Abstract
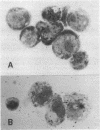
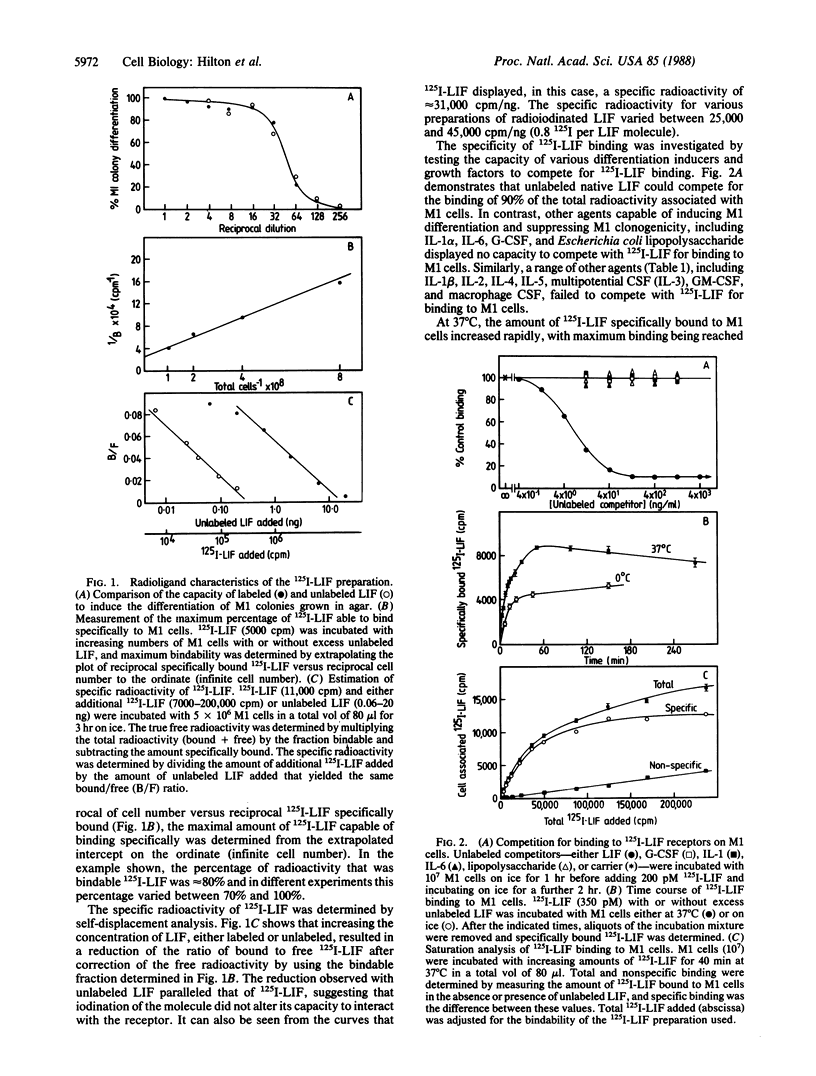
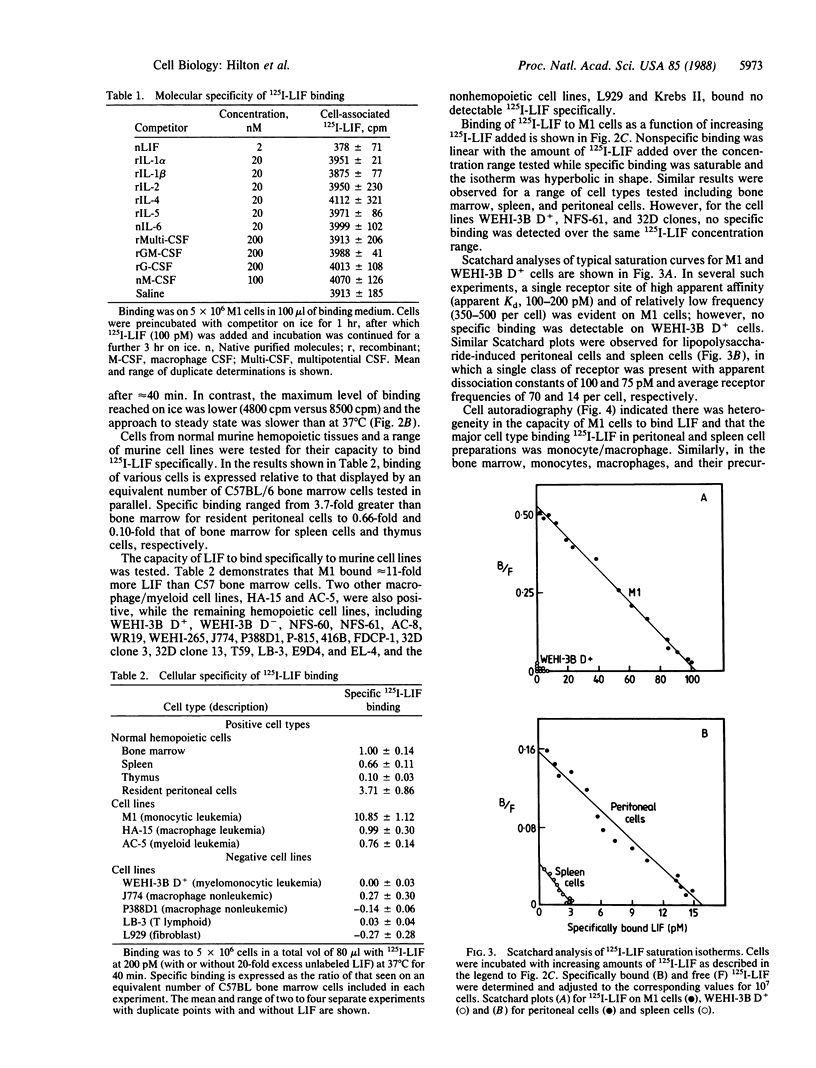
Leukemia inhibitory factor (LIF), a glycoprotein capable of suppressing the clonogenicity and inducing the differentiation of the murine myeloid leukemia cell line M1, was radioiodinated to a high specific radioactivity with retention of full biological activity. Binding of 125I-labeled LIF to M1 cells reached a steady state at 37 degrees C after approximately equal to 40 min and was in competition with unlabeled LIF but not granulocyte colony-stimulating factor or a range of other cytokines or differentiation-inducing agents. Specific binding was demonstrable to cells from a range of murine hemopoietic tissues including the bone marrow, the spleen, and the peritoneal cavity. Autoradiography revealed macrophages, monocytes, and their precursors to be the major cell types responsible for 125I-labeled LIF binding within these tissues. Receptors on M1 cells were of high affinity (apparent Kd, 100-200 pM) and few in number (300-500 per cell).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calvo J. C., Radicella J. P., Charreau E. H. Measurement of specific radioactivities in labelled hormones by self-displacement analysis. Biochem J. 1983 May 15;212(2):259–264. doi: 10.1042/bj2120259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras M. A., Bale W. F., Spar I. L. Iodine monochloride (IC1) iodination techniques. Methods Enzymol. 1983;92:277–292. [PubMed] [Google Scholar]
- Gearing D. P., Gough N. M., King J. A., Hilton D. J., Nicola N. A., Simpson R. J., Nice E. C., Kelso A., Metcalf D. Molecular cloning and expression of cDNA encoding a murine myeloid leukaemia inhibitory factor (LIF). EMBO J. 1987 Dec 20;6(13):3995–4002. doi: 10.1002/j.1460-2075.1987.tb02742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilbert L. J., Stanley E. R. Specific interaction of murine colony-stimulating factor with mononuclear phagocytic cells. J Cell Biol. 1980 Apr;85(1):153–159. doi: 10.1083/jcb.85.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozumi M. Established leukemia cell lines: their role in the understanding and control of leukemia proliferation. Crit Rev Oncol Hematol. 1985;3(3):235–277. doi: 10.1016/s1040-8428(85)80028-7. [DOI] [PubMed] [Google Scholar]
- Kawano M., Hirano T., Matsuda T., Taga T., Horii Y., Iwato K., Asaoku H., Tang B., Tanabe O., Tanaka H. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988 Mar 3;332(6159):83–85. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Regulation of cell-surface receptors for hematopoietic differentiation-inducing protein MGI-2 on normal and leukemic myeloid cells. Int J Cancer. 1987 Oct 15;40(4):532–539. doi: 10.1002/ijc.2910400417. [DOI] [PubMed] [Google Scholar]
- Metcalf D. Clonal analysis of the action of GM-CSF on the proliferation and differentiation of myelomonocytic leukemic cells. Int J Cancer. 1979 Nov 15;24(5):616–623. doi: 10.1002/ijc.2910240515. [DOI] [PubMed] [Google Scholar]
- Metcalf D. Clonal analysis of the response of HL60 human myeloid leukemia cells to biological regulators. Leuk Res. 1983;7(2):117–132. doi: 10.1016/0145-2126(83)90002-4. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Hilton D. J., Nicola N. A. Clonal analysis of the actions of the murine leukemia inhibitory factor on leukemic and normal murine hemopoietic cells. Leukemia. 1988 Apr;2(4):216–221. [PubMed] [Google Scholar]
- Metcalf D. How many cancers are reversible or suppressible? Pathology. 1983 Jan;15(1):1–3. doi: 10.3109/00313028309061392. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The Wellcome Foundation lecture, 1986. The molecular control of normal and leukaemic granulocytes and macrophages. Proc R Soc Lond B Biol Sci. 1987 May 22;230(1261):389–423. doi: 10.1098/rspb.1987.0026. [DOI] [PubMed] [Google Scholar]
- Nicola N. A. Granulocyte colony-stimulating factor and differentiation-induction in myeloid leukemic cells. Int J Cell Cloning. 1987 Jan;5(1):1–15. doi: 10.1002/stem.5530050102. [DOI] [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D. Binding of 125I-labeled granulocyte colony-stimulating factor to normal murine hemopoietic cells. J Cell Physiol. 1985 Aug;124(2):313–321. doi: 10.1002/jcp.1041240222. [DOI] [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D. Binding of the differentiation-inducer, granulocyte-colony-stimulating factor, to responsive but not unresponsive leukemic cell lines. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3765–3769. doi: 10.1073/pnas.81.12.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D. Biochemical properties of differentiation factors for murine myelomonocytic leukemic cells in organ conditioned media--separation from colony-stimulating factors. J Cell Physiol. 1981 Nov;109(2):253–264. doi: 10.1002/jcp.1041090208. [DOI] [PubMed] [Google Scholar]
- Old L. J. Tumor necrosis factor (TNF). Science. 1985 Nov 8;230(4726):630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- Onozaki K., Urawa H., Tamatani T., Iwamura Y., Hashimoto T., Baba T., Suzuki H., Yamada M., Yamamoto S., Oppenheim J. J. Synergistic interactions of interleukin 1, interferon-beta, and tumor necrosis factor in terminally differentiating a mouse myeloid leukemic cell line (M1). Evidence that interferon-beta is an autocrine differentiating factor. J Immunol. 1988 Jan 1;140(1):112–119. [PubMed] [Google Scholar]
- Oppenheim J. J., Matsushima K., Yoshimura T., Leonard E. J. The activities of cytokines are pleiotropic and interdependent. Immunol Lett. 1987 Dec;16(3-4):179–183. doi: 10.1016/0165-2478(87)90145-3. [DOI] [PubMed] [Google Scholar]
- Sachs L. Control of normal cell differentiation and the phenotypic reversion of malignancy in myeloid leukaemia. Nature. 1978 Aug 10;274(5671):535–539. doi: 10.1038/274535a0. [DOI] [PubMed] [Google Scholar]
- Tomida M., Yamamoto-Yamaguchi Y., Hozumi M. Characterization of a factor inducing differentiation of mouse myeloid leukemic cells purified from conditioned medium of mouse Ehrlich ascites tumor cells. FEBS Lett. 1984 Dec 10;178(2):291–296. doi: 10.1016/0014-5793(84)80619-5. [DOI] [PubMed] [Google Scholar]
- Tomida M., Yamamoto-Yamaguchi Y., Hozumi M., Okabe T., Takaku F. Induction by recombinant human granulocyte colony-stimulating factor of differentiation of mouse myeloid leukemic M1 cells. FEBS Lett. 1986 Oct 27;207(2):271–275. doi: 10.1016/0014-5793(86)81503-4. [DOI] [PubMed] [Google Scholar]
- Tomida M., Yamamoto-Yamaguchi Y., Hozumi M. Purification of a factor inducing differentiation of mouse myeloid leukemic M1 cells from conditioned medium of mouse fibroblast L929 cells. J Biol Chem. 1984 Sep 10;259(17):10978–10982. [PubMed] [Google Scholar]
- Tsuda H., Neckers L. M., Pluznik D. H. Colony stimulating factor-induced differentiation of murine M1 myeloid leukemia cells is permissive in early G1 phase. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4317–4321. doi: 10.1073/pnas.83.12.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker F., Burgess A. W. Specific binding of radioiodinated granulocyte-macrophage colony-stimulating factor to hemopoietic cells. EMBO J. 1985 Apr;4(4):933–939. doi: 10.1002/j.1460-2075.1985.tb03721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto-Yamaguchi Y., Tomida M., Hozumi M. Specific binding of a factor inducing differentiation to mouse myeloid leukemic M1 cells. Exp Cell Res. 1986 May;164(1):97–102. doi: 10.1016/0014-4827(86)90457-x. [DOI] [PubMed] [Google Scholar]