Abstract
Rationale
Lamina-Associated Polypeptide 2α (LAP2α) is a mammalian chromatin-binding protein that interacts with a fraction of A-type lamins in the nuclear interior. As mutations in lamins and LAP2α lead to cardiac disorders in humans, we hypothesized that these factors may play important roles in heart development and adult tissue homeostasis.
Objective
We asked whether the presence of LAP2α was required for normal cardiac function.
Methods and results
To study the molecular mechanisms of the disease, we analyzed heart structure and function in complete and conditional Lap2α−/− mice as well as Lap2α−/−/Mdx mutants. Unlike conditional deletion of LAP2α in late embryonic striated muscle, its complete knockout caused systolic dysfunction in young mice, accompanied by sporadic fibrosis in old animals, as well as deregulation of major cardiac transcription factors GATA4 and MEF2c. Activation of compensatory pathways, including downregulation of β-adrenergic receptor signaling, resulted in reduced responsiveness of the myocardium to chronic β-adrenergic stimulation and stalled the progression of LAP2α-deficient hearts from hypertrophy towards cardiac failure. Dystrophin deficiency in an Mdx background resulted in a transient rescue of the Lap2α−/− phenotype.
Conclusion
Our data suggest a novel role of LAP2α in the maintenance of cardiac function under normal and stress conditions.
Keywords: Lamins, LAP2α, dilated cardiomyopathy, β-adrenergic receptors
Introduction
Dilated cardiomyopathy (DCM) is a primary myocardial disease characterized by dilation and impaired contraction of one or both heart ventricles. One of the genes most frequently involved in the development of DCM is LMNA, which encodes the nuclear intermediate filament proteins lamin A and lamin C1. Mutations in lamin A/C cause the most severe forms of DCM, posing a high risk of heart failure in symptomatic patients1. Besides heart muscle disease, mutations in LMNA cause a variety of pathological conditions in skeletal muscle, skin, nerve, bone and adipose tissue, known as laminopathies2, emphasizing the importance of the search for molecular disease mechanisms.
Recently, research on laminopathies has focused on lamin A/C-interacting proteins, whose mutations have been linked to a similar spectrum of human disorders3. One of the best studied lamin A/C-binding partners is Lamina Associated Polypeptide 2α (LAP2α), an unusual splice variant of the mammalian LAP2 gene4. All LAP2 proteins (α, β, γ, δ, ε, ζ) share a common chromatin-binding structural motif called the LEM (LAP2-Emerin-MAN1) domain at their N-terminus. The C-terminus of most LAP2 variants comprises a transmembrane region, which targets them to the inner nuclear membrane, where they serve mainly structural roles5. LAP2α lacks the common LAP2 transmembrane domain and possesses an additional chromatin-binding region at its C-terminal end, which mediates targeting to the nuclear interior4. In the nucleoplasm LAP2α specifically interacts with a fraction of lamin A/C via its unique C-terminal tail6. Together, LAP2α and lamin A/C influence various nuclear processes, such as epigenetic chromatin regulation, gene expression and signal transduction7. In particular, LAP2α-lamin A/C complexes have been found to control the balance between proliferation and differentiation of early progenitor cells in regenerative tissues by affecting the E2F/retinoblastoma pathway8.
Interestingly, a mutation in LAP2α (c. 2068C>T, p. R690C), which lowers its binding affinity for lamin A/C in vitro, has also been linked to DCM9. As changes in both LAP2α and lamin A/C cause pathological heart conditions, we hypothesized that an intact LAP2α-lamin A/C complex may be necessary for proper cardiac function and that mislocalization or absence of one of the components would lead to disease.
Most Lmna transgenic mice generated so far show complex phenotypes, including various stages of heart failure10, 11. To see whether the presence of LAP2α is also important for normal cardiac output, we analyzed heart structure and function in previously generated Lap2α−/− mice8. Here we describe a new mouse model of cardiomyopathy and provide novel insights into mechanisms governing heart development and tissue homeostasis.
Materials and Methods
Mice
Lap2α−/− mice were kept on a mixed Mus musculus C57BL/6×129 genetic background8. Compound mutant Lap2α−/−/Mdx animals were obtained by crossing Lap2α−/− mice with the Mdx line12. Generation of conditional LAP2α knockout mice is described in Online Figure I. All histological and physiological analyses were done by observers blinded for the genotype, as well as treatment of the animal. Mice aged 2 days were considered as newborn, 10 weeks as young and 10–12 months as old.
Mice were kept and handled in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals. Experiments were performed according to permissions from Austrian authorities.
Echocardiography
Mice were anesthetized with 5% isoflurane/O2 and maintained on 2% mixture during the experiment. Trans-thoracic echocardiography was performed using Vingmed Vivid Five (GE Technology) equipped with a 10-MHz linear transducer13.
Gravimetric, histological and biochemical analyses
were performed according to standard procedures described in more detail in the Online Supplement.
Isoproterenol (ISO)-induced heart failure model
Mice anesthesia and echocardiography were performed as described above. Alzet® mini-osmotic pumps (1007D), filled with 15 mg/kg/day isoproterenol/PBS (Sigma Aldrich), were implanted according to manufacturer’s instructions. 6 old male Lap2α+/+ and 7 Lap2α−/− mice were infused with ISO for 7 days and 2 littermate couples received only PBS (sham).
Statistical analysis
Experimental outliers were identified using Grubb’s test and excluded from further analyses. One mouse was removed from the initial echocardiography data (FS% = 20.76, FS%mean = 35.54, SD = 5.39, n = 12) and one littermate pair from the MEF2c mRNA expression analysis (KO/WT = 4.82, KO/WTmean = 1.80, SD = 1.74, n = 5). Data were analyzed either by paired Student’s t-test, one-way ANOVA or two-way ANOVA (followed by Boniferroni post hoc test for multiple comparisons) where appropriate, using Microsoft Excel XP. P<0.05 was considered significant. Presented values are means ± standard error (SE).
Results
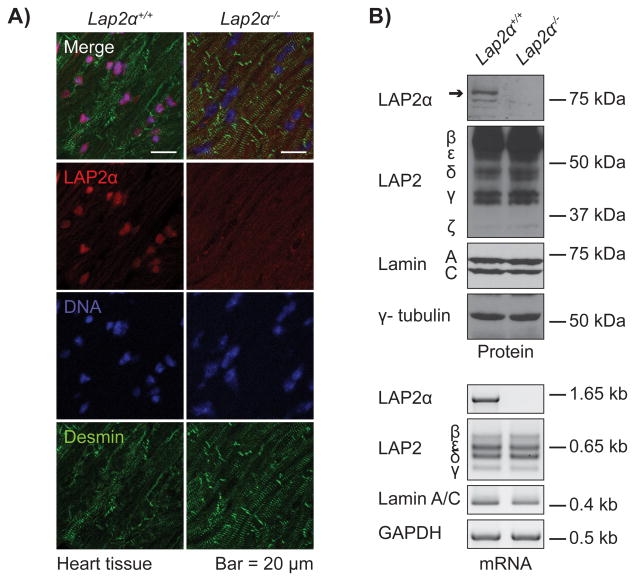
LAP2α-deficient mice used in this study were generated by Cre recombinase-mediated excision of the LAP2α-specific exon 4 of the Lap2 gene in the germline8. To confirm the absence of the gene product, we performed western blot, semi-quantitative PCR (semiqPCR) and immunofluorescence analyses of Lap2α−/− heart muscle tissue. LAP2α was detectable neither at an mRNA nor a protein level, while the expression, as well as localization of lamin A/C and other LAP2 isoforms were not significantly altered (Figure 1, Online Figure II).
Figure 1. LAP2α is absent from cardiac tissue of Lap2α−/− mice.
A) Immunofluorescence analysis of Lap2α+/+ and Lap2α−/− heart tissue. B) LAP2α mRNA and protein are absent from heart muscle tissue of Lap2α−/− mice, while the expression of lamin A/C and alternative LAP2 splice variants remains unaltered. (Western blot and semiqRT-PCR analyses; samples were normalized for endogenous γ-tubulin and Glyceraldehyde 3-phosphate Dehydrogenase (GAPDH) content respectively; n = 3 male + 3 female littermate pairs).
Absence of LAP2α causes ventricular systolic dysfunction in mice
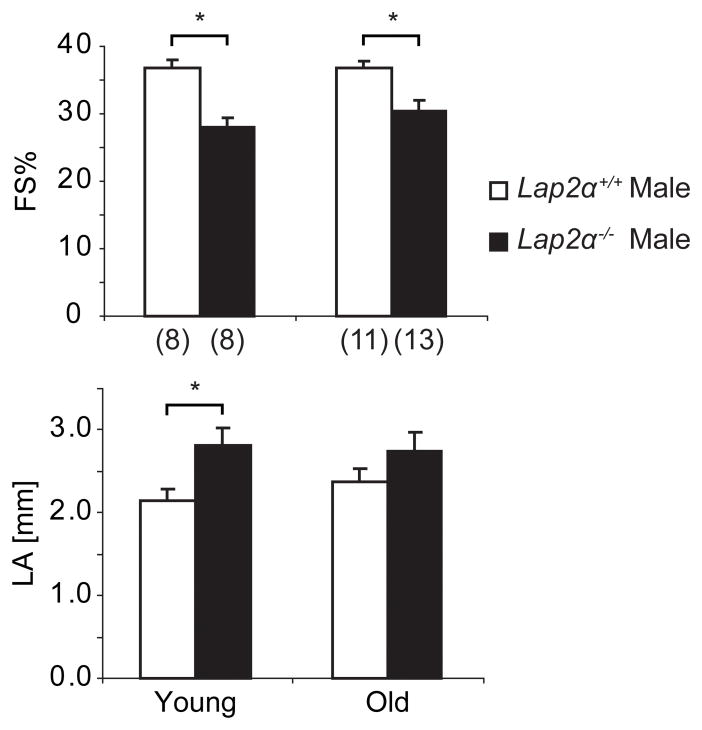
The symptoms in LAP2α- and most lamin A/C-linked human cardiomyopathies appear predominantly in adults5, 9. In mouse lamin A/C-linked laminopathy models, however, heart defects develop in young animals and cause early mortality10, 11. As Lap2α−/− mice are grossly indistinguishable from their WT littermates and have a normal life expectancy8, we analyzed heart function in young, as well as old animals. Echocardiography in 10 weeks old Lap2α−/− mice revealed ventricular systolic dysfunction, characterized by significantly decreased left ventricular fractional shortening (FS%) and ejection fraction (EF%) values. Moreover, enlarged left atria (LA) in male LAP2α-deficient mice emphasized the defective cardiac phenotype, indicating a possible left ventricular and left atrial volume overload (Figure 2, Online Table I). The FS% and EF% values remained similarly depressed in old Lap2α−/− male mice (aged 10–12 months), suggesting that the functional defect was not progressive. Interestingly, cardiac parameters in Lap2α−/− females appeared largely comparable to WT at both ages (Online Table I).
Figure 2. Loss of LAP2α impairs heart function in mice.
Echocardiography data show left ventricular systolic dysfunction in young (10 weeks) and old (10–12 months) male Lap2α−/− mice. (FS% – fractional shortening; LA – left atrium diameter; (n) - number of mice; *p<0.05 ANOVA, values are means ± SE).
Old Lap2α−/− mice present only sporadic cases of cardiac fibrosis
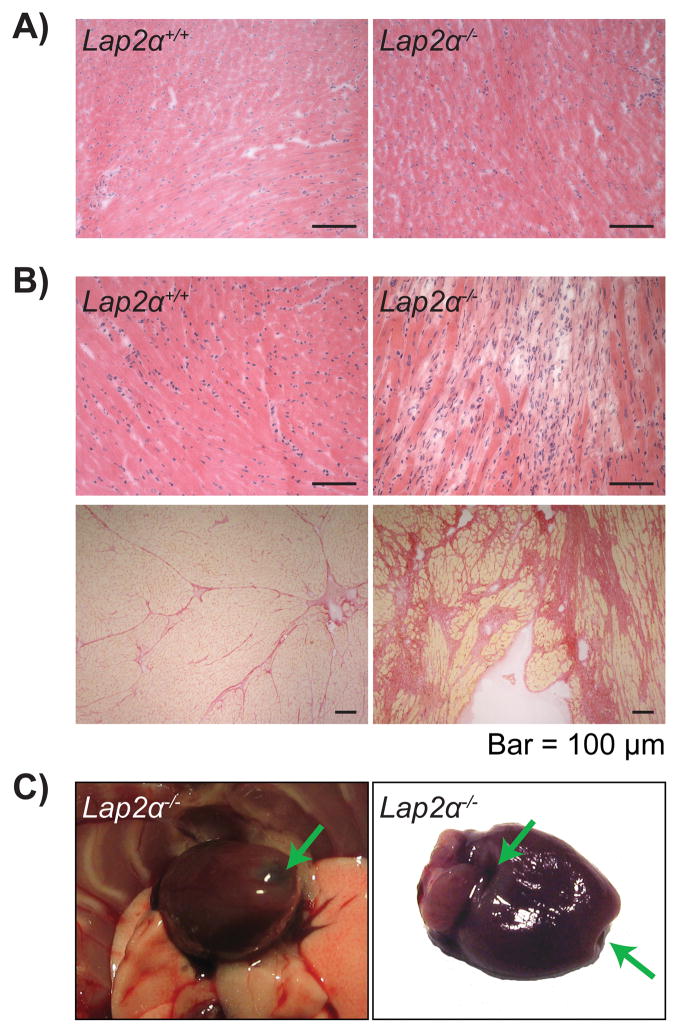
To see whether the functional defect in Lap2α−/− hearts was accompanied by structural changes, we performed gravimetric and histological analyses. Lap2α−/− hearts had normal morphology and similar heart weight/body weight (HW/BW) indexes compared to WT littermates in newborn, young and old animals (data not shown). Furthermore, no overt histological pathologies were detectable in young Lap2α−/− mice (Figure 3A). Old Lap2α−/− hearts, however, showed high phenotypic variability in the degree of fibrosis, as assessed by the extent of interstitial collagen deposition in the left ventricle. Although the overall difference in the extent of cardiac fibrosis between Lap2α−/− and WT mice was found to be statistically insignificant (Online Figure III), out of 11 Lap2α−/− tested mice, 18% developed extensive subendocardial fibrosis of the left ventricle (Figure 3B). In addition to fibrosis, one mouse presented regions of extremely thin, transparent myocardium which collapsed in the absence of internal blood pressure (Figure 3C). In contrast, WT animals did not exhibit signs of increased fibrosis at any age.
Figure 3. Old Lap2α−/− mice show sporadic cases of fibrosis.
A) Young male Lap2α−/− myocardium is histologically indistinguishable from the WT. B) 18% of the old male Lap2α−/− hearts show disperse fibrotic foci (upper panels haematoxylin & eosin-stained, lower panels Picrosirius Red-stained heart sections). C) Thinning of the old Lap2α−/− myocardium – arrows mark the transparent fibrotic regions of the heart.
De-regulated expression of major cardiac transcription factors in male LAP2α-deficient mice
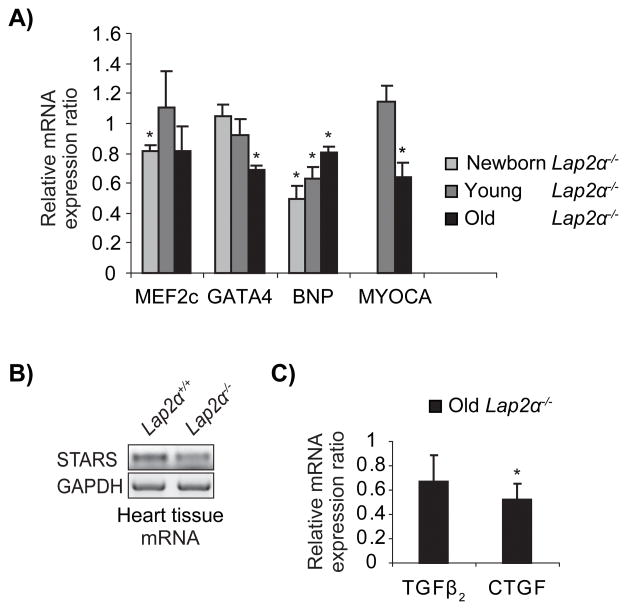
In an attempt to identify the molecular mechanisms leading to systolic dysfunction in Lap2α−/− mice, we analyzed the expression of several markers connected to myocardial remodeling. Two major cardiac transcription factors, GATA4 and MEF2c, as well as their downstream targets – brain natriuretic peptide (BNP) and proteins involved in Serum Response Factor (SRF)-mediated transcription of immediate early and muscle-specific genes, Myocardin A and Striated Muscle Activator of Rho Signaling (STARS)14–18 – showed deregulated expression patterns in male Lap2α−/− mice (Figure 4A, B). Whereas MEF2c expression was repressed in newborn Lap2α−/− hearts and reached WT levels by the age of 10 weeks, GATA4, Myocardin A and STARS were down-regulated only in old hearts. In addition, consistent with the reported cooperative regulation of BNP expression by both GATA4 and MEF2c18, BNP mRNA levels were significantly lower in LAP2α-deficient hearts compared to WT at all ages (Figure 4A). The expression of other MEF2c and GATA4 targets, such as α- and β-myosin heavy chain and atrial natriuretic factor (ANF)19 was not significantly changed in Lap2α−/− mice (data not shown). The expression of the aforementioned factors in female Lap2α−/− hearts was comparable to WT (data not shown). Our data show that loss of LAP2α affects the expression of GATA4 and MEF2c, as well as some of their downstream targets, which in turn may influence the expression of a plethora of genes involved in cardiovascular development and stress-induced hypertrophic growth.
Figure 4. Deregulated expression of major cardiac transcription factors in LAP2α-deficient mice.
A) MEF2c, GATA4 and their downstream targets, BNP and Myocardin A (MYOCA), are downregulated on mRNA level at specific stages in Lap2α−/− mice. B) STARS mRNA levels are lower only in old LAP2α-deficient hearts. C) Old Lap2α−/− mice exhibit decreased CTGF expression and normal TGFβ2 mRNA content. (Quantitative PCR (qPCR) and semiqPCR analyses of heart tissue. n = 4 – 5 male littermate pairs of each age. One outlier pair was excluded according to Grubb’s test. Samples were normalized for endogenous GAPDH levels in semiqPCR and HPRT (Hypoxanthine-Guanine Phosphoribosyltransferase) levels in qPCR and analyzed according to Pfaffl method44. Each knockout (KO) sample was compared to its respective WT littermate sample. Values are mean KO/WT expression ratios ± SE. *p<0.05, ANOVA).
Since old Lap2α−/− mice showed occasional fibrosis, we analyzed the expression of known fibrotic markers in WT and LAP2α-deficient hearts (Figure 4C). Interestingly, loss of LAP2α caused a downregulation of connective tissue growth factor (CTGF), an inducer of fibroblast proliferation and extracellular matrix synthesis20, but affected only mildly the expression of its activator, the transforming growth factor β2 (TGFβ2). Altogether, our data demonstrate that loss of LAP2α in mice leads to deregulated expression of genes involved in cardiac remodeling and fibrosis.
Lap2α−/− mice show a blunted response to chronic isoproterenol infusion
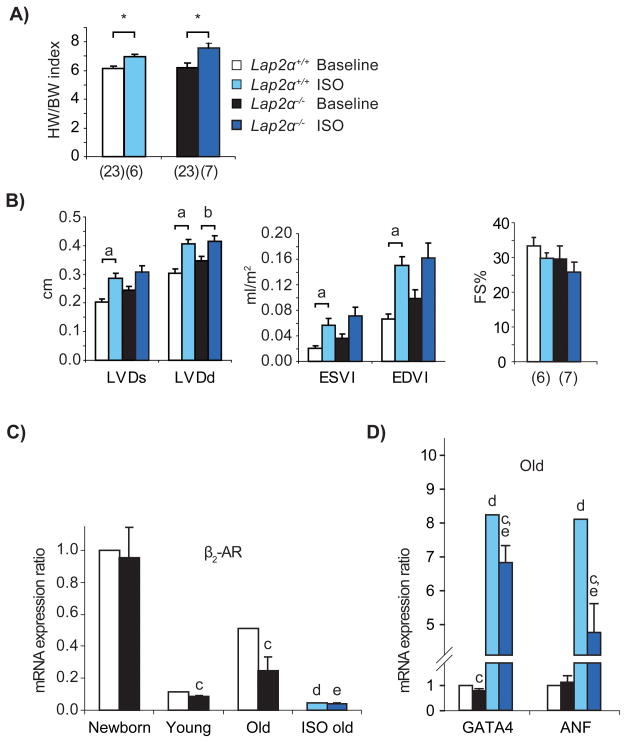
GATA4 and MEF2c play important roles during cardiomyocyte hypertrophy21, 22. To see whether their deregulation in Lap2α−/− hearts affects their response to cardiac hypertrophic stimuli, we subjected Lap2α−/− mice to chronic infusion of the β-adrenergic agonist isoproterenol (ISO) for 7 days. To detect ISO-induced changes in heart function and structure, we performed echocardiography before and after the treatment, as well as gravimetric and histological analyses at the end of the experiment. As shown by the HW/BW indexes, ISO administration caused a similar degree of cardiac hypertrophy in Lap2α+/+ and Lap2α−/− mice (Figure 5A), indicating that the hypertrophic growth response is not grossly affected by the loss of LAP2α. Similarly, the extent of subendocardial fibrosis caused by chronic ISO infusion23 was comparable in Lap2α+/+ and Lap2α−/− mice (Online Figure IV).
Figure 5. Lap2α−/− mice show a blunted response to chronic isoproterenol infusion.
A) Lap2α+/+ and Lap2α−/− male mice exhibit a similar increase in heart weight/body weight (HW/BW) indexes after 7 days of ISO treatment. (*p<0.05 ANOVA). B) Echocardiography reveals the absence of significant left ventricular chamber dilation in Lap2α−/− mice and preserved basal systolic function in both genotypes. [LVDd/s – left ventricular diameter in diastole/systole; ESVl – end-systolic left ventricular volume index; EDVl – end-diastolic left ventricular volume index; (n) - sample size. Data were analyzed using Student’s paired t-test (comparisons within one genotype before and after the treatment) and one way-ANOVA (comparisons between the two genotypes). (a) p<0.05, (b) p = 0.05]. C) Relative baseline mRNA levels of β2-AR are lower in Lap2α−/− hearts compared to WT, whereas ISO treatment causes its downregulation in both genotypes. D) ISO-induced increase in expression of fetal and pro-fibrotic genes is diminished in the absence of LAP2α. (qPCR analyses of heart tissue, n = 5 ISO-treated + 4 – 5 untreated male littermate pairs of each age; ANOVA, p<0.05 for (c) WT vs. KO of the respective age, (d) WT baseline vs. WT ISO-treated and (e) KO baseline vs. KO ISO-treated animals, mean ± SE).
In addition to hypertrophy, ISO treatment caused dilation of left heart ventricles, as shown by echocardiography (Figure 5B, Online Table II). In WT animals, systolic and diastolic left ventricular diameters (LVD) and the corresponding ventricular volumes exhibited a significant increase, indicating the progression from cardiac hypertrophy towards heart failure. In contrast, left ventricles of Lap2α−/− mice were already slightly enlarged at the baseline and showed only milder additional dilation upon ISO treatment, reaching end point sizes similar to WT. HW/BW indexes and echocardiography parameters of sham (PBS) treated animals were not altered by the procedure (Online Table II). These data point to a blunted cardiac stress response in LAP2α-deficient mice.
FS values in treated animals were only slightly affected, showing that chronic ISO infusion did not significantly impair left ventricular systolic function either in Lap2α+/+ or Lap2α−/− mice (Figure 5B, Online Table II). This is in agreement with previous studies23.
The preserved basal systolic function in ISO-induced cardiac hypertrophy has been associated with downregulation of β-adrenergic receptor (β-AR)-mediated inotropic responses23. An ISO-induced downregulation of β-AR has also been shown to cause a blunted reactivity to its subsequent re-administration23. Therefore, we hypothesized that the reduced responsiveness of Lap2α−/− myocardium to ISO might also be a consequence of similar de-sensitization of the β-AR signaling pathway. To test this, we analyzed the expression of β2-AR in LAP2α-deficient and WT mice at baseline, as well as after ISO treatment. As expected, Lap2α−/− hearts showed lower baseline expression levels of β2-AR mRNA and protein in young and old mice (Figure 5C, Online Figure V). Importantly, the difference in relative β2-AR mRNA levels in untreated LAP2α knockout versus WT hearts increased with age (Figure 5C), suggesting that the downregulation of β-AR signaling in Lap2α−/− mice is an aging-dependent phenomenon and may be a consequence of heart function impairment. ISO treatment caused an additional downregulation of β2-AR mRNA in Lap2α−/− hearts, albeit not as extensive as in the WT situation (~71% in WT vs. ~39% in Lap2α−/−), resulting in comparable end-point expression levels in both genotypes (Figure 5C).
Development of β-AR-mediated hypertrophy is associated with the activation of the fetal cardiac transcriptional program23, including the expression of embryonic transcription factors ANF and GATA424. In accordance with this, ANF and GATA4 mRNA levels were upregulated in ISO-treated hearts of both Lap2α+/+ and Lap2α−/− mice (Figure 5D). However, consistent with the reduced β-AR levels in old Lap2α−/− mice, the increase in ISO-induced ANF expression was significantly lower in LAP2α-deficient myocardium compared to WT. Relative GATA4 levels were also substantially lower in Lap2α−/− than in WT hearts after ISO treatment, although the ISO-mediated increase of GATA4 expression was similar in both genotypes. Overall, these results suggest that loss of LAP2α affects cardiac-specific transcription and progression of ISO-induced hypertrophy.
Absence of dystrophin delays the onset of ventricular dysfunction in Lap2α−/−/Mdx mice
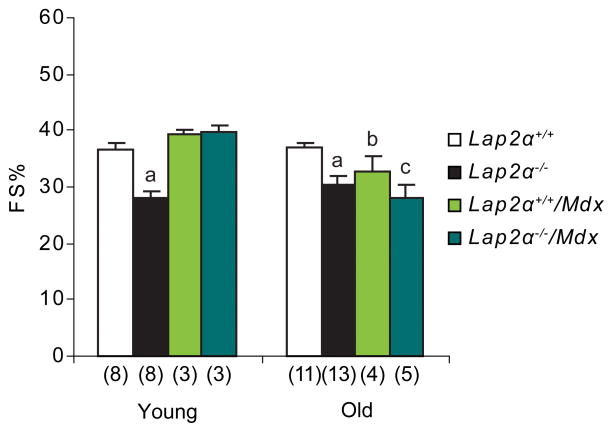
Since baseline systolic dysfunction in Lap2α−/− mice was not progressive and did not affect their life span, we thought that the effect of LAP2α loss might be augmented in a cardiomyopathy background. Therefore, we crossed Lap2α−/− mice with Mdx mutants12, which develop skeletal muscle dystrophy and DCM at an advanced age due to the absence of dystrophin25, 26.
The homozygous Lap2α−/−/Mdx mutant animals were born according to Mendelian ratios and appeared grossly indistinguishable from their Mdx littermates. Unexpectedly, echocardiography in young Lap2α−/−/Mdx mice showed normal heart function, with FS values similar to WT and Mdx (Figure 6, Online Table III). This suggests that the absence of dystrophin can pre-sensitize the heart and turn on compensatory pathways leading to a transient rescue of the Lap2α−/− phenotype. Nevertheless, old compound mutant animals, as well as Mdx mice, developed similar heart function defects as Lap2α−/− mice (Figure 6, Online Table I, III).
Figure 6. Absence of dystrophin delays the onset of ventricular dysfunction in Lap2α−/−/Mdx mice.
FS% values in young male Lap2α−/−/Mdx mice are higher compared to Lap2α−/− animals, whereas old mice of both genotypes show similar systolic dysfunction (ANOVA; p<0.05 for (a) WT vs. KO, (b) WT vs. Mdx and (c) WT vs. Lap2α−/−/Mdx mice; (n) - sample size; mean ± SE).
LAP2α is dispensable in late-embryonic and adult cardiomyocytes
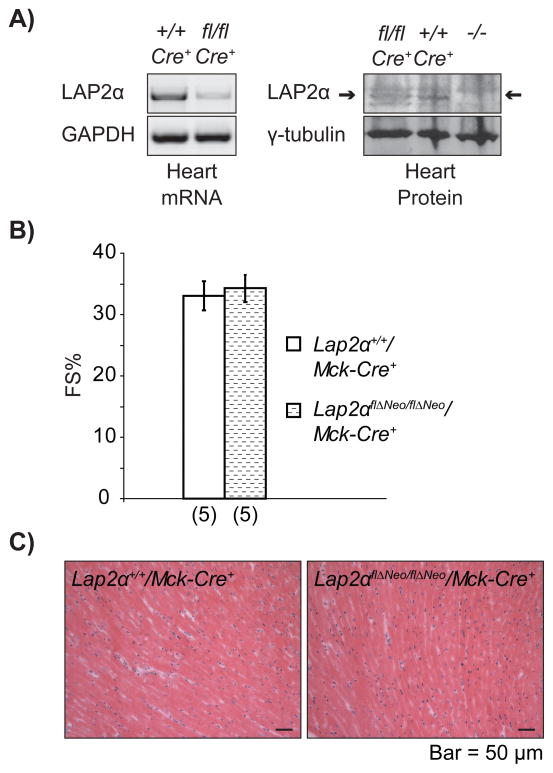
LAP2α is highly expressed in proliferating tissues27, 28. Since the vast majority of adult cardiomyocytes persists in a post-mitotic stage29, and only low levels of LAP2α are present in nuclei throughout adult myocardium9 (Figure 1), we thought that LAP2α may exhibit its major activity during early cardiomyocyte proliferation and myocardial growth, when its expression is clearly detectable (Online Figure VI). To address this question, we generated conditional knockout mice by crossing Lap2αflΔNeo/flΔNeo animals with mice expressing Cre recombinase under the control of striated muscle-specific muscle creatine kinase (Mck) promoter30 (Online Figure I). In this system, the expression of Cre recombinase and the consequent deletion of LAP2α are turned on in heart and skeletal muscle during later stages of mouse embryonic development and striated muscle differentiation. Lap2αflΔNeo/flΔNeo/Mck-Cre+ mice were born at Mendelian ratios and did not demonstrate any overt phenotype. LAP2α mRNA and protein levels were significantly reduced in Lap2αflΔNeo/flΔNeo/Mck-Cre+ hearts, indicating an efficient recombination in the myocardium (Figure 7A). In contrast, other tissues like spleen, which do not express MCK31, had normal levels of LAP2α (our unpublished data). Echocardiography in Lap2αflΔNeo/flΔNeo/Mck-Cre+ mice showed normal heart function (Figure 7B, Online Table IV) and histological, as well as morphometric analyses, did not reveal any pathological heart phenotype (Figure 7C and data not shown). In accordance, the expression levels of MEF2c and GATA4 mRNA were similar in Lap2αflΔNeo/flΔNeo/Mck-Cre+ and Lap2α+/+/Mck-Cre+ hearts (data not shown). Thus, LAP2α expression in late-embryonic and adult cardiomyocytes is dispensable for normal heart function, but may be important during earlier stages of heart development (before E13.5 when the Mck promoter becomes active) or in non-striated muscle cells of the heart.
Figure 7. LAP2α is dispensable in late-embryonic and adult cardiomyocytes.
A) Reduced LAP2α mRNA and protein levels in Lap2αflΔNeo/flΔNeo/Mck-Cre+ [fl/fl] vs. Lap2α+/+/Mck-Cre+ heart tissue [+/+]. (Samples were normalized to GAPDH in semiqPCR and to γ-tubulin in western blot analyses). B) Normal FS% values in young male Lap2αflΔNeo/flΔNeo/Mck-Cre+ mice (p>0.05, ANOVA; (n) - sample size, mean ± SE). C) Normal heart structure in conditional striated muscle-specific LAP2α knockout mice.
Discussion
In this study we describe a new mouse model of cardiomyopathy caused by the absence of LAP2α, a major binding partner of lamin A/C in the nucleoplasm6. Together with previous studies linking lamin A/C and lamin-binding inner nuclear membrane proteins, emerin and nesprin, to congenital heart disorders1, 3, 9, 32, our data suggest a major role of Atype lamin complexes in normal heart function.
A mutation in the α-specific exon 4 of the human LAP2 gene has previously been linked to familial DCM9. Here we show that the absence of LAP2α in mice also leads to a heart disease. Interestingly, only male Lap2α−/− mice showed a heart defect, whereas female animals exhibited normal cardiac function implicating gender-specific factors in the development of the disease. Similar gender-related phenotype variations were described in other cardiomyopathy mouse models33, including mice carrying the H222P-Lmna mutation34. Although the exact background of this phenomenon is still unknown, gender based differences in cardiac dysfunction were linked to the activity of steroid hormones35. At present no data about the risk of heart failure in male versus female carriers of LAP2α mutations in humans are available.
In an attempt to disclose the molecular pathways of the disease, we analyzed the expression of factors involved in the development and remodeling of the myocardium. Lap2α−/− mice showed deregulated expression of major heart transcription factors, MEF2c14 and GATA417, as well as some of their downstream targets, at different life stages. Both GATA4 and MEF2c play essential roles in embryonic heart development and hypertrophic growth14, 17, 21, 22. Since MEF2c is required for normal heart development (see review14), its downregulation in the absence of LAP2α might compromise the early stages of cardiac development in Lap2α−/− mice.
The appearance of systolic dysfunction in young Lap2α−/− mice and the delayed decrease in GATA4 levels only in old mice suggests that this deregulation may be a consequence rather than the cause of the disease. Despite lower GATA4 expression levels, Lap2α−/− hearts were able to undergo hypertrophic growth. In support of our data, mice with reduced GATA4 levels (G4D mice) show a similar heart function defect at baseline, as well as the ability to grow under hypertrophic conditions21. Accordingly, G4D mice develop fibrosis only after pressure overload as a consequence of increased stress-induced cardiomyocyte death21.
GATA4 and MEF2c synergistically activate the expression of BNP18, a cardiac hormone involved in the regulation of blood pressure and fluid-electrolyte balance, which also plays a role in the inhibition of cardiac fibroblast proliferation and extracellular matrix production36. Decreased levels of BNP in the absence of LAP2α could potentially explain the observed occurrence of cardiac fibrosis in Lap2α−/− mice. Interestingly, BNP-deficient mice (Nppb−/−), which exhibit normal heart morphology and hypertrophic growth after ventricular pressure overload, show a higher incidence of fibrosis (~50%) in male vs. female mice at the age of 15 weeks37.
The blunted response of LAP2α-deficient hearts to chronic isoproterenol infusion points to the existence of compensatory pathways activated in response to changes in cardiac function in Lap2α−/− mice. The attenuated hypertrophic growth and lack of fibrosis observed in β-AR knockout mice after pressure overload38 indicate that the downregulation of β-AR signaling found in Lap2α−/− hearts might be a part of this process.
The variability in the extent of fibrosis at baseline, as well as after chronic ISO infusion, might be explained by the observed deregulated expression of pro- (CTGF) and anti-fibrotic (BNP) factors in Lap2α−/− mice. The observed downregulation of CTGF in old LAP2α-deficient hearts might be a part of an extensive compensatory mechanism activated in response to loss of LAP2α to protect the myocardium from further tissue deterioration and loss of function.
Since LAP2α is highly expressed in proliferating tissues and only weakly in post-mitotic tissues9, 27, 28, such as heart muscle29, we hypothesized that LAP2α might be required during cardiac development and/or postnatal myocardial remodeling, mediated by putative cardiac stem cells39. Therefore, we generated conditional knockout mice which lose LAP2α during later stages of embryonic development and adult striated muscle differentiation30. Lap2αflΔNeo/flΔNeo/Mck-Cre+ mice showed normal heart function and normal levels of GATA4 and MEF2c, supporting the model according to which the defect in complete knockout mice might arise during early embryonic development and at early stages of muscle differentiation, before the Mck promoter becomes active, and/or it might be a consequence of LAP2α loss from heart stem- and non-striated muscle cells.
We have previously shown that LAP2α retains a sub-fraction of the nuclear lamin A/C pool inside the nucleoplasm in proliferating skin fibroblasts and intestinal cells. In contrast, nucleoplasmic lamin A/C is lost in non-dividing differentiated cells8, implicating the nucleoplasmic pool of lamin A/C in regulation of the transition from proliferating to the differentiated state. The localization of lamin A/C has also been shown to change in cardiomyocytes during aging and development, going from being mainly nucleoplasmic to the nuclear periphery40. The elongated shape and different orientation of cardiomyocyte nuclei within heart tissue, however, precluded the detection of potential changes in lamin A/C localization in LAP2α-deficient tissue. In view of the lack of heart defects in muscle-specific conditional LAP2α knockout mice, there is a possibility that a potential mislocalization of lamin A/C in LAP2α-deficient cardiomyocyte precursor or non-striated muscle cells may be the primary cause of the Lap2α−/− cardiac defect. Alternatively lack of LAP2α may change the function, rather than the localization of lamin A/C, such as binding to epigenetic modifiers and components of different signaling cascades7, which in turn may lead to cardiomyopathy. This model is consistent with previous reports that have linked lamin A/C-related cardiomyopathies to changes in signaling pathways, such as TGFβ, PI3-kinase and MAPK41, which influence MEF2c and GATA4 expression during development and hypertrophic growth42, 43 and are also affected by β-AR signaling23.
In summary, we show that the absence of LAP2α causes a baseline ventricular systolic dysfunction in male mice and activates compensatory pathways that prevent further decline of heart function under chronic stress conditions. The origins of these defects, which could lie in impaired proliferation and/or differentiation of early embryonic cardiomyocytes, resident cardiac stem cells or non-muscle cardiac tissue, remain to be discovered.
Supplementary Material
Acknowledgments
Funding
This work was supported by grants from the Austrian Science Research Fund [grant number FWF P17871] and the EURO-Laminopathies research project of the European Commission [Contract LSHM-CT-2005-018690] to RF, the Austrian Society of Cardiology (to B.M.) and the Hans und Blanca Moser-Foundation (to B.J.H.).
We wish to thank C. Ronald Kahn, Department of Medicine, Joslin Diabetes Center, Boston MA for kindly providing Mck-Cre mice and Reginald Bittner, Medical University Vienna for Mdx mice.
Non-standard Abbreviations and Acronyms
- ANF
Atrial Natriuretic Factor
- β-AR
β-Adrenergic Receptor
- BNP
Brain Natriuretic Peptide
- CTGF
Connective Tissue Growth Factor
- DCM
Dilated Cardiomyopathy
- EF%
Ejection Fraction
- EDVI
End-Diastolic Left Ventricular Volume Index
- ESVI
End-Systolic Left Ventricular Volume Index
- FS%
Fractional Shortening
- GAPDH
Glyceraldehyde 3-Phosphate Dehydrogenase
- HPRT
Hypoxanthine-Guanine Phosphoribosyltransferase
- HW/BW
Heart Weight/Body Weight
- ISO
Isoproterenol
- IVS
Interventricular Septum
- LA
Left Atrium
- LAP2α
Lamina-Associated Polypeptide 2α
- LEM
LAP2-Emerin-MAN
- LVD
Left Ventricular Diameter
- LVPW
Left Ventricular Posterior Wall Thickness
- MAPK
Mitogen Activated Protein Kinase
- MEF2c
Myocyte Enhancer Factor 2c
- PI3-kinase
Phosphoinositide 3-kinase
- SE
Standard Error, SRF, Serum Response Factor
- STARS
Striated Muscle Activator of Rho Signaling
- TGFβ
Transforming Growth Factor β
- WT
Wild Type
Footnotes
Conflicts of interest
None.
References
- 1.Sylvius N, Tesson F. Lamin A/C and cardiac diseases. Curr Opin Cardiol. 2006;21:159–165. doi: 10.1097/01.hco.0000221575.33501.58. [DOI] [PubMed] [Google Scholar]
- 2.Capell BC, Collins FS. Human laminopathies: nuclei gone genetically awry. Nat Rev Genet. 2006;7:940–952. doi: 10.1038/nrg1906. [DOI] [PubMed] [Google Scholar]
- 3.Worman HJ, Bonne G. “Laminopathies”: a wide spectrum of human diseases. Exp Cell Res. 2007;313:2121–2133. doi: 10.1016/j.yexcr.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vlcek S, Korbei B, Foisner R. Distinct functions of the unique C terminus of LAP2alpha in cell proliferation and nuclear assembly. J Biol Chem. 2002;277:18898–18907. doi: 10.1074/jbc.M200048200. [DOI] [PubMed] [Google Scholar]
- 5.Schirmer EC, Foisner R. Proteins that associate with lamins: many faces, many functions. Exp Cell Res. 2007;313:2167–2179. doi: 10.1016/j.yexcr.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Dechat T, Korbei B, Vaughan OA, Vlcek S, Hutchison CJ, Foisner R. Lamina-associated polypeptide 2alpha binds intranuclear A-type lamins. J Cell Sci. 2000;113(Pt 19):3473–3484. doi: 10.1242/jcs.113.19.3473. [DOI] [PubMed] [Google Scholar]
- 7.Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker DK, Solimando L, Goldman RD. Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev. 2008;22:832–853. doi: 10.1101/gad.1652708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naetar N, Korbei B, Kozlov S, Kerenyi MA, Dorner D, Kral R, Gotic I, Fuchs P, Cohen TV, Bittner R, Stewart CL, Foisner R. Loss of nucleoplasmic LAP2alpha-lamin A complexes causes erythroid and epidermal progenitor hyperproliferation. Nat Cell Biol. 2008;10:1341–1348. doi: 10.1038/ncb1793. [DOI] [PubMed] [Google Scholar]
- 9.Taylor MR, Slavov D, Gajewski A, Vlcek S, Ku L, Fain PR, Carniel E, Di Lenarda A, Sinagra G, Boucek MM, Cavanaugh J, Graw SL, Ruegg P, Feiger J, Zhu X, Ferguson DA, Bristow MR, Gotzmann J, Foisner R, Mestroni L. Thymopoietin (lamina-associated polypeptide 2) gene mutation associated with dilated cardiomyopathy. Hum Mutat. 2005;26:566–574. doi: 10.1002/humu.20250. [DOI] [PubMed] [Google Scholar]
- 10.Nikolova V, Leimena C, McMahon AC, Tan JC, Chandar S, Jogia D, Kesteven SH, Michalicek J, Otway R, Verheyen F, Rainer S, Stewart CL, Martin D, Feneley MP, Fatkin D. Defects in nuclear structure and function promote dilated cardiomyopathy in lamin A/C-deficient mice. J Clin Invest. 2004;113:357–369. doi: 10.1172/JCI19448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart CL, Kozlov S, Fong LG, Young SG. Mouse models of the laminopathies. Exp Cell Res. 2007;313:2144–2156. doi: 10.1016/j.yexcr.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bulfield G, Siller WG, Wight PA, Moore KJ. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci U S A. 1984;81:1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuba K, Zhang L, Imai Y, Arab S, Chen M, Maekawa Y, Leschnik M, Leibbrandt A, Markovic M, Schwaighofer J, Beetz N, Musialek R, Neely GG, Komnenovic V, Kolm U, Metzler B, Ricci R, Hara H, Meixner A, Nghiem M, Chen X, Dawood F, Wong KM, Sarao R, Cukerman E, Kimura A, Hein L, Thalhammer J, Liu PP, Penninger JM. Impaired heart contractility in Apelin gene-deficient mice associated with aging and pressure overload. Circ Res. 2007;101:e32–42. doi: 10.1161/CIRCRESAHA.107.158659. [DOI] [PubMed] [Google Scholar]
- 14.Black BL. Transcriptional pathways in second heart field development. Semin Cell Dev Biol. 2007;18:67–76. doi: 10.1016/j.semcdb.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuwahara K, Teg Pipes GC, McAnally J, Richardson JA, Hill JA, Bassel-Duby R, Olson EN. Modulation of adverse cardiac remodeling by STARS, a mediator of MEF2 signaling and SRF activity. J Clin Invest. 2007;117:1324–1334. doi: 10.1172/JCI31240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pipes GC, Creemers EE, Olson EN. The myocardin family of transcriptional coactivators: versatile regulators of cell growth, migration, and myogenesis. Genes Dev. 2006;20:1545–1556. doi: 10.1101/gad.1428006. [DOI] [PubMed] [Google Scholar]
- 17.Xin M, Davis CA, Molkentin JD, Lien CL, Duncan SA, Richardson JA, Olson EN. A threshold of GATA4 and GATA6 expression is required for cardiovascular development. Proc Natl Acad Sci U S A. 2006;103:11189–11194. doi: 10.1073/pnas.0604604103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morin S, Charron F, Robitaille L, Nemer M. GATA-dependent recruitment of MEF2 proteins to target promoters. Embo J. 2000;19:2046–2055. doi: 10.1093/emboj/19.9.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pikkarainen S, Tokola H, Kerkela R, Ruskoaho H. GATA transcription factors in the developing and adult heart. Cardiovasc Res. 2004;63:196–207. doi: 10.1016/j.cardiores.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 20.Chen MM, Lam A, Abraham JA, Schreiner GF, Joly AH. CTGF expression is induced by TGF- beta in cardiac fibroblasts and cardiac myocytes: a potential role in heart fibrosis. J Mol Cell Cardiol. 2000;32:1805–1819. doi: 10.1006/jmcc.2000.1215. [DOI] [PubMed] [Google Scholar]
- 21.Bisping E, Ikeda S, Kong SW, Tarnavski O, Bodyak N, McMullen JR, Rajagopal S, Son JK, Ma Q, Springer Z, Kang PM, Izumo S, Pu WT. Gata4 is required for maintenance of postnatal cardiac function and protection from pressure overload-induced heart failure. Proc Natl Acad Sci U S A. 2006;103:14471–14476. doi: 10.1073/pnas.0602543103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Oort RJ, van Rooij E, Bourajjaj M, Schimmel J, Jansen MA, van der Nagel R, Doevendans PA, Schneider MD, van Echteld CJ, De Windt LJ. MEF2 activates a genetic program promoting chamber dilation and contractile dysfunction in calcineurin-induced heart failure. Circulation. 2006;114:298–308. doi: 10.1161/CIRCULATIONAHA.105.608968. [DOI] [PubMed] [Google Scholar]
- 23.Osadchii OE. Cardiac hypertrophy induced by sustained beta-adrenoreceptor activation: pathophysiological aspects. Heart Fail Rev. 2007;12:66–86. doi: 10.1007/s10741-007-9007-4. [DOI] [PubMed] [Google Scholar]
- 24.Pikkarainen S, Tokola H, Majalahti-Palviainen T, Kerkela R, Hautala N, Bhalla SS, Charron F, Nemer M, Vuolteenaho O, Ruskoaho H. GATA-4 is a nuclear mediator of mechanical stretch-activated hypertrophic program. J Biol Chem. 2003;278:23807–23816. doi: 10.1074/jbc.M302719200. [DOI] [PubMed] [Google Scholar]
- 25.Quinlan JG, Hahn HS, Wong BL, Lorenz JN, Wenisch AS, Levin LS. Evolution of the mdx mouse cardiomyopathy: physiological and morphological findings. Neuromuscul Disord. 2004;14:491–496. doi: 10.1016/j.nmd.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science. 1989;244:1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- 27.Parise P, Finocchiaro G, Masciadri B, Quarto M, Francois S, Mancuso F, Muller H. Lap2alpha expression is controlled by E2F and deregulated in various human tumors. Cell Cycle. 2006;5:1331–1341. doi: 10.4161/cc.5.12.2833. [DOI] [PubMed] [Google Scholar]
- 28.Ishijima Y, Toda T, Matsushita H, Yoshida M, Kimura N. Expression of thymopoietin beta/lamina-associated polypeptide 2 (TP beta/LAP2) and its family proteins as revealed by specific antibody induced against recombinant human thymopoietin. Biochem Biophys Res Commun. 1996;226:431–438. doi: 10.1006/bbrc.1996.1373. [DOI] [PubMed] [Google Scholar]
- 29.MacLellan WR, Schneider MD. Genetic dissection of cardiac growth control pathways. Annu Rev Physiol. 2000;62:289–319. doi: 10.1146/annurev.physiol.62.1.289. [DOI] [PubMed] [Google Scholar]
- 30.Bruning JC, Michael MD, Winnay JN, Hayashi T, Horsch D, Accili D, Goodyear LJ, Kahn CR. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell. 1998;2:559–569. doi: 10.1016/s1097-2765(00)80155-0. [DOI] [PubMed] [Google Scholar]
- 31.Lyons GE, Muhlebach S, Moser A, Masood R, Paterson BM, Buckingham ME, Perriard JC. Developmental regulation of creatine kinase gene expression by myogenic factors in embryonic mouse and chick skeletal muscle. Development. 1991;113:1017–1029. doi: 10.1242/dev.113.3.1017. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Q, Bethmann C, Worth NF, Davies JD, Wasner C, Feuer A, Ragnauth CD, Yi Q, Mellad JA, Warren DT, Wheeler MA, Ellis JA, Skepper JN, Vorgerd M, Schlotter-Weigel B, Weissberg PL, Roberts RG, Wehnert M, Shanahan CM. Nesprin-1 and -2 are involved in the pathogenesis of Emery Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Hum Mol Genet. 2007;16:2816–2833. doi: 10.1093/hmg/ddm238. [DOI] [PubMed] [Google Scholar]
- 33.Du XJ. Gender modulates cardiac phenotype development in genetically modified mice. Cardiovasc Res. 2004;63:510–519. doi: 10.1016/j.cardiores.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 34.Arimura T, Helbling-Leclerc A, Massart C, Varnous S, Niel F, Lacene E, Fromes Y, Toussaint M, Mura AM, Keller DI, Amthor H, Isnard R, Malissen M, Schwartz K, Bonne G. Mouse model carrying H222P-Lmna mutation develops muscular dystrophy and dilated cardiomyopathy similar to human striated muscle laminopathies. Hum Mol Genet. 2005;14:155–169. doi: 10.1093/hmg/ddi017. [DOI] [PubMed] [Google Scholar]
- 35.Murphy E, Steenbergen C. Gender-based differences in mechanisms of protection in myocardial ischemia-reperfusion injury. Cardiovasc Res. 2007;75:478–486. doi: 10.1016/j.cardiores.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 36.Kapoun AM, Liang F, O’Young G, Damm DL, Quon D, White RT, Munson K, Lam A, Schreiner GF, Protter AA. B-type natriuretic peptide exerts broad functional opposition to transforming growth factor-beta in primary human cardiac fibroblasts: fibrosis, myofibroblast conversion, proliferation, and inflammation. Circ Res. 2004;94:453–461. doi: 10.1161/01.RES.0000117070.86556.9F. [DOI] [PubMed] [Google Scholar]
- 37.Tamura N, Ogawa Y, Chusho H, Nakamura K, Nakao K, Suda M, Kasahara M, Hashimoto R, Katsuura G, Mukoyama M, Itoh H, Saito Y, Tanaka I, Otani H, Katsuki M. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc Natl Acad Sci U S A. 2000;97:4239–4244. doi: 10.1073/pnas.070371497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kiriazis H, Wang K, Xu Q, Gao XM, Ming Z, Su Y, Moore XL, Lambert G, Gibbs ME, Dart AM, Du XJ. Knockout of beta(1)- and beta(2)-adrenoceptors attenuates pressure overload-induced cardiac hypertrophy and fibrosis. Br J Pharmacol. 2008;153:684–692. doi: 10.1038/sj.bjp.0707622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 40.Afilalo J, Sebag IA, Chalifour LE, Rivas D, Akter R, Sharma K, Duque G. Age-related changes in lamin A/C expression in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2007;293:H1451–1456. doi: 10.1152/ajpheart.01194.2006. [DOI] [PubMed] [Google Scholar]
- 41.Marmiroli S, Bertacchini J, Beretti F, Cenni V, Guida M, De Pol A, Maraldi NM, Lattanzi G. A-type lamins and signaling: The PI 3-kinase/Akt pathway moves forward. J Cell Physiol. 2009 doi: 10.1002/jcp.21807. [DOI] [PubMed] [Google Scholar]
- 42.Kerkela R, Pikkarainen S, Majalahti-Palviainen T, Tokola H, Ruskoaho H. Distinct roles of mitogen-activated protein kinase pathways in GATA-4 transcription factor-mediated regulation of B-type natriuretic peptide gene. J Biol Chem. 2002;277:13752–13760. doi: 10.1074/jbc.M105736200. [DOI] [PubMed] [Google Scholar]
- 43.Potthoff MJ, Olson EN. MEF2: a central regulator of diverse developmental programs. Development. 2007;134:4131–4140. doi: 10.1242/dev.008367. [DOI] [PubMed] [Google Scholar]
- 44.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.