Abstract
Background and purpose
The relative risk of ischemic stroke associated with transient ischemic attack (TIA) is not well-defined because most studies of stroke after TIA did not include comparison groups. We sought to estimate short term and long term relative risks of ischemic stroke associated with clinically diagnosed TIA.
Methods
We used data from a population-based case-control study. Cases were hypertensive men and women, and postmenopausal women, ages 30–79, with incident ischemic stroke. Controls were sampled within strata of age, sex, hypertension status, and calendar year. The index date was the stroke date for cases and a random date for controls. Clinically diagnosed TIA was ascertained from medical records. We used logistic regression to calculate odds ratios (ORs).
Results
The study included 1,914 stroke cases and 9,874 controls. Clinically diagnosed TIA was present in 215 (11.2%) cases and 252 (2.5%) controls. Analyses focused on the most recent TIA before index date. For TIA <1 month before index date, the adjusted OR for stroke was 30.4 (95% CI: 10.4, 89.4); for TIA 1–3 months before index date, it was 18.9 (8.58, 41.6); for TIA 4–6 months before index date, it was 3.16 (1.27, 7.82); and for TIA more than five years before index date, it was 1.87 (1.22, 2.85).
Conclusions
The relative risk of ischemic stroke was high for TIA diagnosed within the past three months, and moderately high for TIA diagnosed more than five years in the past, compared with no history of clinically diagnosed TIA.
Keywords: stroke, transient ischemic attack, epidemiology, case-control study
Introduction
Transient ischemic attack (TIA) is a risk factor for ischemic stroke, and clinically diagnosed TIA is an opportunity for stroke prevention. Stroke rates after TIA are well-characterized, especially over short intervals. Meta-analyses of cohorts of clinically diagnosed TIA patients have shown the short term risk of stroke after TIA to be approximately 3% at two days, 5% at seven days, 8% at 30 days, and 9% at 90 days.1, 2 Some of the studies included in these meta-analyses followed TIA patients for longer periods, finding stroke risks of 7–21% at one year after TIA.3–5 However, most studies of TIA and stroke risk have focused on TIA patients without reference to comparison groups of TIA-free individuals. Therefore, the relative risk of stroke in TIA patients compared with TIA-free individuals is not as well-defined.
Studies comparing stroke rates after TIA with expected stroke rates based on age and sex have indicated that the relative risk of stroke after TIA is highest during the early period following TIA, then declines over time, but remains elevated for several years.6, 7 One study with concurrent follow-up of TIA patients and TIA-free participants found that TIA was associated with more than twice the risk of ischemic stroke over many years of follow-up, but did not assess short term and long term relative risks separately.8 To refine our knowledge of the temporal relationship between TIA and ischemic stroke risk, we sought to estimate short term and long term relative risks of incident ischemic stroke associated with clinically diagnosed TIA in a general population in routine care.
Methods
Setting and design
The setting for this study was Group Health (GH), an integrated health care system in western Washington State. The data for these analyses were obtained as part of an ongoing population-based case-control study of myocardial infarction (MI) and stroke conducted among GH members from 1989 onward.9, 10 The study was approved by the GH Human Subjects Review Committee. Waiver of consent was granted for patients with language or cognitive difficulty and for patients who had died. All other participants provided verbal consent by telephone or written consent.
Study participants
Men and women with pharmacologically treated hypertension, and postmenopausal women, ages 30–79, with four or more GH visits were eligible to be included in the study and comprised the study population. Eligible cases and controls who refused to give permission to use their medical records were excluded from the study. 93.5% of all eligible stroke cases and 89.7% of all eligible controls were included in the study.
Cases were eligible participants with a confirmed incident fatal or nonfatal ischemic stroke that occurred between July 1, 1989, and December 31, 2005. Diagnostic criteria for stroke were adapted from the Cardiovascular Health Study.11 Stroke was defined as the rapid onset of a neurologic deficit or subarachnoid hemorrhage, with deficits persisting for at least 24 hours (unless death ensued within 24 hours of symptoms or there was evidence of an ischemic or hemorrhagic lesion on computed tomography [CT] or magnetic resonance imaging [MRI] consistent with the symptoms), and no underlying brain trauma, tumor, or infection to cause the symptoms. Ischemic stroke was defined as a focal deficit without evidence of blood on CT or MRI (except bleeding secondary to ischemia), or surgery or autopsy evidence of infarction. Incident ischemic or hemorrhagic stroke cases were identified by ICD-9 codes (430 through 438) and ICD-10 codes (G45 and I60 through I64) from GH hospitalization discharge records, which included hospital stays at GH facilities and at non-GH facilities, and from Washington State death records matched to GH membership files. Trained medical record abstractors classified stroke cases as ischemic or hemorrhagic using data from hospitalization records or death certificates. When abstractors could not determine the type of stroke based on physician diagnoses or imaging reports, a study physician in consultation with a neurologist reviewed the documentation to classify stroke type. A total of 2,470 strokes were identified. Only confirmed ischemic strokes (n=1,914) were included in these analyses; hemorrhagic strokes (n=535) and unclassified strokes (n=21) were excluded. The index date for each case was the date of the stroke.
Controls were a random sample of GH members, frequency matched by age (by decade), sex, hypertension status, and calendar year to cases of incident MI identified as part of the overall MI and stroke case-control study mentioned above.9, 10 Controls included in these analyses met the same eligibility criteria as ischemic stroke cases and had no history of stroke. The index date for each control was a randomly selected date within the calendar year from which they were selected as a control.
Exposure and covariates
Exposure and covariate data were collected by abstractors from the GH ambulatory medical record using a standard protocol with structured forms. Data collection from the medical record was restricted to information recorded before the index date. Additional information on smoking status was obtained from telephone interviews conducted with consenting controls and surviving cases. Information on estrogen replacement therapy was obtained from the GH computerized pharmacy database.
The exposure of interest was history of TIA. Uniform diagnostic criteria were not used. Instead, TIA diagnosis was based on physician diagnosis as recorded in the medical record. Abstractors recorded whether the medical record, covering a median of 18.7 years of clinical care, contained a clinical diagnosis of TIA at any time prior to the index date, but not including the index date. Therefore, none of the TIAs were diagnosed retrospectively after admission for stroke. Abstractors also recorded whether TIA was “probable/definite” or “possible” according to the treating physician (these were treated as mutually exclusive categories), and the month and year of the most recent clinically diagnosed TIA. In these analyses, TIA refers to clinically diagnosed probable or definite TIA; participants with a diagnosis of possible TIA were considered not to have had TIA.
Covariates included in these analyses were the matching factors: age, sex, presence of pharmacologically treated hypertension on the index date, and calendar year; and the following ischemic stroke risk factors: last systolic blood pressure (SBP) before index date, history of coronary artery disease (CHD; defined as myocardial infarction, angina, coronary angioplasty, or coronary artery bypass grafting), chronic congestive heart failure (CHF), atrial fibrillation (classified as current or past), peripheral vascular disease (defined as claudication or a peripheral vascular disease procedure such as angioplasty or bypass grafting of peripheral vessels), carotid endarterectomy, prosthetic heart valves, medically treated diabetes, current smoking, and current estrogen use (women only; defined as having received at least one estrogen prescription before the index date with enough estrogen pills to last until the index date, assuming 80% compliance with prescribing instructions12). Covariate data collected for the time period prior to the index date were not necessarily restricted to the time period prior to the most recent TIA.
Statistical analyses
We used multiple logistic regression to model the association between clinically diagnosed TIA and incident ischemic stroke. Odds ratios (ORs) from these models can be interpreted as rate ratios because controls were sampled according to person-time at risk for ischemic stroke (risk set sampling).13 First we calculated the OR of ischemic stroke for ever having had a clinically diagnosed TIA. Then we created indicator variables for the following mutually exclusive categories of months from most recent clinically diagnosed TIA to index date: <1, 1–3, 4–6, 7–12, 13–24, 25–36, 37–48, 49–60, and >60 months. For this categorization, if the year of the most recent clinically diagnosed TIA was available but the month was missing (3 cases and 10 controls), we considered the TIA to have occurred in the sixth month of the year. We used these indicator variables to calculate the ORs of ischemic stroke for TIA having been clinically diagnosed most recently in each of the time periods, with no history of clinically diagnosed TIA as the reference group. Models were adjusted for the matching factors and additional ischemic stroke risk factors listed above.
We explored whether the pattern of short term and long term relative risks differed across strata defined by age group (ages 30–69; ages 70–79), sex, and treated hypertension status (among women only; all of the men in the study had treated hypertension). The numbers of cases and controls with past TIA were too small in some of the time intervals described above to assess interactions across all the time intervals, so for these exploratory analyses we collapsed the time intervals from most recent TIA to index date into one short term category (≤3 months) and one long term category (≥4 months), and created interaction terms for these categories with age group, sex, and hypertension.
Results
The study included 1,914 incident ischemic stroke cases (mean age 69.7 years; 1,282 [67.0%] women) and 9,874 controls (mean age 65.9 years; 5,304 [53.7%] women). Ischemic stroke cases compared with controls were more likely to have cardiovascular conditions such as CHD, chronic CHF, and atrial fibrillation, and to have a greater burden of vascular risk factors such as diabetes and current smoking (Table 1).
Table 1.
Characteristics of ischemic stroke cases and controls, 1989 – 2005.
| Characteristic | Ischemic stroke cases (n = 1,914) | Controls (n = 9,874) | p value* |
|---|---|---|---|
| Age (years), mean (sd) | 69.7 (8.5) | 65.9 (9.7) | <0.001 |
| Women, % | 67.0 | 53.7 | <0.001 |
| Treated hypertension, % | 75.8 | 73.6 | 0.051 |
| Systolic blood pressure (mmHg), mean (sd) | 145.4 (22.7) | 138.6 (19.4) | <0.001 |
| Coronary heart disease, % | 31.3 | 19.6 | <0.001 |
| Chronic congestive heart failure, % | 12.7 | 4.2 | <0.001 |
| Atrial fibrillation | |||
| Current, % | 14.1 | 4.4 | <0.001 |
| Past, % | 5.2 | 2.1 | <0.001 |
| Peripheral vascular disease, % | 9.3 | 4.1 | <0.001 |
| Carotid endarterectomy, % | 1.8 | 0.9 | <0.001 |
| Prosthetic heart valves, % | 1.3 | 0.5 | <0.001 |
| Diabetes, % | 29.2 | 11.8 | <0.001 |
| Current smoker, % | 15.3 | 11.9 | <0.001 |
| Current estrogen use (women), % | 17.8 | 20.0 | 0.068 |
T tests for differences of means; chi square tests for differences of proportions.
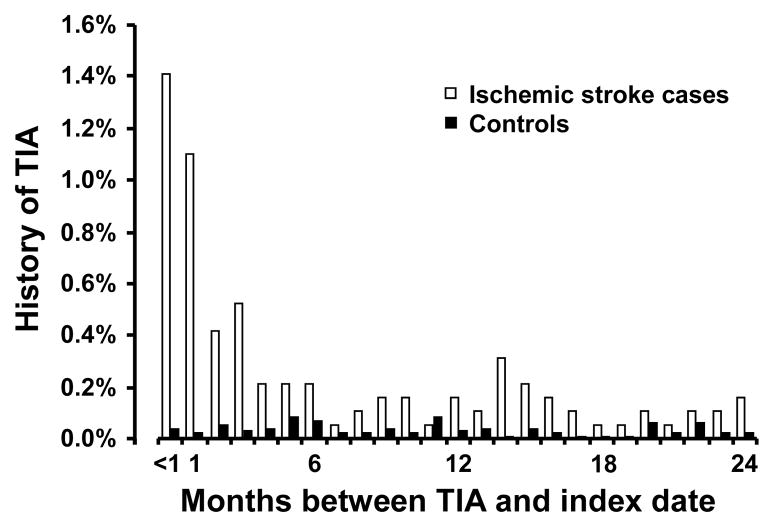
There were 215 (11.2%) ischemic stroke cases and 252 (2.5%) controls with clinically diagnosed TIA prior to the index date (Table 2). Among ischemic stroke cases with a history of clinically diagnosed TIA, the temporal distribution of the most recent TIA diagnosis was heavily concentrated in the few months prior to the index date; whereas among controls this distribution was relatively uniform and the prevalence was much lower (Figure 1).
Table 2.
History of clinically diagnosed transient ischemic attack (TIA) in ischemic stroke cases (n = 1,914) and controls (n = 9,874).
| Ischemic stroke cases | Controls | ||||||
|---|---|---|---|---|---|---|---|
| History of clinically diagnosed TIA | n | (%) | n | (%) | OR* | (95% CI) | p value |
| Never, n (%) | 1,699 | (88.8) | 9,622 | (97.5) | 1 | reference | |
| Ever, n (%) | 215 | (11.2) | 252 | (2.5) | 3.85 | (3.08, 4.82) | <0.001 |
| Most recent TIA prior to index date† | |||||||
| < 1 month, n (%) | 27 | (1.41) | 4 | (0.04) | 30.4 | (10.4, 89.4) | <0.001 |
| 1–3 months, n (%) | 39 | (2.04) | 10 | (0.10) | 18.9 | (8.58, 41.6) | <0.001 |
| 4–6 months, n (%) | 12 | (0.63) | 19 | (0.19) | 3.16 | (1.27, 7.82) | 0.013 |
| 7–12 months, n (%) | 13 | (0.68) | 21 | (0.21) | 1.88 | (0.97, 3.64) | 0.062 |
| 13–24 months, n (%) | 29 | (1.52) | 32 | (0.32) | 4.70 | (2.67, 8.26) | <0.001 |
| 25–36 months, n (%) | 17 | (0.89) | 19 | (0.19) | 3.00 | (1.34, 6.68) | 0.007 |
| 37–48 months, n (%) | 12 | (0.63) | 25 | (0.25) | 2.42 | (1.14, 5.10) | 0.021 |
| 49–60 months, n (%) | 16 | (0.84) | 23 | (0.23) | 2.74 | (1.31, 5.70) | 0.007 |
| > 60 months, n (%) | 42 | (2.19) | 96 | (0.97) | 1.87 | (1.22, 2.85) | 0.004 |
Odds ratios are adjusted for age, sex, hypertension status, calendar year, systolic blood pressure, coronary heart disease, chronic congestive heart failure, atrial fibrillation, peripheral vascular disease, carotid endarterectomy, prosthetic heart valves, medically treated diabetes, current smoking, and current estrogen use.
Data on the timing of the most recent TIA in relation to the index date was available for 207 (96.3%) of ischemic stroke cases with TIA and 249 (98.8%) of controls with TIA. Eight ischemic stroke cases with TIA and three controls with TIA had no data on the timing of the most recent TIA and were dropped from analyses of time intervals from TIA to index date.
Figure 1.
Time from most recent clinically diagnosed transient ischemic attack (TIA) to index date, for ischemic stroke cases and controls with TIA most recently diagnosed within 24 months prior to index date. Open bars represent ischemic stroke cases; solid bars represent controls.
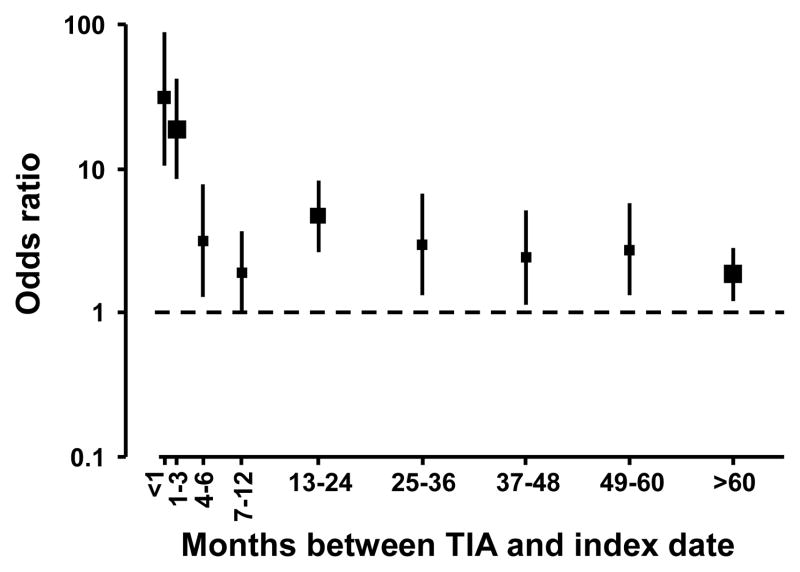
The OR of ischemic stroke for having clinically diagnosed TIA at any time in the past was 4.21 (95% CI: 3.45, 5.14), adjusted for the matching factors age, sex, hypertension status, and calendar year; and was 3.85 (95% CI: 3.08, 4.82) adjusted for additional ischemic stroke risk factors. Analyses accounting for the timing of the TIA diagnosed most recently prior to the index date revealed a high short term relative risk and moderately high long term relative risk for ischemic stroke (Figure 2). The fully-adjusted ORs were 30.4 (95% CI: 10.4, 89.4) for most recent TIA diagnosed <1 month before index date, 18.9 (95% CI: 8.58, 41.6) for 1–3 months, and 3.16 (95% CI: 1.27, 7.82) for 4–6 months before index date. For most recent TIA diagnosed during five additional intervals encompassing seven months to five years before index date, the fully-adjusted ORs ranged from 1.88 (95% CI: 0.97, 3.64) to 4.70 (95% CI: 2.67, 8.26). Finally, for most recent TIA diagnosed more than five years before index date, the fully-adjusted OR was 1.87 (95% CI: 1.22, 2.85).
Figure 2.
Odds ratios of ischemic stroke for having a history of clinically diagnosed transient ischemic attack (TIA), by time intervals from most recent TIA to index date. Reference group had no history of clinically diagnosed TIA. Marker size is proportional to the number of ischemic stroke cases. Odds ratios are adjusted for age, sex, hypertension status, calendar year, systolic blood pressure, coronary heart disease, chronic congestive heart failure, atrial fibrillation, peripheral vascular disease, carotid endarterectomy, prosthetic heart valves, medically treated diabetes, current smoking, and current estrogen use.
In exploratory analyses, when we collapsed the time intervals from most recent TIA to index date into one short term category (≤3 months) and one long term category (≥4 months), the ORs were 22.2 (95% CI: 11.8, 41.9) for most recent TIA ≤3 months before index date and 2.58 (95% CI: 2.00, 3.32) for most recent TIA ≥4 months before index date. This pattern of high short term relative risk and moderately high long term relative risk was similar across all strata we examined. We observed no significant differences in models of the short term and long term ORs for younger (ages 30–69) versus older (ages 70–79) age groups (p = 0.24), for women versus men (p = 0.06), or for non-hypertensive women versus hypertensive women (p = 0.12).
Discussion
In this population of men and women with treated hypertension, and postmenopausal women, TIA diagnosed most recently within the past three months was associated with short term ischemic stroke risk 20 to 30 times greater than ischemic stroke risk for patients without a history of clinically diagnosed TIA. Ischemic stroke risk was also elevated, though to a lesser degree, for TIA diagnosed four months to five years in the past. For patients with TIA diagnosed more than five years in the past, long term ischemic stroke risk was nearly twice as high as for patients without a history of clinically diagnosed TIA.
The short term and long term relative risks we observed in our study were similar to the relative risks observed in prior studies.6–8 In the Oxfordshire Community Stroke Project, the observed stroke incidence among TIA patients was compared with the expected stroke incidence based on age and sex.6 The age- and sex-adjusted relative risk of stroke after TIA was estimated as 80.0 during the first month, 13.4 during the first year, and ranged from 4.7 to 6.4 during five subsequent years, similar to the temporal pattern observed in our study. In Rochester, Minnesota, the observed stroke incidence among TIA patients compared with the expected stroke incidence based on age and sex yielded relative risk estimates of 16.5 during the first year after TIA and 9.5 during the full follow-up period averaging seven years after TIA.7 In the Rotterdam Study, the relative risk of stroke after TIA was estimated using a comparison cohort of TIA-free individuals and adjustment for many potential confounders.8 The relative risk of ischemic stroke over an average of ten years of follow-up, adjusted for age, sex, and a propensity score based on TIA and stroke risk factors, was estimated as 2.5, similar to the long term relative risk seen in our study.
A strength of our study was that ischemic stroke cases and controls were drawn from a defined population with clinically diagnosed TIA documented prospectively in medical records prior to the occurrence of stroke. Other strengths of our study were a high proportion of participation among eligible participants in a general population in routine care, data collection according to the same structured protocol for cases and controls, and adjustment for many stroke and TIA risk factors.
A limitation of our study was the lack of more detailed information about TIA history among the ischemic stroke cases and controls. We did not have data on specific TIA characteristics that would have allowed uniform application of TIA diagnostic criteria; instead we relied on the treating physician’s diagnosis of TIA as documented in the medical record. We also did not have results of neuroimaging performed at the time of clinical evaluation for TIA, although neuroimaging has been recognized as an important component of accurate TIA diagnosis.14 We did not have the date of the first ever clinically diagnosed TIA or the total number of TIAs in the past (we only had the month and year of the most recent TIA). Therefore, we could not address the question of stroke risk associated with first TIA. However, another study of TIA history in stroke patients found that the distribution of time from most recent TIA to stroke was similar to the distribution of time from first TIA to stroke, with both the first TIA and the most recent TIA tending to have occurred in the recent period prior to stroke rather than in the distant past.15 Another limitation of our study was the possibility of a diagnostic bias, where a past diagnosis of TIA may make patients more likely to seek care for stroke symptoms and physicians more likely to diagnose stroke given certain symptoms. Other limitations of our study were that non-hypertensive men were not included, and covariate data collected for the time period prior to the index date were not necessarily restricted to the time period before the most recent TIA. Finally, the numbers of TIAs occurring during some of the discrete time intervals prior to stroke were small, therefore the adjustment for confounding was not as good for the smaller categories as for the larger categories.
TIA and ischemic stroke share the same set of risk factors, therefore an association between TIA and ischemic stroke could arise simply because of these shared factors. However, when we adjusted our models for risk factors including age, sex, hypertension, cardiovascular disease history, diabetes, current smoking, and estrogen use, the association between clinically diagnosed TIA and ischemic stroke remained strong, suggesting that clinically diagnosed TIA was not solely a marker for stroke risk factors documented in the medical record, but was also a marker for underlying ischemic stroke risk that was not discernable based on the other factors documented in the medical record. Factors that could partially explain the association between TIA and ischemic stroke include subclinical vascular disease manifestations, such as carotid artery plaques or stenoses.
We did not evaluate the impact of clinically diagnosed TIA on subsequent risk factor evaluation and management among our study participants. Stroke prevention efforts initiated or enhanced after TIA may have increased during the years of our study (1989–2005), as TIA was becoming more widely recognized as an emergency opportunity for stroke prevention.16 To the extent that ischemic stroke was actually prevented after TIA in the population we studied, our results would have underestimated the association between TIA and ischemic stroke.
The results of our study emphasize that both short term and long term ischemic stroke risks are relevant to clinically diagnosed TIA patients. Based on a growing body of evidence from observational and experimental studies, recent guidelines emphasize the urgency of evaluating TIA patients and implementing treatment and risk factor control for stroke prevention.17, 18 Based on our results, the opportunity for more effective stroke prevention would appear to be greatest in the short term, within the first three months after TIA diagnosis, but prevention opportunities would likely persist through the long term, even five years or more after clinically diagnosed TIA.
Acknowledgments
We thank the participants and staff of the Heart and Vascular Health Studies. This research was supported in part by grants HL043201, HL068639, HL073410, HL074745, HL085251, and HL087652 from the National Heart, Lung, and Blood Institute (NHLBI). EL Thacker was supported by NHLBI training grant T32 HL007902. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or the National Institutes of Health.
Footnotes
Conflicts of interest/disclosures: None.
References
- 1.Wu CM, McLaughlin K, Lorenzetti DL, Hill MD, Manns BJ, Ghali WA. Early risk of stroke after transient ischemic attack: a systematic review and meta-analysis. Arch Intern Med. 2007;167:2417–2422. doi: 10.1001/archinte.167.22.2417. [DOI] [PubMed] [Google Scholar]
- 2.Giles MF, Rothwell PM. Risk of stroke early after transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol. 2007;6:1063–1072. doi: 10.1016/S1474-4422(07)70274-0. [DOI] [PubMed] [Google Scholar]
- 3.Hill MD, Yiannakoulias N, Jeerakathil T, Tu JV, Svenson LW, Schopflocher DP. The high risk of stroke immediately after transient ischemic attack: a population-based study. Neurology. 2004;62:2015–2020. doi: 10.1212/01.wnl.0000129482.70315.2f. [DOI] [PubMed] [Google Scholar]
- 4.Correia M, Silva MR, Magalhaes R, Guimaraes L, Silva MC. Transient ischemic attacks in rural and urban northern Portugal: incidence and short-term prognosis. Stroke. 2006;37:50–55. doi: 10.1161/01.STR.0000195209.26543.8f. [DOI] [PubMed] [Google Scholar]
- 5.Lisabeth LD, Ireland JK, Risser JM, Brown DL, Smith MA, Garcia NM, Morgenstern LB. Stroke risk after transient ischemic attack in a population-based setting. Stroke. 2004;35:1842–1846. doi: 10.1161/01.STR.0000134416.89389.9d. [DOI] [PubMed] [Google Scholar]
- 6.Dennis M, Bamford J, Sandercock P, Warlow C. Prognosis of transient ischemic attacks in the Oxfordshire Community Stroke Project. Stroke. 1990;21:848–853. doi: 10.1161/01.str.21.6.848. [DOI] [PubMed] [Google Scholar]
- 7.Whisnant JP, Matsumoto N, Elveback LR. Transient cerebral ischemic attacks in a community. Rochester, Minnesota, 1955 through 1969. Mayo Clin Proc. 1973;48:194–198. [PubMed] [Google Scholar]
- 8.Bos MJ, van Rijn MJ, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Incidence and prognosis of transient neurological attacks. JAMA. 2007;298:2877–2885. doi: 10.1001/jama.298.24.2877. [DOI] [PubMed] [Google Scholar]
- 9.Klungel OH, Heckbert SR, Longstreth WT, Jr, Furberg CD, Kaplan RC, Smith NL, Lemaitre RN, Leufkens HG, de Boer A, Psaty BM. Antihypertensive drug therapies and the risk of ischemic stroke. Arch Intern Med. 2001;161:37–43. doi: 10.1001/archinte.161.1.37. [DOI] [PubMed] [Google Scholar]
- 10.Psaty BM, Heckbert SR, Koepsell TD, Siscovick DS, Raghunathan TE, Weiss NS, Rosendaal FR, Lemaitre RN, Smith NL, Wahl PW, Wagner EH, Furberg CD. The risk of myocardial infarction associated with antihypertensive drug therapies. JAMA. 1995;274:620–625. [PubMed] [Google Scholar]
- 11.Price TR, Psaty B, O’Leary D, Burke G, Gardin J. Assessment of cerebrovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:504–507. doi: 10.1016/1047-2797(93)90105-d. [DOI] [PubMed] [Google Scholar]
- 12.Heckbert SR, Weiss NS, Koepsell TD, Lemaitre RN, Smith NL, Siscovick DS, Lin D, Psaty BM. Duration of estrogen replacement therapy in relation to the risk of incident myocardial infarction in postmenopausal women. Arch Intern Med. 1997;157:1330–1336. [PubMed] [Google Scholar]
- 13.Rodrigues L, Kirkwood BR. Case-control designs in the study of common diseases: updates on the demise of the rare disease assumption and the choice of sampling scheme for controls. Int J Epidemiol. 1990;19:205–213. doi: 10.1093/ije/19.1.205. [DOI] [PubMed] [Google Scholar]
- 14.Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, Hatsukami TS, Higashida RT, Johnston SC, Kidwell CS, Lutsep HL, Miller E, Sacco RL. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40:2276–2293. doi: 10.1161/STROKEAHA.108.192218. [DOI] [PubMed] [Google Scholar]
- 15.Rothwell PM, Warlow CP. Timing of TIAs preceding stroke: time window for prevention is very short. Neurology. 2005;64:817–820. doi: 10.1212/01.WNL.0000152985.32732.EE. [DOI] [PubMed] [Google Scholar]
- 16.Johnston SC. Editorial comment: transient ischemic attacks are emergencies. Stroke. 2005;36:724. [PubMed] [Google Scholar]
- 17.Johnston SC, Nguyen-Huynh MN, Schwarz ME, Fuller K, Williams CE, Josephson SA, Hankey GJ, Hart RG, Levine SR, Biller J, Brown RD, Jr, Sacco RL, Kappelle LJ, Koudstaal PJ, Bogousslavsky J, Caplan LR, van Gijn J, Algra A, Rothwell PM, Adams HP, Albers GW. National Stroke Association guidelines for the management of transient ischemic attacks. Ann Neurol. 2006;60:301–313. doi: 10.1002/ana.20942. [DOI] [PubMed] [Google Scholar]
- 18.Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, Goldstein LB, Gorelick P, Halperin J, Harbaugh R, Johnston SC, Katzan I, Kelly-Hayes M, Kenton EJ, Marks M, Schwamm LH, Tomsick T. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Circulation. 2006;113:e409–449. [PubMed] [Google Scholar]