Abstract
Rationale
We previously showed that transgenic mice expressing Ca2+/calmodulin-dependent protein kinase II δC (CaMKII-TG) develop dilated cardiomyopathy associated with increased ryanodine receptors (RyR2) phosphorylation, enhanced sarcoplasmic reticulum (SR) Ca2+ leak and lowering of SR Ca2+ load. We hypothesized that phospholamban (PLN) ablation would restore SR Ca2+ load and prevent the decreased ventricular contractility, dilation and mortality seen in CaMKII-TG.
Objective
Our objective was to generate CaMKII-TG mice lacking PLN, determine if the maladaptive effects of cardiac CaMKIIδC expression were corrected and establish the mechanistic basis for these changes.
Methods and Results
CaMKII-TG were crossed with PLN knockout (PLN-KO) mice to generate KO/TG mice. Myocytes from wild type (WT), CaMKII-TG, PLN-KO and KO/TG were compared. The decreased SR Ca2+ load and twitch Ca2+ transients seen in CaMKII-TG were normalized in KO/TG. Surprisingly the heart failure phenotype was exacerbated as indicated by increased left ventricular dilation, decreased ventricular function, increased apoptosis and greater mortality. In KO/TG myocytes SR Ca2+ sparks and leak were significantly increased, presumably due to the combined effects of restored SR Ca2+ load and RyR2 phosphorylation. Mitochondrial Ca2+ loading was increased in cardiomyocytes from KO/TG vs. WT or CaMKII-TG mice and this was dependent upon elevated SR Ca2+ sparks. Cardiomyocytes from KO/TG showed poor viability, improved by inhibiting SR Ca2+ release and mitochondrial Ca2+ loading.
Conclusions
Normalizing cardiomyocyte SR Ca2+ loading in the face of elevated CaMKII and RyR2 phosphorylation leads to enhanced SR Ca2+ leak and mitochondrial Ca2+ elevation, associated with exacerbated cell death, heart failure and mortality.
Keywords: calcium, Ca2+/calmodulin-dependent protein kinase II (CaMKII), phospholamban (PLN), heart failure
Introduction
Ca2+ is a critical second messenger in cardiac function. The Ca2+/calmodulin-dependent protein kinase II (CaMKII) is regulated by and involved in control of Ca2+ cycling in the myocardium. Several years ago we generated transgenic mice expressing CaMKIIδ, the predominant cardiac CaMKII isoform. Mice expressing the CaMKIIδC splice variant in the myocardium (CaMKII-TG) develop heart failure (HF) associated with ventricular dilation, marked decreases in fractional shortening, and mortality.1 We established that there was increased phosphorylation of the cardiac ryanodine receptor (RyR2) at the CaMKII site, associated with increased sarcoplasmic reticulum (SR) Ca2+ spark frequency and increased diastolic SR Ca2+ leak. More recently we generated CaMKIIδ knockout mice and further demonstrated that HF induced by pressure overload was dependent on CaMKIIδ-mediated RyR2 phosphorylation and increased Ca2+ sparks.2
The increased diastolic Ca2+ leak seen in the CaMKII-TG resulted in profound SR Ca2+ depletion and reduced Ca2+ transients and contractions. Accordingly, we hypothesized that diminished SR Ca2+ load could be responsible for the decreased contractile function and concomitant morbidity that characterized these mice. Cardiac SR Ca2+ uptake is regulated through the interaction of phospholamban (PLN) with the SR Ca2+ ATPase (SERCA2a).3 PLN knockout mice (PLN-KO) exhibit enhanced myocardial contractility,4 which can be explained by the increased SERCA2a pump activity and higher SR Ca2+ load in cardiomyocytes isolated from these mice.5 PLN ablation was shown to rescue both the functional and structural cardiomyopathy seen in mice in which the muscle LIM protein (MLP) is genetically deleted.6 Rescue of this genetic model of dilated cardiomyopathy and HF led to the proposal that ablation or inhibition of PLN would have therapeutic value in treatment of HF of various etiologies. Subsequently PLN-KO was shown to rescue cardiac hypertrophy and ventricular dysfunction in calsequestrin- and β1-adrenergic receptor-overexpressing mice.7,8 In light of the cellular changes observed in the CaMKII-TG mice, in particular the prominent SR Ca2+ depletion, we postulated that cardiac dysfunction would be improved if SR Ca2+ was normalized by PLN ablation.
Experiments reported here examined the effects of crossing CaMKII-TG1 with PLN-KO mice.4 Our studies demonstrated the desired and predicted effect of PLN ablation on SR Ca2+, e.g. complete rescue of the SR Ca2+ depletion seen in the CaMKII-TG. Additionally, contractile function of isolated myocytes (as reflected by Ca2+ transients) was improved. Unexpectedly, however, rather than improving the in vivo HF phenotype, the PLN-KO/CaMKII-TG (KO/TG) crosses showed exaggerated HF, with a more rapid onset of lethality and further decreases in contractile function.
The basis for the exaggerated HF phenotype, observed in the absence of changes in other Ca2+ handling proteins, is examined in the studies presented here. The data demonstrate that in KO/TG myocytes there are greater increases in SR Ca2+ sparks and leak, reflective of the now combined effects of increased SR Ca2+ load and RyR2 phosphorylation. Cardiomyocyte apoptosis is also increased in KO/TG. Using isolated cardiomyocytes, we observed increased mitochondrial Ca2+ in KO/TG and determined that the combined effects of increased SR Ca2+ release and mitochondrial Ca2+ loading contribute to diminished cardiomyocyte viability. We suggest that interventions that increase SR Ca2+ in the face of enhanced diastolic Ca2+ leak predispose to cardiomyocyte cell death and likely other Ca2+ mediated toxicities that compromise survival.
Methods
Mice
CaMKII-TG mice1 were crossed with PLN-KO mice4 and heterozygous PLN offspring carrying the CaMKII transgene inbred with the ones without the transgene. Four genotypes of mice including wild type (WT), PLN-KO, CaMKII-TG, and PLN ablation with CaMKIIδC overexpression (KO/TG) were used for experiments. All mice used in the present study were of mixed gender (more male than female) at 8 weeks of age, unless otherwise noted. All procedures were performed in accordance with Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee.
Ca2+ Measurements of Adult Mouse Ventricular Myocytes
Mouse ventricular myocytes were isolated and loaded with indo-1-AM to assess SR Ca2+ content and twitch [Ca2+]i transients, and with fluo-4-AM for SR Ca2+ spark measurements as described previously.9 Ca2+ sparks were measured in intact myocytes and were evaluated as described (IDL 5.3 computer software).9 Ca2+ spark amplitudes were normalized (F/F0) to fluorescence baseline (F0). Duration of the Ca2+ sparks was taken as the full duration above half maximum (FDHM) and width was the full spatial size above half maximum (FWHM). A crude index of diastolic SR Ca2+ leak was calculated as Ca2+ spark frequency (CaSpF) × FDHM × FWHM × amplitude. Some myocytes were pre-incubated for 30 min with a cell permeable myristoylated CaMKII inhibitor, autocamtide-2 related inhibitory peptide II (AIP, Calbiochem # 189485), before measurements.
Mitochondrial Ca2+ Measurements of Adult Mouse Ventricular Myocytes
Mitochondrial Ca2+ was measured indirectly by examining the FCCP-induced increase in [Ca2+]i in myocytes loaded with 10 µM fura-2-AM (Molecular Probes; 30 min plus 10 min de-esterification). Fura-2 was excited at 340 ± 10 nm and 380 ± 10 nm, and emitted fluorescence (535 ± 20 nm) was recorded at 100 Hz. Background-subtracted ratio fluorescence (R = F340/F380) was converted to [Ca2+]i using [Ca2+]i = Kdβ(R-Rmin)/(Rmax –R). Rmin, Rmax, Kd and β were determined experimentally. To assess baseline SR Ca2+ content myocytes were first paced at 0.5 Hz to steady-state, and 10 mM caffeine was rapidly applied. After caffeine washout, myocytes were paced at 0.5 Hz to re-attain the same steady state. They were then exposed to 0Na+/0Ca2+/0K+ solution to block the Na+/Ca2+ exchanger (NCX) and Na+/K+ pump and treated with 10 µM thapsigargin for ~45 seconds to block the SERCA pump.10 Under these conditions, when 1 µM FCCP is applied to abolish the mitochondrial membrane potential, mitochondrial Ca2+ is released and the rise in [Ca2+]i is indicative of mitochondrial Ca2+ content. Oligomycin (1 µM) was included with FCCP to block rapid ATP consumption. SR Ca2+ load assessed by application of 10 mM caffeine at the end of the protocol showed that the SR was still well Ca2+-loaded (and subsequent relaxation and [Ca2+]i decline) confirm that ATP was not dissipated by the protocol. The SR cannot be reloaded after this depletion, confirming the thapsigargin-dependent SR Ca2+-ATPase inhibition.10
Transthoracic Echocardiography
Transthoracic echocardiography was performed in mice using an Agilent Technologies Sonos 5500 system with a 15 MHz transducer as described.1
Western Blotting
Western blot analysis was carried out in cardiac homogenates as described previously.1 The antibodies used for immunoblotting were as followings: rabbit anti-calsequestrin (CsQ), rabbit anti-SERCA2, mouse anti-RyR, mouse anti-phospho-CaMKII (Affinity Bioreagents), rabbit Ser2809 phospho-RyR2 antibody (Badrilla, UK), rabbit Ser2815 phospho-RyR2 antibody (a gift from A.R. Marks, Columbia University), and anti-NCX (monoclonal R3F1, a gift from K.D. Philipson, UCLA).
TUNEL Staining
Transverse sections o f mouse hearts were labeled with fluorescein-terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling (TUNEL) using a kit from Roche (Lewes, UK) according to the manufacturer’s instructions. Slides were mounted with Vectashield mounting media containing DAPI to stain for the nuclei. Wheat germ agglutinin (WGA) was used to stain cell membranes and cardiomyocytes identified by their centrally located nuclei. Labeled nuclei were counted to determine the apoptotic index (number of labeled nuclei/105 total myocyte nuclei).
Cell Viability Study in Adult Mouse Ventricular Myocytes
Ventricular myocytes were isolated as described previously.11 After isolation, cells were plated for 1 hour on laminin-coated dishes in MEM-HBSS (Hanks’ Balanced Salt Solutions) medium containing 5% serum. Cells were then washed, and serum-free medium added along with vehicle, 2 µM KN-93, 100 nM ryanodine, 5 µM Ru-360 or 5 µM cyclosporine A. Using a grid marking system, 8 fields were chosen per dish and the number of rod shaped living cells was counted at various times over a 12 hour time course. At the 12 hour time point, living cells were quantitated by trypan blue exclusion.
Statistical Analysis
All data are reported as mean ± SEM. Statistical significance of differences between groups was determined using one-way ANOVA with Tukey post-hoc test. P value <0.05 was considered statistically significant.
Results
SR Ca2+ Load and Ca2+ Transients in Isolated Cardiomyocytes
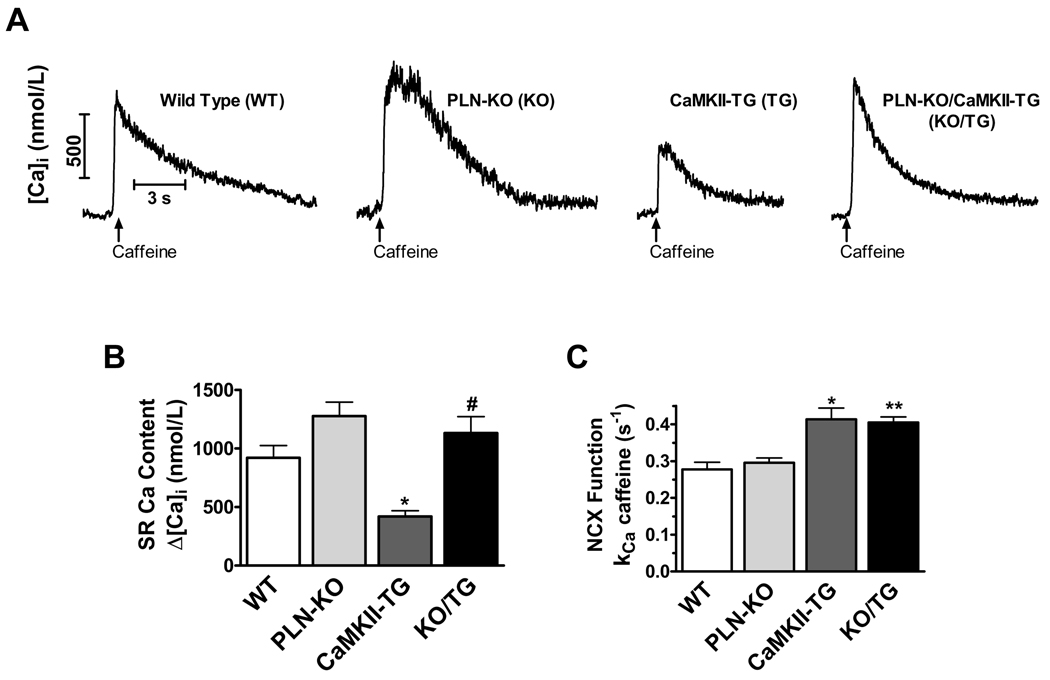
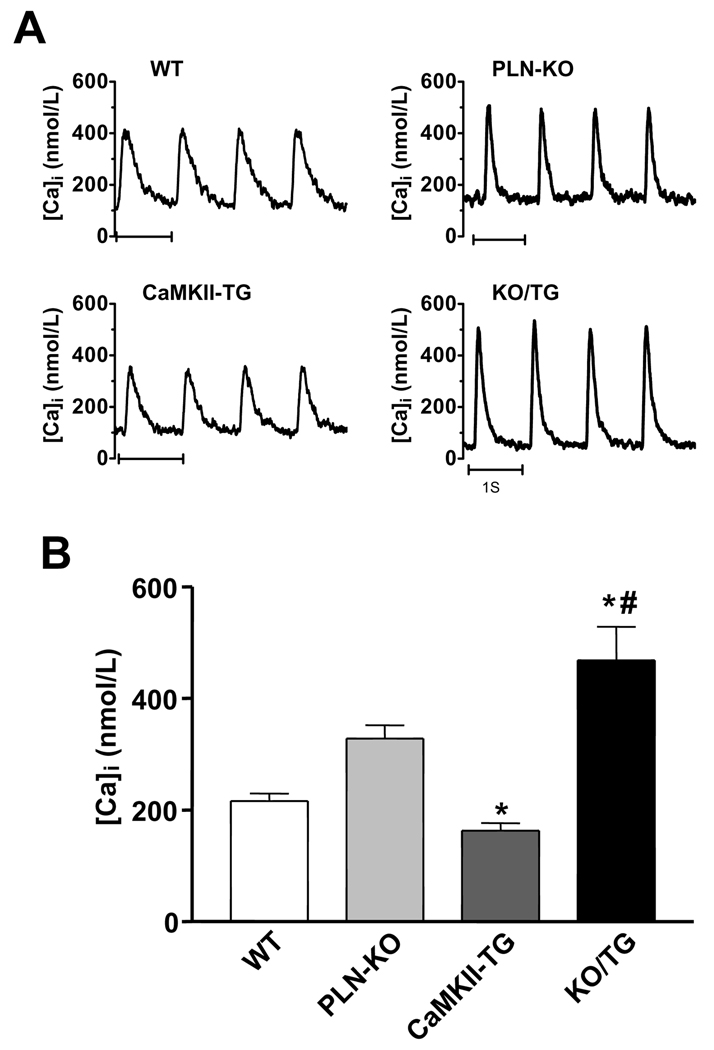
To determine whether PLN ablation normalized the depressed SR Ca2+ load observed in the CaMKIIδC TG mice, SR Ca2+ content was assessed by quantifying caffeine-induced Ca2+ transients after 1 Hz steady state stimulation (Fig. 1A). Consistent with our previous reports, myocytes isolated from PLN-KO showed increased SR Ca2+ load5 while those from CaMKII-TG mice exhibited significantly depressed SR Ca2+ load9 (Fig. 1A–B). SR Ca2+ load was restored to levels equivalent to those of WT mice in myocytes from the KO/TG crosses (Fig. 1A–B). NCX function, assessed as the rate constant of [Ca2+]i decline (kCa) in the presence of caffeine, was significantly faster in CaMKII-TG vs. WT as shown previously,9 and PLN ablation did not normalize this difference in NCX function (Fig. 1C). Twitch Ca2+ transient amplitude (Δ[Ca2+]i at 1 Hz) was also restored in KO/TG vs. CaMKII-TG myocytes (Fig. 2). In fact, Ca2+ transients in KO/TG were greater than those in WT, likely reflecting the combination of restored SR Ca2+ content along with CaMKII-dependent enhancement of fractional SR Ca2+ release.9,12
Figure 1. PLN ablation in CaMKIIδc TG mice repletes SR Ca2+ load in isolated cardiomyocytes.
A, Representative caffeine-induced Ca2+ transient traces. B, Average Δ [Ca2+]i for caffeine-induced Ca2+ transients (SR Ca2+ load). *P<0.05 vs. WT. # P<0.05 vs. CaMKII-TG. C, Average rate constant kCa for [Ca2+]i decline during caffeine exposure (which indicates NCX activity). *P<0.05 vs. WT. **P<0.01 vs. WT. The number of cells studied: WT n=6, PLN-KO n=21, CaMKII-TG n=9, and KO/TG n=9.
Figure 2. PLN ablation in CaMKIIδc TG mice normalizes twitch Ca2+ transients in isolated cardiomyocytes.
A, Representative twitch [Ca2+]i transient traces. B, Average Δ [Ca2+]i for twitches-induced Ca2+ transients. *P<0.05 vs. WT. # P<0.05 vs. CaMKII-TG. The number of cells studied: WT n=6, PLN-KO n=21, CaMKII-TG n=9, and KO/TG n=9.
In Vivo Global Cardiac Function
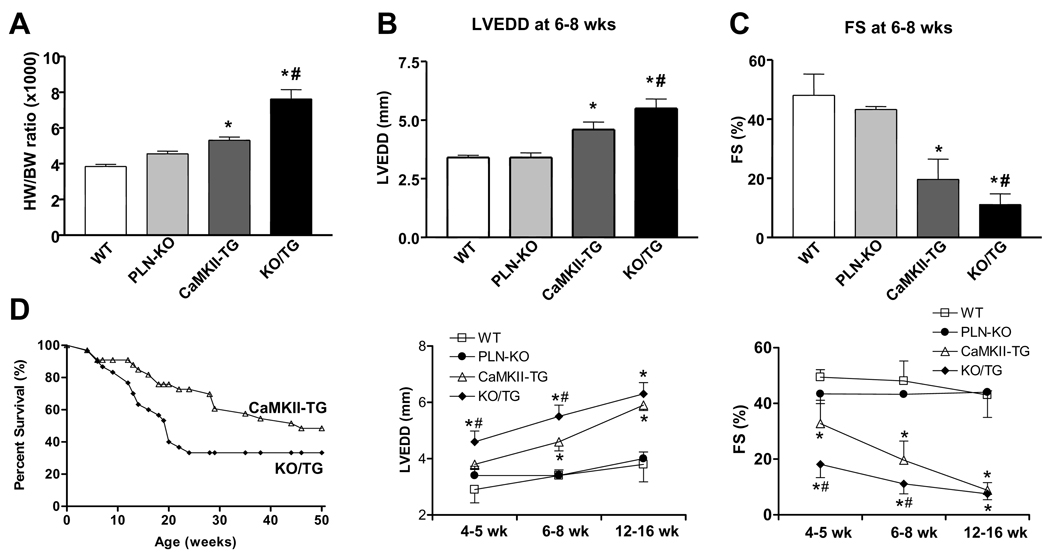
To determine whether the restoration of myocyte Ca2+ handling was associated with a diminished hypertrophic/HF phenotype and improved hemodynamic function, we measured the heart/body weight (HW/BW) ratio and assessed in vivo cardiac diameters and function by echocardiography. As reported previously, CaMKII-TG mice showed significant cardiac enlargement at 8 weeks of age as assessed by HW/BW ratio;1 surprisingly the increase in HW/BW was even greater in KO/TG mice (Fig. 3A) as was cell size (not shown). Left ventricular dilation and cardiac dysfunction were also worsened in KO/TG mice relative to CaMKII-TG, as revealed by significantly greater left ventricular end diastolic dimension (LVEDD), diminished factional shortening (FS), and posterior wall thinning at 6–8 weeks (Fig. 3B–C and Online Figure I). Differences between KO/TG and CaMKII-TG mice were most significant at earlier stages (4–5 weeks), becoming less divergent at 12–16 weeks (Fig. 3B–C and Online Figure I).
Figure 3. PLN ablation in CaMKIIδc TG mice exaggerates the phenotype of dilated cardiomyopathy.
A, Heart weight/body weight (HW/BW) ratio at 8 wks. B and C, Averaged echocardiographic parameters at different ages. Data are presented for B, left ventricular end-diastolic diameter (LVEDD), and for C, calculated percent fractional shortening (FS). n=4 to 6 mice per group at each age; *P<0.05 vs. WT. # P<0.05 vs. CaMKII-TG. D, Survival curves for CaMKII-TG and PLN-KO/CaMKII-TG mice. PLN ablation accentuates premature death in CaMKIIδC TG mice. Numbers of mice were as follows: CaMKII-TG, n=16; KO/TG, n=22. P <0.01 KO/TG vs. CaMKII-TG.
Survival Analyses
As described previously,1 CaMKIIδC TG mice showed premature death. KO/TG mice demonstrated worsened mortality compared to CaMKII-TG mice with 70% of the KO/TG mice dying by 24 weeks of age (Fig. 3D).
Expression and Phosphorylation of Ca2+ Regulatory Proteins
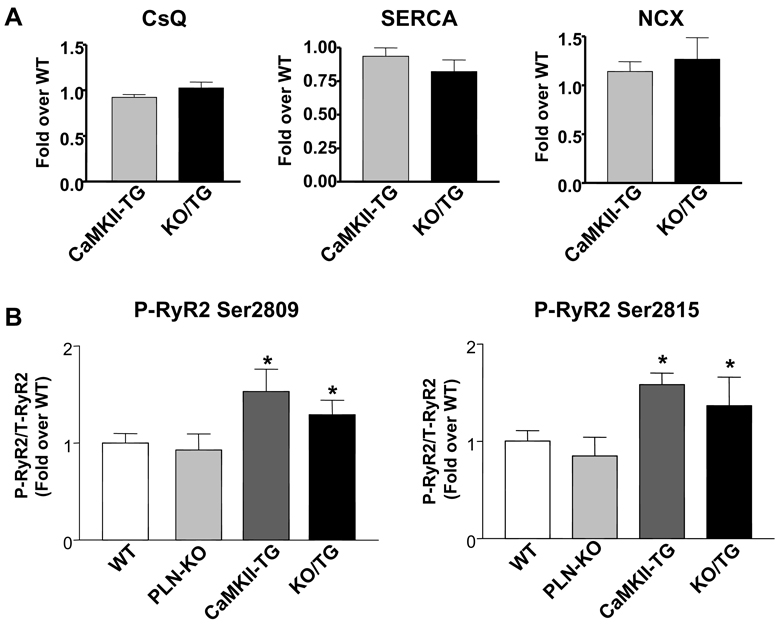
Western blot analyses were performed to assess the expression levels of calsequestrin (CsQ), SERCA2a and NCX in ventricular homogenates from CaMKII-TG and KO/TG mice. As previously reported,9 in CaMKII-TG vs. WT we found reduced SERCA2a and elevated NCX expression, but unaltered CsQ expression (data not shown). There were no significant differences in the expression of these major Ca2+ regulatory proteins in KO/TG versus CaMKII-TG mice (Fig. 4A). Phosphorylation of the RyR2, assessed using antibodies to the CaMKII phosphorylation sites (Ser2815 and Ser2809), was increased significantly, but not differentially, in CaMKII-TG and KO/TG mice (Fig. 4B). These data indicate that the exaggerated phenotype observed in KO/TG mice is not secondary to changes in key Ca2+ handling proteins or greater RyR2 phosphorylation.
Figure 4. Quantitative immunoblotting of major Ca2+ handling proteins in mouse ventricular homogenates.
A, Expression levels of calsequestrin (CsQ), SR Ca2+ ATPase (SERCA) and Na+/Ca2+ exchanger (NCX) did not change significantly between the CaMKII-TG and the PLN-KO/CaMKII-TG. B, Phosphorylation of ryanodine receptors (RyR2) at Ser2809 and Ser2815 (normalized to total RyR2) was increased significantly but not differentially in CaMKIIδc TG and PLN-KO/CaMKII-TG mice. n=4 mice per group; *P<0.05 vs. WT.
SR Ca2+ Sparks and Ca2+ Leak in Isolated Cardiomyocytes
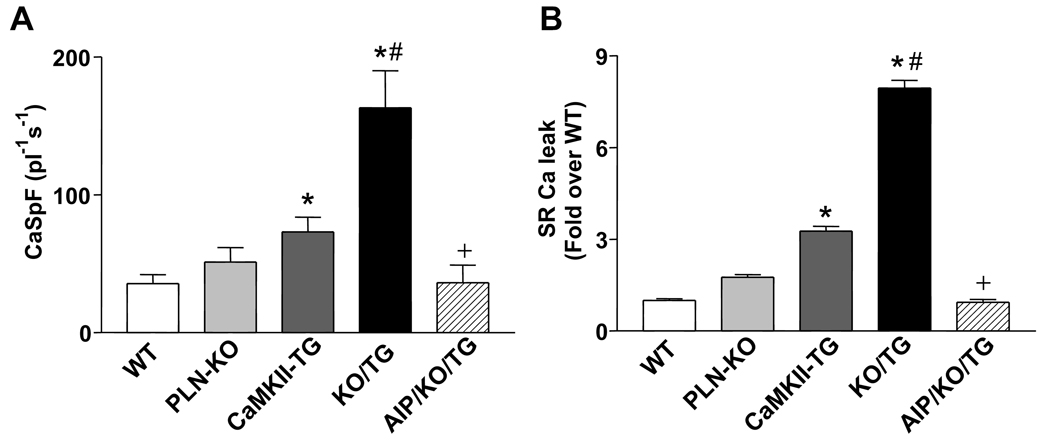
We previously demonstrated increased Ca2+ spark frequency in CaMKII-TG vs. WT myocytes.9 To determine whether the enhanced SR Ca2+ content seen with PLN ablation was associated with a further increase in SR Ca2+ sparks and leak, Ca2+ spark frequency (CaSpF), duration (FDHM), spatial width (FWHM) and amplitude were assessed. CaSpF increased dramatically in KO/TG compared to CaMKII-TG. This was entirely blocked by pretreatment with a CaMKII inhibitory peptide, AIP (Fig. 5A) indicating that it resulted from ongoing CaMKII activity. The overall diastolic SR Ca2+ leak index (CaSpF × FDHM × FWHM × amplitude) was also significantly increased in KO/TG mice (7.5-fold relative to WT and 2.5-fold relative to CaMKII-TG) (Fig. 5B). The augmented SR Ca2+ sparks and leak in KO/TG crosses can be explained in terms of the combined effects of normalized SR Ca2+ load in the face of enhanced RyR2 phosphorylation. Notably enhanced SR Ca2+ loading, Ca2+ spark frequency and Ca2+ transients are observed in KO/TG myocytes despite lower diastolic [Ca2+]i levels measured under comparable conditions (Online Figure II).
Figure 5. Enhanced SR Ca2+ spark frequency and Ca2+ leak in intact cardiomyocytes from PLN-KO/CaMKII-TG mice.
A, SR Ca spark frequency (CaSpF). *P<0.05 vs. WT. # P<0.05 vs. CaMKII-TG. +P<0.05 vs. KO/TG. B, SR Ca2+ leak. *P<0.05 vs. WT. # P<0.05 vs. CaMKII-TG. +P<0.05 vs. KO/TG.
Apoptosis in Heart Sections
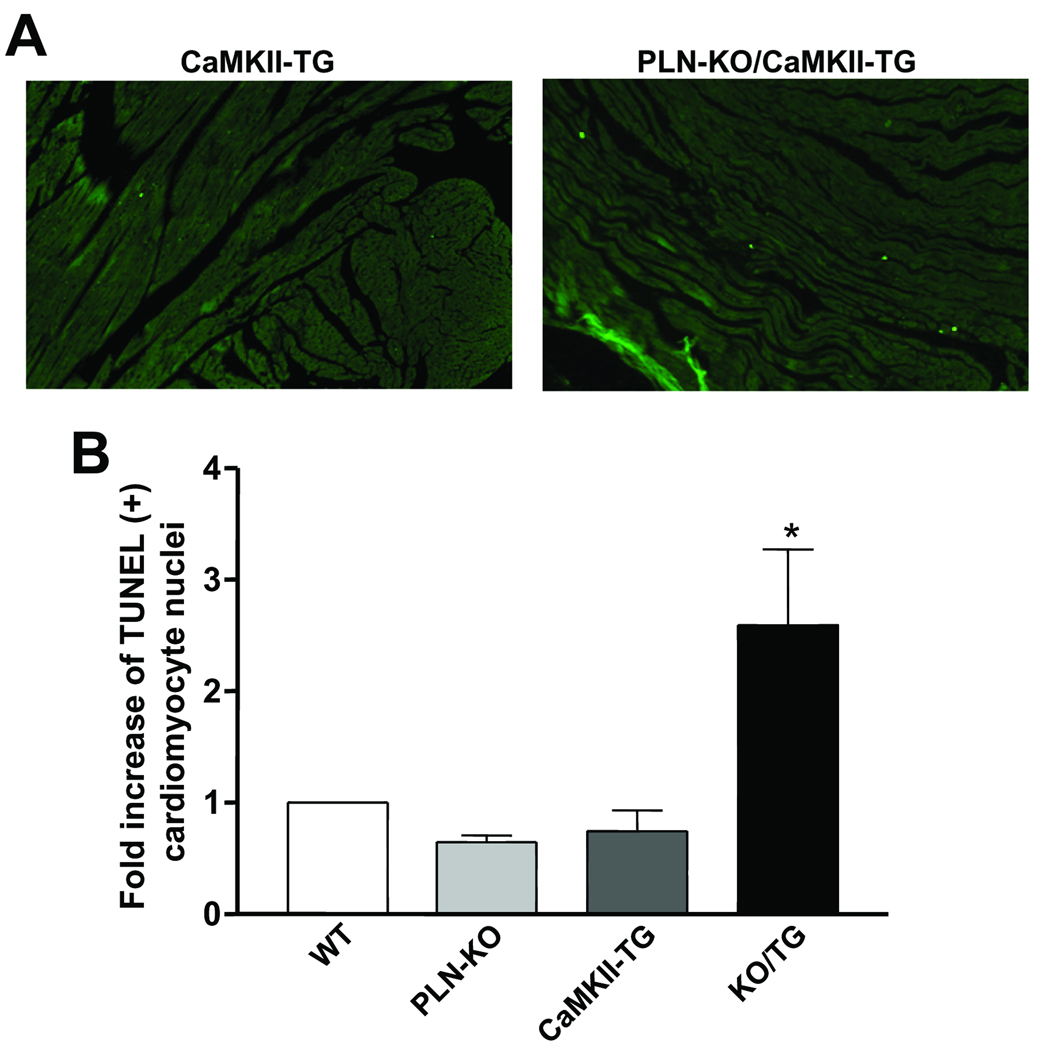
Cardiomyocyte dropout could explain why there is reduced cardiac function, ventricular thinning and worsened HF despite enhanced Ca2+ transients in KO/TG myocytes. TUNEL staining was performed to assess apoptosis in paraffin sections prepared from WT, PLN-KO, CaMKII-TG, and KO/TG mice. DAPI staining and WGA were used to identify cardiomyocytes (see Methods). TUNEL labeled cardiomyocyte nuclei were counted to determine the apoptotic index (number of labeled nuclei/105 total myocyte nuclei). As shown in Fig. 6, the number of TUNEL positive cardiomyocytes was significantly increased (~2.6 fold) in KO/TG mouse hearts compared with WT or CaMKII-TG mice.
Figure 6. Apoptosis occurs more frequently in PLN-KO/CaMKII-TG cardiomyocytes than in CaMKIIδc TG.
A, Representative TUNEL staining images in CaMKII-TG and PLN-KO/CaMKII-TG mouse heart sections. B, Average TUNEL positive myocytes. n=4 mice per group; *P<0.05 vs. WT. Data were normalized to WT; the average number from all experiments was ~75 TUNEL positive nuclei/105 myocytes in the KO/TG group.
Cell Viability Studies
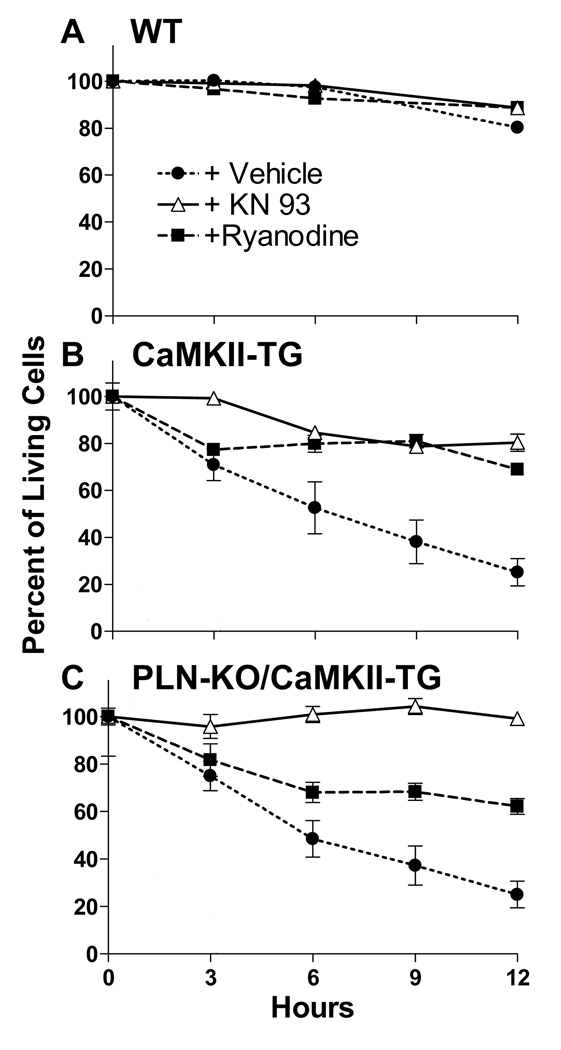
The assessment of TUNEL staining captures a single time point, but suggests that the viability of cardiomyocytes is compromised in the KO/TG line. To further examine cell viability, we isolated and monitored cells by morphology and trypan blue exclusion at various times (Fig. 7). To test whether CaMKII or SR Ca2+ release were important in regulating cell viability, cells were pretreated with 2 µM KN-93 (to inhibit CaMKII) or 100 nM ryanodine (to deplete SR Ca2+ and prevent Ca2+ sparks). WT myocytes remained viable over a 12 hour period and survival was not appreciably altered by KN-93 or ryanodine (Fig. 7A). In contrast, the percent of live myocytes from both CaMKII-TG and KO/TG mice decreased dramatically over 12 hours (to less than 30%). Inhibition of CaMKII with KN-93 or of SR Ca2+ leak with ryanodine decreased the rate of myocyte death in both CaMKII-TG and KO/TG mice (Fig. 7B–C), suggesting that the CaMKII-mediated increase in SR Ca2+ release contributes to diminished myocyte viability.
Figure 7. Inhibition of SR Ca2+ leak by KN-93 or ryanodine decreases the rate of death in isolated cardiomyocytes from PLN-KO/CaMKII-TG mice.
Isolated cardiomyocytes were plated and incubated in the presence and absence of inhibitors, and cell viability assessed at various times using morphology and at 12 hours by trypan blue exclusion. A, WT myocytes showed only slight decreases in viability over 12 hours after plating. B–C, The percent of living myocytes from both CaMKII-TG and PLN-KO/CaMKII-TG mice decreased dramatically over 12 hours. Inhibition of CaMKII with KN-93 or of SR Ca2+ leak with ryanodine decreased the rate of myocyte death in both CaMKII-TG and PLN-KO/CaMKII-TG mice.
Mitochondrial Ca2+ in Isolated Cardiomyocytes
The dramatic increase in diastolic Ca2+ sparks could increase mitochondrial Ca2+ loading and thereby contribute to decreased cardiomyocyte viability. To explore this possibility we assessed mitochondrial Ca2+ content in isolated adult myocytes from WT, CaMKII-TG or KO/TG mice as detailed in Methods (Fig. 8A). The protocol was designed to allow mitochondrial Ca2+ loading to occur during regular pulses (see initial cytosolic [Ca2+]i transients) and a 40 s rest period where Ca2+ sparks could occur. The release of mitochondrial Ca2+ was then induced using FCCP, under conditions where the released Ca2+ is trapped in the cytosol (SERCA, NCX and Na+/K+-ATPase inhibited) and quantitated by the rise in [Ca2+]i. Fig. 8A demonstrates that the FCCP-induced rise in [Ca2+]i in KO/TG (black trace) is substantially larger than that in WT (gray trace), reflecting a greater mitochondrial Ca2+ load. Mitochondrial Ca2+ load was more than 4 fold higher in KO/TG vs. WT or CaMKII-TG myocytes (Fig. 8B).
Figure 8. Involvement of mitochondrial Ca2+ in myocyte death in PLN-KO/CaMKII-TG mice.
A, Mitochondrial Ca2+ was measured as shown in the protocol. NT: no treatment; Thaps: thapsigargin. B, Mitochondrial Ca2+ was increased in cardiomyocytes isolated from PLN-KO/CaMKII-TG mice. *P<0.05 vs. WT. Mitochondrial Ca2+ in PLN-KO/CaMKII-TG myocytes returned to control levels with reduced external Ca2+ (0.3 mM) and CaMKII inhibitor AIP. C, Reduced external Ca2+ and CaMKII inhibition with AIP returned SR Ca2+ content and Ca2+ transients to near control myocyte levels. D, Inhibition of the mitochondrial uniporter by Ru-360 or inhibition of the mitochondrial permeability transition pore by cyclosporine A decreased the rate of death in isolated cardiomyocytes from PLN-KO/CaMKII-TG mice at 12 hours. *P<0.05 vs. WT.
To more directly test the relationship between Ca2+ sparks and mitochondrial Ca2+ loading in KO/TG myocytes, external Ca2+ was reduced (to 0.3 mM) and CaMKII inhibited (with AIP). This restored SR Ca2+ content and Ca2+ transients (Fig. 8C), as well as Ca2+ spark frequency (not shown) in KO/TG myocytes to near WT levels. Strikingly, mitochondrial Ca2+ was not increased in KO/TG myocytes under these conditions (Fig. 8B).
The role of mitochondrial Ca2+ overloading in myocyte viability was examined by treating cells with an inhibitor of the mitochondrial uniporter (Ru-360) or the mitochondrial permeability transition (PT)-pore (cyclosporine A). Both agents significantly enhanced survival of KO/TG cardiomyocytes (Fig. 8D), implicating mitochondrial Ca2+ overloading and the PT-pore in the diminished viability of these vs. WT cells. On the other hand, neither agent increased the survival of CaMKII-TG myocytes (Fig. 8D), suggesting that mitochondrial Ca2+ overloading is less critical to the viability of the CaMKII-TG cells.
Discussion
PLN as a target for HF treatment
The SR Ca2+ content of cardiomyocytes is a key determinant of SR Ca2+ release and resultant Ca2+ transients.13 SR Ca2+ uptake is decreased in many animal models of HF and human failing heart, and this has been suggested to cause contractile dysfunction by decreasing the stored Ca2+ available for release. Ca2+ uptake into the SR is mediated by the SR Ca2+ ATPase (SERCA2a) and regulated by PLN, an endogenous inhibitor of SERCA2a whose activity is regulated by phosphorylation. Ablation or inhibition of PLN has been proposed as a viable therapeutic strategy for treating HF by restoring SR Ca2+ load;14,15 this notion is supported by a number of reports, including the rescue of cardiomyopathy and HF in MLP KO6 and calsequestrin or β1 transgenic mice;7,8 and the suppression of HF progression in cardiomyopathic hamsters16 and in rats following myocardial infarction.17 In sharp contrast, in the present study as in several other genetic hypertrophy and HF models,18,19 PLN ablation clearly restored myocyte Ca2+ stores, Ca2+ transients and contraction, but failed to rescue in vivo cardiac function or the HF phenotype. Thus, although depressed SR Ca2+ uptake is an important feature of HF (and a target to correct cellular Ca2+ dysregulation), restoration of SR Ca2+ handling does not appear to rescue all sequelae associated with HF development. The dissociation between improved cardiomyocyte SR Ca2+ handling and the in vivo loss of ventricular function and survival in the KO/TG mice provided an opportunity to explore, at the cellular level, why restoring SR Ca2+ content fails to improve, and indeed may worsen, global cardiac function.
Ca2+, mitochondria and cell death
SR Ca2+ sparks, elementary events of diastolic SR Ca2+ release, have been reported to be enhanced by both CaMKII overexpression and PLN ablation.9,20 The CaMKII effect is primarily due to an activating effect on RyR2,12 while PLN-KO enhances Ca2+ sparks indirectly by increasing SR Ca2+ content, further enhancing RyR2 opening due to a regulatory effect of intra-SR [Ca2+].12,21 Here we demonstrate that SR Ca2+ sparks are massively increased in the KO/TG myocytes due to the combination of CaMKII mediated RyR2 phosphorylation and elevated SR Ca2+ load.
Numerous studies have demonstrated that mitochondrial Ca2+ overload results in opening of the permeability transition (PT)-pore and that this leads to either apoptotic or necrotic cell death in various cell types including cardiomyocytes.22–27 Cardiomyocyte loss by apoptosis has been recognized as a major factor contributing to HF development.28,29 Necrosis also contributes to ventricular remodeling induced by ischemic damage or in HF.29–31 Enhancement of cardiomyocyte Ca2+ influx, as observed in transgenic mice expressing the sarcolemmal L-type Ca2+ channel, is one mechanism for eliciting Ca2+ overload, mitochondrial PT-pore opening and necrotic myocyte death.32 Mitochondria take up cytosolic Ca2+ via a Ca2+-uniporter and a close relationship between SR and mitochondria, in which Ca2+ released from the SR is efficiently transmitted to the mitochondria in cardiomyocytes, has been reported.33 We suggest that the dramatic increase in Ca2+ sparks in the KO/TG myocyte leads, either directly or indirectly, to increased mitochondrial Ca2+ loading, PT-pore opening, and subsequent cardiomyocyte loss. This mechanism is supported by data demonstrating that mitochondrial Ca2+ loading and cell death are diminished following inhibition of Ca2+ sparks or mitochondrial Ca2+ uptake. Loss of functional cardiomyocytes could explain why the KO/TG hearts perform more poorly than the CaMKII-TG heart, despite improved cardiomyocyte Ca2+ transients.
Sympathetic tone, CaMKII and HF
In HF there is typically increased sympathetic tone and PKA activation,34 as well as increased expression and activation of CaMKII,34–37 and enhanced RyR2 phosphorylation and SR Ca2+ leak.34,38 Under these conditions, where phosphorylated RyR2 may already enhance diastolic SR Ca2+ release, increased adrenergic signaling or therapeutic attempts to replete SR Ca2+ stores could exacerbate increased SR Ca2+ leak (as with PLN ablation). As suggested above the increased leak could trigger mitochondrial Ca2+ overloading with concomitant cardiomyocyte death.27,32 This may also help to explain data demonstrating that CaMKII activation contributes to cardiomyocyte cell death induced by β-adrenergic stimulation and to isoproterenol-induced apoptosis or cardiomyopathy in vivo.39–41
Increased SR Ca2+ leak induced by concomitant increases in SR Ca2+ content and RyR2 phosphorylation, could also predispose to arrhythmias, a possible basis for the observed increase in mortality in the KO/TG mice. While arrhythmias may not occur in all forms of HF in which SERCA function is restored,42 arrhythmias are seen in the CaMKII-TG.43 The notion that arrhythmias would be enhanced by the increased Ca2+ leak44,45 is further supported by our recent demonstration that isoproterenol treatment is highly arrhythmogenic in CaMKII-TG myocytes in vitro and in vivo.46
In conclusion, enhanced SR Ca2+ uptake via PLN ablation can improve myocyte Ca2+ transients in transgenic mice that develop CaMKII-mediated heart failure. Unfortunately, the enhanced SR Ca2+ content exacerbates the already high level of diastolic Ca2+ spark activity, which may be more arrhythmogenic and further worsen overall heart function (via mitochondrial Ca2+ loading and cell death). The data are consistent with the working hypothesis that in the face of phosphorylated activated RyR2 channels, repletion of Ca2+ stores through PLN ablation (or during sympathetic activation) can exacerbate SR Ca2+ leak and thereby increase mitochondrial Ca2+ mediated cell death or activate other Ca2+ dependent processes that contribute to cardiac dysfunction.
Supplementary Material
Acknowledgment
We thank Dr. A. R. Marks for providing Ser2815 phospho-RyR2 antibody.
Sources of Funding
This work was supported by NIH grants HL46345 (JHB), HL80101 (to JHB and DMB), HL30077 (DMB) and HL26057 (EGK). Dr. T. Zhang was supported by a Scientist Development Grant from American Heart Association. S. Mishra was supported by a Pharmacological Sciences Training Grant GM007752.
Non-standard Abbreviations and Acronyms
- AIP
autocamtide-2 related inhibitory peptide II
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- CaSpF
Ca2+ spark frequency
- CsQ
calsequestrin
- FS
factional shortening
- HF
heart failure
- HW/BW
heart/body weight ratio
- KO
knockout
- LVEDD
left ventricular end diastolic dimension
- NCX
Na+/Ca2+ exchanger
- PLN
phospholamban
- PT-pore
permeability transition pore
- RyR
ryanodine receptor
- SERCA
sarcoplasmic reticulum Ca2+ ATPase
- SR
sarcoplasmic reticulum
- TG
transgenic
- TUNEL
transferase-mediated biotinylated UTP nick end labeling
- WGA
wheat germ agglutinin
- WT
wild type
Footnotes
Subject Codes: [130] Animal models of human disease; [136] Calcium cycling/excitation-contraction coupling; [138] Cell signalling/signal transduction; [145] Genetically altered mice; [148] Heart failure - basic studies.
Disclosures
None.
References
- 1.Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J, Jr, Bers DM, Brown JH. The δC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res. 2003;92:912–919. doi: 10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- 2.Ling H, Zhang T, Pereira L, Means CK, Cheng H, Gu Y, Dalton ND, Peterson KL, Chen J, Bers D, Heller Brown J. Requirement for Ca2+/calmodulin-dependent kinase II in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice. J Clin Invest. 2009;119:1230–1240. doi: 10.1172/JCI38022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kranias EG. Regulation of Ca2+ transport by cyclic 3',5'-AMP-dependent and calcium-calmodulin-dependent phosphorylation of cardiac sarcoplasmic reticulum. Biochim Biophys Acta. 1985;844:193–199. doi: 10.1016/0167-4889(85)90090-4. [DOI] [PubMed] [Google Scholar]
- 4.Luo W, Grupp IL, Harrer J, Ponniah S, Grupp G, Duffy JJ, Doetschman T, Kranias EG. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of beta-agonist stimulation. Circ Res. 1994;75:401–409. doi: 10.1161/01.res.75.3.401. [DOI] [PubMed] [Google Scholar]
- 5.Li L, Chu G, Kranias EG, Bers DM. Cardiac myocyte calcium transport in phospholamban knockout mouse: relaxation and endogenous CaMKII effects. Am J Physiol. 1998;274:H1335–H1347. doi: 10.1152/ajpheart.1998.274.4.H1335. [DOI] [PubMed] [Google Scholar]
- 6.Minamisawa S, Hoshijima M, Chu G, Ward CA, Frank K, Gu Y, Martone ME, Wang Y, Ross J, Jr, Kranias EG, Giles WR, Chien KR. Chronic phospholamban-sarcoplasmic reticulum calcium ATPase interaction is the critical calcium cycling defect in dilated cardiomyopathy. Cell. 1999;99:1–20. doi: 10.1016/s0092-8674(00)81662-1. [DOI] [PubMed] [Google Scholar]
- 7.Sato Y, Kiriazis H, Yatani A, Schmidt AG, Hahn H, Ferguson DG, Sako H, Mitarai S, Honda R, Mesnard-Rouiller L, Frank KF, Beyermann B, Wu G, Fujimori K, Dorn GW, 2nd, Kranias EG. Rescue of contractile parameters and myocyte hypertrophy in calsequestrin overexpressing myocardium by phospholamban ablation. J Biol Chem. 2001;276:9392–9399. doi: 10.1074/jbc.M006889200. [DOI] [PubMed] [Google Scholar]
- 8.Engelhardt S, Hein L, Dyachenkow V, Kranias EG, Isenberg G, Lohse MJ. Altered calcium handling is critically involved in the cardiotoxic effects of chronic beta-adrenergic stimulation. Circulation. 2004;109:1154–1160. doi: 10.1161/01.CIR.0000117254.68497.39. [DOI] [PubMed] [Google Scholar]
- 9.Maier LS, Zhang T, Chen L, DeSantiago J, Brown JH, Bers DM. Transgenic CaMKIIδC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res. 2003;92:904–911. doi: 10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
- 10.Bassani JW, Bassani RA, Bers DM. Twitch-dependent SR Ca2+ accumulation and release in rabbit ventricular myocytes. Am J Physiol. 1993;265:C533–C540. doi: 10.1152/ajpcell.1993.265.2.C533. [DOI] [PubMed] [Google Scholar]
- 11.Means CK, Xiao CY, Li Z, Zhang T, Omens JH, Ishii I, Chun J, Brown JH. Sphingosine 1-phosphate S1P2 and S1P3 receptor-mediated Akt activation protects against in vivo myocardial ischemia-reperfusion injury. Am J Physiol. 2007;292:H2944–H2951. doi: 10.1152/ajpheart.01331.2006. [DOI] [PubMed] [Google Scholar]
- 12.Guo T, Zhang T, Mestril R, Bers DM. Ca2+/Calmodulin-dependent protein kinase II phosphorylation of ryanodine receptor does affect calcium sparks in mouse ventricular myocytes. Circ Res. 2006;99:398–406. doi: 10.1161/01.RES.0000236756.06252.13. [DOI] [PubMed] [Google Scholar]
- 13.Bers DM. Altered cardiac myocyte Ca2+ regulation in heart failure. Physiology. 2006;21:380–387. doi: 10.1152/physiol.00019.2006. [DOI] [PubMed] [Google Scholar]
- 14.del Monte F, Harding SE, Dec GW, Gwathmey JK, Hajjar RJ. Targeting phospholamban by gene transfer in human heart failure. Circulation. 2002;105:904–907. doi: 10.1161/hc0802.105564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chien KR, Ross J, Jr., Hoshijima M. Calcium and heart failure: the cycle game. Nat Med. 2003;9:508–509. doi: 10.1038/nm0503-508. [DOI] [PubMed] [Google Scholar]
- 16.Hoshijima M, Ikeda Y, Iwanaga Y, Minamisawa S, Date MO, Gu Y, Iwatate M, Li M, Wang L, Wilson JM, Wang Y, Ross J, Jr., Chien KR. Chronic suppression of heart-failure progression by a pseudophosphorylated mutant of phospholamban via in vivo cardiac rAAV gene delivery. Nat Med. 2002;8:864–871. doi: 10.1038/nm739. [DOI] [PubMed] [Google Scholar]
- 17.Iwanaga Y, Hoshijima M, Gu Y, Iwatate M, Dieterle T, Ikeda Y, Date MO, Chrast J, Matsuzaki M, Peterson KL, Chien KR, Ross J., Jr Chronic phospholamban inhibition prevents progressive cardiac dysfunction and pathological remodeling after infarction in rats. J Clin Invest. 2004;113:727–736. doi: 10.1172/JCI18716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song Q, Schmidt AG, Hahn HS, Carr AN, Frank B, Pater L, Gerst M, Young K, Hoit BD, McConnell BK, Haghighi K, Seidman CE, Seidman JG, Dorn GW, Kranias EG. Rescue of cardiomyocyte dysfunction by phospholamban ablation does not prevent ventricular failure in genetic hypertrophy. J Clin Invest. 2003;111:859–867. doi: 10.1172/JCI16738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janczewski AM, Zahid M, Lemster BH, Frye CS, Gibson G, Higuchi Y, Kranias EG, Feldman AM, McTiernan CF. Phospholamban gene ablation improves calcium transients but not cardiac function in a heart failure model. Cardiovasc Res. 2004;62:468–480. doi: 10.1016/j.cardiores.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Huser J, Bers DM, Blatter LA. Subcellular properties of [Ca2+]i transients in phospholamban-deficient mouse ventricular cells. Am J Physiol. 1998;274:H1800–H1811. doi: 10.1152/ajpheart.1998.274.5.H1800. [DOI] [PubMed] [Google Scholar]
- 21.Gyorke I, Gyorke S. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys J. 1998;75:2801–2810. doi: 10.1016/S0006-3495(98)77723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong Z, Saikumar P, Weinberg JM, Venkatachalam MA. Calcium in cell injury and death. Annu Rev Pathol. 2006;1:405–434. doi: 10.1146/annurev.pathol.1.110304.100218. [DOI] [PubMed] [Google Scholar]
- 23.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341:233–249. [PMC free article] [PubMed] [Google Scholar]
- 24.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 25.Kroemer G, Dallaporta B, Resche-Rignon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 26.Adams JW, Pagel AL, Means CK, Oksenberg D, Armstrong RC, Brown JH. Cardiomyocyte apoptosis induced by Gαq signaling is mediated by permeability transition pore formation and activation of the mitochondrial death pathway. Circ Res. 2000;87:1180–1187. doi: 10.1161/01.res.87.12.1180. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Zhang X, Kubo H, Harris DM, Mills GD, Moyer J, Berretta R, Potts ST, Marsh JD, Houser SR. Ca2+ influx-induced sarcoplasmic reticulum Ca2+ overload causes mitochondrial-dependent apoptosis in ventricular myocytes. Circ Res. 2005;97:1009–1017. doi: 10.1161/01.RES.0000189270.72915.D1. [DOI] [PubMed] [Google Scholar]
- 28.Foo RS, Mani K, Kitsis RN. Death begets failure in the heart. J Clin Invest. 2005;115:565–571. doi: 10.1172/JCI24569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dorn GW., 2nd Apoptotic and non-apoptotic programmed cardiomyocyte death in ventricular remodelling. Cardiovasc Res. 2009;81:465–473. doi: 10.1093/cvr/cvn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baines CP. The mitochondrial permeability transition pore and ischemia-reperfusion injury. Basic Res Cardiol. 2009;104:181–188. doi: 10.1007/s00395-009-0004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldspink DF, Burniston JG, Tan LB. Cardiomyocyte death and the ageing and failing heart. Exp Physiol. 2003;88:447–458. doi: 10.1113/eph8802549. [DOI] [PubMed] [Google Scholar]
- 32.Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, Jaleel N, Chua BH, Hewett TE, Robbins J, Houser SR, Molkentin JD. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest. 2007;117:2431–2444. doi: 10.1172/JCI31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szalai G, Csordas G, Hantash BM, Thomas AP, Hajnoczky G. Calcium signal transmission between ryanodine receptors and mitochondria. J Biol Chem. 2000;275:15305–15313. doi: 10.1074/jbc.275.20.15305. [DOI] [PubMed] [Google Scholar]
- 34.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 35.Bossuyt J, Helmstadter K, Wu X, Clements-Jewery H, Haworth RS, Avkiran M, Martin JL, Pogwizd SM, Bers DM. CaMKIIδ and PKD overexpression reinforce the HDAC5 redistribution in heart failure. Circ Res. 2008;102:695–702. doi: 10.1161/CIRCRESAHA.107.169755. [DOI] [PubMed] [Google Scholar]
- 36.Hoch B, Meyer R, Hetzer R, Krause E-G, Karczewski P. Identification and expression of δ-isoforms of the multifunctional Ca2+/calmodulin dependent protein kinase in failing and nonfailing human myocardium. Circ Res. 1999;84:713–721. doi: 10.1161/01.res.84.6.713. [DOI] [PubMed] [Google Scholar]
- 37.Kirchhefer U, Schmitz W, Scholz H, Neumann J. Acitivity of cAMP-dependent protein kinase and Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human hearts. Cardiovasc Res. 1999;42:254–261. doi: 10.1016/s0008-6363(98)00296-x. [DOI] [PubMed] [Google Scholar]
- 38.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 39.Zhu WZ, Wang SQ, Chakir K, Yang D, Zhang T, Brown JH, Devic E, Kobilka BK, Cheng H, Xiao RP. Linkage of β1-adrenergic stimulation to apoptotic heart cell death through protein kinase A-independent activation of Ca2+/calmodulin kinase II. J Clin Invest. 2003;111:617–625. doi: 10.1172/JCI16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, Price EE, Jr, Thiel W, Guatimosim S, Song LS, Madu EC, Shah AN, Vishnivetskaya TA, Atkinson JB, Gurevich VV, Salama G, Lederer WJ, Colbran RJ, Anderson ME. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 41.Yang Y, Zhu WZ, Joiner ML, Zhang R, Oddis CV, Hou Y, Yang J, Price EE, Gleaves L, Eren M, Ni G, Vaughan DE, Xiao RP, Anderson ME. Calmodulin kinase II inhibition protects against myocardial cell apoptosis in vivo. Am J Physiol. 2006;291:H3065–H3075. doi: 10.1152/ajpheart.00353.2006. [DOI] [PubMed] [Google Scholar]
- 42.Kawase Y, Ly HQ, Prunier F, Lebeche D, Shi Y, Jin H, Hadri L, Yoneyama R, Hoshino K, Takewa Y, Sakata S, Peluso R, Zsebo K, Gwathmey JK, Tardif JC, Tanguay JF, Hajjar RJ. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J Am Coll Cardiol. 2008;51:1112–1119. doi: 10.1016/j.jacc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 43.Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, Maier SK, Zhang T, Hasenfuss G, Brown JH, Bers DM, Maier LS. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest. 2006;116:3127–3138. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chelu MG, Wehrens XH. Sarcoplasmic reticulum calcium leak and cardiac arrhythmias. Biochem Soc Trans. 2007;35:952–956. doi: 10.1042/BST0350952. [DOI] [PubMed] [Google Scholar]
- 45.Chopra N, Kannankeril PJ, Yang T, Hlaing T, Holinstat I, Ettensohn K, Pfeifer K, Akin B, Jones LR, Franzini-Armstrong C, Knollmann BC. Modest reductions of cardiac calsequestrin increase sarcoplasmic reticulum Ca2+ leak independent of luminal Ca2+ and trigger ventricular arrhythmias in mice. Circ Res. 2007;101:617–626. doi: 10.1161/CIRCRESAHA.107.157552. [DOI] [PubMed] [Google Scholar]
- 46.Sag CM, Wadsack DP, Khabbazzadeh S, Abesser M, Grefe C, Neumann K, Opiela M, Backs J, Olson EN, Brown JH, Neef S, Maier SK, Maier LS. Calcium/calmodulin-dependent protein kinase II contributes to cardiac arrhythmogenesis in heart failure. Circ Heart Fail. 2009;2 doi: 10.1161/CIRCHEARTFAILURE.109.865279. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.