Abstract
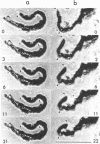
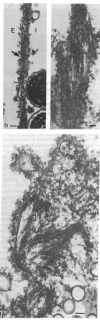
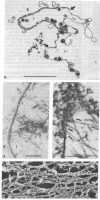
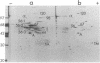
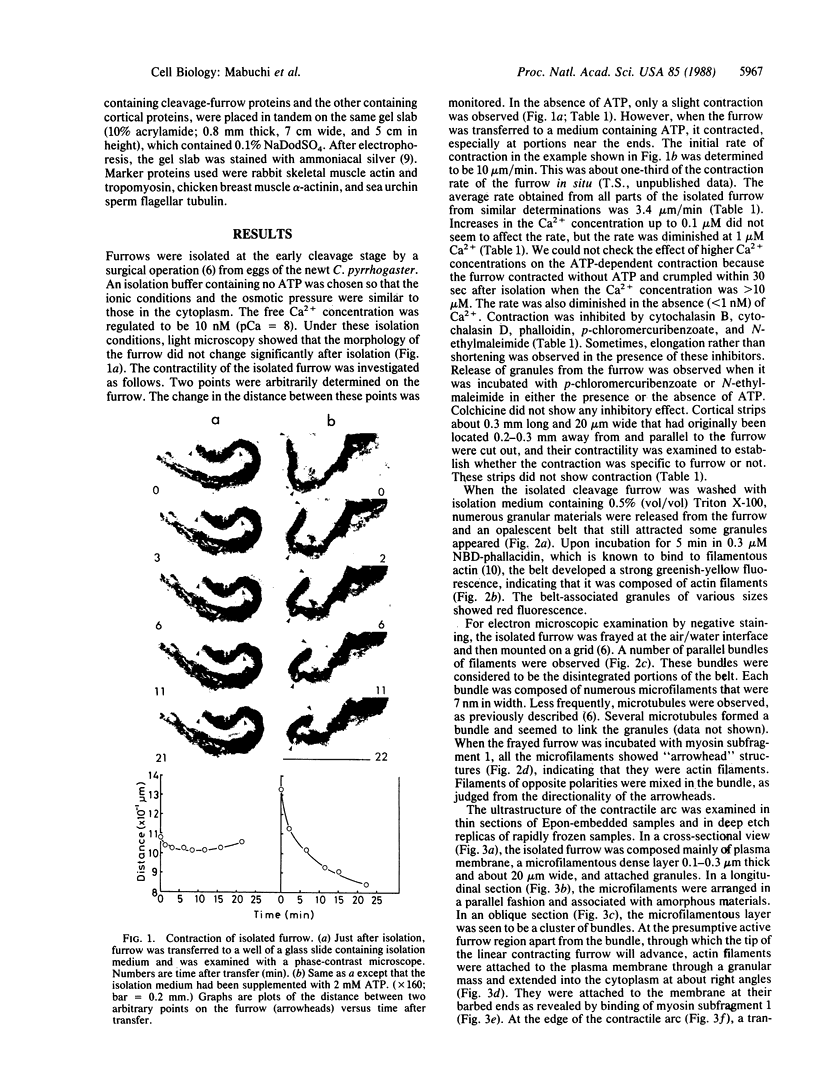
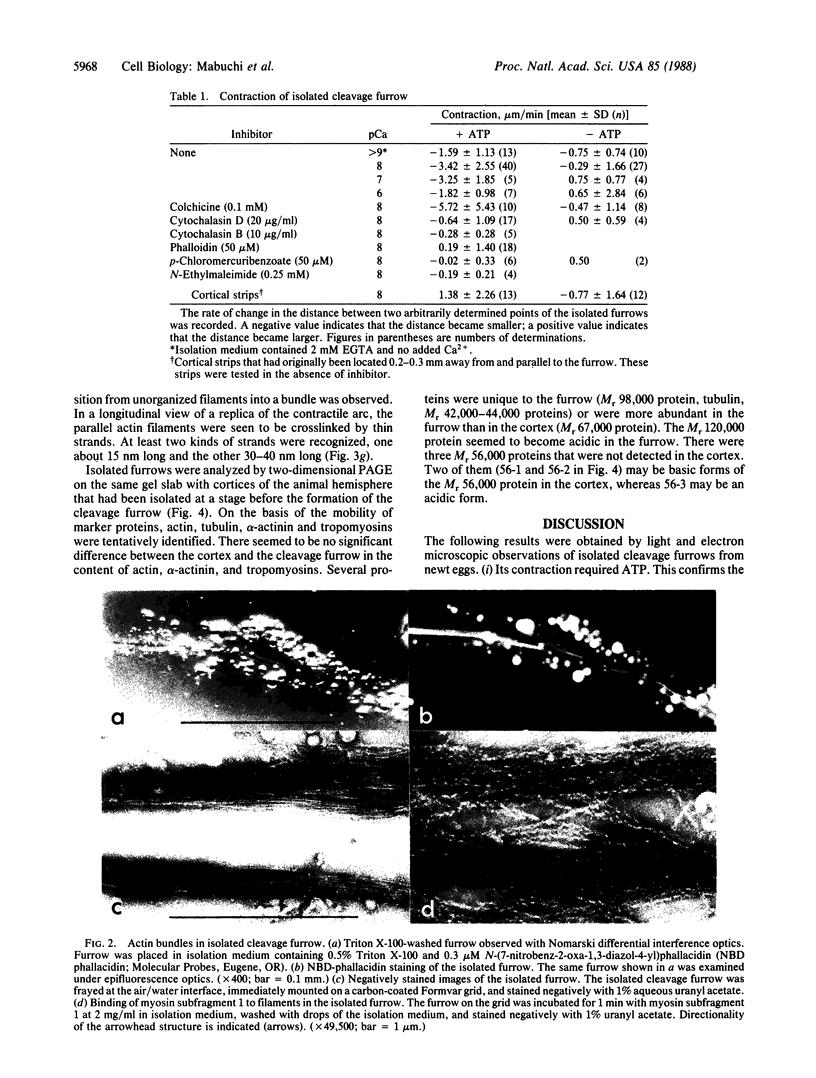
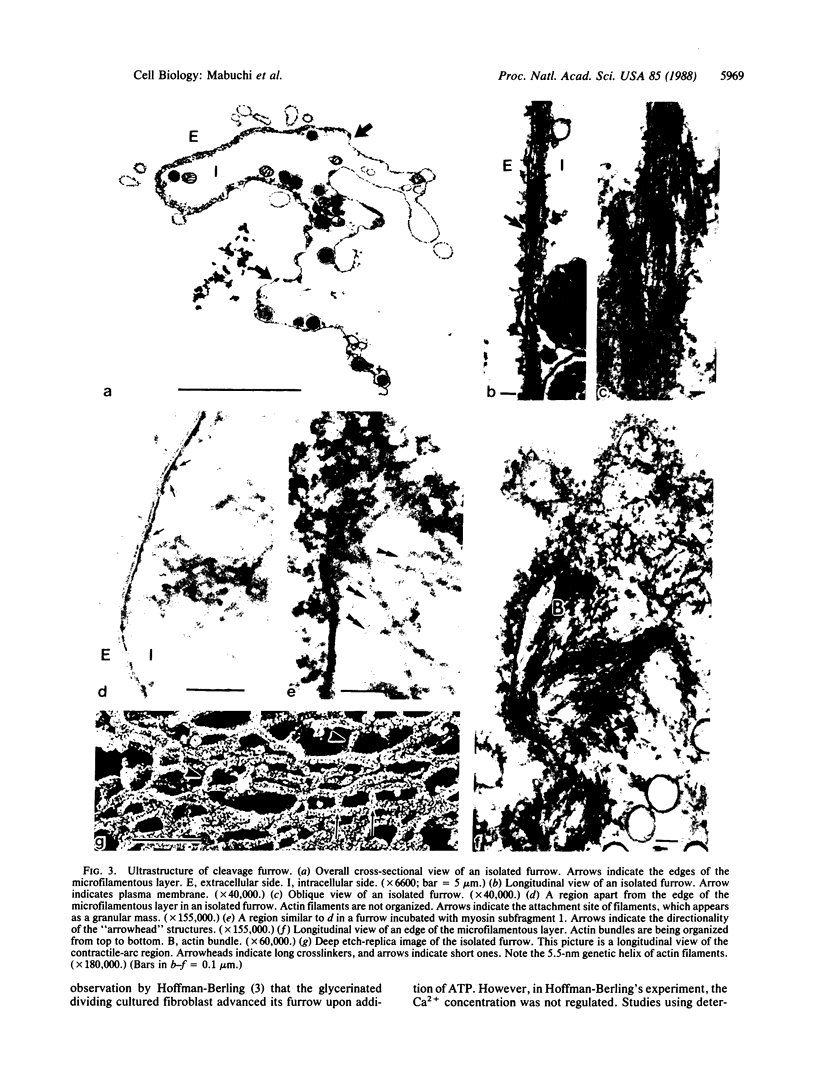
The cleavage-furrow region was isolated surgically from newt eggs at the early stage of the first cleavage. The isolated furrow contracted in the presence of ATP at a Ca2+ concentration of 10 or 100 nM, although the speed was less than that of the furrow in vivo. Cytochalasin B, cytochalasin D, phalloidin, p-chloromercuribenzoate, and N-ethyl-maleimide interfered with the contraction, but colchicine did not. The furrow contained bundles of actin filaments of opposite polarities oriented parallel to the long axis of the furrow; these bundles may be the main component of the contractile arc. From electron microscopic observation of thin sections of the furrow, it was suggested that the actin bundles of the contractile arc were organized from preexisting cortical filaments that were connected to the plasma membrane by granular materials at their barbed ends. Contractile-arc actin filaments were revealed to be crosslinked by thin strands by the rapid freezing/deep etching-replication technique. Two-dimensional polyacrylamide gel electrophoresis showed that several proteins found in the furrow cortex are absent from the cortical layer before the cleavage furrow is formed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aubin J. E., Weber K., Osborn M. Analysis of actin and microfilament-associated proteins in the mitotic spindle and cleavage furrow of PtK2 cells by immunofluorescence microscopy. A critical note. Exp Cell Res. 1979 Nov;124(1):93–109. doi: 10.1016/0014-4827(79)90260-x. [DOI] [PubMed] [Google Scholar]
- Barak L. S., Yocum R. R., Webb W. W. In vivo staining of cytoskeletal actin by autointernalization of nontoxic concentrations of nitrobenzoxadiazole-phallacidin. J Cell Biol. 1981 May;89(2):368–372. doi: 10.1083/jcb.89.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cande W. Z. A permeabilized cell model for studying cytokinesis using mammalian tissue culture cells. J Cell Biol. 1980 Nov;87(2 Pt 1):326–335. doi: 10.1083/jcb.87.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cande W. Z., McDonald K., Meeusen R. L. A permeabilized cell model for studying cell division: a comparison of anaphase chromosome movement and cleavage furrow constriction in lysed PtK1 cells. J Cell Biol. 1981 Mar;88(3):618–629. doi: 10.1083/jcb.88.3.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara K., Pollard T. D. Fluorescent antibody localization of myosin in the cytoplasm, cleavage furrow, and mitotic spindle of human cells. J Cell Biol. 1976 Dec;71(3):848–875. doi: 10.1083/jcb.71.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFMANN-BERLING H. Adenosintriphosphat als Betriebsstoff von Zellbewegungen. Biochim Biophys Acta. 1954 Jun;14(2):182–194. doi: 10.1016/0006-3002(54)90157-2. [DOI] [PubMed] [Google Scholar]
- Kiehart D. P., Mabuchi I., Inoué S. Evidence that myosin does not contribute to force production in chromosome movement. J Cell Biol. 1982 Jul;94(1):165–178. doi: 10.1083/jcb.94.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuchi I. A myosin-like protein in the cortical layer of cleaving starfish eggs. J Biochem. 1974 Jul;76(1):47–55. doi: 10.1093/oxfordjournals.jbchem.a130558. [DOI] [PubMed] [Google Scholar]
- Mabuchi I. Biochemical aspects of cytokinesis. Int Rev Cytol. 1986;101:175–213. doi: 10.1016/s0074-7696(08)60249-1. [DOI] [PubMed] [Google Scholar]
- Mabuchi I., Okuno M. The effect of myosin antibody on the division of starfish blastomeres. J Cell Biol. 1977 Jul;74(1):251–263. doi: 10.1083/jcb.74.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi V. T., Furthmayr H., Tomita M. The red cell membrane. Annu Rev Biochem. 1976;45:667–698. doi: 10.1146/annurev.bi.45.070176.003315. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Perry M. M., John H. A., Thomas N. S. Actin-like filaments in the cleavage furrow of newt egg. Exp Cell Res. 1971 Mar;65(1):249–253. doi: 10.1016/s0014-4827(71)80075-7. [DOI] [PubMed] [Google Scholar]
- Sanger J. M., Sanger J. W. Banding and polarity of actin filaments in interphase and cleaving cells. J Cell Biol. 1980 Aug;86(2):568–575. doi: 10.1083/jcb.86.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder T. E. The egg cortex in early development of sea urchins and starfish. Dev Biol (N Y 1985) 1986;2:59–100. doi: 10.1007/978-1-4613-2141-5_3. [DOI] [PubMed] [Google Scholar]
- Tilney L. G. The role of actin in nonmuscle cell motility. Soc Gen Physiol Ser. 1975;30:339–388. [PubMed] [Google Scholar]
- Tsukita S., Tsukita S., Ishikawa H. Cytoskeletal network underlying the human erythrocyte membrane. Thin-section electron microscopy. J Cell Biol. 1980 Jun;85(3):567–576. doi: 10.1083/jcb.85.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S., Tsukita S., Usukura J., Ishikawa H. ATP-dependent structural changes of the outer dynein arm in Tetrahymena cilia: a freeze-etch replica study. J Cell Biol. 1983 May;96(5):1480–1485. doi: 10.1083/jcb.96.5.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto Y., Hiramoto Y. Cleavage in a saponin model of the sea urchin egg. Cell Struct Funct. 1985 Mar;10(1):29–36. doi: 10.1247/csf.10.29. [DOI] [PubMed] [Google Scholar]
- Yumura S., Fukui Y. Reversible cyclic AMP-dependent change in distribution of myosin thick filaments in Dictyostelium. Nature. 1985 Mar 14;314(6007):194–196. doi: 10.1038/314194a0. [DOI] [PubMed] [Google Scholar]