mediated decrease in SBRS and parasympathetic tone but did not affect sympathetic drive (n=14/group). Statistical significance: *P<0.05 vs. NT + Ang II; †P<0.05 vs. saline for the same genotype and §P<0.05 vs. SA + Ang II.
To address the participation of Ang-(1–7) in the prevention of hypertension in this model, we used D-Ala7-Ang-(1–7) infusion, to chronically block the Ang-(1–7) receptor while infusing mice with Ang II. Interestingly, the Ang-(1–7) receptor antagonist totally reversed the anti-hypertensive effect of ACE2 over-expression, therefore facilitating the development of hypertension (145 ±3 mmHg, n=6) in SA mice (Figure 2A). D-Ala7-Ang-(1–7) alone did not alter BP. Moreover, the Ang-(1–7) receptor antagonist failed to reverse the Ang II-mediated increase in water intake in SA mice (Figure 2B). Altogether, our data suggest that Ang-(1–7) mediates the reduction of BP in SA transgenic mice, while this peptide has no effect on BP in normotensive mice and does not trigger water intake.
Brain-targeted ACE2 over-expression reinforces spontaneous baroreflex sensitivity and parasympathetic tone
To dissect the physiological mechanisms involved in BP regulation in SA mice, spontaneous baroreflex sensitivity (SBRS) was assessed. Baseline (saline-infused mice) SBRS was similar between SA (2.7 ±0.3 msec/mmHg, n=8) and NT littermates (2.6 ±0.3 msec/mmHg, n=6, P>0.05) (Figure 2C). After 1 week of Ang II infusion, SBRS was already significantly reduced in NT mice (1.6 ±0.2 msec/mmHg, n=11 P<0.05) and remained low after 2-week infusion (1.8 ±0.2 msec/mmHg, n=11, P<0.05). In SA mice, SBRS shows a slight but non-significant reduction at 1 week (2.0 ±0.3 msec/mmHg, n=10, P>0.05) and remained normal until the end of the protocol (2.5 ±0.2 msec/mmHg, n=14). In addition, as another mechanism controlling BP, autonomic function was assessed using classic pharmacological blockers of sympathetic and/or parasympathetic drive. Baseline parasympathetic and sympathetic tones were not significantly different between SA (Changes in heart rate, ΔHR [bpm]: +206 ±11, n=15 and −117 ±18, n=9) and NT (ΔHR [bpm]: +219 ±9, n=7 and −93 ±34, n=6; P>0.05) mice, respectively (Figure 2, D and E). Ang II infusion significantly reduced the parasympathetic tone in NT (ΔHR: +145 ±6 bpm, n=6, P<0.001) but not in SA (ΔHR: 186 ±13, n=6, P>0.05) mice (Figure 2D). However, sympathetic tone was similarly increased in both genotypes following Ang II infusion (NT: −168 ±16, n=11; SA: −209 ±30 bpm, n=13) (Figure 2E). Ganglionic blockade reduced HR to the same extent in both genotypes in mice infused with saline (NT: 393 ±37, n=6; SA: 396 ±25 bpm, n=9) and Ang II (NT: 333 ±17, n=13; SA: 363 ±16 bpm, n=13), suggesting that intrinsic heart rate was not affected by ACE2 overexpression. Taken together; these data suggest that ACE2 over-expression in SA mice prevents the Ang II-mediated decreases in both SBRS and parasympathetic tone, without significantly affecting sympathetic outflow.
ACE2 and Angiotensin receptors expression in the brainstem
We previously showed that ACE2-mediated reduction of the pressor response to Ang II is associated with down-regulation of AT1 receptor expression in the SFO.5 To further elucidate the mechanisms involved in the reduction of neurogenic hypertension in SA mice, we examined Ang receptors expression in the brainstem where Ang II levels appear to be reduced in SA mice (Table 1). ACE2 overexpression led to AT1 receptors down-regulation in both NTS (Table 2, Figure 3A) and RVLM (Online Figure II). Moreover, this was associated with up-regulation of AT2 and Mas receptors in SA brainstem, compared to NT mice. The resulting elevated AT2/AT1 (Figure 3B) and Mas/AT1 (Figure 3C) receptors ratios also remained higher in SA mice following Ang II infusion, suggesting that SA mice could be less responsive to Ang II stimulation. In addition, Ang-(1–7) receptor blockade resulted in a significant increase in AT1 receptors immunostaining (Table 2, P<0.05) contributing to the reduction of the AT2/AT1 (Figure 3B) and Mas/AT1 (Figure 3C) receptors ratios. Together, these data suggest that ACE2 overexpression confers a protective effect to SA mice by modulating Ang receptors expression in the brainstem.
Table 2.
Quantification of brainstem AT1, AT2 and Mas receptors immune-staining.
| AT1 | AT2 | Mas | |
|---|---|---|---|
| Nucleus of Tractus Solitarius | |||
| NT+saline | 1.0 ±0.27 | 1.0 ±0.27 | 1.0 ±0.66 |
| SA+saline | 0.28 ±0.11* | 2.9 ±0.29* | 3.88 ±0.88* |
| NT+Ang II | 0.57 ±0.29 | 0.95 ±0.40 | 0.80 ±0.11 |
| SA+Ang II | 0.11 ±0.03 | 0.77 ±0.14† | 0.71 ±0.03 |
| SA+Ang II+D-ala7-Ang-(1–7) | 8.96 ±0.83§ | 1.98 ±0.22 | 0.81 ±0.14 |
| Rostral Ventrolateral Medulla | |||
| NT+saline | 1.0 ±0.09 | 1.0 ±0.24 | 1.0 ±0.20 |
| SA+saline | 1.53 ±0.21 | 3.2 ±0.24* | 4.05 ±0.34* |
| NT+Ang II | 1.39 ±0.31 | 1.12 ±0.13 | 1.13 ±0.19 |
| SA+Ang II | 0.92 ±0.15 | 0.78 ±0.16† | 1.09 ±0.07 |
| SA+Ang II+D-ala7-Ang-(1–7) | 3.57 ±0.82§ | 1.43 ±0.26 | 0.69 ±0.07§ |
Data represent the relative receptor density normalized to NT+saline. Values are expressed as mean ±SEM.
P<0.05 vs. NT for the same treatment;
P<0.05 vs. saline for the same genotype and
P<0.05 vs. SA+Ang II.
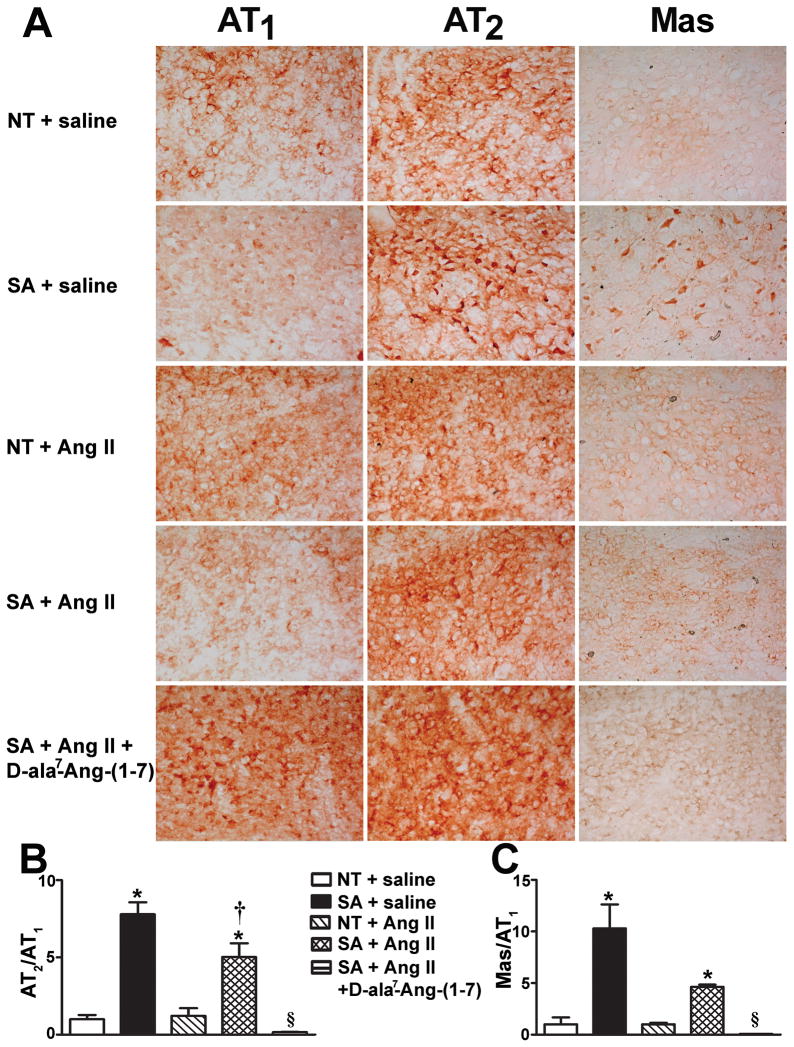
Figure 3.
Angiotensin AT1, AT2 and Mas receptors expression in the NTS. (A) Representative immunohistochemistry pictures for AT1, AT2 and Mas receptors (n=3/group). At baseline, brain AT2/AT1 (B) and Mas/AT1 (C) receptor ratios were significantly (P<0.05) increased in SA compared to NT mice. Following Ang II infusion, the both ratios remained higher in SA mice but were dramatically (P<0.05) reduced after Ang-II+D-ala7-Ang-(1–7). *P<0.05 vs. NT for the same treatment; †P<0.05 vs. saline for the same genotype and §P<0.05 vs. SA + Ang II.
ACE2 and NOS expression
Activation of Ang receptors has been shown to regulate NOS expression and phosphorylation thus modulating NO release in vitro and in vivo.15–17 To address the molecular mechanisms by which ACE2 prevents the development of hypertension and the Ang II-mediated decreases in SBRS and parasympathetic tone in SA mice, we investigated the consequences of ACE2 over-expression on NOS expression and phosphorylation.
Using immunohistochemistry, total eNOS, Ser1177-phosphorylated-eNOS, Thr495-phosphorylated-eNOS and nNOS expression were assessed in mouse brainstem. At baseline, ACE2 over-expression was associated with significant (P<0.05) up-regulation of nNOS and total eNOS in the brainstem (Figure 4A, B, C, and Online Figure III). Moreover, phosphorylated-eNOS-Ser1177/Thr495 ratio, an index of phosphorylation vs. dephosphorylation, was significantly elevated in the NTS (Figure 4A, D, P<0.05). Ang II infusion dramatically reduced NOS expression in the brainstem but it remained significantly higher in the dorsal medulla of SA compared to NT mice (Figure 4, P<0.05). Interestingly, Ang II+D-Ala7-Ang-(1–7) dramatically decreased the phosphorylated-eNOS-Ser1177/Thr495 ratio (Figure 4D, P<0.05) in SA mice, suggesting that eNOS phosphorylation is regulated by Ang-(1–7) in our model. Altogether, these data suggest that ACE2 exerts its modulatory effects partly through up-regulation of NOS expression and phosphorylation, thus preventing the development of neurogenic hypertension.
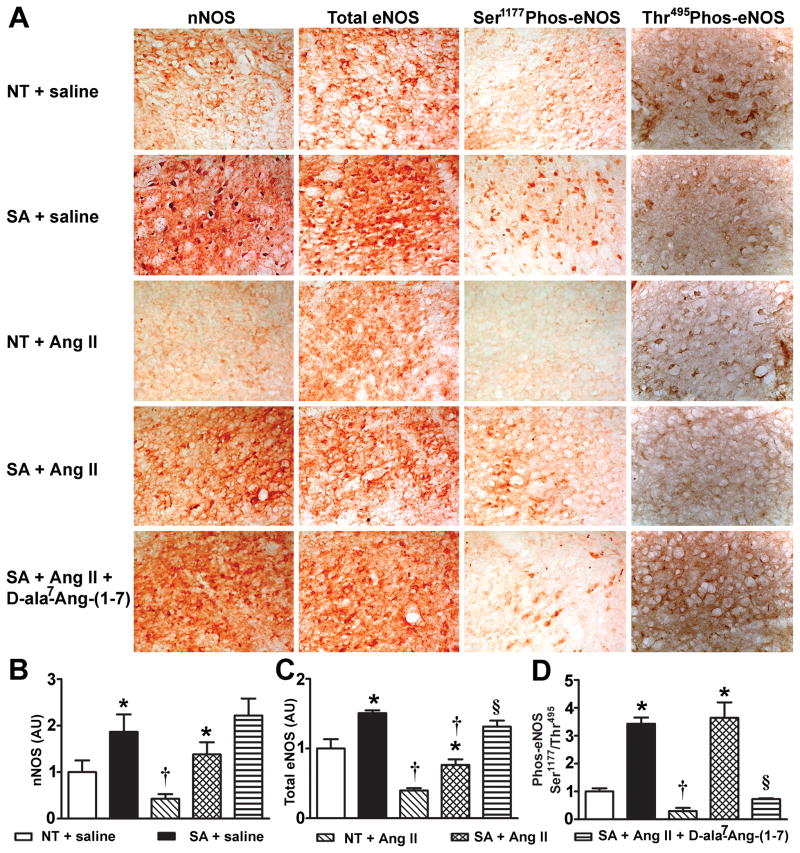
Figure 4.
NOS expression in the NTS. (A) Representative immunohistochemistry pictures for nNOS, total eNOS, phos-eNOS Ser1177 and phos-eNOS Thr495 (n=3/group). At baseline, nNOS (B) and total eNOS (C), were significantly (P<0.05) increased in the NTS of SA compared to NT mice and remained elevated following Ang II infusion. Blockade of the Ang-(1–7) receptor did not change nNOS and eNOS expression in SA mice (P>0.05). (D) Baseline phos-eNOS Ser1177/Thr495 ratio, an index of phosphorylation vs. dephosphorylation, was significantly (P<0.05) increased in the NTS of SA compared to NT mice. It remained higher in SA mice following Ang II infusion, but was dramatically (P<0.05) reduced after Ang-II+D-ala7-Ang-(1–7). Statistical significance: *P<0.05 vs. NT for the same treatment; †P<0.05 vs. saline for the same genotype and §P<0.05 vs. SA + Ang II.
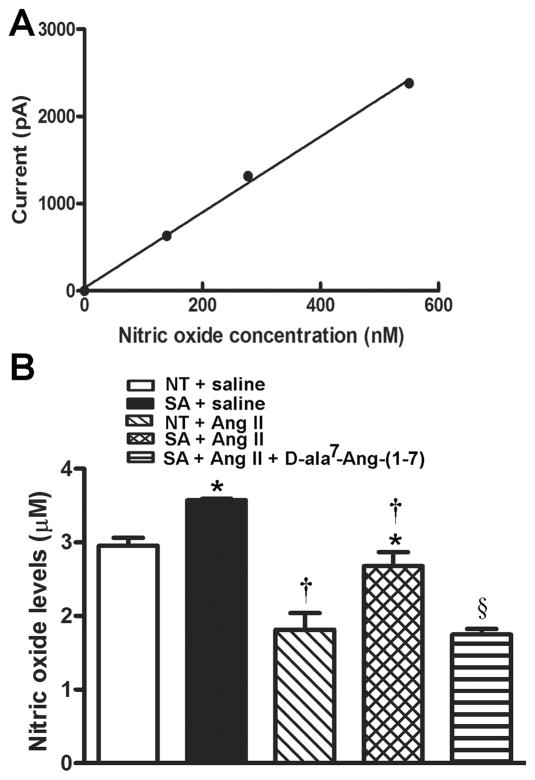
ACE2 over-expression increases brain NO levels
NO is known to be released following stimulation of AT2 or Mas receptors in the brain18 and the periphery.19 To confirm the pivotal role of NO in our model, we used a free radical analyzer to directly measure NO release in the cerebrospinal fluid. Following calibration of the NO probe (Figure 5A), baseline NO release was shown to be enhanced in SA mice (3.57 ±0.02 vs. 2.95 ±0.11 μM, P<0.05) compared to controls (Figure 5B). Ang-II infusion resulted in a ~50% reduction in NO release (1.82 ±0.22 μM) while only ~20% was depleted in transgenic mice (2.68 ±0.19 μM, Figure 5B). However, blockade of the Ang-(1–7) receptor in Ang II-infused SA mice further reduced NO levels to the same extent (1.75 ±0.08 μM) as in Ang II-infused NT mice (Figure 5B). These data suggest that ACE2 over-expression reinforces Ang-(1–7)-mediated NO release in the brain, which might contribute to the reversal of neurogenic hypertension.
Figure 5.
Nitric oxide levels in the CSF. (A) The NO standard curve was generated by using SNAP. (B) Baseline NO levels were significantly higher in SA compare to NT mice (P<0.05). The 2-week Ang II infusion significantly decreased NO levels in both SA and NT mice (P<0.05), although they remained higher in SA compared to NT mice (P<0.05). Ang II + D-ala7-Ang-(1–7) infusion further decreased NO levels compared to Ang II infusion alone. Statistical significance: *P<0.05 vs. NT for the same treatment; †P<0.05 vs. saline for the same genotype and §P<0.05 vs. SA + Ang II.
Discussion
In addition to the systemic RAS, local systems are present in various tissues and have important physiological and pathophysiological roles.1,2 All RAS components have been identified in the brain and play important functions in cardiovascular diseases and BP regulation.1 As a new member of the RAS, ACE2 has been suggested to counterbalance the effects of the ACE/Ang II/AT1 receptor axis and to play a pivotal role in the maintenance of cardiovascular function. However, although evidence for a central role of ACE2 have emerged,5,13,20 further understanding of its importance depends on the availability of tools to manipulate its expression in the brain. As important means to investigate gene function, transgenic models have been used in the last 2 decades and have proven their value in dissecting the brain RAS.21 Here, we describe the characterization of a new transgenic mouse model, with brain-targeted expression of hACE2 under the control of a neuron-specific promoter. More importantly, we show evidence that brain-targeted ACE2 over-expression reverses the development of neurogenic hypertension, possibly through regulation of Ang receptors, up-regulation of NOS expression and enhanced NO release in the brain.
Several studies have focused on the beneficial effects of peripheral ACE2 in the regulation of cardiovascular function, showing an association between reduction of BP and increased ACE2 in heart and kidney of SHR,12 as well as the beneficial effects of ACE inhibitors and AT1 receptor blockers in increasing cardiac ACE2.10 Further evidence of the protective role of ACE2 against cardiac hypertrophy and fibrosis,11 but also in reducing BP and improving vascular function, have recently emerged.22
In the CNS, virus-mediated gene delivery5,13 and pharmacological20 studies support a role for ACE2 in BP regulation and baroreflex function. However, while these studies suggest a pivotal role for ACE2 in the SFO, NTS and RVLM, the technical difficulties inherent to virus-mediated expression, including short duration, and the restricted availability of pharmacological agents affecting ACE2, have limited the investigation of the potential benefits of this enzyme in preventing diseases associated with the hyperactive RAS.
To clarify and further dissect the functional role of ACE2 in BP regulation and its potential for gene therapy, we engineered a new transgenic mouse model with chronic expression of hACE2 targeted to neurons in the CNS. While SA transgenic mice harbor normal resting hemodynamic, autonomic and baroreflex function, the altered balances between receptors and Ang peptides levels are evidence that, when overexpressed, ACE2 can modulate the RAS, supporting the idea of a compensatory function in specific conditions. Our data show that neuron-targeted ACE2 over-expression reverses the effects of chronic administration of Ang II, thus preventing both hypertension and enhanced drinking behavior in the Ang II “slow pressor” model. In this model, infusion of a low concentration of Ang II is most effective at reaching the brain, via the blood brain barrier-deficient circumventricular organs (e.g. SFO and area postrema), and acting on nuclei controlling BP rather than directly affecting peripheral vasculature, therefore leading to neurogenic hypertension via increased sympathetic outflow.23 However, it is important to note that like in other Ang II-mediated hypertension,24 a peripheral component (first week of infusion) exists in the “slow-pressor” model and that this early rise in BP is not affected by ACE2 overexpression in the brain.
Although inhibition of the pressor response to acute Ang II essentially involved ACE2-mediated Ang II hydrolysis and AT1 receptors down-regulation, further reducing Ang II downstream signaling,5 the reversal of neurogenic hypertension in SA mice emphasizes an important role for Ang-(1–7). Indeed, blockade of Ang-(1–7) receptors permitted the development of hypertension in this model. Moreover, unlike in NT, infusion of Ang II did not result in SBRS and parasympathetic tone reduction in SA mice, consistent with the ability of Ang-(1–7) to reinforce baroreflex sensitivity and vagal tone.8,20,25 However, despite a trend to, the Ang-(1–7) antagonist did not reverse the protective effects of ACE2 over-expression on SBRS and parasympathetic tone following Ang II infusion suggesting the involvement of both Ang II and Ang-(1–7) in this modulation. Evidence of ACE2 beneficial effects on the mechanisms controlling BP was recently substantiated by baroreflex sensitivity decline following ACE2 blockade in the NTS.20 Surprisingly, hACE2 over-expression in SA mice did not prevent the Ang II-mediated increase in sympathetic tone. However, this is somehow consistent with previous data showing that blockade of endogenous Ang-(1–7) in the paraventricular nucleus, reduced renal sympathetic tone thus suggesting that Ang-(1–7) may also participate in the maintenance of sympathetic outflow.26 Alternatively, this absence of reduction in sympathetic drive could be due to the lack of sensitivity of our pharmacological approach since urinary norepinephrine levels are reduced in Ang II-infused SA mice (Feng et al., unpublished data).
Brain AT1 receptors are well known for promoting enhanced sympathetic tone leading to the development of hypertension and chronic heart failure.27 Studies have shown that Mas and AT2 receptors oppose AT1 receptors both in the brain and the periphery.10,28,29 We previously showed that acute ACE2 over-expression in the brain resulted in AT1 receptors down-regulation.5 Here, we confirm and extend this observation by also noting that both Mas and AT2 receptors were up-regulated in the presence of over-expressed ACE2. However, the AT1 receptor expression patterns were different in NTS and RVLM. In NTS, AT1 receptors were down-regulated in SA mice; while unchanged in RVLM. Moreover, ACE2 over-expression prevented the reversal of AT2/AT1 ratio by Ang II in the NTS but not RVLM, suggesting a different regulation of these receptors among the brain nuclei. Overall, the balance between AT2/AT1 and Mas/AT1 was dramatically altered, shifting the equilibrium from increased sympathetic outflow and neurogenic hypertension (AT1 activation) towards enhanced vagal tone and BP normalization. Similar findings were recently reported by Ferreira et al., showing that the ACE2 activator XNT increased the Mas/AT1 receptors ratio in pulmonary hypertension.30 An imbalance between AT1 and AT2 in the brainstem has also been reported to affect sympathetic tone and overexpression of AT2 receptors in the RVLM was shown to promote sympatho-inhibition.31,32 Interestingly, we observed that both Ang II and the Ang-(1–7) receptor blocker reverse the increased AT2/AT1 and Mas/AT1 ratios. While increased AT1 receptors level, and therefore a reduction of the ratios, might be explained by a positive feedback of Ang II on its main receptor, the mechanism(s) by which D-ala7-Ang-(1–7) may affect the receptors balance is less clear. Clark et al. reported down-regulation of AT1 receptors by Ang-(1–7) through a cyclooxygenase-dependent pathway in the kidney, indicating the participation of Ang-(1–7) and probably NO in these regulations.33 Our data suggest that the regulation of these receptors is probably involving several mechanisms and our observation are the sum of positive and negative feeedbacks. Clearly, more investigation is needed to elucidate these receptors interactions.
The present data suggest ACE2 over-expression in the brain resulted in AT1 receptors down-regulation in SA mice, leading to reduced Ang II signaling. Indeed, the Ang II-induced drinking response was significantly blunted in SA mice and could not be restored to the level observed in NT mice following Ang-(1–7) receptor blockade. As first shown by Fitzsimons,34 this suggests that Ang-(1–7) is not involved in water intake, and that the impaired drinking response is due to a reduction in Ang II levels and downstream signaling. These are further evidence of the dichotomy between BP and body fluid regulation in the CNS.
In addition to modulating baroreflex and autonomic function, Ang-(1–7) has been reported to increase NO release in the brain18 where this neuromediator can modulate the regulation of baroreflex, autonomic function and BP.35–37 Ang-(1–7) has been shown to activate eNOS via an Akt-dependent pathway in vitro15 and to produce hypotensive effects by generating NO via activation of nNOS in the CVLM.36
Interestingly, we observed that over-expression of ACE2 in the brain of SA mice is associated with increased eNOS and nNOS levels in key nuclei involved in the central regulation of BP, like the NTS and RVLM. More importantly, ACE2 over-expression prevented the Ang II-induced decrease in NOS expression in the NTS, suggesting that the protective effects of ACE2 could be linked to increase NO availability in this area.
Neurons in the NTS receive and integrate inputs from the periphery and higher brain structures, and subsequently influence the activity of the principal brain nuclei governing efferent parasympathetic and sympathetic drive.38 Consistent with our observations, nNOS expression was reduced in the brainstem of a myocardial infarction mouse model, while adenovirus-mediated eNOS expression resulted in robust NO production and attenuation of the enhanced sympathetic nerve activity.39 However, while eNOS in the RVLM has been reported to improve baroreflex function,37 it has opposite effects in the NTS.40,41 Therefore, enhanced expression of eNOS in the NTS of SA mice may not be responsible for the improved baroreflex gain in these animals, but this could be achieved by increased nNOS in the ventral medulla.36 Direct measurement in SA mice reveals increased NO levels in the cerebrospinal fluid, suggesting that the overall improvements in autonomic and baroreflex function, observed in this model, could originate from various brain regions. Again, more work is needed to dissect this mechanism.
One concern with SA mice is the widespread high level expression of ACE2 throughout the brain. Although we previously showed that endogenous ACE2 expression is also prevalent in the CNS, it is possible that SA mice express the enzyme in places where it is not normally. However, for the enzyme to be active in such places it would need to have access to its substrate and the target Ang receptor, making it unlikely for the activation of non-physiological pathways. In summary, we have generated a new transgenic mouse model with over-expression of ACE2 in the brain. While these mice have normal cardiovascular parameters, they exhibit altered NOS and Ang receptors expression at baseline. Importantly, brain-targeted ACE2 over-expression reverses neurogenic hypertension, partially by preventing the decrease in both SBRS and parasympathetic tone. Ang-(1–7) plays a pivotal role in this reversal, promoting NOS activation and leading to enhanced NO release in the CNS. In addition to generating a new transgenic mouse model that will be critical to further dissect the role of ACE2 in the brain, we provide evidence of the mechanism of action by which ACE2 prevents the development of neurogenic hypertension, therefore supporting its beneficial role as a potential drug target for the treatment of hypertension.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Rhoda Reddix, Peter J. Hickman, Sharell M. Bindom and Bhavana Singh for technical assistance. Transgenic mice were generated at the University of Iowa Transgenic Animal Facility directed by Dr. Curt D. Sigmund and supported in part by grants from the NIH and from the Roy J. and Lucille A. Carver College of Medicine. We wish to thank Norma Sinclair, Patricia Yarolem and Joanne Schwarting for their technical expertise in generating transgenic mice. Angiotensin peptides levels were assessed at the Hypertension Core Laboratory at Wake Forest University School of Medicine directed by Dr. Bridget K. Brosnihan.
Sources of Funding
This work was supported, in part, by an American Heart Association Postdoctoral Fellowship to Dr. Yumei Feng and NIH grants NS052479, RR018766 and HL093178 to Dr. Eric Lazartigues.
Non-standard abbreviations and acronyms
- ACE2
Angiotensin converting enzyme type 2
- Ang
Angiotensin
- BP
blood pressure
- CNS
central nervous system
- CSF
cerebrospinal fluid
- eNOS
endothelial nitric oxide synthase
- hACE2
human ACE2
- ICV
intracerebroventricular
- NO
nitric oxide
- NOS
nitric oxide synthase
- nNOS
neuronal nitric oxide synthase
- NT
non-transgenic littermates
- NTS
nucleus of tractus solitarius
- RAS
renin-angiotensin system
- RVLM
rostral ventrolateral medulla
- SA
syn-hACE2 transgenic mice
- SBRS
spontaneous baroreceptor reflex sensitivity
- SFO
subfornical organ
- SHR
spontaneously hypertensive rats
Footnotes
Disclosures
None.
References
- 1.Paul M, Poyan Mehr A, Kreutz R. Physiology of Local Renin-Angiotensin Systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 2.Bader M, Ganten D. Update on tissue renin–angiotensin systems. J Mol Med. 2008;86:615–621. doi: 10.1007/s00109-008-0336-0. [DOI] [PubMed] [Google Scholar]
- 3.Lazartigues E, Feng Y, Lavoie JL. The two fACEs of the tissue renin-angiotensin systems: implication in cardiovascular diseases. Curr Pharm Des. 2007;13:1231–1245. doi: 10.2174/138161207780618911. [DOI] [PubMed] [Google Scholar]
- 4.Xia H, Lazartigues E. Angiotensin-converting enzyme 2 in the brain: properties and future directions. J Neurochem. 2008;107:1482–1494. doi: 10.1111/j.1471-4159.2008.05723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng Y, Yue X, Xia H, Bindom SM, Hickman PJ, Filipeanu CM, Wu G, Lazartigues E. Angiotensin-Converting Enzyme 2 Overexpression in the Subfornical Organ Prevents the Angiotensin II-Mediated Pressor and Drinking Responses and Is Associated With Angiotensin II Type 1 Receptor Downregulation. Circ Res. 2008;102:729–736. doi: 10.1161/CIRCRESAHA.107.169110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doobay MF, Talman LS, Obr TD, Tian X, Davisson RL, Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol. 2007;292:R373–R381. doi: 10.1152/ajpregu.00292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 8.Campagnole-Santos MJ, Heringer SB, Batista EN, Khosla MC, Santos RA. Differential baroreceptor reflex modulation by centrally infused angiotensin peptides. Am J Physiol. 1992;263:R89–R94. doi: 10.1152/ajpregu.1992.263.1.R89. [DOI] [PubMed] [Google Scholar]
- 9.Dobruch J, Paczwa P, £oń S, Khosla M, Szczepańska-Sadowska E. Hypotensive function of the brain angiotensin-(1–7) in Sprague Dawley and renin transgenic rats. J Physiol Pharmacol. 2003;54:371–381. [PubMed] [Google Scholar]
- 10.Ferrario CM. Angiotensin-Converting Enzyme 2 and Angiotensin-(1–7): An Evolving Story in Cardiovascular Regulation. Hypertension. 2006;47:515–521. doi: 10.1161/01.HYP.0000196268.08909.fb. [DOI] [PubMed] [Google Scholar]
- 11.Diez-Freire C, Vazquez J, Correa de Adjounian MF, Ferrari MFR, Yuan L, Silver X, Torres R, Raizada MK. ACE2 gene transfer attenuates hypertension-linked pathophysiological changes in the SHR. Physiol Genomics. 2006;27:12–19. doi: 10.1152/physiolgenomics.00312.2005. [DOI] [PubMed] [Google Scholar]
- 12.Zhong JC, Huang DY, Yang YM, Li YF, Liu GF, Song XH, Du K. Upregulation of angiotensin-converting enzyme 2 by all-trans retinoic acid in spontaneously hypertensive rats. Hypertension. 2004;44:907–912. doi: 10.1161/01.HYP.0000146400.57221.74. [DOI] [PubMed] [Google Scholar]
- 13.Yamazato M, Yamazato Y, Sun C, Diez-Freire C, Raizada MK. Overexpression of Angiotensin-Converting Enzyme 2 in the Rostral Ventrolateral Medulla Causes Long-Term Decrease in Blood Pressure in the Spontaneously Hypertensive Rats. Hypertension. 2007;49:926–931. doi: 10.1161/01.HYP.0000259942.38108.20. [DOI] [PubMed] [Google Scholar]
- 14.Donoghue M, Wakimoto H, Maguire CT, Acton S, Hales P, Stagliano N, Fairchild-Huntress V, Xu J, Lorenz JN, Kadambi V, Berul CI, Breitbart RE. Heart block, ventricular tachycardia, and sudden death in ACE2 transgenic mice with downregulated connexins. J Mol Cell Cardiol. 2003;35:1043–1053. doi: 10.1016/s0022-2828(03)00177-9. [DOI] [PubMed] [Google Scholar]
- 15.Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM. Angiotensin-(1–7) Through Receptor Mas Mediates Endothelial Nitric Oxide Synthase Activation via Akt-Dependent Pathways. Hypertension. 2007;49:185–192. doi: 10.1161/01.HYP.0000251865.35728.2f. [DOI] [PubMed] [Google Scholar]
- 16.Yayama K, Okamoto H. Angiotensin II-induced vasodilation via type 2 receptor: Role of bradykinin and nitric oxide. International Immunopharmacology. 2008;8:312–318. doi: 10.1016/j.intimp.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Stennett AK, Qiao X, Falone AE, Koledova VV, Khalil RA. Increased vascular angiotensin type 2 receptor expression and NOS-mediated mechanisms of vascular relaxation in pregnant rats. Am J Physiol Heart Circ Physiol. 2009;296:H745–755. doi: 10.1152/ajpheart.00861.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calka J, Block CH. Angiotensin-(1–7) and nitric oxide synthase in the hypothalamo-neurohypophysial system. Brain Res Bull. 1993;30:677–685. doi: 10.1016/0361-9230(93)90099-w. [DOI] [PubMed] [Google Scholar]
- 19.Pörsti IBA, Busse R, Hecker M. Release of nitric oxide by angiotensin-(1–7) from porcine coronary endothelium: implications for a novel angiotensin receptor. Br J Pharmacol. 1994;111:652–654. doi: 10.1111/j.1476-5381.1994.tb14787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diz DI, Garcia-Espinosa MA, Gegick S, Tommasi EN, Ferrario CM, Ann Tallant E, Chappell MC, Gallagher PE. Injections of angiotensin-converting enzyme 2 inhibitor MLN4760 into nucleus tractus solitarii reduce baroreceptor reflex sensitivity for heart rate control in rats. Exp Physiol. 2008;93:694–700. doi: 10.1113/expphysiol.2007.040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davisson RL. Physiological genomic analysis of the brain renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol. 2003;285:R498–R511. doi: 10.1152/ajpregu.00190.2003. [DOI] [PubMed] [Google Scholar]
- 22.Alenina N, Xu P, Rentzsch B, Patkin EL, Bader M. Genetically altered animal models for Mas and angiotensin-(1–7) Exp Physiol. 2008;93:528–537. doi: 10.1113/expphysiol.2007.040345. [DOI] [PubMed] [Google Scholar]
- 23.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res. 2004;95:210–216. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]
- 24.Lazartigues E, Lawrence AJ, Lamb FS, Davisson RL. Renovascular Hypertension in Mice With Brain-Selective Overexpression of AT1a Receptors Is Buffered by Increased Nitric Oxide Production in the Periphery. Circ Res. 2004;95:523–531. doi: 10.1161/01.RES.0000140892.86313.c2. [DOI] [PubMed] [Google Scholar]
- 25.Alzamora AC, Santos RAS, Campagnole-Santos MJ. Baroreflex modulation by angiotensins at the rat rostral and caudal ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1027–1034. doi: 10.1152/ajpregu.00852.2004. [DOI] [PubMed] [Google Scholar]
- 26.Gomes da Silva AQ, Sousa dos Santos RA, Peliky Fontes MA. Blockade of Endogenous Angiotensin-(1–7) in the Hypothalamic Paraventricular Nucleus Reduces Renal Sympathetic Tone. Hypertension. 2005;46:341–348. doi: 10.1161/01.HYP.0000179216.04357.49. [DOI] [PubMed] [Google Scholar]
- 27.Zucker IH, Schultz HD, Patel KP, Wang W, Gao L. The Regulation of Central Angiotensin Type 1 Receptors and Sympathetic Outflow in Heart Failure. Am J Physiol Heart Circ Physiol. 2009:00073.02009. doi: 10.1152/ajpheart.00073.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Widdop RE, Matrougui K, Levy BI, Henrion D. AT2 Receptor-Mediated Relaxation Is Preserved After Long-Term AT1 Receptor Blockade. Hypertension. 2002;40:516–520. doi: 10.1161/01.hyp.0000033224.99806.8a. [DOI] [PubMed] [Google Scholar]
- 29.Santos RAS, Ferreira AJ. Angiotensin-(1–7) and the renin-angiotensin system. Curr Opin Nephrol Hypertens. 2007;16:122–128. doi: 10.1097/MNH.0b013e328031f362. [DOI] [PubMed] [Google Scholar]
- 30.Ferreira AJ, Shenoy V, Yamazato Y, Sriramula S, Francis J, Yuan L, Castellano RK, Ostrov DA, Oh SP, Katovich MJ, Raizada MK. Evidence for Angiotensin-converting Enzyme 2 as a Therapeutic Target for the Prevention of Pulmonary Hypertension. Am J Respir Crit Care Med. 2009;179:1048–1054. doi: 10.1164/rccm.200811-1678OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao L, Wang W, Wang W, Li H, Sumners C, Zucker IH. Effects of Angiotensin Type 2 Receptor Overexpression in the Rostral Ventrolateral Medulla on Blood Pressure and Urine Excretion in Normal Rats. Hypertension. 2008;51:521–527. doi: 10.1161/HYPERTENSIONAHA.107.101717. [DOI] [PubMed] [Google Scholar]
- 32.Gao L, Wang W-Z, Wang W, Zucker IH. Imbalance of Angiotensin Type 1 Receptor and Angiotensin II Type 2 Receptor in the Rostral Ventrolateral Medulla: Potential Mechanism for Sympathetic Overactivity in Heart Failure. Hypertension. 2008;52:708–714. doi: 10.1161/HYPERTENSIONAHA.108.116228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark MA, Tallant EA, Tommasi E, Bosch S, Diz DI. Angiotensin-(1–7) Reduces Renal Angiotensin II Receptors through a Cyclooxygenase-Dependent Mechanism. [Article] J Cardiovasc Pharmacol. 2003;41 doi: 10.1097/00005344-200302000-00017. [DOI] [PubMed] [Google Scholar]
- 34.Fitzsimons JT. The effect on drinking of peptide precursors and of shorter chain peptide fragments of angiotensin II injected into the rat’s diencephalon. J Physiol. 1971;214:295–303. doi: 10.1113/jphysiol.1971.sp009433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paton JFR, Kasparov S, Paterson DJ. Nitric oxide and autonomic control of heart rate: a question of specificity. Trends Neurosci. 2002;25:626–631. doi: 10.1016/s0166-2236(02)02261-0. [DOI] [PubMed] [Google Scholar]
- 36.Alzamora AC, Santos RAS, Campagnole-Santos MJ. Hypotensive effect of ANG II and ANG-(1–7) at the caudal ventrolateral medulla involves different mechanisms. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1187–1195. doi: 10.1152/ajpregu.00580.2001. [DOI] [PubMed] [Google Scholar]
- 37.Kishi T, Hirooka Y, Kimura Y, Sakai K, Ito K, Shimokawa H, Takeshita A. Overexpression of eNOS in RVLM improves impaired baroreflex control of heart rate in SHRSP. Rostral ventrolateral medulla. Stroke-prone spontaneously hypertensive rats. Hypertension. 2003;41:255–260. doi: 10.1161/01.hyp.0000050649.30821.cb. [DOI] [PubMed] [Google Scholar]
- 38.Chapleau MW, Abboud FM. Neuro-cardiovascular regulation: from molecules to man. Ann N Y Acad Sci. 2001;940:xiii–xxii. [PubMed] [Google Scholar]
- 39.Sakai K, Hirooka Y, Shigematsu H, Kishi T, Ito K, Shimokawa H, Takeshita A, Sunagawa K. Overexpression of eNOS in brain stem reduces enhanced sympathetic drive in mice with myocardial infarction. Am J Physiol Heart Circ Physiol. 2005;289:H2159–2166. doi: 10.1152/ajpheart.00408.2005. [DOI] [PubMed] [Google Scholar]
- 40.Paton JF, Deuchars J, Ahmad Z, Wong LF, Murphy D, Kasparov S. Adenoviral vector demonstrates that angiotensin II-induced depression of the cardiac baroreflex is mediated by endothelial nitric oxide synthase in the nucleus tractus solitarii of the rat. J Physiol. 2001;531:445–458. doi: 10.1111/j.1469-7793.2001.0445i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paton J, Wang S, Polson J, Kasparov S. Signalling across the blood brain barrier by angiotensin II: novel implications for neurogenic hypertension. J Mol Med. 2008;86:705–710. doi: 10.1007/s00109-008-0324-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.