Abstract
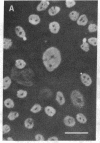
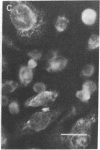
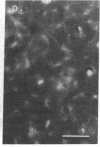
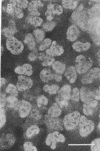
To facilitate understanding of the mechanisms underlying pulmonary diseases, including lung cancer and cystic fibrosis, we have transformed and characterized cultures of human tracheal epithelial cells. Cells were transfected by calcium phosphate precipitation with a plasmid containing a replication-defective simian virus 40 (SV40) genome. Colonies of cells with enhanced growth potential were isolated and analyzed for transformation- and epithelial-specific characteristics. Precrisis cells were observed to express the SV40 large tumor antigen, produce cytokeratins, have microvilli, and form tight junctions. After crisis, cells continued to express the SV40 large tumor antigen as well as epithelial-specific cytokeratins and to display the apical membrane microvilli. Apical membrane Cl channels were opened in postcrisis cells exposed to 50 microM forskolin. These channels showed electrical properties similar to those observed in primary cultures. The postcrisis cells have been in culture for greater than 250 generations and are potentially "immortal." In addition to providing a useful in vitro model for the study of ion transport by human airway epithelial cells, the cells can be used to examine stages of neoplastic progression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks-Schlegel S. P., Howley P. M. Differentiation of human epidermal cells transformed by SV40. J Cell Biol. 1983 Feb;96(2):330–337. doi: 10.1083/jcb.96.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brash D. E., Reddel R. R., Quanrud M., Yang K., Farrell M. P., Harris C. C. Strontium phosphate transfection of human cells in primary culture: stable expression of the simian virus 40 large-T-antigen gene in primary human bronchial epithelial cells. Mol Cell Biol. 1987 May;7(5):2031–2034. doi: 10.1128/mcb.7.5.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. E. In vitro transformation of human epithelial cells. Biochim Biophys Acta. 1986;823(3):161–194. doi: 10.1016/0304-419x(86)90001-6. [DOI] [PubMed] [Google Scholar]
- Chou J. Y. Establishment of clonal human placental cells synthesizing human choriogonadotropin. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1854–1858. doi: 10.1073/pnas.75.4.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defendi V., Naimski P., Steinberg M. L. Human cells transformed by SV40 revisited: the epithelial cells. J Cell Physiol Suppl. 1982;2:131–140. doi: 10.1002/jcp.1041130517. [DOI] [PubMed] [Google Scholar]
- Deftos L. J., Rabson A. S., Buckle R. M., Aurbach G. D., Potts J. T., Jr Parathyroid hormone production in vitro by human parathyroid cells transformed by Simian virus 40. Science. 1968 Jan 26;159(3813):435–436. doi: 10.1126/science.159.3813.435. [DOI] [PubMed] [Google Scholar]
- Emura M., Mohr U., Kakunaga T., Hilfrich J. Growth inhibition and transformation of a human fetal tracheal epithelial cell line by long-term exposure to diethylnitrosamine. Carcinogenesis. 1985 Aug;6(8):1079–1085. doi: 10.1093/carcin/6.8.1079. [DOI] [PubMed] [Google Scholar]
- Estivill X., Farrall M., Scambler P. J., Bell G. M., Hawley K. M., Lench N. J., Bates G. P., Kruyer H. C., Frederick P. A., Stanier P. A candidate for the cystic fibrosis locus isolated by selection for methylation-free islands. 1987 Apr 30-May 6Nature. 326(6116):840–845. doi: 10.1038/326840a0. [DOI] [PubMed] [Google Scholar]
- Finkbeiner W. E., Nadel J. A., Basbaum C. B. Establishment and characterization of a cell line derived from bovine tracheal glands. In Vitro Cell Dev Biol. 1986 Oct;22(10):561–567. doi: 10.1007/BF02623514. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Rechkemmer G., Shoemaker R. L. Altered regulation of airway epithelial cell chloride channels in cystic fibrosis. Science. 1986 Aug 1;233(4763):558–560. doi: 10.1126/science.2425436. [DOI] [PubMed] [Google Scholar]
- GIRARDI A. J., JENSEN F. C., KOPROWSKI H. SV40-INDUCED TRANFORMATION OF HUMAN DIPLOID CELLS: CRISIS AND RECOVERY. J Cell Physiol. 1965 Feb;65:69–83. doi: 10.1002/jcp.1030650110. [DOI] [PubMed] [Google Scholar]
- Gluzman Y., Frisque R. J., Sambrook J. Origin-defective mutants of SV40. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):293–300. doi: 10.1101/sqb.1980.044.01.033. [DOI] [PubMed] [Google Scholar]
- Gruenert D. C., Cleaver J. E. Repair of psoralen-induced cross-links and monoadducts in normal and repair-deficient human fibroblasts. Cancer Res. 1985 Nov;45(11 Pt 1):5399–5404. [PubMed] [Google Scholar]
- Gruenert D. C., Kapp L. N., Cleaver J. E. Inhibition of DNA synthesis by psoralen-induced lesions in xeroderma pigmentosum and Fanconi's anemia fibroblasts. Photochem Photobiol. 1985 May;41(5):543–550. doi: 10.1111/j.1751-1097.1985.tb03524.x. [DOI] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Li M., McCann J. D., Liedtke C. M., Nairn A. C., Greengard P., Welsh M. J. Cyclic AMP-dependent protein kinase opens chloride channels in normal but not cystic fibrosis airway epithelium. Nature. 1988 Jan 28;331(6154):358–360. doi: 10.1038/331358a0. [DOI] [PubMed] [Google Scholar]
- Little C. D., Nau M. M., Carney D. N., Gazdar A. F., Minna J. D. Amplification and expression of the c-myc oncogene in human lung cancer cell lines. Nature. 1983 Nov 10;306(5939):194–196. doi: 10.1038/306194a0. [DOI] [PubMed] [Google Scholar]
- Milo G. E., Noyes I., Donahoe J., Weisbrode S. Neoplastic transformation of human epithelial cells in vitro after exposure to chemical carcinogens. Cancer Res. 1981 Dec;41(12 Pt 1):5096–5102. [PubMed] [Google Scholar]
- Nau M. M., Brooks B. J., Battey J., Sausville E., Gazdar A. F., Kirsch I. R., McBride O. W., Bertness V., Hollis G. F., Minna J. D. L-myc, a new myc-related gene amplified and expressed in human small cell lung cancer. Nature. 1985 Nov 7;318(6041):69–73. doi: 10.1038/318069a0. [DOI] [PubMed] [Google Scholar]
- Neutra M. R. The use of human intestinal mucosa in organ culture for the study of cystic fibrosis. Birth Defects Orig Artic Ser. 1980;16(2):261–273. [PubMed] [Google Scholar]
- Reddel R. R., Ke Y., Gerwin B. I., McMenamin M. G., Lechner J. F., Su R. T., Brash D. E., Park J. B., Rhim J. S., Harris C. C. Transformation of human bronchial epithelial cells by infection with SV40 or adenovirus-12 SV40 hybrid virus, or transfection via strontium phosphate coprecipitation with a plasmid containing SV40 early region genes. Cancer Res. 1988 Apr 1;48(7):1904–1909. [PubMed] [Google Scholar]
- Rhim J. S., Fujita J., Arnstein P., Aaronson S. A. Neoplastic conversion of human keratinocytes by adenovirus 12-SV40 virus and chemical carcinogens. Science. 1986 Apr 18;232(4748):385–388. doi: 10.1126/science.2421406. [DOI] [PubMed] [Google Scholar]
- Rhim J. S., Jay G., Arnstein P., Price F. M., Sanford K. K., Aaronson S. A. Neoplastic transformation of human epidermal keratinocytes by AD12-SV40 and Kirsten sarcoma viruses. Science. 1985 Mar 8;227(4691):1250–1252. doi: 10.1126/science.2579430. [DOI] [PubMed] [Google Scholar]
- Ruscetti F. W., Chou J. Y., Gallo R. C. Human trophoblasts: cellular source of colony-stimulating activity in placental tissue. Blood. 1982 Jan;59(1):86–90. [PubMed] [Google Scholar]
- Sack G. H., Jr Human cell transformation by simian virus 40--a review. In Vitro. 1981 Jan;17(1):1–19. doi: 10.1007/BF02618025. [DOI] [PubMed] [Google Scholar]
- Schoumacher R. A., Shoemaker R. L., Halm D. R., Tallant E. A., Wallace R. W., Frizzell R. A. Phosphorylation fails to activate chloride channels from cystic fibrosis airway cells. Nature. 1987 Dec 24;330(6150):752–754. doi: 10.1038/330752a0. [DOI] [PubMed] [Google Scholar]
- Scott D. M., MacDonald C., Brzeski H., Kinne R. Maintenance of expression of differentiated function of kidney cells following transformation by SV40 early region DNA. Exp Cell Res. 1986 Oct;166(2):391–398. doi: 10.1016/0014-4827(86)90485-4. [DOI] [PubMed] [Google Scholar]
- Small M. B., Gluzman Y., Ozer H. L. Enhanced transformation of human fibroblasts by origin-defective simian virus 40. Nature. 1982 Apr 15;296(5858):671–672. doi: 10.1038/296671a0. [DOI] [PubMed] [Google Scholar]
- Stampfer M. R., Bartley J. C. Induction of transformation and continuous cell lines from normal human mammary epithelial cells after exposure to benzo[a]pyrene. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2394–2398. doi: 10.1073/pnas.82.8.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taya Y., Hosogai K., Hirohashi S., Shimosato Y., Tsuchiya R., Tsuchida N., Fushimi M., Sekiya T., Nishimura S. A novel combination of K-ras and myc amplification accompanied by point mutational activation of K-ras in a human lung cancer. EMBO J. 1984 Dec 1;3(12):2943–2946. doi: 10.1002/j.1460-2075.1984.tb02236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M. J. An apical-membrane chloride channel in human tracheal epithelium. Science. 1986 Jun 27;232(4758):1648–1650. doi: 10.1126/science.2424085. [DOI] [PubMed] [Google Scholar]
- Welsh M. J. Electrolyte transport by airway epithelia. Physiol Rev. 1987 Oct;67(4):1143–1184. doi: 10.1152/physrev.1987.67.4.1143. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Liedtke C. M. Chloride and potassium channels in cystic fibrosis airway epithelia. 1986 Jul 31-Aug 6Nature. 322(6078):467–470. doi: 10.1038/322467a0. [DOI] [PubMed] [Google Scholar]
- Widdicombe J. H. Cystic fibrosis and beta-adrenergic response of airway epithelial cell cultures. Am J Physiol. 1986 Oct;251(4 Pt 2):R818–R822. doi: 10.1152/ajpregu.1986.251.4.R818. [DOI] [PubMed] [Google Scholar]
- Widdicombe J. H., Welsh M. J., Finkbeiner W. E. Cystic fibrosis decreases the apical membrane chloride permeability of monolayers cultured from cells of tracheal epithelium. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6167–6171. doi: 10.1073/pnas.82.18.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
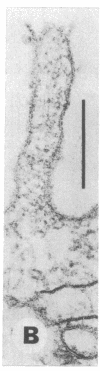
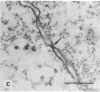
- Yoakum G. H., Lechner J. F., Gabrielson E. W., Korba B. E., Malan-Shibley L., Willey J. C., Valerio M. G., Shamsuddin A. M., Trump B. F., Harris C. C. Transformation of human bronchial epithelial cells transfected by Harvey ras oncogene. Science. 1985 Mar 8;227(4691):1174–1179. doi: 10.1126/science.3975607. [DOI] [PubMed] [Google Scholar]