Abstract
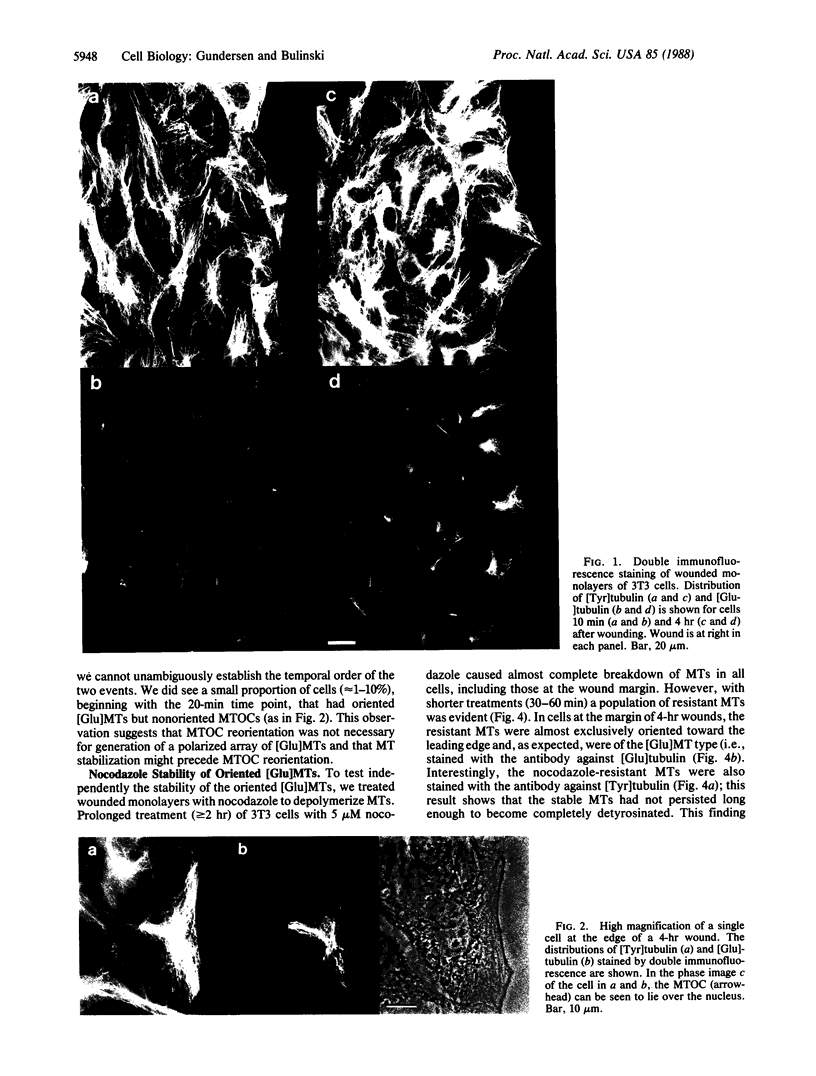
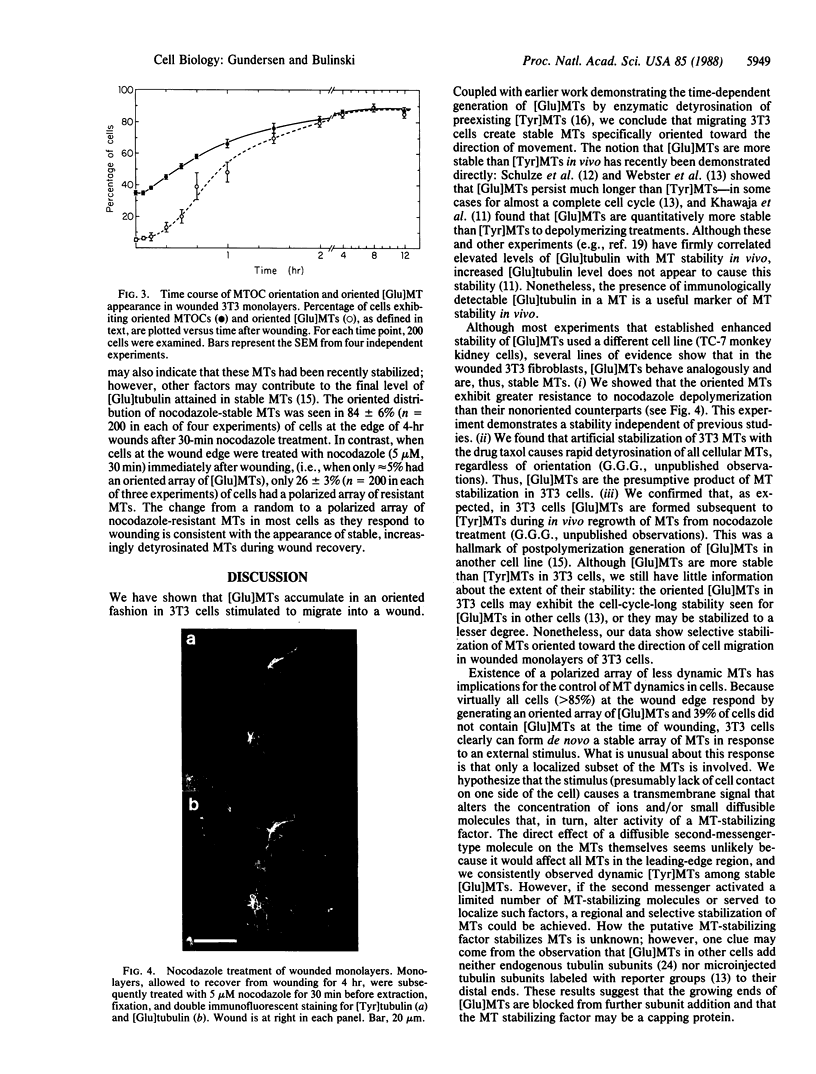
A small subset of the microtubule (MT) array in many cultured cells does not exhibit the rapid turnover (t 1/2 approximately equal to 10 min) shown by most cellular MTs. The function of the stable class of MTs is unknown and has been confounded by the apparent lack of organization of stable MTs within cells. Using an antibody against detyrosinated tubulin, a post-translationally modified form of tubulin that accumulates in stable MTs, we localized the stable MTs in mouse 3T3 cells induced to initiate directional migration by experimental wounding of confluent monolayers. Immediately after monolayer wounding, the distribution of stable MTs in cells at the wound edge resembled that in cells in the monolayer interior; most cells either contained randomly distributed stable MTs or lacked them entirely. However, by 20 min after wounding, cells at the wound margin began to generate an asymmetric MT array, with virtually all stable MTs oriented toward the cell edge in contact with the wound. Two hours after monolayer wounding, greater than or equal to 80% of cells at the wound margin had generated this polarized array of stable MTs, and the array was maintained for at least 12 hr. MTs in the polarized array showed enhanced resistance to depolymerization by nocodazole, thus providing an independent test of their stability. Formation of the polar array of stable MTs appeared to precede onset of cell migration and closely paralleled reorientation of the MT-organizing center. These results show that cultured cells can remodel their MT array rapidly in response to an extracellular signal and suggest that selective stabilization of MTs is an early event in the generation of cellular asymmetry.
Full text
PDF


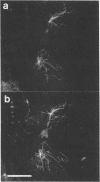
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. D., Weiss D. G., Hayden J. H., Brown D. T., Fujiwake H., Simpson M. Gliding movement of and bidirectional transport along single native microtubules from squid axoplasm: evidence for an active role of microtubules in cytoplasmic transport. J Cell Biol. 1985 May;100(5):1736–1752. doi: 10.1083/jcb.100.5.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argaraña C. E., Barra H. S., Caputto R. Release of [14C]tyrosine from tubulinyl-[14C]tyrosine by brain extract. Separation of a carboxypeptidase from tubulin-tyrosine ligase. Mol Cell Biochem. 1978 Feb 24;19(1):17–21. doi: 10.1007/BF00231230. [DOI] [PubMed] [Google Scholar]
- Bergmann J. E., Kupfer A., Singer S. J. Membrane insertion at the leading edge of motile fibroblasts. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1367–1371. doi: 10.1073/pnas.80.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley B. R., Cox S. M., Pepper D. A., Wible L., Brenner S. L., Pardue R. L. Tubulin assembly sites and the organization of cytoplasmic microtubules in cultured mammalian cells. J Cell Biol. 1981 Sep;90(3):554–562. doi: 10.1083/jcb.90.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuens G., Gundersen G. G., Nuydens R., Cornelissen F., Bulinski J. C., DeBrabander M. Ultrastructural colocalization of tyrosinated and detyrosinated alpha-tubulin in interphase and mitotic cells. J Cell Biol. 1986 Nov;103(5):1883–1893. doi: 10.1083/jcb.103.5.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. D. The role of three cytoplasmic fibers in BHK-21 cell motility. I. Microtubules and the effects of colchicine. J Cell Biol. 1971 Dec;51(3):752–762. doi: 10.1083/jcb.51.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlieb A. I., May L. M., Subrahmanyan L., Kalnins V. I. Distribution of microtubule organizing centers in migrating sheets of endothelial cells. J Cell Biol. 1981 Nov;91(2 Pt 1):589–594. doi: 10.1083/jcb.91.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen G. G., Bulinski J. C. Microtubule arrays in differentiated cells contain elevated levels of a post-translationally modified form of tubulin. Eur J Cell Biol. 1986 Dec;42(2):288–294. [PubMed] [Google Scholar]
- Gundersen G. G., Kalnoski M. H., Bulinski J. C. Distinct populations of microtubules: tyrosinated and nontyrosinated alpha tubulin are distributed differently in vivo. Cell. 1984 Oct;38(3):779–789. doi: 10.1016/0092-8674(84)90273-3. [DOI] [PubMed] [Google Scholar]
- Gundersen G. G., Khawaja S., Bulinski J. C. Postpolymerization detyrosination of alpha-tubulin: a mechanism for subcellular differentiation of microtubules. J Cell Biol. 1987 Jul;105(1):251–264. doi: 10.1083/jcb.105.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khawaja S., Gundersen G. G., Bulinski J. C. Enhanced stability of microtubules enriched in detyrosinated tubulin is not a direct function of detyrosination level. J Cell Biol. 1988 Jan;106(1):141–149. doi: 10.1083/jcb.106.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin J. V., Wright B., Milstein C. Rat monoclonal antitubulin antibodies derived by using a new nonsecreting rat cell line. J Cell Biol. 1982 Jun;93(3):576–582. doi: 10.1083/jcb.93.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner M., Mitchison T. Beyond self-assembly: from microtubules to morphogenesis. Cell. 1986 May 9;45(3):329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- Kumar N., Flavin M. Modulation of some parameters of assembly of microtubules in vitro by tyrosinolation of tubulin. Eur J Biochem. 1982 Nov;128(1):215–222. doi: 10.1111/j.1432-1033.1982.tb06954.x. [DOI] [PubMed] [Google Scholar]
- Kupfer A., Louvard D., Singer S. J. Polarization of the Golgi apparatus and the microtubule-organizing center in cultured fibroblasts at the edge of an experimental wound. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2603–2607. doi: 10.1073/pnas.79.8.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malech H. L., Root R. K., Gallin J. I. Structural analysis of human neutrophil migration. Centriole, microtubule, and microfilament orientation and function during chemotaxis. J Cell Biol. 1977 Dec;75(3):666–693. doi: 10.1083/jcb.75.3.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T., Kirschner M. Microtubule assembly nucleated by isolated centrosomes. Nature. 1984 Nov 15;312(5991):232–237. doi: 10.1038/312232a0. [DOI] [PubMed] [Google Scholar]
- Osborn M., Webster R. E., Weber K. Individual microtubules viewed by immunofluorescence and electron microscopy in the same PtK2 cell. J Cell Biol. 1978 Jun;77(3):R27–R34. doi: 10.1083/jcb.77.3.r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschal B. M., Vallee R. B. Retrograde transport by the microtubule-associated protein MAP 1C. Nature. 1987 Nov 12;330(6144):181–183. doi: 10.1038/330181a0. [DOI] [PubMed] [Google Scholar]
- Piperno G., LeDizet M., Chang X. J. Microtubules containing acetylated alpha-tubulin in mammalian cells in culture. J Cell Biol. 1987 Feb;104(2):289–302. doi: 10.1083/jcb.104.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinnerthaler G., Geiger B., Small J. V. Contact formation during fibroblast locomotion: involvement of membrane ruffles and microtubules. J Cell Biol. 1988 Mar;106(3):747–760. doi: 10.1083/jcb.106.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapp B. J., Vale R. D., Sheetz M. P., Reese T. S. Single microtubules from squid axoplasm support bidirectional movement of organelles. Cell. 1985 Feb;40(2):455–462. doi: 10.1016/0092-8674(85)90160-6. [DOI] [PubMed] [Google Scholar]
- Schulze E., Asai D. J., Bulinski J. C., Kirschner M. Posttranslational modification and microtubule stability. J Cell Biol. 1987 Nov;105(5):2167–2177. doi: 10.1083/jcb.105.5.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze E., Kirschner M. Dynamic and stable populations of microtubules in cells. J Cell Biol. 1987 Feb;104(2):277–288. doi: 10.1083/jcb.104.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze E., Kirschner M. Microtubule dynamics in interphase cells. J Cell Biol. 1986 Mar;102(3):1020–1031. doi: 10.1083/jcb.102.3.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W. C., Asai D. J., Carney D. H. Heterogeneity among microtubules of the cytoplasmic microtubule complex detected by a monoclonal antibody to alpha tubulin. J Cell Biol. 1984 Mar;98(3):1017–1025. doi: 10.1083/jcb.98.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D., Reese T. S., Sheetz M. P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985 Aug;42(1):39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiliev J. M., Gelfand I. M., Domnina L. V., Ivanova O. Y., Komm S. G., Olshevskaja L. V. Effect of colcemid on the locomotory behaviour of fibroblasts. J Embryol Exp Morphol. 1970 Nov;24(3):625–640. [PubMed] [Google Scholar]
- Webster D. R., Gundersen G. G., Bulinski J. C., Borisy G. G. Differential turnover of tyrosinated and detyrosinated microtubules. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9040–9044. doi: 10.1073/pnas.84.24.9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehland J., Willingham M. C., Sandoval I. V. A rat monoclonal antibody reacting specifically with the tyrosylated form of alpha-tubulin. I. Biochemical characterization, effects on microtubule polymerization in vitro, and microtubule polymerization and organization in vivo. J Cell Biol. 1983 Nov;97(5 Pt 1):1467–1475. doi: 10.1083/jcb.97.5.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]