Abstract
With improved survival afforded by highly-active antiretroviral therapy (HAART), CKD has emerged as one of the primary comorbid conditions affecting human immunodeficiency virus (HIV)-infected individuals. Although CKD in HIV-infected individuals is classically thought of as a consequence of advanced HIV infection such as in the case of HIV-associated nephropathy (HIVAN), several factors likely contribute to the development CKD in HIV infection. These factors include genetic predisposition, age-related decline in kidney function, HAART-related metabolic changes, exposure to multiple nephrotoxic medications, and concurrent conditions such as hepatitis C or illicit drug use. Similar to the general population, proteinuria and impaired kidney function are associated with faster progression to acquired immune deficiency syndrome (AIDS) and death. Given the prevalence and impact of kidney disease on the course of HIV infection and its management, current guidelines recommend screening all HIV-infected individuals for kidney disease. This review focuses on the current guidelines for kidney disease screening and discusses traditional as well as promising strategies for detecting CKD in this vulnerable population.
Index words: HIV infection, proteinuria, estimated GFR, MDRD equation, cystatin C
Introduction
More than a decade after the introduction of HAART in 1996, approximately 1.2 million Americans are living with HIV.(1) With improved survival among HAART users, advancing age, (2) and HAART-related metabolic effects such as hypertension,(3,4) diabetes mellitus,(5 6) and dyslipidemia,(7,8) traditional chronic medical illnesses such as CKD have become increasingly important comorbidities.(9,10) In fact, the overall proportion of ESRD attributed to HIV infection within the U.S. has nearly doubled in the last decade.(11) However, this figure likely underestimates the burden of CKD, as it does not account for other causes of kidney disease in HIV infection.(12,13) In a study of HIV-positive women during the early HAART era, 3.5% were found to have serum creatinine levels of 1.4 mg/dL or greater.(14) However, the prevalence of CKD in the later HAART era remains unclear. Recent studies show that the prevalence of impaired kidney function, as defined by an estimated glomerular filtration rate (GFR) <60 mL/min/1.73 m2, may be as low as 2.4% and as high as 10%.(15-17)
Several concurrent pathological changes are frequently observed in renal biopsies obtained from HIV-infected persons.(18) These changes are the result of several co-existing factors, such as advanced HIV-disease, diabetes, hypertension, and hepatitis C infection, which simultaneously contribute to the development and progression of kidney disease in the setting of HIV infection. The majority of CKD cases in HIV infection are purportedly due to HIVAN; however, up to 50% of kidney diseases in HIV-infected persons result from a wide array of non-HIVAN pathology, ranging from glomerulonephritides to diabetic nephropathy.(18) In the later HAART era, in which earlier antiretroviral initiation is being advocated,(19) the relative contribution of these latter entities to HIV-related kidney disease is likely to evolve, with a diminishing number of HIVAN cases.
As in the general population, proteinuria and decreased kidney function portend worse outcomes. Among HIV-positive individuals, proteinuria and impaired kidney function are associated with faster progression to AIDS and death.(14,20) The impact of CKD on mortality in HIV-infected individuals increases proportionately with lower levels of kidney function, such that HIV-infected persons with estimated GFRs <15 mL/min/1.73 m2 are nearly six times more likely to die compared to those with estimated GFRs >60 mL/min/1.73 m2.(21) Insufficient HAART use and doses in HIV-infected persons with CKD may contribute to these observed differences in mortality risk.(21)
Although nearly one-third of HIV-infected persons have abnormal kidney function,(22) a recent study suggests that only a minority of affected individuals are recognized as having kidney disease.(17) Given the detrimental association of CKD with poorer outcomes in HIV infection and the implications of kidney function for HAART use and dosing, early recognition of CKD and diagnosis of the underlying cause is imperative in the management of individuals with HIV infection. Early recognition of CKD involves not only detection of proteinuria and examination of the urine sediment, but also estimation of kidney function. Current methods of kidney function estimates have not been thoroughly validated in HIV infection; however, studies are now underway to do so. Ultimately, the affected individual may require a kidney biopsy to determine the underlying cause of kidney disease. Clinical information gleaned from the composite of these evaluations may facilitate HAART management in those with indications for HAART and may lead to focused interventions to attenuate further kidney injury.(23) Ultimately, these individuals may benefit from the use of emerging biomarkers discovered as a result of renal tissue proteomic techniques.(24)
Guidelines for CKD Screening
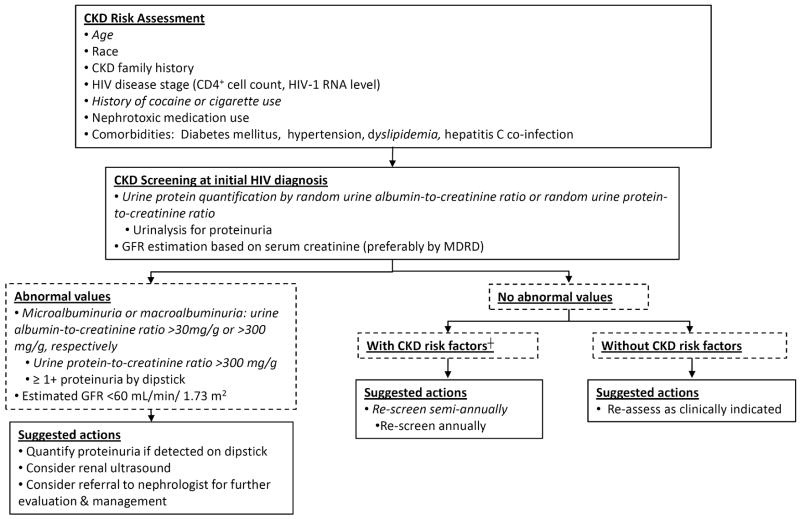
Early detection and diagnosis of kidney disease are essential for preventing or slowing further decline in kidney function and improving outcomes in HIV-infected persons. The NKF defines CKD by the presence of an abnormal urinalysis, typically in the form of proteinuria, and/or an estimated GFR <60 mL/min/1.73 m2 of at least 3 months duration.(25) In agreement with the NKF, current guidelines provided by the HIV Medicine Association of the Infectious Diseases Society of America (IDSA) advocate evaluation for both proteinuria and kidney dysfunction as CKD screening in HIV-infected individuals.(26) Figure 1 displays a modified approach to CKD screening in HIV-positive persons based on these guidelines. The IDSA guidelines highlight the largest subset of HIV-infected individuals at higher risk for developing kidney disease; however, persons currently using injection drugs, especially cocaine,(27,28) those with a strong family history of kidney disease,(29) dyslipidemia,(30) or cigarette use,(31) and those who are receiving certain medications should also be monitored for the development of CKD.
Figure 1. IDSA guidelines(26) for CKD screening in HIV infection with alternatives (italicized) based on the authors' opinions and the National Kidney Foundation guidelines(25).
Abbreviations: CKD, chronic kidney disease; GFR, glomerular filtration rate; MDRD, Modification of Diet in Renal Disease † CKD risk factors: African American, CD4+ cell count <200 cells/mm3, HIV-1 RNA >14,000 copies/ml, diabetes, hypertension, dyslipidemia, cocaine/cigarette use, hepatitis co-infection
(Adapted with permission from AIDS Patient Care and STDs 21:813-824, 2007)
Although there is a dearth of evidence to guide how frequent these assessments should be, individuals with underlying impaired kidney function and those initiated on drugs with potential renal toxicities likely benefit from more vigilant renal follow-up. Persons receiving tenofovir, in particular, may benefit from more frequent assessments of their kidney function, serum phosphate levels, and urinalyses to monitor for early signs of nephrotoxicity.(32,33) In the following sections, we will review additional methods available for assessing proteinuria and estimating kidney function, providing alternatives to the IDSA guidelines for CKD screening.
Assessment of Proteinuria
Proteinuria is one of the earliest indicators of various types of kidney disease, indicating a disturbance of glomerular basement membrane permeability(34) or renal tubular reabsorption.(35) Proteinuria is commonly found in HIV-infected individuals, with up to one-third having microalbuminuria despite normal serum creatinine levels.(36,37) The severity of proteinuria is prognostic of CKD progression in the general population.(38) In HIV-infected persons, proteinuria is associated with faster progression to AIDS and death.(14,20)
A 24-hour urine collection for proteinuria is generally considered the gold standard for proteinuria measurement; however, this method is subject to inaccuracies as a result of over- or under-collection.(25) Therefore, clinicians frequently rely upon urine dipsticks or random urine protein quantifications. The IDSA guidelines advocate use of urine dipsticks for proteinuria screening.(26) However, the NKF recommends screening for proteinuria using urine albumin-based methods such as albumin-specific dipsticks and random (preferably first morning void) urine albumin-to-creatinine ratios.(25) Additionally, individuals found to have proteinuria by dipstick should undergo confirmatory protein quantification within 3 months.(25) Although urine dipsticks are considered effective screening tools, falsely negative results may occur with dilute urine or with proteinuria consisting primarily of proteins other than albumin.(25) In a study of 166 HIV-infected persons who underwent both a urine dipstick and a spot urine protein-to-creatinine ratio within a 48-hour period, 21% of those with low but clinically significant levels of proteinuria (300 to 999 mg/g creatinine) were not detected by urine dipstick.(39) Urine protein quantifications provide more accurate assessments that may be monitored over time and have been shown to correlate well with 24-hour urinary protein excretion under various renal conditions.(40,41) Among individuals found to have albuminuria or proteinuria, subsequent monitoring may be performed using spot urine albumin-to-creatinine ratio or spot urine protein-to-creatinine ratio.(25) It is important to note that the use of spot urine samples to approximate 24-hour urinary protein excretion should be avoided in individuals with acute kidney failure, extremes of muscle mass, and advanced CKD in which urine creatinine excretion (on which this approximation is based) is unreliable and may provide spurious results.(42) In these particular scenarios, a 24-hour urine collection provides a more accurate assessment of urinary protein excretion.
Based on the convenience of collection compared with 24-hour estimations, and accuracy compared with the semi-quantitative urine dipstick, we suggest the use of either a spot protein-to-creatinine or spot albumin-to-creatinine ratio for kidney disease screening.
Estimation of Glomerular Filtration Rate
Accurately assessing kidney function is essential to clinical management of HIV-infected individuals. GFR is regarded as the best overall measure of kidney function. The gold standard measurement of GFR is the timed urinary clearance of inulin.(43) Although other exogenous substances provide similar results, the expense and burden on patients and clinical staff of using inulin or other exogenous substances limit their clinical practicality.(43) Therefore, GFR-estimating equations using endogenous substances are more commonly used. Table 1 summarizes currently available GFR-estimating equations using endogenous substances.
Table 1. GFR-estimating equations based on serum creatinine, serum cystatin C, or both.
| Biomarker | Equation | Clinical Performance |
|---|---|---|
| Abbreviated MDRD(50) | eGFR = 186 × SCr − 1.154 × age − 0.203 × 0.742 if female × 1.212 if black |
|
| Re-expressed MDRD(51) | eGFR = 175 × standardized SCr − 1.154 × age − 0.203 × 0.742 if female × 1.212 if black |
|
| Cockcroft-Gault(48) | CrCl = ([140 ‒ age] × weight)/(72 × SCr) × 0.85 if female |
|
| CKD-EPI(56) | eGFR = 141 × min(SCr/κ, 1)α × max(SCr/κ, 1) ‒ 1.209 × 0.933 age × 1.018 if female × 1.159 if black where κ is 0.7 for males and 0.9 for females; α is −0.411 for males and −0.329 for females; min indicates minimum of SCr/κ or 1; and max indicates maximum of SCr/κ or 1. |
|
| Cystatin C only(73) | eGFR = 127.7 × CysC − 1.17 × age − 0.13 × 0.91 if female × 1.06 if black |
|
| Serum creatinine and cystatin C(73) | eGFR = 177.6 × SCr − 0.65 × CysC − 0.57 × age − 0.20 × 0.82 if female × 1.11 if black |
|
Abbreviations: eGFR, estimated glomerular filtration rate; SCr, serum creatinine; CrCl, estimated creatinine clearance; CysC, serum cystatin C. Units: SCr in mg/dL, CysC in mg/L, age in years, weight in kg.
Practice guidelines by the NKF recommend using serum creatinine-based estimates of GFR.(25) Serum creatinine originates from muscle catabolism, and its production varies by nutritional status, dietary intake, and inflammatory status.(44,45) It is filtered by renal glomeruli but is also secreted and reabsorbed downstream by renal tubules. The renal secretion and reabsorption of creatinine vary intra- and inter-individually.(44) In addition, the earlier assays, which utilize the Jaffe rather than the enzymatic method to measure serum creatinine, suffer from poor inter-laboratory calibration, especially at lower serum creatinine levels.(46, 47) In severe kidney disease, the effects of these factors on serum creatinine levels are trivial. However, at milder levels of kidney function impairment, these effects are more substantial.(46) Therefore, use of serum creatinine values alone to estimate kidney function is generally avoided.(25) Instead, equations for estimating GFR that account for age, sex, and race are preferred.(25) The two equations that have been used most commonly in clinical practice are the Cockcroft-Gault formula and the Modification of Diet in Renal Disease (MDRD) equation.(48,49) The Cockcroft-Gault formula was developed based on 24-hour urinary creatinine clearance measurements obtained from hospitalized Caucasian men.(48) It is often used as an estimate of kidney function in pharmacokinetic studies and as such is widely used to adjust drug dosing for kidney function. Conversely, the original MDRD equation was developed utilizing iothalamate clearance measurements from more than 1000 individuals with chronically impaired kidney function. This study included no diabetic participants. Moreover, older persons and those infected with HIV or with severe proteinuria were excluded from the MDRD study.(49) Since its initial publication, the abbreviated MDRD equation, using only four of the variables included in the original equation,(50) and the re-expressed MDRD equation, to account for standardized serum creatinine measurements,(51) have been proposed and used in clinical practice. Since these equations were developed in limited study populations, they only partially adjust for the effects of extra-renal factors on serum creatinine.(52) Consequently, the Cockcroft-Gault and MDRD equations have diminished precision and increased bias at increasing GFR levels, especially at GFR >60 mL/min/1.73 m2.(53,54) At these levels of kidney function, the Cockcroft-Gault equation may over- or underestimate actual GFR (45,55), while the MDRD equation yields estimates that tend to underestimate actual GFR.(45,53) The newly published CKD-EPI equation developed by the CKD Epidemiology Collaboration seems to provide GFR estimates with decreased bias and improved accuracy compared to the re-expressed MDRD equation.(56) At GFR >60 mL/min/1.73 m2, the median bias provided by the CKD-EPI equation was 3.5 versus 10.6 by the re-expressed MDRD equation. Moreover, 88.3% of the GFR estimates based on the CKD-EPI equation were within 30% of the measured GFR compared with only 84.7% of the estimates based on the MDRD equation.(56) However, this newer formula has yet to gain widespread clinical application.
Serum creatinine-based estimated GFR among HIV-infected persons may be particularly biased due to associated altered metabolism, body mass abnormalities, and exposure to multiple medications known to affect renal tubular creatinine secretion.(57,58) The three early studies that compared Cockcroft-Gault GFR estimates to 24-hour creatinine clearance measurements yielded conflicting results and were small in nature, with sample sizes <50.(59-61) Furthermore, 24-hour creatinine clearance measurements themselves are frequently plagued with inaccuracies as a result of over- or under-collection and variable renal tubular creatinine secretion. A more recent study of 27 HIV-positive participants compared Cockcroft-Gault and MDRD GFR estimates with 99mTc-pentetate-based GFR measurements.(62) The median GFR in this population was 91 mL/min/1.73 m2 with only three participants having GFR <60 mL/min/1.73 m2. As in the general population, the precision of both GFR-estimating methods diminished with increasing GFR. Overall, the MDRD equation provided more accurate GFR estimates than the Cockcroft-Gault equation. Whereas 89% of the MDRD-based GFR estimates fell within 30% of the measured GFR, only 70% of the Cockcroft-Gault-based GFR estimates were within 30% of the measured GFR.(62) Although this study was limited by its small sample size, it supports the IDSA recommendation of using the MDRD equation rather than the Cockcroft-Gault formula in HIV-infected persons.(26) However, larger validation studies are still needed to determine the generalizability of either equation to the HIV-positive population at large. Additional studies are also needed to assess the clinical usefulness of the new CKD-EPI equation in the setting of HIV infection.
Due to the limitations presented by serum creatinine-based estimates of GFR, investigators have sought alternative biomarkers that more accurately estimate kidney function. Serum cystatin C is a novel biomarker which may provide a better estimate.(63) Cystatin C, a cystine protease inhibitor produced by almost all human cells at a constant rate, is freely filtered by glomeruli, subsequently reabsorbed, and then catabolized by renal tubules.(64,65) However, recent studies in the general U.S. population and CKD populations demonstrate that serum cystatin C, like serum creatinine, is affected by age, gender, and race.(66,67) Furthermore, studies have variably shown an association of inflammation with serum cystatin C levels.(68) Unlike serum creatinine, serum cystatin C is thought to be independent of body mass.(69)
A meta-analysis of 46 studies comparing serum cystatin C and serum creatinine with measured GFR found that the correlations (0.82 vs 0.74, respectively) and the area under the curve from a receiver-operator curve analysis (0.93 vs 0.84) were greater between GFR and 1/serum cystatin than between GFR and 1/serum creatinine.(63) Many of the studies indicate that serum cystatin C has a distinct advantage in detecting mild kidney dysfunction.(70-72) More recently, the CKD Epidemiology Collaboration has published GFR-estimating equations based on serum creatinine, serum cystatin C, and both. These equations (shown in Table 1) were developed based on individuals screened for three CKD studies in the U.S. and were validated internally with one-third of the studies' data and validated externally in a French clinical population.(73) The proportions of GFR estimates that were within 30% of the iothalamate GFR were 81, 83, and 89%, respectively, for GFR-estimating equations containing serum cystatin C alone; cystatin C with age, sex, and race; and serum cystatin C combined with serum creatinine, age, sex and race. This study illustrates that GFR estimates based on serum cystatin C have similar accuracy to those based on serum creatinine without dependence upon muscle mass, and that GFR estimated by the combination of serum cystatin C and creatinine yields the highest accuracy.(73) Prospective observational studies have also shown that serum cystatin C is a stronger predictor of death than either serum creatinine or serum creatinine-based GFR.(74-76)
An increasing number of studies have evaluated the utility of serum cystatin C as an indicator of kidney function in the HIV-positive population; however, they have largely been limited by the lack of a gold-standard measurement of GFR. In the Fat Redistribution and Metabolic Change in HIV Infection (FRAM) cohort, serum cystatin C was higher in HIV-infected participants compared with healthy controls. Furthermore, among HIV-infected participants, serum cystatin C correlated with several risk factors for kidney disease (eg, hypertension, hepatitis C co-infection).(77) A similar trend was observed in the Nutrition for Healthy Living study in which serum cystatin C-based GFR provided significantly higher prevalence estimates of impaired kidney function compared with MDRD-based estimated GFR.(15) In the Strategies for Management of Antiretroviral Therapy (SMART) trial, which was a randomized, controlled clinical trial comparing episodic antiretroviral therapy guided by CD4 cell counts versus continuous therapy for HIV viral load suppression, investigators showed that participants who received interrupted treatment had higher levels of serum cystatin C during follow-up compared with those who received continuous antiretroviral therapy.(78) This result supported the clinical findings of worse fatal and nonfatal renal disease in the interrupted-treatment group.(79) Although these studies indicate that serum cystatin C levels are reflective of kidney function, none had actual GFR measurements for comparison, and concerns about the independent effect of HIV infection itself of serum cystatin C levels have recently surfaced. In a small study by Barraclough and colleagues, which compared various methods of estimating GFR to 99mTc-pentetate-based measured GFR, the cystatin C-based GFR estimates had the largest bias (median bias -29 mL/min/1.73 m2) of all the methods evaluated. Furthermore, only 41% of the cystatin C-based GFR estimates were within 30% of the measured GFR.(62) These findings may reflect the larger effects of extra-renal factors on serum cystatin C levels in the setting of HIV infection or, alternatively, they may reflect the investigators' choice of a cystatin C-based GFR equation void of adjustments for age, gender, and race. Additional studies that evaluate cystatin C-based GFR equations that adjust for these factors, such as that developed by the CKD Epidemiology Collaboration, are needed. Until adequate validation of these equations occurs in HIV-infected patients, the re-expressed MDRD equation should be the preferred GFR-estimating equation used in this patient population, despite its shortcomings, since it is the most widely accepted GFR-estimating equation in clinical practice and appears to be the most reliable of the available formulas studied thus far in HIV-positive persons.
Novel Biomarkers in CKD
Effective CKD screening and management should ideally involve accurate, non-invasive indicators that reflect the pathophysiologic mechanisms underlying CKD. Given the inherent limitations of proteinuria assessments and GFR estimations, several potential novel biomarkers that correlate with histopathological findings are actively being investigated for their utility in determining the underlying renal pathological processes and in predicting CKD progression prior to the development of overt clinical evidence of CKD. These include neutrophil gelatin-associated lipocalin (NGAL), asymmetric dimethylarginine (ADMA), and liver-type fatty acid binding protein (L-FABP).(80)
NGAL is produced by neutrophils and various epithelial cell types, including those composing the renal distal tubules.(81) NGAL has been shown as an early, sensitive marker of acute kidney injury; however, more recent studies have also supported NGAL's possible role as a marker of kidney function and a prognostic indicator of CKD progression in individuals with varying renal pathological processes.(80) In small studies evaluating HIV-negative individuals with CKD, urinary NGAL positively correlated with proteinuria severity and inversely with estimated GFR.(82) In a study of 96 participants with pre-existing CKD, baseline serum and urinary NGAL levels independently predicted declines in estimated GFR over a mean follow-up period of 18.5 months.(83) The data on NGAL as a marker of kidney function in HIV-infected patients is limited to a pediatric study that demonstrated progressive decreases in urinary NGAL levels with HAART initiation in a child with biopsy-proven HIVAN.(84) However, it is unclear whether these changes are necessarily due to improvement in renal function or instead reflect changes in the child's immune system. The single study that has compared serum NGAL levels in HIV-positive individuals to HIV-negative controls showed that HIV-infected persons have lower NGAL levels compared to healthy controls.(85) Among HIV-infected participants who experienced virological response (HIV RNA <200 copies/mL) to HAART, serum NGAL levels increased to near normal levels; a similar response was not observed in HAART non-responders. The results of this study imply that serum NGAL levels are altered in HIV infection due to diminished neutrophil numbers and function (85) and are consistent with prior observations of increased NGAL expression by epithelial cells and neutrophils in the setting of systemic inflammation and bacterial infection.(86-88) However, this study did not evaluate kidney function.(85)
Another promising biomarker for CKD is ADMA, which functions as a nitric oxide synthase inhibitor and reflects endothelial function.(85) In HIV-negative persons with CKD, ADMA levels have been associated with faster progression to dialysis and death in individuals with CKD.(89,90) Similarly, L-FABP, a marker of proximal tubule integrity, has also been associated with progressive CKD.(85) Among 120 HIV-negative individuals with non-diabetic CKD, urinary L-FABP concentrations correlated with proteinuria and serum creatinine levels. Moreover, individuals who had progressive CKD had higher urinary L-FABP levels compared to those with non-progressive CKD, despite similar levels of proteinuria and serum creatinine.(91) No studies to date have evaluated urinary ADMA and L-FABP as CKD biomarkers in HIV-positive persons. The field of tissue proteomics is quickly advancing and may yield additional promising biomarkers in the near future.(24) Such molecular analyses in HIV-positive persons with kidney disease are underway.
Conclusion
CKD has arisen as one of the leading non-infectious conditions affecting HIV-infected persons in the later HAART era as a consequence of aging, other chronic conditions such as diabetes and hypertension, and exposure to a myriad of potentially nephrotoxic drugs. Several readily-available screening tools enable clinicians to detect early decrements in kidney function with the primary aim of averting further kidney damage. Although these screening tools have not been thoroughly evaluated in the HIV-positive population, increasing recognition of CKD as a major comorbidity in this population has spurred a number of investigations to validate these methods. Emerging biomarkers for CKD that reflect underlying pathological findings may ultimately augment efforts at detecting and diagnosing CKD in HIV-infected individuals.
Acknowledgments
Dr. Estrella is supported by the NIH-NIDDK grant 1K23DK081317-01A1.
Footnotes
Disclosure: Dr. Fine has received consulting fees and speaker honoraria from GlaxoSmithKline
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.UNAIDS. Report on the global AIDS epidemic. UNAIDS; Geneva: 2006. [Google Scholar]
- 2.Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report 2005. Vol. 17. Atlanta: U. S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2006. [Google Scholar]
- 3.Seaberg EC, Munoz A, Lu M, et al. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS. 2005;19:953–960. doi: 10.1097/01.aids.0000171410.76607.f8. [DOI] [PubMed] [Google Scholar]
- 4.Chow DC, Souza SA, Chen R, Richmond-Crum SM, Grandinetti A, Shikuma C. Elevated blood pressure in HIV-infected individuals receiving highly active antiretroviral therapy. HIV Clin Trials. 2003;4:414–416. doi: 10.1310/5E7Q-PGWB-16UE-J48U. [DOI] [PubMed] [Google Scholar]
- 5.Koster JC, Remedi MS, Qiu H, Nichols CG, Hruz PW. HIV protease inhibitors acutely impair glucose-stimulated insulin release. Diabetes. 2003;52:1695–1700. doi: 10.2337/diabetes.52.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown T, Cole SR, Li X, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the Multicenter AIDS Cohort Study. Arch Intern Med. 2005;165:1179–1184. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 7.Riddler SA, Smit E, Cole SR, et al. Impact of HIV infection and HAART on serum lipids in men. JAMA. 2003;289:2978–2982. doi: 10.1001/jama.289.22.2978. [DOI] [PubMed] [Google Scholar]
- 8.Periard D, Telenti A, Sudre P, et al. Atherogenic dyslipidemia in HIV-infected individuals treated with protease inhibitors. Circulation. 1999;100:700–705. doi: 10.1161/01.cir.100.7.700. [DOI] [PubMed] [Google Scholar]
- 9.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352:48. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz EJ, Szczech LA, Ross MJ, Klotman ME, Winston JA, Klotman PE. Highly active antiretroviral therapy and the epidemic of HIV+ end-stage renal disease. J Am Soc Nephrol. 2005;16:2412–2420. doi: 10.1681/ASN.2005040340. [DOI] [PubMed] [Google Scholar]
- 11.Khan S, Haragsim L, Laszik ZG. HIV-associated nephropathy. Adv Chronic Kidney Dis. 2006;13:307–313. doi: 10.1053/j.ackd.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Eggers PW, Kimmel PL. Is there an epidemic of HIV infection in the US ESRD program? J Am Soc Nephrol. 2004;15:2477–2485. doi: 10.1097/01.ASN.0000138546.53152.A7. [DOI] [PubMed] [Google Scholar]
- 13.Szczech LA, Gupta SK, Habash R, et al. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int. 2004;66:1145–1152. doi: 10.1111/j.1523-1755.2004.00865.x. [DOI] [PubMed] [Google Scholar]
- 14.Gardner LI, Holmberg SD, Williamson JM, et al. Development of proteinuria or elevated serum creatinine and mortality in HIV-infected women. J Acquir Immune Defic Syndr. 2003;32:203–209. doi: 10.1097/00126334-200302010-00013. [DOI] [PubMed] [Google Scholar]
- 15.Jones CY, Jones CA, Wilson IB, et al. Cystatin C and creatinine in an HIV cohort: the nutrition for healthy living study. Am J Kidney Dis. 2008;51:914–924. doi: 10.1053/j.ajkd.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Overton ET, Nurutdinova D, Freeman J, Seyfried W, Mondy KE. Factors associated with renal dysfunction within an urban HIV-infected cohort in the era of highly active antiretroviral therapy. HIV Med. 2009;10:343–350. doi: 10.1111/j.1468-1293.2009.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernando SK, Finkelstein FO, Moore BA, Weissman S. Prevalence of chronic kidney disease in an urban HIV infected population. Am J Med Sci. 2008;355:89–94. doi: 10.1097/MAJ.0b013e31812e6b34. [DOI] [PubMed] [Google Scholar]
- 18.Estrella MM, Fine DM, Gallant JE, et al. HIV type 1 RNA level as a clinical indicator of renal pathology in HIV-infected patients. Clin Infect Dis. 2006;43:377–380. doi: 10.1086/505497. [DOI] [PubMed] [Google Scholar]
- 19.Hammer SM, Eron JJ, Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300:555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 20.Szczech LA, Hoover DR, Feldman JG, et al. Association between renal disease and outcomes among HIV-infected women receiving or not receiving antiretroviral therapy. Clin Infect Dis. 2004;39:1199–1206. doi: 10.1086/424013. [DOI] [PubMed] [Google Scholar]
- 21.Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Hernandez GT, O'Hare AM. Lower rates of antiretroviral therapy among HIV-infected patients with chronic kidney disease. Clin Infect Dis. 2007;45:1633–1639. doi: 10.1086/523729. [DOI] [PubMed] [Google Scholar]
- 22.Gupta SK, Mamlin BW, Johnson CS, Dollins MD, Topf JM, Dubé MP. Prevalence of proteinuria and the development of chronic kidney disease in HIV-infected patients. Clin Nephrol. 2004;61:1–6. doi: 10.5414/cnp61001. [DOI] [PubMed] [Google Scholar]
- 23.Fine DM, Perazella MA, Lucas GM, Atta MG. Kidney biopsy in HIV: beyond HIV-associated nephropathy. Am J Kidney Dis. 2008;51:504–514. doi: 10.1053/j.ajkd.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Sedor JR. Tissue proteomics: a new investigative tool for renal biopsy analysis. Kidney Int. 2009;75:876–879. doi: 10.1038/ki.2009.54. [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 26.Gupta SK, Eustace JA, Winston JA, et al. Guidelines for the management of chronic kidney disease in HIV-infected patients: recommendations of the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2005;40:1559–1585. doi: 10.1086/430257. [DOI] [PubMed] [Google Scholar]
- 27.Bakir AA, Dunea G. Drugs of abuse and renal disease. Curr Opin Nephrol Hypertens. 1996;5:122–126. doi: 10.1097/00041552-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Fine DM, Garg N, Haas M, et al. Cocaine use and hypertensive renal changes in HIV-infected individuals. Clin J Am Soc Nephrol. 2007;2:1125–1130. doi: 10.2215/CJN.02450607. [DOI] [PubMed] [Google Scholar]
- 29.Freedman BI, Soucie JM, Stone SM, Pegram S. Familial clustering of end-stage renal disease in blacks with HIV-associated nephropathy. Am J Kidney Dis. 1999;34:254–258. doi: 10.1016/s0272-6386(99)70352-5. [DOI] [PubMed] [Google Scholar]
- 30.Appel GB, Radhakrishnan J, Avram MM, et al. Analysis of metabolic parameters as predicators of risk in the RENAAL study. Diabetes Care. 2003;26:1402–1407. doi: 10.2337/diacare.26.5.1402. [DOI] [PubMed] [Google Scholar]
- 31.Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyrén O. Association between smoking and chronic renal failure in a nationwide population-based case-control study. J Am Soc Nephrol. 2004;15:2178–2185. doi: 10.1097/01.ASN.0000135048.35659.10. [DOI] [PubMed] [Google Scholar]
- 32.Zimmermann AE, Pizzoferrato T, Bedford J, Morris A, Hoffman R, Braden G. Tenofovir-associated acute and chronic kidney disease: a case of multiple drug interactions. Clin Infect Dis. 2006;42:283–290. doi: 10.1086/499048. [DOI] [PubMed] [Google Scholar]
- 33.Winston A, Amin J, Mallon P, et al. Minor changes in calculated creatinine clearance and anion-gap are associated with tenofovir disoproxil fumarate-containing highly active antiretroviral therapy. HIV Med. 2006;7:105–111. doi: 10.1111/j.1468-1293.2006.00349.x. [DOI] [PubMed] [Google Scholar]
- 34.Moller CC, Pollak MR, Reiser J. The genetic basis of human glomerular disease. Adv Chronic Kidney Dis. 2006;13:166–173. doi: 10.1053/j.ackd.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 35.Russo LM, Sandoval RM, McKee M, et al. The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: retrieval is disrupted in nephrotic states. Kidney Int. 2007;71:501–513. doi: 10.1038/sj.ki.5002041. [DOI] [PubMed] [Google Scholar]
- 36.Kimmel PL, Umana WO, Bosch JP. Abnormal urinary protein excretion in HIV-infected patients. Clin Nephrol. 1993;39:17–21. [PubMed] [Google Scholar]
- 37.Busch HW, Riechmann S, Heyen P, et al. Albuminuria in HIV-infected patients. AIDS Res Hum Retroviruses. 1994;10:717–720. doi: 10.1089/aid.1994.10.717. [DOI] [PubMed] [Google Scholar]
- 38.Zandi-Nejad K, Eddy AA, Glassock RJ, Brenner BM. Why is proteinuria an ominous biomarker of progressive kidney disease? Kidney Int Suppl. 2004;92:S76. doi: 10.1111/j.1523-1755.2004.09220.x. [DOI] [PubMed] [Google Scholar]
- 39.Siedner MJ, Atta MG, Lucas GM, Perazella MA, Fine DM. Poor validity of urine dipstick as a screening tool for proteinuria in HIV-positive patients. J Acquir Immune Defic Syndrom. 2008;47:261–263. doi: 10.1097/QAI.0b013e31815ac4ad. [DOI] [PubMed] [Google Scholar]
- 40.Ginsberg JM, Chang BS, Matarese RA, Garella S. Use of single voided urine samples to estimate quantitative proteinuria. N Engl J Med. 1983;309:1543–1546. doi: 10.1056/NEJM198312223092503. [DOI] [PubMed] [Google Scholar]
- 41.Price CP, Newall RG, Boyd JC. Use of protein:creatinine ratio measurements on random urine samples for prediction of significant proteinuria: a systematic review. Clin Chem. 2005;51:1577–1586. doi: 10.1373/clinchem.2005.049742. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen MT, Maynard SE, Kimmel PL. Misapplications of commonly used kidney equations: renal physiology in practice. Clin J Am Soc Nephrol. 2009;4:528–534. doi: 10.2215/CJN.05731108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaspari F, Perico N, Remuzz G. Measurement of glomerular filtration rate. Kidney Int Suppl. 1997;63:S151–S154. [PubMed] [Google Scholar]
- 44.Levey AS. Measurement of renal function in chronic renal disease (clinical conference) Kidney Int. 1990;38:167–184. doi: 10.1038/ki.1990.182. [DOI] [PubMed] [Google Scholar]
- 45.Verhave JC, Fesler P, Ribstein J, du Cailar G, Mimran A. Estimation of renal function in subjects with normal serum creatinine levels: influence of age and body mass index. Am J Kidney Dis. 2005;46:233–241. doi: 10.1053/j.ajkd.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 46.Coresh J, Astor BC, McQuillan G, et al. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis. 2002;39:920–292. doi: 10.1053/ajkd.2002.32765. [DOI] [PubMed] [Google Scholar]
- 47.Ross JW, Miller WG, Myers GL, Praestgaard J. The accuracy of laboratory measurements in clinical chemistry: a study of 11 routine chemistry analytes in the College of American Pathologists Chemistry Survey with fresh frozen serum, definitive methods, and reference methods. Arch Pathol Lab Med. 1998;122(608):587. [PubMed] [Google Scholar]
- 48.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 49.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 50.Levey AS, Greene T, Kusek JW, Beck GJ, Group MS. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000;11:115A. [Google Scholar]
- 51.Levey AS, Coresh J, Greene T, et al. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 52.Coresh J, Stevens LA. Kidney function estimating equations: where do we stand? Curr Opin Nephrol Hypertens. 2006;15:276–284. doi: 10.1097/01.mnh.0000222695.84464.61. [DOI] [PubMed] [Google Scholar]
- 53.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141:929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 54.Bostom AG, Kronenberg F, Ritz E. Predictive performance of renal function equations for patients with chronic kidney disease and normal serum creatinine levels. J Am Soc Nephrol. 2002;13:2140–2144. doi: 10.1097/01.asn.0000022011.35035.f3. [DOI] [PubMed] [Google Scholar]
- 55.Lin J, Knight EL, Hogan ML, Singh AK. A comparison of prediction equations for estimating glomerular filtration rate in adults without kidney disease. J Am Soc Nephrol. 2003;14:2573–2580. doi: 10.1097/01.asn.0000088721.98173.4b. [DOI] [PubMed] [Google Scholar]
- 56.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roy MT, First MR, Myre SA, Cacini W. Effect of co-trimoxazole and sulfamethoxazole on serum creatinine in normal subjects. Ther Drug Monit. 1982;4:77–79. doi: 10.1097/00007691-198204000-00011. [DOI] [PubMed] [Google Scholar]
- 58.Maia BS, Engelson ES, Wang J, Kotler DP. Antiretroviral therapy affects the composition of weight loss in HIV infection: implications for clinical nutrition. Clin Nutr. 2005;24:971–978. doi: 10.1016/j.clnu.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 59.Huang E, Hewitt RG, Shelton M, Morse GD. Comparison of measured and estimated creatinine clearance in patients with advanced HIV disease. Pharmacotherapy. 1996;16:222–229. [PubMed] [Google Scholar]
- 60.Noormohamed SE, Katseres JK, Stapleton JT. Poor correlation between published methods to predict creatinine clearance and measured creatinine clearance in asymptomatic HIV infected individuals. Ren Fail. 1998;20:627–633. doi: 10.3109/08860229809045156. [DOI] [PubMed] [Google Scholar]
- 61.Smith BL, Sarnoski TP, Dennis S, Luke DR. Failure of predicted creatinine clearance equations in HIV-seropositive patients. Int J Clin Pharmacol Ther Toxicol. 1992;30:394–399. [PubMed] [Google Scholar]
- 62.Barraclough K, Er L, Ng F, Harris M, Montaner J, Levin A. A comparison of the predictive performance of different methods of kidney function estimation in a well-characterized HIV-infected population. Nephron Clin Pract. 2009;111:c39–48. doi: 10.1159/000178978. [DOI] [PubMed] [Google Scholar]
- 63.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40:221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 64.Laterza OF, Price CP, Scott MG, Cystatin C. an improved estimator of glomerular filtration rate? Clin Chem. 2002;48:699–707. [PubMed] [Google Scholar]
- 65.Grubb A. Diagnostic value of analysis of cystatin C and protein HC in biological fluids. Clin Nephrol. 1992;38(Suppl 1):S20–S27. [PubMed] [Google Scholar]
- 66.Kottgen A, Selvin E, Stevens LA, Levey AS, Van Lente F, Coresh J. Serum cystatin C in the United States: the Third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2008;51:385–394. doi: 10.1053/j.ajkd.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 67.Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75:652–660. doi: 10.1038/ki.2008.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Knight EL, Verhave JC, Spiegelman D, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65:1416–1421. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 69.Baxmann AC, Ahmed MS, Marques NC, et al. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol. 2008;3:348–354. doi: 10.2215/CJN.02870707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O'Riordan SE, Webb MC, Stowe JH, et al. Cystatin C improves the detection of mild renal dysfunction in older patients. Ann Clin Biochem. 2003;40:648–655. doi: 10.1258/000456303770367243. [DOI] [PubMed] [Google Scholar]
- 71.Coll E, Botey A, Alvarez L, et al. Serum cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am J Kidney Dis. 2000;36:29–34. doi: 10.1053/ajkd.2000.8237. [DOI] [PubMed] [Google Scholar]
- 72.Christensson AG, Grubb A, Nilsson JA, Norrgren K, Sterner G, Sundkvist G. Serum cystatin C advantageous compared with serum creatinine in the detection of mild but no severe diabetic nephropathy. J Intern Med. 2004;256:510–518. doi: 10.1111/j.1365-2796.2004.01414.x. [DOI] [PubMed] [Google Scholar]
- 73.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis of cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145:237–246. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 75.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 76.Larsson A, Helmersson J, Hansson LO, Basu S. Increased serum cystatin C is associated with increased mortality in elderly men. Scand J Clin Lab Invest. 2005;65:301–305. doi: 10.1080/00365510510013839. [DOI] [PubMed] [Google Scholar]
- 77.Odden MC, Scherzer R, Bacchetti P, et al. Cystatin C level as a marker of kidney function in human immunodeficiency virus infection: the FRAM study. Arch Intern Med. 2007;167:2213–2219. doi: 10.1001/archinte.167.20.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mocroft A, Wyatt C, Szczech L, et al. Interruption of antiretroviral therapy is associated with increased plasma cystatin C. AIDS. 2009;23:71–82. doi: 10.1097/QAD.0b013e32831cc129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 80.Nickolas TL, Barasch J, Devarajan P. Biomarkers in acute and chronic kidney disease. Curr Opin Nephrol Hypertens. 2008;17:127–132. doi: 10.1097/MNH.0b013e3282f4e525. [DOI] [PubMed] [Google Scholar]
- 81.Yang J, Mori K, Li JY, Barasch J. Iron, lipocalin, and kidney epithelia. Am J Physiol Renal Physiol. 2003;285:F9–F18. doi: 10.1152/ajprenal.00008.2003. [DOI] [PubMed] [Google Scholar]
- 82.Bolignano D, Coppolino G, Campo S, et al. Urinary neutrophil gelatinase-associated lipocalin (NGAL) is associated with severity of renal disease in proteinuric patients. Nephrol Dial Transplant. 2008;23:414–416. doi: 10.1093/ndt/gfm541. [DOI] [PubMed] [Google Scholar]
- 83.Bolignano D, Lacquaniti A, Coppolino G, et al. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:337–344. doi: 10.2215/CJN.03530708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Soler-Garcia AA, Johnson D, Hathout Y, Ray PE. Iron-related proteins: candidate urine biomarkers in childhood HIV-associated renal diseases. Clin J Am Soc Nephrol. 2009;4:763–771. doi: 10.2215/CJN.0200608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Landro L, Damas JK, Flo TH, et al. Decreased serum lipocalin-2 levels inhuman immunodeficiency virus-infected patients: increase during highly active anti-retroviral therapy. Clin Exp Immunol. 2008;152:57–63. doi: 10.1111/j.1365-2249.2008.03592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Borregaard N, Sehested M, Nielsen BS, Borregaard N, Rygaard J, Danø K. Biosynthesis of granule proteins in normal human bone marrow cells. Gelatinase is a marker of terminal neutrophil differentiation. Blood. 1995;85:812–917. [PubMed] [Google Scholar]
- 87.Xu S, Venge P. Lipocalins as biochemical markers of disease. Biochem Biophys Acta. 2000;1482:298–307. doi: 10.1016/s0167-4838(00)00163-1. [DOI] [PubMed] [Google Scholar]
- 88.Hemdahl AL, Gabrielsen A, Zhu C, et al. Expression of neutrophil gelatinase-associated lipocalin in atherosclerosis and myocardial infarction. Arterioscler Thromb Vasc Bio. 2006;26:136–142. doi: 10.1161/01.ATV.0000193567.88685.f4. [DOI] [PubMed] [Google Scholar]
- 89.Ravani P, Tripepi G, Malberti F, Testa S, Mallamaci F, Zoccali C. Asymmetrical dimethylarginine predicts progression to dialysis and death in patients with chronic kidney disease: a competing risks modeling approach. J Am Soc Nephrol. 2005;16:2449–2455. doi: 10.1681/ASN.2005010076. [DOI] [PubMed] [Google Scholar]
- 90.Fliser D, Kronenberg F, Kielstein JT, et al. Asymmetric dimetholarginine and progression of chronic kidney disease: the mild to moderate kidney disease study. J Am Soc Nephrol. 2005;16:2456–2461. doi: 10.1681/ASN.2005020179. [DOI] [PubMed] [Google Scholar]
- 91.Kamijo A, Sugaya T, Hikawa A, et al. Urinary liver-type fatty acid binding protein as a useful biomarker in chronic kidney disease. Mol Cell Biochem. 2006;284:175–82. doi: 10.1007/s11010-005-9047-9. [DOI] [PubMed] [Google Scholar]