Abstract
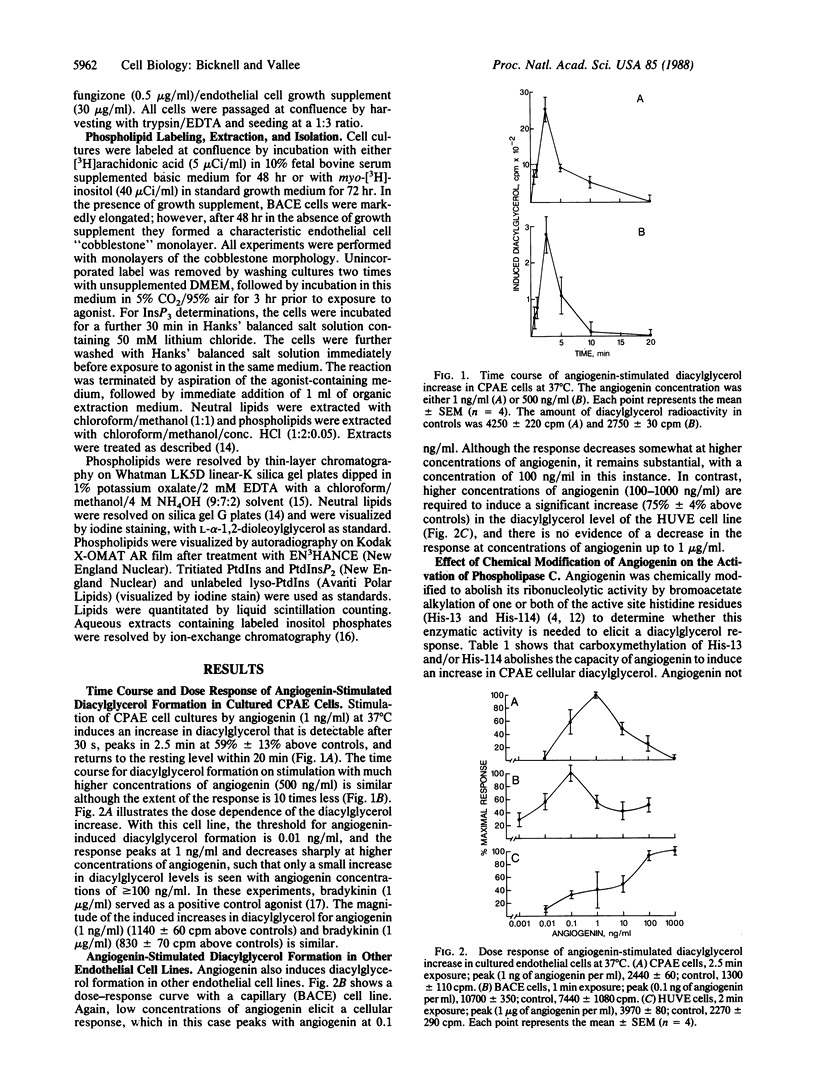
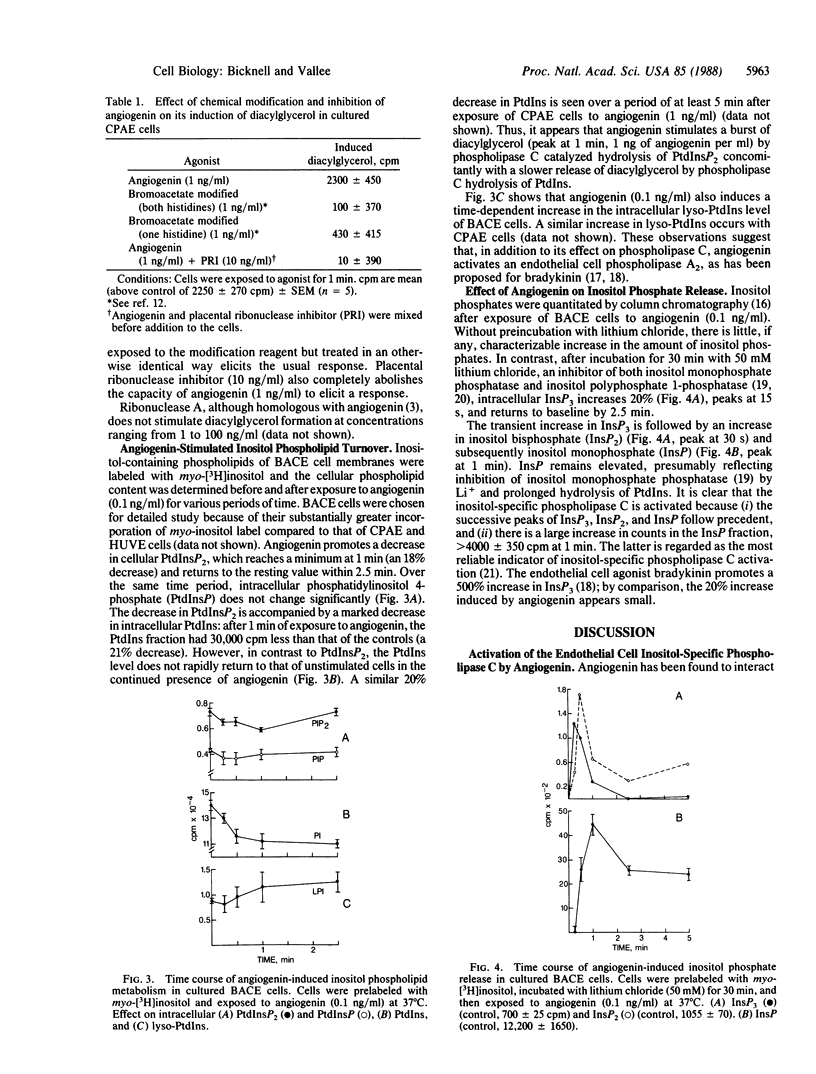
Low concentrations of angiogenin activate the inositol-specific phospholipase C of cultured pulmonary artery, umbilical vein, and capillary endothelial cells, promoting a transient increase in the intracellular levels of 1,2-diacylglycerol and inositol trisphosphate. The response is strongly dose dependent with a maximum in the ng/ml concentration range and, for some cell lines, a marked decrease at concentrations greater than 1 ng/ml; e.g., arterial endothelial cells respond weakly to angiogenin concentrations comparable to that in normal human plasma (approximately equal to 400 ng/ml). Chemical modification of the active site of angiogenin or inhibition with placental ribonuclease inhibitor abolishes its activation of endothelial cell phospholipase C; this correlates with the concomitant loss of both intrinsic ribonucleolytic and angiogenic activity. The response to low concentrations of angiogenin is consistent with its potency of inducing vascularization in classical angiogenesis assays. In vivo, endothelial cells are exposed to concentrations of angiogenin higher than that required to elicit a cellular response; it seems likely, therefore, that expression of a surface receptor or some other process must be rate limiting in the cellular response.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Brooks R. C., Morell P., DeGeorge J. J., McCarthy K. D., Lapetina E. G. Differential effects of phorbol ester and diacylglycerols on inositol phosphate formation in C62B glioma cells. Biochem Biophys Res Commun. 1987 Oct 29;148(2):701–708. doi: 10.1016/0006-291x(87)90933-8. [DOI] [PubMed] [Google Scholar]
- Burns C. P., Rozengurt E. Serum, platelet-derived growth factor, vasopressin and phorbol esters increase intracellular pH in Swiss 3T3 cells. Biochem Biophys Res Commun. 1983 Nov 15;116(3):931–938. doi: 10.1016/s0006-291x(83)80231-9. [DOI] [PubMed] [Google Scholar]
- Davis R. J., Czech M. P. Tumor-promoting phorbol diesters mediate phosphorylation of the epidermal growth factor receptor. J Biol Chem. 1984 Jul 10;259(13):8545–8549. [PubMed] [Google Scholar]
- Denèfle P., Kovarik S., Guitton J. D., Cartwright T., Mayaux J. F. Chemical synthesis of a gene coding for human angiogenin, its expression in Escherichia coli and conversion of the product into its active form. Gene. 1987;56(1):61–70. doi: 10.1016/0378-1119(87)90158-2. [DOI] [PubMed] [Google Scholar]
- Doctrow S. R., Folkman J. Protein kinase C activators suppress stimulation of capillary endothelial cell growth by angiogenic endothelial mitogens. J Cell Biol. 1987 Mar;104(3):679–687. doi: 10.1083/jcb.104.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Fett J. W., Bethune J. L., Vallee B. L. Induction of angiogenesis by mixtures of two angiogenic proteins, angiogenin and acidic fibroblast growth factor, in the chick chorioallantoic membrane. Biochem Biophys Res Commun. 1987 Aug 14;146(3):1122–1131. doi: 10.1016/0006-291x(87)90764-9. [DOI] [PubMed] [Google Scholar]
- Fett J. W., Strydom D. J., Lobb R. R., Alderman E. M., Bethune J. L., Riordan J. F., Vallee B. L. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry. 1985 Sep 24;24(20):5480–5486. doi: 10.1021/bi00341a030. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Fràter-Schröder M., Risau W., Hallmann R., Gautschi P., Böhlen P. Tumor necrosis factor type alpha, a potent inhibitor of endothelial cell growth in vitro, is angiogenic in vivo. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5277–5281. doi: 10.1073/pnas.84.15.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Sastre F., Folch-Pi J. Thin-layer chromatography of the phosphoinositides. J Lipid Res. 1968 Jul;9(4):532–533. [PubMed] [Google Scholar]
- Griendling K. K., Rittenhouse S. E., Brock T. A., Ekstein L. S., Gimbrone M. A., Jr, Alexander R. W. Sustained diacylglycerol formation from inositol phospholipids in angiotensin II-stimulated vascular smooth muscle cells. J Biol Chem. 1986 May 5;261(13):5901–5906. [PubMed] [Google Scholar]
- Hallcher L. M., Sherman W. R. The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J Biol Chem. 1980 Nov 25;255(22):10896–10901. [PubMed] [Google Scholar]
- Hong S. L., Deykin D. Activation of phospholipases A2 and C in pig aortic endothelial cells synthesizing prostacyclin. J Biol Chem. 1982 Jun 25;257(12):7151–7154. [PubMed] [Google Scholar]
- Inhorn R. C., Majerus P. W. Inositol polyphosphate 1-phosphatase from calf brain. Purification and inhibition by Li+, Ca2+, and Mn2+. J Biol Chem. 1987 Nov 25;262(33):15946–15952. [PubMed] [Google Scholar]
- Jaffe E. A., Grulich J., Weksler B. B., Hampel G., Watanabe K. Correlation between thrombin-induced prostacyclin production and inositol trisphosphate and cytosolic free calcium levels in cultured human endothelial cells. J Biol Chem. 1987 Jun 25;262(18):8557–8565. [PubMed] [Google Scholar]
- Lambert T. L., Kent R. S., Whorton A. R. Bradykinin stimulation of inositol polyphosphate production in porcine aortic endothelial cells. J Biol Chem. 1986 Nov 15;261(32):15288–15293. [PubMed] [Google Scholar]
- Leibovich S. J., Polverini P. J., Shepard H. M., Wiseman D. M., Shively V., Nuseir N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature. 1987 Oct 15;329(6140):630–632. doi: 10.1038/329630a0. [DOI] [PubMed] [Google Scholar]
- Levin D., London I. M. Regulation of protein synthesis: activation by double-stranded RNA of a protein kinase that phosphorylates eukaryotic initiation factor 2. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1121–1125. doi: 10.1073/pnas.75.3.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T. W., Wysolmerski R. B. Ca2+-dependent and Ca2+-independent pathways for release of arachidonic acid from phosphatidylinositol in endothelial cells. J Biol Chem. 1987 Sep 25;262(27):13086–13092. [PubMed] [Google Scholar]
- Mehmet H., Morris C. M., Taylor-Papadimitriou J., Rozengurt E. Interferon inhibition of DNA synthesis in Swiss 3T3 cells: dissociation from protein kinase C activation. Biochem Biophys Res Commun. 1987 Jun 30;145(3):1026–1032. doi: 10.1016/0006-291x(87)91538-5. [DOI] [PubMed] [Google Scholar]
- Montesano R., Orci L. Tumor-promoting phorbol esters induce angiogenesis in vitro. Cell. 1985 Sep;42(2):469–477. doi: 10.1016/0092-8674(85)90104-7. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Tertoolen L. G., de Laat S. W. Phorbol ester and diacylglycerol mimic growth factors in raising cytoplasmic pH. Nature. 1984 Nov 22;312(5992):371–374. doi: 10.1038/312371a0. [DOI] [PubMed] [Google Scholar]
- Moscat J., Moreno F., García-Barreno P. Mitogenic activity and inositide metabolism in thrombin-stimulated pig aorta endothelial cells. Biochem Biophys Res Commun. 1987 Jun 30;145(3):1302–1309. doi: 10.1016/0006-291x(87)91579-8. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Owen N. E. Effect of TPA on ion fluxes and DNA synthesis in vascular smooth muscle cells. J Cell Biol. 1985 Aug;101(2):454–459. doi: 10.1083/jcb.101.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryshyn R., Chen J. J., London I. M. Growth-related expression of a double-stranded RNA-dependent protein kinase in 3T3 cells. J Biol Chem. 1984 Dec 10;259(23):14736–14742. [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena M., Smith K. A. Phorbol esters, phospholipase C, and growth factors rapidly stimulate the phosphorylation of a Mr 80,000 protein in intact quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7244–7248. doi: 10.1073/pnas.80.23.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro R., Riordan J. F., Vallee B. L. Characteristic ribonucleolytic activity of human angiogenin. Biochemistry. 1986 Jun 17;25(12):3527–3532. doi: 10.1021/bi00360a008. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Strydom D. J., Olson K. A., Vallee B. L. Isolation of angiogenin from normal human plasma. Biochemistry. 1987 Aug 11;26(16):5141–5146. doi: 10.1021/bi00390a037. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Vallee B. L. Human placental ribonuclease inhibitor abolishes both angiogenic and ribonucleolytic activities of angiogenin. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2238–2241. doi: 10.1073/pnas.84.8.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro R., Weremowicz S., Riordan J. F., Vallee B. L. Ribonucleolytic activity of angiogenin: essential histidine, lysine, and arginine residues. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8783–8787. doi: 10.1073/pnas.84.24.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnett-Smith J. W., Rozengurt E. Diacylglycerol treatment rapidly decreases the affinity of the epidermal growth factor receptors of Swiss 3T3 cells. J Cell Physiol. 1985 Jul;124(1):81–86. doi: 10.1002/jcp.1041240114. [DOI] [PubMed] [Google Scholar]
- St Clair D. K., Rybak S. M., Riordan J. F., Vallee B. L. Angiogenin abolishes cell-free protein synthesis by specific ribonucleolytic inactivation of ribosomes. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8330–8334. doi: 10.1073/pnas.84.23.8330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strydom D. J., Fett J. W., Lobb R. R., Alderman E. M., Bethune J. L., Riordan J. F., Vallee B. L. Amino acid sequence of human tumor derived angiogenin. Biochemistry. 1985 Sep 24;24(20):5486–5494. doi: 10.1021/bi00341a031. [DOI] [PubMed] [Google Scholar]
- Vara F., Schneider J. A., Rozengurt E. Ionic responses rapidly elicited by activation of protein kinase C in quiescent Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2384–2388. doi: 10.1073/pnas.82.8.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyta J. C., Via D. P., Butterfield C. E., Zetter B. R. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984 Dec;99(6):2034–2040. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap W. H., Teo T. S., McCoy E., Tan Y. H. Rapid and transient rise in diacylglycerol concentration in Daudi cells exposed to interferon. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7765–7769. doi: 10.1073/pnas.83.20.7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap W. H., Teo T. S., Tan Y. H. An early event in the interferon-induced transmembrane signaling process. Science. 1986 Oct 17;234(4774):355–358. doi: 10.1126/science.2429366. [DOI] [PubMed] [Google Scholar]
- Yin E. T., Galanos C., Kinsky S., Bradshaw R. A., Wessler S., Lüderitz O., Sarmiento M. E. Picogram-sensitive assay for endotoxin: gelation of Limulus polyphemus blood cell lysate induced by purified lipopolysaccharides and lipid A from Gram-negative bacteria. Biochim Biophys Acta. 1972 Jan 28;261(1):284–289. doi: 10.1016/0304-4165(72)90340-6. [DOI] [PubMed] [Google Scholar]



