Abstract
Objectives
To determine whether there is differential response to placebo or citalopram among older patients with and without deficient response inhibition.
Design
8-week, double-blind, placebo controlled trial.
Setting
Outpatient psychiatry.
Participants
Unipolar depressed patients aged 75 years and older.
Intervention
citalopram (20–40 mg/d) or placebo pill.
Measurements
Baseline Stroop Color Word Test and weekly 24-item Hamilton Rating Scale for Depression assessments.
Results
Citalopram treated patients with deficient response inhibition did significantly worse than placebo treated patients with deficient response inhibition. Conversely, citalopram treated patients without deficient response inhibition did significantly better than placebo treated patients without deficient response inhibition.
Conclusion
Patients with late-life depression and deficient response inhibition respond worse to SSRI than placebo. These findings suggest that there may be a deleterious interaction between deficient response inhibition and antidepressant medication in late-life depression and that the mechanism of SSRI and placebo response is different.
Keywords: executive dysfunction, response inhibition, treatment outcome, geriatric depression, late-life depression, citalopram
The executive functions represent a broad, multifaceted class of functions consisting of mental planning and organization, cognitive flexibility, set development and shifting, error monitoring, and response inhibition (1). Deficits in the executive functioning are common in late-life depression (defined as depression occurring among patients age 60 and older) and are associated with poor response to antidepressant treatment and increased rate of relapse, and recurrence (2–5).
Baldwin, et al (6) reported that deficits in Stroop and verbal fluency performance were higher among antidepressant non-responders compared to responders. In another study, late-life depressed patients with deficits in Stroop performance had lower remission rates to citalopram than late-life depressed patients without deficits in Stroop performance (2). Sneed, et. al (5) also showed that deficits in Stroop performance predicted poor response in an 8-week placebo-controlled trial of citalopram. Furthermore, it was demonstrated that this effect was not accounted for by deficits in other domains of cognitive functioning (7). In another study, depressed patients who remained symptomatic following antidepressant treatment showed increased perseveration and greater deficits in initiation (assessed using the Mattis Dementia Rating Scale) as compared to patients who achieved remission (3). Increased reaction time in the conflict condition of the Attention Network Test predicted prolonged time to remission depression among geriatric depressed patients (4). Finally, in a sample of non-demented elderly depressed treated with citalopram, impairment in initiation and increased perseveration (assessed using the Mattis Dementia Rating Scale) predicted both relapse and recurrence (8).
It appears that depressed older adults with deficits on tasks tapping executive functions respond less well to antidepressant medication than older depressed adults without these deficits. However, it is unclear whether patients with deficits on executive tasks respond the same to placebo or medication. The purpose of the present study is to extend our previous report and to determine whether there is a difference in outcome between treatment conditions (placebo or citalopram) among patients with and without deficits on the Stroop, a response inhibition task tapping a central component of the executive functions. To accomplish this goal, we use data from the Old-Old study (9), a randomized, double-blind, placebo-controlled trial of citalopram in depressed adults age 75 and older.
METHOD
The procedures used in the multi-site, randomized, placebo-controlled trial (RCT) have been previously described (9). Briefly, 174 community dwelling men and women 75 years or older meeting DSM-IV criteria (based on SCID interview) for non-psychotic unipolar depression (single or recurrent) with a baseline 24-item Hamilton Rating Scale for Depression (HRSD) score ≥ 20 participated in this 8 week RCT. All patients began the trial with a one-week single-blind placebo lead-in with the baseline visit conducted at the end of the lead-in. Patients were randomized to citalopram 20 mg/d or matched placebo only if they continued to meet inclusion and exclusion criteria at the end of the placebo lead-in. At the end of week four, patients with a HRSD score > 10 had the dose increased to two pills per day, i.e., 40 mg of citalopram or 2 placebo pills.
Patients were excluded if they met National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association criteria for probable Alzheimer’s disease or probable vascular dementia, had an MMSE score ≤18, or had bipolar disorder, obsessive-compulsive disorder, psychotic disorder, or current substance abuse or substance dependence within the past year (other than nicotine) by DSM-IV criteria. Participants did not receive additional treatment during this study.
Response inhibition, a fundamental executive function (10), was measured using a Stroop test that was adapted from standard color/word versions of the task (11), using a single item presentation and a button press response. The computerized task was programmed in the PsyScope (v1.1) programming language and presented on a Macintosh laptop computer, with responses recorded via an external keypad. Subjects responded “1” for red, “2” for blue, and “3” for green on a numeric keypad, using index, middle and ring fingers. Before each of the three conditions of the task, study participants were screened for visual acuity via a practice trial in which subjects were shown the three colors (red, blue, green) to determine if they could discriminate them. The three blocks of trials were administered in a fixed order. In the first block (word only condition), printed color names (Red, Blue, Green) were presented (in black). In the second block (color only condition), a string of XXXs was presented in different colors (Red, Blue, Green). In the third block, color names were again presented, but this time printed in incongruous colors. Subjects were given auditory feedback on each trial (beep for correct; buzz for error). Stimuli were presented individually and cleared after subject response, with a 50-ms ISI. Word and Color blocks included 45 stimulus trials (0.5–1.0 min run time each); the Color/Word block included 90 trials (1.0–2.0 min run time). Percent interference (percent increase in median reaction time in the color/word condition relative to the color condition) was used to summarize performance, and patients were classified as having or not having deficient response inhibition (DRI) if they performed in the highest (slowest) quartile. This version of the Stroop task has been used in previous studies in both depressed and non-depressed samples and produces consistent interference effects (12, 13).
Data Analysis
Multiple regression (14, 15) was used to test for differences at endpoint HRSD scores (week 8) between the four groups of patients while covarying for baseline HRSD scores, gender, age, and site of study. Dummy variable coding was used to designate each of the four groups (placebo treated patients without DRI, placebo treated patients with DRI, citalopram treated patients without DRI, and citalopram treated patients with DRI) with 1 indicating membership and 0 indicating non-membership. Each covariate was centered at its respective mean so that the intercept corresponded to the mean of the reference group at endpoint and the unstandardized regression weights reflect the difference between the groups included in the model and the reference group (excluded from the model). This dummy-variable coding approach to endpoint analysis is equivalent to conducting a general linear model with a main effect for drug, a main effect for the presence or absence of response inhibition, and their interaction (14, 16). All analyses were evaluated at the 5% level.
Prior to testing our hypotheses, we also used the PROC MI and MIANALYZE procedures in SAS to perform multiple imputation on any missing HRSD scores over the course of the study. Multiple imputation is a simulation technique that replaces each missing datum with a set of m > 1 plausible values (17). In our case, we chose m = 5, which generates 5 imputed data sets and is sufficient to obtain excellent results in most applications (18). The m complete data sets are analyzed using standard statistical analyses. The results from the analyses from the m complete data sets are combined by using Rubin’s rules (17, 18) to generate valid statistical inferences that reflect uncertainty due to missing values, and therefore, improve both the accuracy and often the statistical power of results. This approach represents an improvement over endpoint analyses based on last observation carried forward (LOCF) data, which makes the unlikely assumption that data are missing completely at random and patient response is constant from the last observation to the end of the trial, assumptions that are likely to lead to biased results (19).
RESULTS
Table 1 presents basic demographic and clinical characteristics of the total sample, the placebo and citalopram subsamples, and the four groups of patients classified according to treatment condition and DRI status at baseline. Twenty-one percent of the citalopram treated patients met criteria for DRI and twenty-three percent of the placebo treated patients met criteria. The average study participant was approximately 79 years old, 58% of the sample consisted of women, the average study participant completed 13.74 years of education, the average age-at-onset of depression was 68, 42% of the sample had recurrent depressive episodes, the average Cumulative Illness rating Scale –Geriatrics was 7.08, and the average depression severity rating at baseline for the sample as a whole was 24.31 on the 24-item HRSD. There were no differences in any of these variables between the two treatment conditions or between the four groups based on treatment condition and DRI status except for baseline MMSE. As can be seen in Table 1, baseline MMSE scores were slightly higher in the citalopram group as compared to the placebo group, t(156)= −2.53, p =.01. In the analysis of the four groups based on treatment condition and DRI status, there was a statistically significant omnibus test, F(3,154)−2.80, p = .042, but the differences between the four groups were not large enough to yield statistically significant post-comparisons.
Table 1.
Baseline Clinical and Demographic Characteristics for total sample, citalopram and placebo subsamples, and the four groups of patients classified according treatment condition and response inhibition status at baseline.
| Variable | Total Sample (n=174) |
Citalopram (n=84) |
Placebo (n=90) |
Placebo, No DRI (n=61) |
Placebo, DRI (n=21) |
Citalopram, No DRI (n=58) |
Citalopram, DRI (n=18) |
|---|---|---|---|---|---|---|---|
| Age | 79.57 (4.37) | 79.82 (3.99) | 79.33 (4.71) | 79.33 (5.08) | 79.38 (4.04) | 78.95 (2.97) | 82.05 (5.59) |
| Women | .58 | .54 | .62 | .40 | .19 | .48 | .44 |
| Site | 6.59 (4.14) | 6.78 (4.20) | 6.38 (4.10) | 6.85 (4.24) | 6.57 (4.18) | 6.33 (4.19) | 6.56 (3.90) |
| Education | 13.74 (3.31) | 13.79 (2.77) | 13.70 (3.78) | 13.85 (3.88) | 13.24 (3.52) | 13.69 (2.50) | 14.11 (3.56) |
| MMSE | 28.02 (2.15) | 28.46 (1.54) | 27.61 (2.53) | 27.80 (2.26) | 27.05 (3.19) | 28.45 (1.48) | 28.50 (1.76) |
| Age-at-Onset | 68.06 (18.73) | 67.31 (20.41) | 68.76 (17.10) | 69.17 (16.53) | 67.50 (19.10) | 69.70 (16.10) | 59.89 (29.56) |
| Recurrent MDD | .42 | .39 | .45 | .49 | .33 | .40 | .39 |
| CIRS-G | 7.08 (3.80) | 7.38 (3.83) | 6.80 (3.79) | 6.72 (4.00) | 7.05 (3.14) | 7.34 (4.11) | 7.50 (2.83) |
| HAMD 0 | 24.31 (4.21) | 24.17 (3.99) | 24.46 (4.45) | 24.48 (4.40) | 23.29 (2.26) | 24.90 (4.47) | 23.06 (4.21) |
| HAMD 8 | 13.77 (7.37) | 13.95 (7.50) | 13.54 (7.26) | 15.00 (7.42) | 10.94 (7.09) | 12.07 (6.31) | 17.50 (8.31) |
Note. MMSE = baseline Mini Mental Status Exam score; CIRS-G = baseline Cumulative Rating Scale - Geriatrics score; HAMD 0 and HAMD 8 = baseline and endpoint Hamilton Depression Rating Scale scores, respectively; DRI = deficient response inhibition; MDD = major depressive disorder.
Table 2 reports the unstandardized regression coefficients for the regression analysis comparing the four groups on endpoint depression severity scores adjusting for baseline depression severity, age, gender, and site of study. As can be seen from Table 2, there was no difference in endpoint HRSD scores between patients with and without DRI in the placebo condition. However, this model reveals that patients without DRI assigned to citalopram scored lower at endpoint than those assigned to placebo. The unadjusted difference between the placebo and citalopram conditions in endpoint HRSD scores was approximately 3 points (see Table 1), which corresponds to a medium effect (Cohen’s d = 0.43). In the second variation, placebo treated DRI patients had significantly lower scores than citalopram treated DRI patients. The unadjusted difference between placebo and citalopram among patients with DRI was approximately 6.5 points (see Table 1), which corresponds to a large effect (Cohen’s d = 0.88). In the third variation of the model, citalopram treated patients with DRI scored significantly higher than citalopram treated patients without DRI, which has been reported previously (5). Figure 1 depicts the differences in change between the groups in HRSD scores.
Table 2.
Regressed change analyses comparing treatment effects of either placebo or citalopram in patients with and without deficient response inhibition
| Citalopram | ||||||
|---|---|---|---|---|---|---|
| Parameter | B | SE(B) | 95% CI | t | df | p |
| Variation 1 (Reference group = Placebo, No DRI) | ||||||
| Intercept | 15.15 | .96 | 13.27, 17.03 | 15.83 | 5839.7 | .001 |
| Placebo, DRI | −3.37 | 1.86 | −7.02, .26 | −1.82 | 2040.6 | .069 |
| Citalopram, No DRI | −2.57 | 1.30 | −5.13, −.01 | −1.97 | 82407 | .049 |
| Citalopram, RI | 2.23 | 2.16 | −2.06, 6.51 | 1.03 | 104.35 | .305 |
| Variation 2 (Reference group = Placebo, DRI) | ||||||
| Intercept | 11.77 | 1.61 | 8.61, 14.95 | 7.30 | 798.53 | .001 |
| Placebo, No DRI | 3.37 | 1.86 | −.27, 7.02 | 1.82 | 2040.6 | .069 |
| Citalopram, No DRI | .80 | 1.89 | −2.90, 4.51 | .42 | 2163.3 | .671 |
| Citalopram, DRI | 5.60 | 2.50 | .68, 10.52 | 2.25 | 210.1 | .026 |
| Variation 3 (Reference group = Citalopram, No DRI) | ||||||
| Intercept | 12.58 | .99 | 10.62, 14.54 | 12.58 | 351658 | .001 |
| Placebo, No DRI | 2.57 | 1.31 | .01, 5.14 | 1.97 | 82407 | .049 |
| Placebo, DRI | −.80 | 1.88 | −4.51, 2.90 | −.42 | 2163.3 | .671 |
| Citalopram, DRI | 4.80 | 2.19 | .46, 9.13 | 2.20 | 107.4 | .030 |
Note. Dependent variable = Week 8 HRSD score. All models covary for baseline HRSD, site, age, and gender centered at their means (not tabled). DRI = deficient response inhibition; B = unstandardized regression weight, SE = standard error, and CI = confidence interval.
Figure 1.
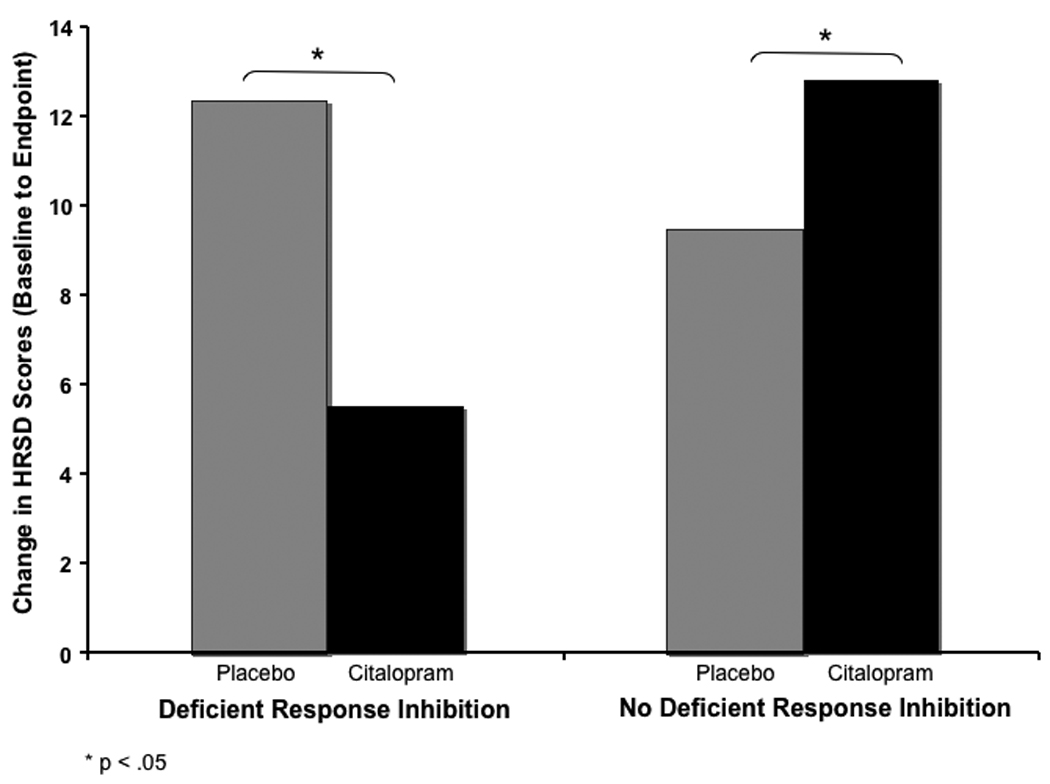
Change in unadjusted HRSD scores between placebo and citalopram conditions among patients with deficient response inhibition, t(82407) = −1.97, p = .049, and without deficient response inhibition, t(210.1) = −2.25, p = .026.
DISCUSSION
Although a number of studies have shown that DRI predicts poor response to antidepressant medication (2–5), this is the first report that we know of showing a significant difference in response between medication and placebo in depressed patients with DRI. Extending our previous report (5), it is not simply that depressed older adults with DRI respond poorly to antidepressant medication, in fact these patients respond less well than they would if they had been treated with placebo.
We also found a significant difference between citalopram and placebo among depressed patients without DRI, which contradicts a previous report indicating no difference between treatment conditions based on the total sample (9). Thus, this study suggests that DRI is a potential confound of treatment response in the analysis of antidepressant medication trials and should be considered when interpreting the results of clinical trials conducted in late-life depression that did not find a significant difference between medication and placebo (9, 20–22). Future studies involving late-life depressed patients should report moderator analyses using a measure of DRI.
Although the mechanism of action of the placebo effect is unknown, the findings from this study indicate that it is different from SSRIs. Brain responses to antidepressant medications have been described as “placebo plus.” In a well known comparison of regional brain glucose metabolism between placebo and SSRI responders, Mayberg et al. (23) found that both placebo and drug response were associated with increased metabolism in the prefrontal cortex, premotor cortex, inferior parietal cortex, posterior insula, and posterior cingulate and decreased metabolism in the subgenual cingulate, hypothalamus, thalamus, and parahippocampus. However, drug response was also associated with metabolism changes in other areas, such as the striatum, which were not associated with placebo response. These data may help explain why drug response should be preferentially reduced compared to placebo response in the current study. Patients with DRI have white matter lesions that may interrupt fronto-striatal pathways, possibly disrupting the connectivity of brain regions known to be associated with drug response over and above the placebo response.
The topic of this report raises the critical issue as to what is meant by the use of the term executive dysfunction in geriatric psychiatry and what aspects of it interact negatively with antidepressant medication. The term executive dysfunction is “vague and ill defined” (27). The executive functions refer to a theorized cognitive system that involves the monitoring and allocation of attentional resources and subsumes a broad class of functions including planning, organization, problem solving, cognitive flexibility such as set shifting and switching, updating and monitoring of working memory representations, error monitoring, and response inhibition (1, 24, 25). Typically, the term executive dysfunction is used despite the fact that only one or two components of the executive functions were assessed in the study or a single composite score is used based on an executive functions screening tool. In the latter case, although a broad array of functions is assessed, it is not clear from the composite score which aspect of executive functioning is affected. Therefore, we recommend when reporting results of studies using tests tapping the executive functions that researchers are specific to the test being used and do not generalize to the broader class of executive functions.
The second issue that is raised is what aspects of executive functioning interact negatively with antidepressant medication. Not all studies demonstrating interference with treatment response have measured the same components of the executive functions or used the same tests. For example, some studies have used the Stroop and the Attention Network Test (2, 5, 6), which are response inhibition tasks, whereas other studies have used the initiation/perseveration subtest of the Dementia Rating Scale (3, 8), which incorporates a number of different components but may be most accurately described as a test of fluency (26). It is not clear what the relationship is between response inhibition tasks and the initiation/perseveration subtest of the Dementia Rating Scale, and furthermore, why they are independently predictive of poor antidepressant treatment response. One possible explanation for the common effect is that both response inhibition and fluency tasks require cognitive flexibility and that deficits in cognitive flexibility predict poor treatment outcome.
As for the mechanism of executive dysfunction interference on antidepressant response, one possibility is disruption of frontostriatal tracts connecting the striatum to the prefrontal cortex, an important locus of central executive functioning as well as emotion regulation (27, 28). Consistent with this hypothesis, patients with late-onset depression and microvascular ischemic disease also tend to show deficits in executive functioning (29–31). Although executive tasks activate a diffuse network of brain areas suggesting that the neuroanotomical basis for the executive functions is widely distributed (24), the central locus of response inhibition appears to be the anterior cingulate cortex (ACC) and dorsolateral prefrontal cortex (DLPFC) as seen on Positron Emission Tomography (PET) and functional magnetic resonance imaging (fMRI) studies (32–35). Specifically, it has been suggested that the ACC is implicated in response-related processes such as conflict detection and monitoring, but that activity in this region decreases as the level of response conflict is reduced with practice and as attentional control is established in the DLPFC (36, 37). Thus, microvascular ischemic disease may interfere with the circuits necessary for both the therapeutic action of antidepressant medication and executive functioning in general and response inhibition in particular. It is interesting to note that Sneed et al. (5) examined the relationship between response inhibition and qualitative ratings of microvascular ischemia using data from the Old-Old Study and found no relationship.
The Stroop Color-Word Test is a complex neuropsychological task that focuses on the slowing of reaction time in the color-word conflict condition relative the color-only condition. As opposed to a specific deficit in response inhibition, it is possible that a generalized slowing of reaction time or a slowing that occurs with greater cognitive demand accounted for the relationship between baseline Stroop performance and antidepressant response. However, we have shown in previous reports that the effect of DRI on antidepressant treatment response is independent of reaction time (Sneed, et al., 2007). We have also shown that poor antidepressant response is not associated with performance on any other neuropsychological task including mental status, psychomotor speed, reaction time, spatial judgment, or memory (7).
This study should be interpreted in the context of several limitations, which are balanced by using data from the only randomized, placebo-controlled clinical trial of antidepressant treatment among depressed patients age 75 or older. One possibility is that these findings do not hold in younger samples. However, the effect of response inhibition on treatment response has been shown in both young-old (2–4, 6, 8) and old-old samples (5). This study represents the first report of its kind and awaits replication in both young-old and old-old samples. Another possibility is that task novelty in the elderly might limit the validity of the computerized Stroop task used in this study. However, these novelty issues are more likely in less educated subjects, and if education predicted treatment response equally as well as Stroop performance, one could make an argument that there is some general novelty or testing familiarity effect. However, we included education as a covariate to test for this possibility and it did not substantively change the findings. Finally, response inhibition was the only component of the executive functions used in this study. The executive functions represent a multifaceted class of functions and, therefore, these findings may not generalize to other tests of executive functioning.
The findings from this study are clinically significant and have important implications for the treatment of late-life depression. We extended our previous report by showing that patients with depression and DRI respond worse than they would have had they been assigned to treatment with placebo. The observed effects were moderate to large in size indicating not only statistical significance but also that the findings are meaningful and detectable in small samples. These findings also imply that the mechanism of SSRI and placebo response is different, which may lead to important breakthroughs with regard to the treatment of depression with cognitive impairment in the elderly.
Acknowledgments
This research was supported by a grant from Forest Laboratories and National Institute of Mental Health grants T32 MH20004 (Steven P. Roose) and K23 MH075006 (Joel R. Sneed).
Footnotes
Disclosures: S.P.R. reports receiving a research grant from Forest Laboratories as well as consultant fees from Forest Laboratories, Wyeth Pharmaceuticals, Sanofi-Anventis, Pfizer, and Sierra Pharmaceuticals. D.P.D. reports receiving a research grant from Eli Lilly and Novartis as well as consultant fees from Glaxosmithkline, Acadia, and Sanofi-Aventis.
REFERENCES
- 1.Podell K, Lovell MR. Neuropsychological assessment. In: Coffey CE, Cummings JL, editors. Textbook of Geriatric Neuropsychiatry. Washington, DC: American Psychiatric Press; 2000. [Google Scholar]
- 2.Alexopoulos G, Kiosses DN, Heo M, Murphy CF, Shanmugham B, Gunning-Dixon F. Executive dysfunction and the course of geriatric depression. Biological Psychiatry. 2005;58:204–210. doi: 10.1016/j.biopsych.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 3.Kalayam B, Alexopoulos GS. Prefrontal dysfunction and treatment response in geriatric depression. Archives of General Psychiatry. 1999;56(8):713–718. doi: 10.1001/archpsyc.56.8.713. [DOI] [PubMed] [Google Scholar]
- 4.Murphy GF, Alexopoulos GS. Attention network dysfunction and treatment of response of geriatric depression. Journal of Clinical and Experimental Neuropsychology. 2006;28:96–100. doi: 10.1080/13803390490918101. [DOI] [PubMed] [Google Scholar]
- 5.Sneed JR, Roose SP, Keilp JG, Krishnan KRR, Alexopoulos GS, Sackeim HA. Response inhibition predicts poor antidepressant treatment response in very old depressed patients. American Journal of Geriatric Psychiatry. 2007;15:553–563. doi: 10.1097/JGP.0b013e3180302513. [DOI] [PubMed] [Google Scholar]
- 6.Baldwin R, Jeffries S, Jackson A, et al. Treatment response in late-onset depression: relationship to neuropsychological, neuroradiological and vascular risk factors. Psychological Medicine. 2004;34(1):125–136. doi: 10.1017/s0033291703008870. [DOI] [PubMed] [Google Scholar]
- 7.Sneed JR, Keilp JG, Brickman AM, Roose SP. The specificity of neuropsychological impairment in predicting antidepressant non-response in the very old depressed. International Journal of Geriatric Psychiatry. 2008;23:319–323. doi: 10.1002/gps.1889. [DOI] [PubMed] [Google Scholar]
- 8.Alexopoulos GS, Meyers BS, Young RC, et al. Executive dysfunction and long-term outcomes of geriatric depression. Archives of General Psychiatry. 2000;57(3):285–290. doi: 10.1001/archpsyc.57.3.285. [DOI] [PubMed] [Google Scholar]
- 9.Roose SP, Sackeim HA, Krishnan KRR, et al. Antidepressant pharmacotherapy in the treatment of depression in the very old: A randomized, placebo-controlled trial. American Journal of Psychiatry. 2004;161(11):2050–2059. doi: 10.1176/appi.ajp.161.11.2050. [DOI] [PubMed] [Google Scholar]
- 10.Lezak MD. Neuropsychological Assessment. NY: Oxford University Press; 1995. [Google Scholar]
- 11.MacLeod C. A half-century of research on the Stroop effect: An integrative review. Psychological Bulletin. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- 12.Keilp JG, Sackeim H, Mann JJ. Correlates of trait impulsiveness in performance measures and neuropsychological tests. Psychiatry Research. 2005;135:191–201. doi: 10.1016/j.psychres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Keilp JG, Gorlyn M, Oquendo MA, Burke AK, Mann JJ. Attention deficit in depressed suicide attempters. Psychiatry Research. 2008;159:7–17. doi: 10.1016/j.psychres.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation for the behavioral sciences. 3rd ed. Mahwah, NJ: Lawrence Erlbaum Publishers; 2003. [Google Scholar]
- 15.Fleiss JL. Design and analysis of clinical experiments. NY: John Wiley & Sons; 1986. [Google Scholar]
- 16.Cohen J. Multiple regression as a general data-analytic system. Psychological Bulletin. 1968;70:426–443. [Google Scholar]
- 17.Schafer JL, Olsen MK. Multiple imputation for multivariate missing-data problems: A data analyst's perspective. Multivariate Behavioral Research. 1998;33(4):545–571. doi: 10.1207/s15327906mbr3304_5. [DOI] [PubMed] [Google Scholar]
- 18.Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- 19.Mallinckrodt CH, Kaiser CJ, Watkin JG, Molenberghs G, Carroll RJ. The effect of correlation structure on treatment contrasts estimated from incomplete clinical trial data with likelihood-based repeated measures compared with last observation carried forward. Clinical Trials. 2004;1:477–489. doi: 10.1191/1740774504cn049oa. [DOI] [PubMed] [Google Scholar]
- 20.Bose A, Dayong L, Gandhi C. Escitalopram in the acute treatment of depressed patients aged 60 years or older. American Journal of Geriatric Psychiatry. 2008;16:14–20. doi: 10.1097/JGP.0b013e3181591c09. [DOI] [PubMed] [Google Scholar]
- 21.Kasper S, de Swart H, Andersen HF. Escitalopram in the treatment of depressed elderly patients. American of Geriatric Psychiatry. 2005;13:884–891. doi: 10.1176/appi.ajgp.13.10.884. [DOI] [PubMed] [Google Scholar]
- 22.Schatzberg A, Roose S. A double-blind, placebo-controlled study of venlafaxine and fluoxetine in geriatric outpatients with major depression. American Journal of Geriatric Psychiatry. 2006;14:361–370. doi: 10.1097/01.JGP.0000194645.70869.3b. [DOI] [PubMed] [Google Scholar]
- 23.Mayberg HS, Silva JA, Brannan SK, et al. The functional neuroanatomy of the placebo effect. Am J Psychiatry. 2002;159(5):728–737. doi: 10.1176/appi.ajp.159.5.728. [DOI] [PubMed] [Google Scholar]
- 24.Garavan H, Ross TJ, Li SJ, Stein EA. A parametric manipulation of central executive functioning. Cerebral Cortex. 2000;10:585–592. doi: 10.1093/cercor/10.6.585. [DOI] [PubMed] [Google Scholar]
- 25.Miyake A, Freidman NP, Emerson MJ, Witzki AA, Howerter A. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 26.Marson DC, Dymak MP, Duke LW, Harrell LE. Subscale validity of the Mattis Dementia Rating Scale. Archives of Clinical Neuropsychology. 1997;12:269–275. [PubMed] [Google Scholar]
- 27.Gunning-Dixon F, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: A prospective MRI study. Neuropsychologia. 2003;41:1929–1941. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- 28.Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: An update. Journal of Psychosomatic Research. 2002;53:647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- 29.Salloway S, Malloy P, Kohn R, et al. MRI and neuropsychological differences in early- and late-life-onset geriatric depression. Neurology. 1996;46(6):1567–1574. doi: 10.1212/wnl.46.6.1567. [DOI] [PubMed] [Google Scholar]
- 30.Alexopoulos GS, Meyers BS, Young RC, Kakuma T, Silbersweig D, Charlson M. Clinically defined vascular depression. American Journal of Psychiatry. 1997;154(4):562–565. doi: 10.1176/ajp.154.4.562. [DOI] [PubMed] [Google Scholar]
- 31.Lesser I, Boone K, Mehringer C, Wohl M, Miller B, Berman N. Cognition and white matter hyperintensities in older depressed patients. American Journal of Psychiatry. 1996;153(10):1280–1287. doi: 10.1176/ajp.153.10.1280. [DOI] [PubMed] [Google Scholar]
- 32.Leung HC, Skudlarski P, Gatenby JC, Peterson BS, Gore JC. An event-related functional MRI study of the stroop color word interference task. Cereb Cortex. 2000;10(6):552–560. doi: 10.1093/cercor/10.6.552. [DOI] [PubMed] [Google Scholar]
- 33.MacDonald AW, III, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 34.Carter CS, Mintun M, Cohen JD. Interference and facilitation effects during selective attention, an H215O PET study of Stroop task performance. NeuroImage. 1995;2:264–272. doi: 10.1006/nimg.1995.1034. [DOI] [PubMed] [Google Scholar]
- 35.Harrison BJ, Shaw M, Yücel M, et al. Functional connectivity during Stroop task performance. Neuroimage. 2005;24(1):181–191. doi: 10.1016/j.neuroimage.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 36.Milham MP, Banich MT, Claus ED, Cohen NJ. Practice-related effects demonstrate complementary roles of anterior cingulate and prefrontal cortices in attentional control. NeuroImage. 2003;18:483–493. doi: 10.1016/s1053-8119(02)00050-2. [DOI] [PubMed] [Google Scholar]
- 37.Erickson KI, Milham MP, Colcombe SJ, et al. Behavioral conflict, anterior cingulate cortex, and experiment duration: implications of diverging data. Hum. Brain Mapp. 2004;21:98–107. doi: 10.1002/hbm.10158. [DOI] [PMC free article] [PubMed] [Google Scholar]


