Abstract
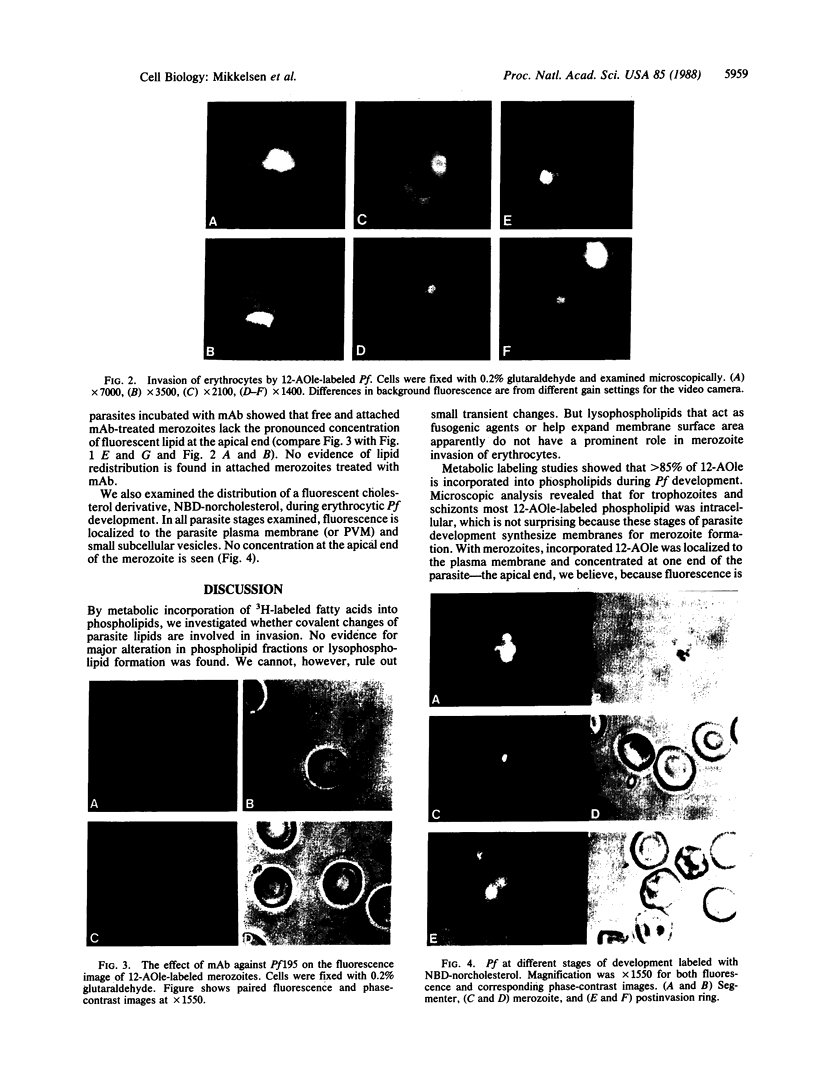
The role of lipids in Plasmodium falciparum invasion of erythrocytes was investigated by biochemical and fluorescent microscopic analysis. Metabolic incorporation of radioactive oleate or palmitate and fractionation of radiolabeled phospholipids by thin-layer chromatography revealed no difference in the major phospholipid classes of schizonts and early ring forms after merozoite invasion. Fluorescent anthroyloxy derivatives of oleate and palmitate were also metabolically incorporated into parasite phospholipids. By microscopic analysis, the fluorescent phospholipids were seen localized in the plasma membrane and, within the merozoite, concentrated near the apical end. During invasion fluorescent phospholipid appeared to be injected from the apical end of the merozoite into the host membrane, both within and outside the parasite-host membrane junctions. After invasion fluorescent lipid was only found in the parasite plasma membrane and/or parasitophorous vacuole membrane. Parallel experiments with a fluorescent cholesterol derivative, incorporated into parasite membranes by exchange, revealed neither heterogeneous distribution of label within the parasite nor evidence for cholesterol transfer from merozoite to host cell membrane. Results suggest that during invasion no major covalent alteration of parasite lipids, such as lysophospholipid formation, occurs. However, invasion and formation of the parasitophorous vacuolar membrane apparently involves insertion of parasite phospholipids into the host membrane.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa M., Miller L. H., Johnson J., Rabbege J. Erythrocyte entry by malarial parasites. A moving junction between erythrocyte and parasite. J Cell Biol. 1978 Apr;77(1):72–82. doi: 10.1083/jcb.77.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aikawa M., Miller L. H., Rabbege J. R., Epstein N. Freeze-fracture study on the erythrocyte membrane during malarial parasite invasion. J Cell Biol. 1981 Oct;91(1):55–62. doi: 10.1083/jcb.91.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister L. H., Butcher G. A., Dennis E. D., Mitchell G. H. Structure and invasive behaviour of Plasmodium knowlesi merozoites in vitro. Parasitology. 1975 Dec;71(3):483–491. doi: 10.1017/s0031182000047247. [DOI] [PubMed] [Google Scholar]
- Bannister L. H., Butcher G. A., Mitchell G. H. Recent advances in understanding the invasion of erythrocytes by merozoites of Plasmodium knowlesi. Bull World Health Organ. 1977;55(2-3):163–169. [PMC free article] [PubMed] [Google Scholar]
- Brown J., Whittle H. C., Berzins K., Howard R. J., Marsh K., Sjoberg K. Inhibition of Plasmodium falciparum growth by IgG antibody produced by human lymphocytes transformed with Epstein-Barr virus. Clin Exp Immunol. 1986 Jan;63(1):135–140. [PMC free article] [PubMed] [Google Scholar]
- Dvorak J. A., Miller L. H., Whitehouse W. C., Shiroishi T. Invasion of erythrocytes by malaria merozoites. Science. 1975 Feb 28;187(4178):748–750. doi: 10.1126/science.803712. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Friedman M. J., Fukuda M., Laine R. A. Evidence for a malarial parasite interaction site on the major transmembrane protein of the human erythrocyte. Science. 1985 Apr 5;228(4695):75–77. doi: 10.1126/science.3883494. [DOI] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R. Biosynthesis and processing of a Plasmodium falciparum schizont antigen recognized by immune serum and a monoclonal antibody. J Exp Med. 1982 Nov 1;156(5):1528–1538. doi: 10.1084/jem.156.5.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozbor D., Lagarde A. E., Roder J. C. Human hybridomas constructed with antigen-specific Epstein-Barr virus-transformed cell lines. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6651–6655. doi: 10.1073/pnas.79.21.6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladda R., Aikawa M., Sprinz H. Penetration of erythrocytes by merozoites of mammalian and avian malarial parasites. J Parasitol. 1969 Jun;55(3):633–644. [PubMed] [Google Scholar]
- Langreth S. G. Electron microscope cytochemistry of host-parasite membrane interactions in malaria. Bull World Health Organ. 1977;55(2-3):171–178. [PMC free article] [PubMed] [Google Scholar]
- Lantz C. H., Van Dyke K. Depressed incorporation of purine derivatives into malarial parasite ribonucleic acid by known ribonucleic acid polymerase-inhibiting drugs. Biochem Pharmacol. 1972 Mar 15;21(6):891–894. doi: 10.1016/0006-2952(72)90133-5. [DOI] [PubMed] [Google Scholar]
- McLaren D. J., Bannister L. H., Trigg P. I., Butcher G. A. Freeze fracture studies on the interaction between the malaria parasite and the host erythrocyte in Plasmodium knowlesi infections. Parasitology. 1979 Aug;79(1):125–139. doi: 10.1017/s0031182000052021. [DOI] [PubMed] [Google Scholar]
- Mitchell G. H., Hadley T. J., McGinniss M. H., Klotz F. W., Miller L. H. Invasion of erythrocytes by Plasmodium falciparum malaria parasites: evidence for receptor heterogeneity and two receptors. Blood. 1986 May;67(5):1519–1521. [PubMed] [Google Scholar]
- Nillni E. A., Schmidt-Ullrich R., Mikkelsen R. B., Wallach D. F. Extracellular development of Plasmodium knowlesi erythrocytic stages in an artificial intracellular medium. Mol Biochem Parasitol. 1985 Nov;17(2):219–237. doi: 10.1016/0166-6851(85)90020-9. [DOI] [PubMed] [Google Scholar]
- Okoye V. C., Bennett V. Plasmodium falciparum malaria: band 3 as a possible receptor during invasion of human erythrocytes. Science. 1985 Jan 11;227(4683):169–171. doi: 10.1126/science.3880920. [DOI] [PubMed] [Google Scholar]
- Perkins M. E. Surface proteins of Plasmodium falciparum merozoites binding to the erythrocyte receptor, glycophorin. J Exp Med. 1984 Sep 1;160(3):788–798. doi: 10.1084/jem.160.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmann H., Berzins K., Wahlgren M., Carlsson J., Björkman A., Patarroyo M. E., Perlmann P. Antibodies in malarial sera to parasite antigens in the membrane of erythrocytes infected with early asexual stages of Plasmodium falciparum. J Exp Med. 1984 Jun 1;159(6):1686–1704. doi: 10.1084/jem.159.6.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Ullrich R., Brown J., Whittle H., Lin P. S. Human-human hybridomas secreting monoclonal antibodies to the Mr 195,000 Plasmodium falciparum blood stage antigen. J Exp Med. 1986 Jan 1;163(1):179–188. doi: 10.1084/jem.163.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M. J., Schulman S., Vanderberg J. P. Rhoptry secretion of membranous whorls by Plasmodium berghei sporozoites. J Protozool. 1985 May;32(2):280–283. doi: 10.1111/j.1550-7408.1985.tb03051.x. [DOI] [PubMed] [Google Scholar]
- Stewart M. J., Schulman S., Vanderberg J. P. Rhoptry secretion of membranous whorls by Plasmodium falciparum merozoites. Am J Trop Med Hyg. 1986 Jan;35(1):37–44. doi: 10.4269/ajtmh.1986.35.37. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Van Dyke K., Trush M. A., Wilson M. E., Stealey P. K. Isolation and analysis of nucleotides from erythrocyte-free malarial parasites (Plasmodium berghei) and potential relevance to malaria chemotherapy. Bull World Health Organ. 1977;55(2-3):253–264. [PMC free article] [PubMed] [Google Scholar]
- Vanderberg J. P., Gupta S. K., Schulman S., Oppenheim J. D., Furthmayr H. Role of the carbohydrate domains of glycophorins as erythrocyte receptors for invasion by Plasmodium falciparum merozoites. Infect Immun. 1985 Jan;47(1):201–210. doi: 10.1128/iai.47.1.201-210.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vial H. J., Philippot J. R., Wallach D. F. A reevaluation of the status of cholesterol in erythrocytes infected by Plasmodium knowlesi and P. falciparum. Mol Biochem Parasitol. 1984 Sep;13(1):53–65. doi: 10.1016/0166-6851(84)90101-4. [DOI] [PubMed] [Google Scholar]
- Vial H. J., Philippot J. R., Wallach D. F. A reevaluation of the status of cholesterol in erythrocytes infected by Plasmodium knowlesi and P. falciparum. Mol Biochem Parasitol. 1984 Sep;13(1):53–65. doi: 10.1016/0166-6851(84)90101-4. [DOI] [PubMed] [Google Scholar]
- Vial H. J., Thuet M. J., Philippot J. R. Phospholipid biosynthesis in synchronous Plasmodium falciparum cultures. J Protozool. 1982 May;29(2):258–263. doi: 10.1111/j.1550-7408.1982.tb04023.x. [DOI] [PubMed] [Google Scholar]
- Wunderlich F., Stübig H., Königk E. Intraerythrocytic development of Plasmodium knowlesi: structure, temperature- and Ca2+-response of the host and parasite membranes. J Protozool. 1982 Feb;29(1):49–59. doi: 10.1111/j.1550-7408.1982.tb02879.x. [DOI] [PubMed] [Google Scholar]











