Abstract
We evaluated changes in myostatin, follistatin and MyoD mRNA gene expression using eccentric exercise (EE) and concentric exercise (CE) as probes to better understand the mechanisms of muscle hypertrophy in young women. Twelve women performed single-leg maximal eccentric (n=6, 25±1yr, 59±7kg) or concentric (n=6, 24±1 yr, 65±7kg) isokinetic knee extension exercise for 7 sessions. Muscle biopsies were taken from the vastus lateralis at baseline, 8hrs after the first exercise session, and 8hrs after the 7th exercise session. In the EE group, there were no changes in myostatin and follistatin (p≥0.17), however, MyoD expression increased after one exercise bout (p=0.02). In the CE group, there were no changes in myostatin, follistatin, or MyoD mRNA gene expression (p≥0.07). Differences between the EE and CE groups were not significant (p≥0.05). These data suggests that a single bout or multiple bouts of maximal EE or CE may not significantly alter myostatin or follistatin mRNA gene expression in young women. However MyoD mRNA expression appears to increase only after EE.
Keywords: Eccentric, Concentric, MyoD, Resistance Exercise, Hypertrophy
INTRODUCTION
The process of exercise induced skeletal muscle damage involves multiple signaling mechanisms that ultimately influence muscle hypertrophy. Using isokinetic dynamometry, it is possible to provide controlled muscle stimuli from external forces utilizing exclusively concentric or eccentric contractions. Concentric exercise (CE) and eccentric exercise (EE) elicit different adaptations in skeletal muscle (6, 9). Skeletal muscle loading via high intensity EE produces more muscle damage (5) compared to CE, thus leading to greater hypertrophy (6). Prior investigation describes the effects of these forms of exercise in mediating muscle hypertrophy by inhibition (7) or reduction in myostatin (a negative regulator of muscle mass) mRNA expression. Myostatin may be downregulated after specific types of exercise stimuli (14, 27) and follistatin, a putative inhibitor of myostatin (1), may be upregulated (30) by binding to the ACTIIB myostatin receptor site (16), allowing for greater muscle hypertrophy. Furthermore, myostatininhibits expression of the myogenic regulatory factors, (Myf 5 and MyoD), thus inhibiting differentiation of skeletal muscle myoblasts, inducing early cell cycle arrest and reduced myogenesis (1, 15, 27, 30). Conversely, lower concentrations of myostatin may allow greater activation of myogenic regulatory factors and satellite cells (21) responsible for muscle hypertrophy.
Greater knowledge of the mechanisms of muscle hypertrophy following resistance exercise are important to our understanding of potential anecdotes for increasing muscle mass in women. It is likely that gender differences in muscle mass may be attributed to the influence of sex hormones. Higher testosterone levels are associated with increased muscle mass in men. However, testosterone levels in women are approximately 1/10 that of men; therefore, endogenous testosterone is probably not an influential factor in increasing muscle mass in women. Anabolic hormones are responsible for muscle mass in men, but the precise factors involved in regulating muscle mass in young women are unknown. Understanding the role of myostatin and follistatin in skeletal muscle will help to determine the mechanism(s) by which women regulate muscle mass.
We hypothesized that EE and CE would down regulate myostatin mRNA gene expression and that follistatin and MyoD mRNA gene expression would be upregulated and that there would be a blunted response in the CE group due to the lower exercise-induced muscle damage associated with this type of stimulus. Therefore, we designed a study to investigate the influence of high intensity concentric and eccentric resistance exercise on myostatin and follistatin in young women. The primary goal was to quantify changes in myostatin, follistatin, and MyoD mRNA expression eight hours after a single resistance exercise bout and following seven training sessions using high intensity EE or CE.
METHODS
Experimental Approach to the Problem
All subjects were recruited from the USC Health Science Campus. All clinical procedures were conducted at the University of Southern California General Clinical Research Center (GCRC) except for the exercise testing, which was performed in the Clinical Exercise Research Center of the Division of Biokinesiology and Physical Therapy. Subjects were screened for health conditions prior to entry into the study. All subjects participated in 7 exercise sessions supervised by a certified strength and conditioning specialist.
Subjects
Subjects provided written informed consent approved by University of Southern California Institutional Review Board. We included women 21 to 35 years of age who were recruited from the USC Health Sciences Campus. The subjects were in good health, had negative pregnancy tests, were recreationally active, and completed medical history screening for eligibility to assure absence of underlying medical conditions that could confound results. Exclusion criteria included acute infections, recent surgery, trauma or chronic illness (e.g. diabetes, liver disease, kidney failure, cancer, musculoskeletal disease or injury) that would prevent resistance exercise. Women that did not have regular menstrual cycles (28–32 days), were using oral contraceptives (OCs) that contained anabolic androgens, or had performed regular lower extremity resistance exercise in the preceding 6 months were also excluded.
Procedures
Dual-energy X-ray Absorptiomtry (DEXA)
Prior to biopsy #1, all subjects underwent a total body DEXA (model DPX-IQ 2288 with Smart Scan version 4.7e; Lunar Radiation Corporation, Madison, WI, USA) to assess body fat percentage and lean body mass. Quality assurance was performed using a single acrylic block three times per week to confirm the accuracy and precision of the DEXA system. The same experienced investigator was responsible for performing and analyzing all scans.
Maximal Eccentric or Concentric Exercise
The women were randomly assigned to either the eccentric (n=6) or concentric (n=6) training program (Table 1). Single-leg maximal eccentric or concentric (knee extension) isokinetic loading was performed on the right leg using the Cybex Norm™ dynamometer (Cybex International Inc. Ronkonkoma, New York) at 60°/s. The Cybex was calibrated immediately before the exercise bout. Subjects were coupled to the Cybex by visually aligning the placement of the lateral femoral condyle with the axis of rotation for the Cybex. A strap was placed distally on the dominant leg at the level of the load cell. The load cell was positioned 3 cm proximal to the talocural joint. During the maximal loading, a shoulder harness, hip restraint, and thigh strap (exercised leg) were used to limit excessive movement and secure the participant to the device. The settings were recorded to ensure exact placement for each exercise bout.
Table 1.
Schedule of events
| Visit # | Pre- Ex | Pre- Ex | Pre- Ex | V1 | V2 | V3 | V4 | V5 | V6 | V7 | V8 | V9 | V10 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1RM | Xc | Xc | Xf | ||||||||||
| Exercise | Xb | Xb | Xb | Xb | Xb | Xb | Xb | ||||||
| Blood | X | Xa | Xd | Xe | Xa | Xd | |||||||
| Biopsy | X | Xa | Xa |
Abbreviation: (Pre-Ex) before exercise training
Abbreviation: (V1–V10) is visit 1 to visit 10
Abbreviation: (1RM) one repetition maximum
X 1 week before any exercise (afternoon)
Xa Approximately 8 hours after exercise (afternoon)
Xb Exercise bout (1–3 days between exercise bouts in mornings)
Xc 1 week after biopsy and 1 week before exercise training (morning)
Xd 24 hours after exercise (morning)
Xe 24 hours after exercise bout, but blood taken before next exercise bout (morning)
Xf Final 1RM, one week after final muscle biopsy (morning)
Before completing the exercise protocol, the tester determined the subjects’ eccentric and concentric 1 repetition maximum (1RM). Each participant was familiarized with the equipment immediately prior to the exercise. The 1RM was determined to assess whether the subjects were generating maximal effort during each set. Ten sets of ten repetitions were completed by each participant. Each participant completed 7 exercise bouts beginning one week after their initial muscle biopsy. Each exercise bout was completed in the morning. The subjects typically exercised on a Monday, Wednesday, Friday, unless scheduling conflicts occurred, where the exercise days were slightly modified. A similar training protocol was used in a previous eccentric exercise study (8); this training protocol was less intense; however, the exercise elicited muscle damage as measure by creatine kinase and satellite cell number.
The exercise protocol consisted of maximal repetitions with the group assigned to eccentric exercise performing the eccentric component while the investigator performed the concentric component by moving the limb back to the starting position (15 degrees before full extension); whereas for the concentric group, the participant extended her leg against the load while the tester lowered the load. Each repetition was separated by the time it took the testing investigator to manually return the lever arm back to 75 degrees of knee flexion (i.e. starting position). Each of the ten sets was separated by 20 seconds. Biofeedback was provided on the computer monitor where subjects were instructed to generate as much torque as generated during the 1-RM test. Additionally, verbal encouragement was provided by the investigator during each maximal contraction. Subjects filled out and orally confirmed a rating of perceived soreness scale before each exercise bout.
Muscle Biopsies
Three muscle biopsies of the vastus lateralis were obtained from the right leg. One week prior to the baseline biopsy #1, subjects were required to abstain from recreational activity and did not perform rigorous activity until participating in the maximal eccentric or concentric exercise bout. Biopsy #1 was taken one week before the first exercise bout. Biopsy #2 was performed 8 hours after the participant completed the first exercise bout of unilateral maximal EE or CE of the right quadriceps muscle group. Biopsy #3 was taken 8 hours after the final exercise bout (Table 1) to assess mRNA gene expression (17). Additional ubiquitin proteins will be analyzed with the remaining muscle tissue.
Muscle biopsies were performed between 4:30 and 5:30 pm after vitals were measured and blood specimens were collected. Biopsy specimens were collected using sterile conditions via a 5-mm Stille biopsy needle (Micrins Surgical, Lake Forest, Illinois) from the mid-portion of the vastus lateralis muscle (under local anesthesia) approximately 18 cm proximal to the patella. Biopsy #2 and #3 were performed at a distance of 2–4 cm proximal or distal (randomly assigned) to the pre-exercise biopsy site. Approximately 100–250mg of muscle tissue was obtained from each biopsy. None of the participants experienced adverse events as a result of the muscle biopsy. The participants experienced subtle bruising and muscle soreness that disappeared after a few days.
Muscle Biopsy Specimen Processing
Muscle tissue samples were immediately flash-frozen in liquid nitrogen and stored at −80° C until being processed for analysis. All muscle biopsies were assigned a participant identification number to eliminate investigator bias.
RNA extraction
Total RNA was extracted from muscle using a commercially available kit (Qiagen Kit). The manufacturer’s instructions were followed using the RNeasy fibrous mini protocol for isolation of total RNA from muscle tissue. Briefly, approximately 10 mg of muscle was removed from the freezer and immediately samples were lysed in a guanidine-isothiocyanate-containing lysis buffer (Buffer RLT) and β-Mercaptoethanol (β-ME). The tissue was homogenized using a polytron homogenizer (Kinematica Polytron PT1200C, Switzerland). After dilution of the lysate, the sample was treated with proteinase K for 10 minutes at 55°C before pelleting debris by centrifugation. Ethanol was added to the cleared lysate and RNA was bound to the RNeasy silica-gel membrane. Contaminants were washed away with simple wash spins (Buffers RW1 and RPE), and total RNA was eluted in RNase-free water. The concentration and purity of the RNA was determined using a UV spectrophotometer (Beckman Coulter DU 640, Fullerton, CA) by measuring absorbance at 260 nm and 280 nm.
Reverse Transcription and cDNA synthesis
500 nanograms of total skeletal muscle RNA was reverse transcribed to synthesize cDNA using Taqman reverse transcription reagents (Applied Biosystems, Branchburg, NJ). A reverse transcription (RT) reaction master mixture for one reaction was 50μl total volume (0.5μg of cellular RNA; 5μl 10x reverse transcription buffer; 11μl MgCL2; 10μl deoxynucleotide 5′-triphosphate (dNTPs); 2.5lμl Random Hexamer; 1.25μl RNase inhibitor; DEPC H20 to a final volume of 50uL) and was incubated at 25°C for 10min, 48°C for 30min, 95°C for 5min, and 4°C infinite (reaction done in the Mycycler, BioRad, Hercules, CA).
Oligonucleotide primers for PCR
Oligonucleotide primers were used to amplify the mRNA expression of myostatin, follistatin, and MyoD. Primer sequences were designed using Primer3 program (29). The primer sequences for the specific target mRNAs and melt curve temperatures are shown in Table 2 (18). Melt curves were determined for each primer set to ensure amplification of pure PCR products.
Table 2.
Primer Set Sequences used for real-time PCR
| Target mRNA | PCR Primer Sequence 5′ → 3″ | Melt Curve Temperature |
|---|---|---|
| MyoD | F: AGC ACT ACA GCG GCG ACT | 82°C |
| R: AGG CAG TCT AGG CTC GAC AC | ||
| Myostatin | F: CTG TAA CCT TCC CAG GAC CA | 82°C |
| R: CCC ATC CAA AAG CT CAA AA | ||
| Follistatin | F: AAG ACC GAA CTG AGC AAG GA | 84°C |
| R: TTT TTC CCA GGT CCA CAG TA | ||
| 18s rRNA* | F: CGG CTA CCA CAT CCA AGG AA | 82°C |
| R: TGC TGG CAC CAG ACT TGC CTC |
18s rRNA Sequence (20)
PCR amplification
A quantitative real time PCR (qRT-PCR) method was applied in the present study using 18s ribosomal RNA as an internal standard to determine relative expression levels of mRNAs for myostatin, follistatin, and MyoD. A total of 10ng of cDNA were added to each of the 20μl PCR reaction for myostatin, follistatin, MyoD, 18s rRNA. Specifically, each PCR reaction contained the following mixture: 10μl 2.5x iQSYBRgreen supermix (BioRad, Hercules, CA); 7ul RNase free water, 10ng cDNA and 10 pmol of each primer (F and R) of interest. Each PCR reaction was amplified using BioRad iCycler iQ thermal cycler (BioRad, Hercules, CA). A melting curve analysis was generated by the following amplification profile: 95°C for 3 min, and 60 cycles as follows: 95°C for 15 sec and ramp to 56°C for 1 min, followed by an increase in set-point temperature by 0.5°C every 5 sec for each sample. All cycle threshold values were less than 30. A single melt curve was detected to exclude primer dimmers from real time analysis. Primer efficiency was determined to be between 80–100%. To help control for differences in amplification efficiency during thermocycling, all PCR reactions were prepared from the master mix stock solution. After the melt curves were determined, the amplification profile involved the denaturation step at 95°C for 3 min, and 60 cycles as follows: 95°C for 15 sec and ramp to 56°C for 1 min, followed by a predetermined melt temperature for 10 sec (Table 2).
mRNA Quantification
mRNA expression levels were normalized to the 18s rRNA control gene after determining that there was no change in 18s RNA in response to the exercise bout. Real-time RT-PCR analyses of the following housekeeping genes were also tested: GAPDH and β2- microglobulin (β2M). Exercise appeared to alter gene expression in each of these housekeeping genes; therefore we did not use these controls to normalize the data as demonstrated by a prior studies (12, 14). All data were determined by normalizing the cDNA measured in 8 replicates (4 replicates repeated) for each participant sample to 18s rRNA (internal control) and then averaging the data to account for the change in mRNA expression as a result of the exercise stimulus. The data were then normalized to biopsy #1 (pre-exercise) for each participant to determine the fold change of mRNA gene expression after exercising. All participant samples were then averaged to determine the average mRNA expression prior to and following exercise.
Statistical Analyses
Statistical analyses were performed using SPSS, version 14.0 (SPSS Inc., Chicago, IL). To our knowledge this exercise protocol was not used by other investigators, thus we powered this study from prior literature investigating myogenic factors via muscle biopsy. A priori analysis determined that a sample size of 6 participants in each group would have 80% power to detect a difference in means for myostatin mRNA expression of 1.5 (the difference between group 1 mean (μ1) of 2.8 and group 2 mean (μ2) of 1.3) assuming that the common standard deviation is 0.8 with an effect size of 1.88 using a two group t-test with a 0.05 two-sided significance level. The independent variables included the muscle biopsy time points (biopsies #1, #2, and #3) and the type of exercise (EE and CE). The dependent variables included myostatin, follistatin, and MyoD. A 2 (group) × 3 (biopsy) factorial repeated measures analysis of variance (ANOVA) was used to compare changes in mRNA gene expression between biopsy time points and for each exercise group (EE and CE). Independent t-tests were performed to test group differences (EE versus CE). The alpha level of significance used was P ≤ 0.05 with Bonferroni adjustments. Data were normalized to biopsy #1 (pre-exercise) for each participant to determine the fold change of mRNA gene expression after exercising. All participant samples were then averaged to determine the average mRNA expression prior to and following exercise. Furthermore, the data were log transformed (Log10) to demonstrate both positive and negative values. The statistics were performed on the log transformed data. A priori, a power analysis for change in myostatin demonstrated that a sample size of 6 subjects in each group would have 80% power using a two sided alpha of 0.05 to detect a mean difference of 1.5 and an effect size of 1.88 for myostatin mRNA gene expression.
RESULTS
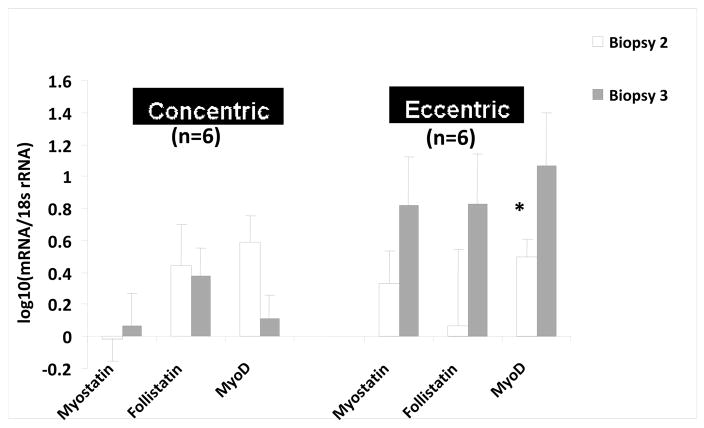
Twelve healthy women 23–28 years of age volunteered to participate. Baseline characteristics of the study subjects were similar (Table 3). Leg extension 1RM torque was similar between groups (EE group 144±26 Nm; CE group 146±20 Nm). The study results indicate that the fold changes for myostatin and follistatin mRNA gene expression were not significantly different between biopsy #1 (pre exercise) and #2 (after one bout of exercise) or between biopsy #1 and biopsy #3 (post training) (p≥0.14); for all paired comparisons, (Fig. 1); whereas, MyoD gene expression increased significantly between biopsy #1 and biopsy #2 in the EE group (p=0.05) but was not significantly different between biopsy #1 and biopsy #3 in the CE and EE groups (p=0.2, p=0.3 respectively) (Fig. 1).
Table 3.
Subject Characteristics
| Training mode | Age (yrs) | Weight (kg) | Height (McMahon, Popovic et al.) | Lean Mass (kg) | Percent Fat (%) |
|---|---|---|---|---|---|
| Concentric (n=6) | 24 ± 1 | 65 ± 7 | 165 ± 3.3 | 41.0 ± 3.6 | 32.4 ± 4.0 |
| Eccentric (n=6) | 25 ± 1 | 59 ± 7 | 168 ± 6.4 | 41.0 ± 2.2 | 28.0 ± 6.0 |
Data are presented as Mean ± Standard Deviation.
Figure 1.
Myostatin, Follistatin and MyoD mRNA gene expression normalized to 18s rRNA after each muscle biopsy. Biopsy #2 and biopsy #3 compared to biopsy #1 for each protein. The data are expressed as fold change. (*) indicates significant change in mRNA gene expression compared to biopsy #1 (P≤0.05). The corresponding P-Values are in the table below each figure. The data was Log10 transformed with biopsy #1 equal to zero (mean ± SE).
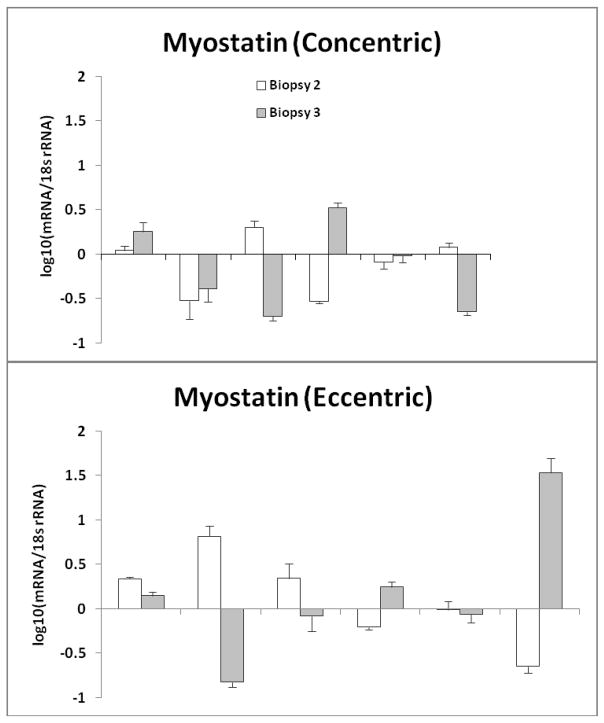
Although changes in myostatin, follistatin, and MyoD mRNA expression did not reach statistical significance, except for MyoD expression between biopsy #1 and biopsy #2 in the EE group, there were interesting individual changes in each group (Fig. 2–4). Changes in mRNA gene expression were compared to biopsy #1. Error bars for each individual were generated after duplicating the results by running the sample in 4-plicate two times. Myostatin mRNA gene expression decreased in biopsy #2 (in two subjects) and biopsy #3 (in three subjects) after CE (Fig. 2a). Whereas, in the EE group, three subjects demonstrated increases in myostatin mRNA gene expression in biopsy #2 and one in biopsy #3 (Fig. 2b), and three subjects showed decreases during one of the two post exercise biopsies. Follistatin increased in three subjects in either biopsy #2 or biopsy #3 after CE (Figure 3a). Follistatin increased in three subjects but decreased in five subjects at either biopsy after EE (Figure 3b).
Figure 2.
a) represents the individual myostatin mRNA response for each participant after concentric exercise b) represents the individual myostatin mRNA response for each participant after eccentric exercise. The data are expressed as fold change, however it was Log10 transformed with biopsy #1 equal to zero (mean ± SE). Error bars were generated after duplicating the results by running the sample in 4-plicate two times.
Figure 4.
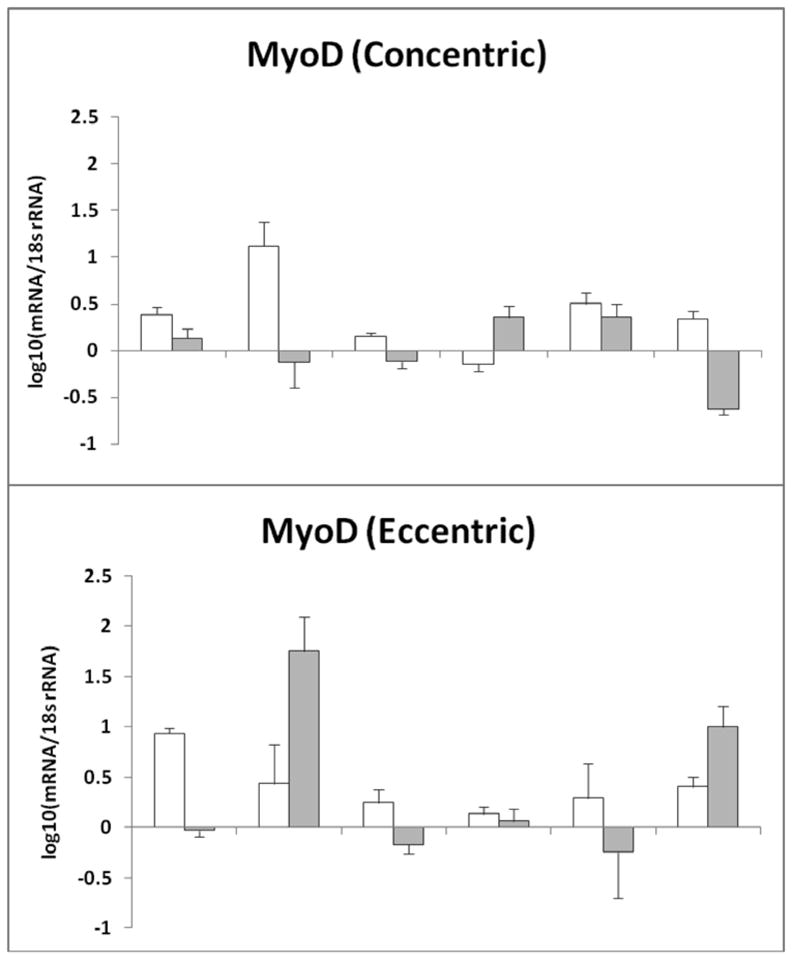
MyoD mRNA gene expression normalized to 18s rRNA after each muscle biopsy a) represents the individual MyoD mRNA response after concentric exercise b) represents the individual MyoD mRNA response after eccentric exercise. The data are expressed as fold change; that was Log10 transformed with biopsy #1 equal to zero (mean ± SE). Error bars were generated after duplicating the results by running the sample in 4-plicate two times.
Figure 3.
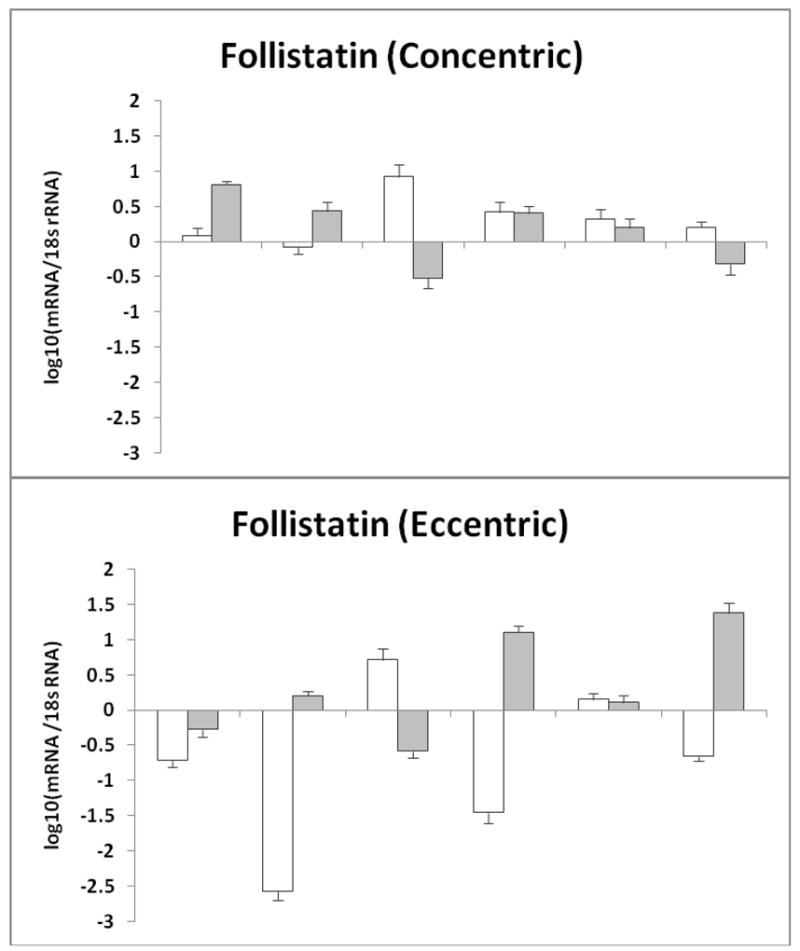
Follistatin mRNA gene expression normalized to 18s rRNA after each muscle biopsy a) represents the individual Follistatin mRNA response after concentric exercise b) represents the individual Follistatin mRNA response after eccentric exercise. The data are expressed as fold change that was Log10 transformed with biopsy #1 equal to zero (mean ± SE). Error bars were generated after duplicating the results by running the sample in 4-plicate two times.
MyoD mRNA gene expression was also elevated after both EE and CE in some subjects at biopsy #2 or biopsy #3 (Fig. 1 and Fig. 4). MyoD gene expression increased in five subjects in the CE group and four subjects from biopsy #1 to biopsy #2 in the EE group; one subject also had an increase at biopsy #3 with EE. There were no significant differences between the EE and CE groups (p≥0.11) (Fig 1).
DISCUSSION
Our study is unique in that we have examined skeletal muscle myostatin, follistatin, and MyoD mRNA gene expression in response to either exclusively EE or CE in young women. After a single bout of EE, MyoD mRNA expression was significantly elevated, however, MyoD did not respond to a single bout of CE or multiple bouts of EE and CE. Although myostatin and follistatin mRNA gene expression levels were not different following one or multiple bouts of EE or CE, there were some large individual changes. An unexpected increase in myostatin and follistatin mRNA expression in the EE group was observed, while the CE group experienced a decline in myostatin mRNA expression (three of six subjects) and an increase in both follistatin and MyoD mRNA expression.
Contrary to our findings in young women, several studies report that young and older trained and untrained men down-regulate myostatin mRNA expression after resistance training (7, 11, 19). Although we did not directly compare men and women, it is possible that differences between gender may be attributed to the increases in myostatin mRNA expression in women demonstrated by our findings. Moreover, myostatin activity could differ between men and women if there are differences in any of the proteins that process, bind, or mediate myostatin signal transduction. Skeletal muscle fiber type may also contribute to gender differences. Myostatin mRNA gene expression is more specific to Type II fibers (20) and there is a greater ratio of Type II fiber mass to Type I fiber mass in men (2) with myostatin mRNA expression being further modified by sex hormones (29). It is possible that some of our subjects had a lower proportion of type II fibers in the vastus lateralis, consequently leading to minimal changes in myostatin mRNA expression (20), in turn affecting follistatin and MyoD mRNA expression. Furthermore, both EE and CE are effective in promoting protein synthesis (33, 34); however, diet and in particular, amino acids and to a lesser extent carbohydrate ingestion have a substantial influence on skeletal muscle hypertrophy (23, 26, 33, 34). Unfortunately, we did not control the subjects’ nutritional status and this could have altered mRNA gene expression in individual cases.
Gender differences in skeletal muscle mass appear to be mediated by higher testosterone levels in men, which has an anabolic effect (3) and exerts its effects by influencing gene expression. Men generally have higher expression of genes encoding mitochondrial proteins, ribosomal proteins and translation initiation factors (31). This supports the contention that anabolic hormones influence gene expression and may explain the differences in findings between our study in young women and other studies in men.
Microarray analyses provide information about gender differences in gene expression. Women have two-fold greater expression of the myostatin ACTIIB receptors than men (31). These data suggest that greater ACTIIB receptors could contribute to increases in myostatin mRNA gene expression which would further support our findings. The ACTIIB receptor genes may also be altered with exercise as some studies indicate that the ACTIIB receptor is either downregulated after resistance training in men (14); or unchanged in a cluster analysis of both men and women (10). Perhaps the ACTIIB receptors in men and women respond differently to resistance training, thus leading to alternate changes in myostatin mRNA expression. These findings may indicate that increased myostatin activity contributes to gender differences in muscle size (31). The results of our investigation were unexpected as previous studies have reported that load mediated myofiber hypertrophy inhibits myostatin gene transcript (13, 28). We utilized a higher intensity exercise protocol than prior studies that elicited satellite cell activation after one bout of eccentric exercise (7, 8) and reductions in myostatin mRNA expression after one bout of conventional resistance exercise (14, 17) yet changes in mRNA expression for each gene were minimal throughout the experimental period. Similar to previous studies that showed decreases in myostatin mRNA expression after a single exercise bout or nine weeks of training (13, 28), we observed a trend in decreasing myostatin mRNA expression in the CE group. In contrast, there was a trend to increasing myostatin and follistatin mRNA expression in the EE group similar to other studies (24, 32). However, the trend of increasing MyoD mRNA gene expression that is typical of increase muscle protein synthesis and hypertrophy was not associated with significant reductions in myostatin mRNA expression as hypothesized. One mechanism that could account for these observations is the individual differences in gene expression that may be based on the inherited organization of genes (25).
A plausible explanation for our findings may be the trend of increasing follistatin mRNA expression in the CE group. Because follistatin acts as an antagonist to myostatin and inhibits myostatin from binding to the ACTIIB receptor site, this may be affecting myostatin’s role as a negative regulator of muscle mass (30). Further, myostatin could be a chalone for skeletal muscle tissue (4, 7), in that skeletal muscle regulates its growth and regeneration (22). Indeed, it is possible that a resistance exercise stimulus might complement myostatin down-regulation to enable skeletal muscle to achieve optimal repair and growth (6) following high intensity exercise. Once muscle repair and growth are successful, skeletal muscle would likely upregulate myostatin expression in order to avoid excessive muscle growth. Thus, it may be that the increased myostatin mRNA expression in our subjects occurred to modulate or prevent excessive muscle cell growth.
In summary, a single bout and multiple bouts of either maximal EE or CE in young women did not significantly alter myostatin or follistatin mRNA expression. Whereas, MyoD mRNA gene expression was significantly elevated after one bout of maximal EE, it was not significantly increased after multiple bouts of EE or CE. Therefore, the type of exercise stimulus did not appear to differentially alter mRNA gene expression of hypertrophic myogenic regulators with the exception of MyoD. Regardless, outcomes of this study suggest that high intensity resistance exercise may not down-regulate myostatin and up-regulate follistatin in young women as we had hypothesized and has been demonstrated in previous studies with men.
PRACTICAL APPLICATIONS
Although skeletal muscle regulators did not differ with eccentric and concentric exercise, results suggest that gender differences may exist in regards to hypertrophic gene expression regulation following resistance exercise. For example, in men, following resistance exercise, myostatin is down-regulated while follistatin is up-regulated. This indicates that resistance exercise promotes muscle hypertrophy in men. However, our findings indicate that this is not the case in women. Gender differences in skeletal muscle regulators must be considered when coaches are designing a hypertrophic resistance training program. Also, it is important to determine the mechanisms of muscle hypertrophy in both men and women, because eventually these concepts will be applied to exercise programs that aim to prevent or reduce muscle wasting as a result of disease and/or aging.
Acknowledgments
The study was funded in part by the USC Clinical Exercise Research Center and the USC General Clinical Research Center MO1 RR 00043 from NCRR/NIH.
We gratefully thank the subjects for their time and dedication to our study. This study was funded in part by the USC Clinical Exercise Research Center and the USC General Clinical Research Center MO1 RR 00043 from NCRR/NIH.
References
- 1.Amthor H, Nicholas G, McKinnell I, Kemp CF, Sharma M, Kambadur R, Patel K. Follistatin complexes Myostatin and antagonises Myostatin-mediated inhibition of myogenesis. Dev Biol. 2004;270:19–30. doi: 10.1016/j.ydbio.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 2.Bamman MM, Hill VJ, Adams GR, Haddad F, Wetzstein CJ, Gower BA, Ahmed A, Hunter GR. Gender differences in resistance-training-induced myofiber hypertrophy among older adults. The journals of gerontology. 2003;58:108–116. doi: 10.1093/gerona/58.2.b108. [DOI] [PubMed] [Google Scholar]
- 3.Bhasin S, Storer TW, Berman N, Yarasheski KE, Clevenger B, Phillips J, Lee WP, Bunnell TJ, Casaburi R. Testosterone replacement increases fat-free mass and muscle size in hypogonadal men. The Journal of clinical endocrinology and metabolism. 1997;82:407–413. doi: 10.1210/jcem.82.2.3733. [DOI] [PubMed] [Google Scholar]
- 4.Bullough WS. Mitotic and functional homeostasis: a speculative review. Cancer Res. 1965;25:1683–1727. [PubMed] [Google Scholar]
- 5.Clarkson PM, Hubal MJ. Exercise-induced muscle damage in humans. Am J Phys Med Rehabil. 2002;81:S52–69. doi: 10.1097/00002060-200211001-00007. [DOI] [PubMed] [Google Scholar]
- 6.Colliander EB, Tesch PA. Effects of eccentric and concentric muscle actions in resistance training. Acta Physiol Scand. 1990;140:31–39. doi: 10.1111/j.1748-1716.1990.tb08973.x. [DOI] [PubMed] [Google Scholar]
- 7.Costa A, Dalloul H, Hegyesi H, Apor P, Csende Z, Racz L, Vaczi M, Tihanyi J. Impact of repeated bouts of eccentric exercise on myogenic gene expression. Eur J Appl Physiol. 2007;101:427–436. doi: 10.1007/s00421-007-0510-z. [DOI] [PubMed] [Google Scholar]
- 8.Dreyer HC, Blanco CE, Sattler FR, Schroeder ET, Wiswell RA. Satellite cell numbers in young and older men 24 hours after eccentric exercise. Muscle Nerve. 2006;33:242–253. doi: 10.1002/mus.20461. [DOI] [PubMed] [Google Scholar]
- 9.Evans WJ. Protein nutrition, exercise and aging. J Am Coll Nutr. 2004;23:601S–609S. doi: 10.1080/07315724.2004.10719430. [DOI] [PubMed] [Google Scholar]
- 10.Hulmi JJ, Ahtiainen JP, Kaasalainen T, Pollanen E, Hakkinen K, Alen M, Selanne H, Kovanen V, Mero AA. Postexercise myostatin and activin IIb mRNA levels: effects of strength training. Med Sci Sports Exerc. 2007;39:289–297. doi: 10.1249/01.mss.0000241650.15006.6e. [DOI] [PubMed] [Google Scholar]
- 11.Hulmi JJ, Kovanen V, Lisko I, Selanne H, Mero AA. The effects of whey protein on myostatin and cell cycle-related gene expression responses to a single heavy resistance exercise bout in trained older men. European journal of applied physiology. 2008;102:205–213. doi: 10.1007/s00421-007-0579-4. [DOI] [PubMed] [Google Scholar]
- 12.Jensky NE, Sims JK, Rice JC, Dreyer HC, Schroeder ET. The influence of eccentric exercise on mRNA expression of skeletal muscle regulators. Eur J Appl Physiol. 2007;101:473–480. doi: 10.1007/s00421-007-0521-9. [DOI] [PubMed] [Google Scholar]
- 13.Kim JS, Cross JM, Bamman MM. Impact of resistance loading on myostatin expression and cell cycle regulation in young and older men and women. Am J Physiol Endocrinol Metab. 2005;288:E1110–1119. doi: 10.1152/ajpendo.00464.2004. [DOI] [PubMed] [Google Scholar]
- 14.Kim JS, Petrella JK, Cross JM, Bamman MM. LOAD-MEDIATED DOWN-REGULATION OF MYOSTATIN mRNA IS NOT SUFFICIENT TO PROMOTE MYOFIBER HYPERTROPHY IN HUMANS: A CLUSTER ANALYSIS. J Appl Physiol. 2007 doi: 10.1152/japplphysiol.01194.2006. [DOI] [PubMed] [Google Scholar]
- 15.Langley B, Thomas M, Bishop A, Sharma M, Gilmour S, Kambadur R. Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J Biol Chem. 2002;277:49831–49840. doi: 10.1074/jbc.M204291200. [DOI] [PubMed] [Google Scholar]
- 16.Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci U S A. 2001;98:9306–9311. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louis ES, Raue U, Yang Y, Jemiolo B, Trappe SW. Time Course of Proteolytic, Cytokine, and Myostatin Gene Expression After Acute Exercise in Human Skeletal Muscle. J Appl Physiol. 2007 doi: 10.1152/japplphysiol.00679.2007. [DOI] [PubMed] [Google Scholar]
- 18.Marx JO, Kraemer WJ, Nindl BC, Larsson L. Effects of aging on human skeletal muscle myosin heavy-chain mRNA content and protein isoform expression. J Gerontol A Biol Sci Med Sci. 2002;57:B232–238. doi: 10.1093/gerona/57.6.b232. [DOI] [PubMed] [Google Scholar]
- 19.Mascher H, Tannerstedt J, Elfegoun T, Ekblom BT, Gustafsson T, Blomstrand E. Repeated resistance exercise training induces different changes in mRNA expression of MAFbx and MuRF-1 in human skeletal muscle. American journal of physiology. 2007 doi: 10.1152/ajpendo.00504.2007. [DOI] [PubMed] [Google Scholar]
- 20.Matsakas A, Bozzo C, Cacciani N, Caliaro F, Reggiani C, Mascarello F, Patruno M. Effect of swimming on myostatin expression in white and red gastrocnemius muscle and in cardiac muscle of rats. Exp Physiol. 2006;91:983–994. doi: 10.1113/expphysiol.2006.033571. [DOI] [PubMed] [Google Scholar]
- 21.McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol. 2003;162:1135–1147. doi: 10.1083/jcb.200207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCroskery S, Thomas M, Platt L, Hennebry A, Nishimura T, McLeay L, Sharma M, Kambadur R. Improved muscle healing through enhanced regeneration and reduced fibrosis in myostatin-null mice. J Cell Sci. 2005;118:3531–3541. doi: 10.1242/jcs.02482. [DOI] [PubMed] [Google Scholar]
- 23.Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and elderly. American journal of physiology. 2004;286:E321–328. doi: 10.1152/ajpendo.00368.2003. [DOI] [PubMed] [Google Scholar]
- 24.Peters D, Barash IA, Burdi M, Yuan PS, Mathew L, Friden J, Lieber RL. Asynchronous functional, cellular and transcriptional changes after a bout of eccentric exercise in the rat. J Physiol. 2003;553:947–957. doi: 10.1113/jphysiol.2003.048462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Psilander N, Damsgaard R, Pilegaard H. Resistance exercise alters MRF and IGF-I mRNA content in human skeletal muscle. J Appl Physiol. 2003;95:1038–1044. doi: 10.1152/japplphysiol.00903.2002. [DOI] [PubMed] [Google Scholar]
- 26.Rasmussen BB, Tipton KD, Miller SL, Wolf SE, Wolfe RR. An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J Appl Physiol. 2000;88:386–392. doi: 10.1152/jappl.2000.88.2.386. [DOI] [PubMed] [Google Scholar]
- 27.Rios R, Carneiro I, Arce VM, Devesa J. Myostatin is an inhibitor of myogenic differentiation. Am J Physiol Cell Physiol. 2002;282:C993–999. doi: 10.1152/ajpcell.00372.2001. [DOI] [PubMed] [Google Scholar]
- 28.Roth SM, Martel GF, Ferrell RE, Metter EJ, Hurley BF, Rogers MA. Myostatin gene expression is reduced in humans with heavy-resistance strength training: a brief communication. Exp Biol Med (Maywood) 2003;228:706–709. doi: 10.1177/153537020322800609. [DOI] [PubMed] [Google Scholar]
- 29.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 30.Thomas M, Langley B, Berry C, Sharma M, Kirk S, Bass J, Kambadur R. Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J Biol Chem. 2000;275:40235–40243. doi: 10.1074/jbc.M004356200. [DOI] [PubMed] [Google Scholar]
- 31.Welle S, Tawil R, Thornton CA. Sex-related differences in gene expression in human skeletal muscle. PLoS ONE. 2008;3:e1385. doi: 10.1371/journal.pone.0001385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willoughby DS. Effects of heavy resistance training on myostatin mRNA and protein expression. Med Sci Sports Exerc. 2004;36:574–582. doi: 10.1249/01.mss.0000121952.71533.ea. [DOI] [PubMed] [Google Scholar]
- 33.Wong TS, Booth FW. Protein metabolism in rat gastrocnemius muscle after stimulated chronic concentric exercise. J Appl Physiol. 1990;69:1709–1717. doi: 10.1152/jappl.1990.69.5.1709. [DOI] [PubMed] [Google Scholar]
- 34.Wong TS, Booth FW. Protein metabolism in rat tibialis anterior muscle after stimulated chronic eccentric exercise. J Appl Physiol. 1990;69:1718–1724. doi: 10.1152/jappl.1990.69.5.1718. [DOI] [PubMed] [Google Scholar]