Abstract
Oxidative stress has been implicated in the pathogenesis of a number of diseases including Alzheimer’s disease (AD). The oxidative stress hypothesis of AD pathogenesis, in part, is based on β-amyloid peptide (Aβ)-induced oxidative stress in both in vitro and in vivo studies. Oxidative modification of the protein may induce structural changes in a protein that might lead to its functional impairment. A number of oxidatively modified brain proteins were identified using redox proteomics in AD, mild cognitive impairment (MCI) and Aβ models of AD, which support a role of Aβ in the alteration of a number of biochemical and cellular processes such as energy metabolism, protein degradation, synaptic function, neuritic growth, neurotransmission, cellular defense system, long term potentiation involved in formation of memory, etc. All the redox proteomics-identified brain proteins fit well with the appearance of the three histopathological hallmarks of AD, i.e., synapse loss, amyloid plaque formation and neurofibrillary tangle formation and suggest a direct or indirect association of the identified proteins with the pathological and/or biochemical alterations in AD. Further, Aβ models of AD strongly support the notion that oxidative stress induced by Aβ may be a driving force in AD pathogenesis. Studies conducted on arguably the earliest stage of AD, MCI, may elucidate the mechanism(s) leading to AD pathogenesis by identifying early markers of the disease, and to develop therapeutic strategies to slow or prevent the progression of AD. In this review, we summarized our findings of redox proteomics identified oxidatively modified proteins in AD, MCI and AD models.
Keywords: Oxidative stress, Alzheimer’s disease, Mild cognitive impairment, Protein oxidation, 4-Hydroxy 2-trans-nonenal, 3-Nitrotyrosine, Redox proteomics
Introduction
Oxidative stress has been implicated in the pathogenesis of a number of diseases including neurodegenerative disorders, cancer, ischemia, etc. [20]. Under physiological conditions, there is a balance between the pro-oxidant and anti-oxidant levels; however, certain environmental factors, stressors, or disease may cause an imbalance leading to increased production of reactive oxygen species (ROS) and reactive nitrogen species (RNS). ROS and RNS may react with biomolecules including proteins, lipids, carbohydrates, DNA and RNA [66] leading to oxidative damage of these biomolecules. Oxidative modification of biomolecules has been shown to lead to cellular dysfunction [2, 24, 97, 101, 102, 148]. The markers of oxidative stress that are commonly studied to determine the level of oxidative stress in biological samples include protein carbonyls, 3-nitrotyrosine (3-NT), thiobarbituric acid reactive substance (TBARS), free fatty acid release, iso- and neuroprostane formation, acrolein and 4-hydroxy-2-nonenal (HNE), advanced glycation end products for carbohydrates, and 8-OH-2′-deoxyguanosine and 8-OH-guanosine and other oxidized bases, and altered DNA repair mechanisms [14, 20, 35, 48, 49, 96, 102, 138, 147, 154, 155, 159, 180].
Protein carbonyls are formed by several different mechanisms including free radical induced scission of the peptide backbone, oxidation of certain amino acid sides chain, HNE or acrolein covalent modification of proteins [30], and advanced glycation end products [102, 115]. Experimentally, levels of protein carbonyls are determined following derivatization of the carbonyl moiety by 2,4-dinitrophenylhydrazine that forms a hydrazone product which can be detected spectrophotometrically or immunochemically [30, 87, 158]. Further, protein oxidation also can be indexed by measuring the levels of 3-NT, a product formed by reaction of tyrosine with breakdown products of peroxynitrite. Peroxynitrite is formed by reaction of nitric oxide and superoxide, and is highly reactive with a half-life of less than 1 s. The levels of protein-bound 3-NT can be determined using immunochemical methods [73, 162].
Lipid peroxidation is indexed by a number of markers including malondialdehyde (MDA), acrolein, and HNE [56, 107, 170]. The products of lipid peroxidation are highly reactive and can bind covalently to proteins by forming adducts with cysteine, lysine, or histidine residues [56] through Michael addition [30]. HNE accumulates in membranes at concentrations of 10 µM to 5 mM in response to oxidative insults [56] and invokes a wide range of biological activities, including inhibition of protein and DNA synthesis [138, 170], stimulation of phospholipases C and D [57], disruption of Ca2+ homeostasis, membrane damage, activation of stress signaling pathways and cell death [101, 165]. Since the cell membrane is rich in polyunsaturated fatty acid, increased levels of ROS can lead to increased production of toxic lipid peroxidation products, which can mediate oxidative stress-induced death in many cell types [164, 170]. Among all the body organs, brain is particularly vulnerable to oxidative damage because of high levels of polyunsaturated fatty acids in addition to its high oxygen consumption and high levels of redox transition metal ions and low levels of antioxidant enzymes. Previous studies have clearly documented increased levels of lipid peroxidation in neurodegenerative disease including AD [25, 113].
Oxidation of proteins can lead to unfolding or conformational changes in the protein, thereby exposing more hydrophobic residues to an aqueous environment, which may lead to loss of structural or functional activity including aggregation and accumulation of oxidized proteins as cytoplasmic inclusions, as reported in AD [150]. Oxidatively modified proteins may have effects on normal physiological processes by disrupting cellular functions such as alterations in protein expression and gene regulation, protein turnover, modulation of cell signaling, induction of apoptosis and necrosis, etc. [30]. In our laboratory, we used a redox proteomics approach to identify specific oxidatively modified brain proteins such as carbonylated, nitrated, HNE-bound, and glutathionylated brain proteins in neurodegenerative diseases including AD and models thereof [8, 9, 27, 32, 33, 35, 125, 132, 133, 155, 159].
Role of Abeta in oxidative stress
Oxidative stress in AD brain
Alzheimer’s disease is histopathologically characterized by the presence of extracellular senile plaque (SP), predominantly consisting of fibrillar amyloid-peptide (Aβ), intracellular neurofibrillary tangles (NFTs), composed of hyperphosphorylated tau protein, and loss of synapses in the selected regions of the brain. However, the pathogenesis of AD largely remains unknown. Mutation of presenilin-1 (PS-1), presenilin-2 (PS-2), and APP genes has been reported to cause inherited AD [83, 153]. In addition, other genes like apolipoprotein E 4 (APOE) gene, endothelial nitric oxide synthase-3 gene, and the alpha-2-macroglobulin gene have been associated with AD [88]. Further, a number of hypotheses were proposed for AD mechanisms, which include: the amyloid cascade, excitotoxicity, oxidative stress, and infammation hypothesis, and all of these hypotheses are based, to some extent, on the role of Aβ [21, 68].
Oxidative stress in AD brain is manifested by decreased levels of antioxidant enzymes and also by increased protein oxidation (including protein carbonyls and 3-NT formation), lipid peroxidation, DNA oxidation, advanced glycation end products, and ROS formation, among other indices [21, 85, 96, 102, 103].
Oxidative stress in MCI brain
Mild cognitive impairment is considered as a boundary or transition stage between normal aging and dementia. MCI is characterized by loss of current memory without dementia or significant impairment of other cognitive functions and with no loss of daily activities [114, 131]. Based on memory impairment, MCI is grouped into two broad groups, i.e., amnestic MCI and non-amnestic MCI. A large number of amnestic MCI subjects have been reported to convert to AD; however, there are cases where an amnestic MCI case has been reported to revert to normal stage. A number of MCI subjects show neuropathological hallmarks similar to AD, such as temporal lobe atrophy, while others showed low CSF-β amyloid levels [38]. In addition, MCI also shows some genetic similarities to AD such as mutations in APOE4 [118, 136].
In MCI patients, plasma levels of non-enzymatic antioxidants and activity of antioxidant enzymes appeared to be decreased when compared to those of controls [65, 140, 160] with no change in the levels of antioxidant protein levels [65]. Further, studies showed increased levels of oxidative damage in MCI indexed by elevated levels of markers such as 8-hydroxy-2-deoxy-guanosine (8-OhdG), 2,6-diamino-4-hydroxy-5-formamidopyrimidine (fapyguanine), 8-hydroxyadenine, 4,6-diamino-5-formamidopyrimidine (fapyadenine) and 5-hydroxycytosine, isoprostanes, HNE, protein carbonyls, 3-NT, etc. [27, 28, 36, 79, 95, 116, 163, 177], which were indicative of oxidative stress in MCI [27, 29, 52, 79] and further supporting the notion that oxidative stress could be an early event in the progression of MCI to AD. Based on the oxidative stress and histopathological similarities, and also involvement of similar genes as risk factors for AD and MCI, studies of MCI may provide clues about AD pathogenesis, progression of AD, and to the development of therapeutics to treat or delay this dementing disorder.
Aβ, a key player of oxidative stress
A 40–42 amino acid peptide, Aβ, is generated by proteolytic cleavage of amyloid precursor protein (APP), a transmembrane protein, by the action of beta- and gamma-secretases. Mutations in APP, presenilin-1 (PS-1) or presenilin-2 (PS-2) have been shown to lead to increased production of Aβ(1–42) and to early onset of AD [144]. Both Aβ (1–40) and (1–42) were found in AD brain and a number of in vitro and in vivo studies showed that Aβ(1–42), a primary component of SP, is more toxic than Aβ (1–40) [8, 10, 11, 17, 111, 112]. In addition, small oligomers of Aβ were suggested to be actual toxic species rather than fibrillar Aβ [51, 54, 84, 119, 171–173]. Many studies performed in our laboratory, focused attention on the neurotoxic mechanisms associated with increased levels of Aβ(1–42), which is the major component of the senile plaques—one of the characteristic features of AD neuropathology. The amyloid β-cascade hypothesis, suitably updated, postulates that Aβ is likely central to the pathogenesis of AD [15, 68]. The mechanisms involved in Aβ -mediated neurotoxicity are unknown, but there is evidence suggesting that oxidative stress plays a key role [14, 21, 68, 102]. Growing attention has focused on oxidative mechanisms of Aβ toxicity as well as the search for novel neuroprotective agents. The ability of toxic Abeta peptides to induce protein oxidation and to inhibit the activity of oxidation-sensitive enzymes is consistent with the hypothesis that Abeta can induce severe oxidative damage. Research from our laboratory and others showed that among the 42 amino acids that were found in Aβ(1–42), methionine located at position 35 is critical for Aβ associated neurotoxicity [18, 41, 45, 75, 76, 117]. The assignment of the critical function to Met35 is based on its ability to undergo a 1-electron oxidation to form a sulfuranyl free radical, which can then undergo free radical chain reaction with allylic H-atoms on unsaturated acyl chains of lipids, thereby greatly increasing free-radical mediated lipid peroxidation and protein oxidation and providing a mechanism for formation of HNE. The substitution of Ile31 by proline, a helix breaking amino acid, i.e., AβI31P, did not cause any protein oxidation or neurotoxicity in neuronal cell culture [76]; this could be due to disruption of the interaction of the carboxyl oxygen of Pro31 with the S-atom of Met35. This interaction is proposed to facilitate the 1-electron oxidation of Met [18]. Studies conducted using computer modeling suggested that the β-sheet conformation of this Aβ peptide contains the sulfur-centered radical cation of Met35, which augment the generation of a α-carbon centered radical on the Gly33 residue, which then leads to the formation of a hydrophobic environment that is ideal for lipid peroxidation in the lipid bilayer [13, 77]. Previous study from our laboratory showed that the presence of VitE blocks Aβ induced neurotoxicity in neural cell culture, consistent with the notion that oxidative stress could be one of the key process in the progression of the AD pathogenesis [178].
The amounts of Aβ and SP were reported to be lower in MCI compared to AD [169]. In a model of AD, a direct correlation of the amount of Aβ to oxidative stress was demonstrated [3]. Therefore, increased oxidative stress caused by Aβ may lead to increased oxidative modification of proteins and lipids, leading to impaired cellular function and cell death, and consequently to cognitive impartment and AD-like pathology. In this review, we have summarized our findings of the oxidatively modified protein in MCI, AD and animal models of AD to gain insight about the role of oxidative stress in the pathogenesis and progression of AD.
Redox proteomics
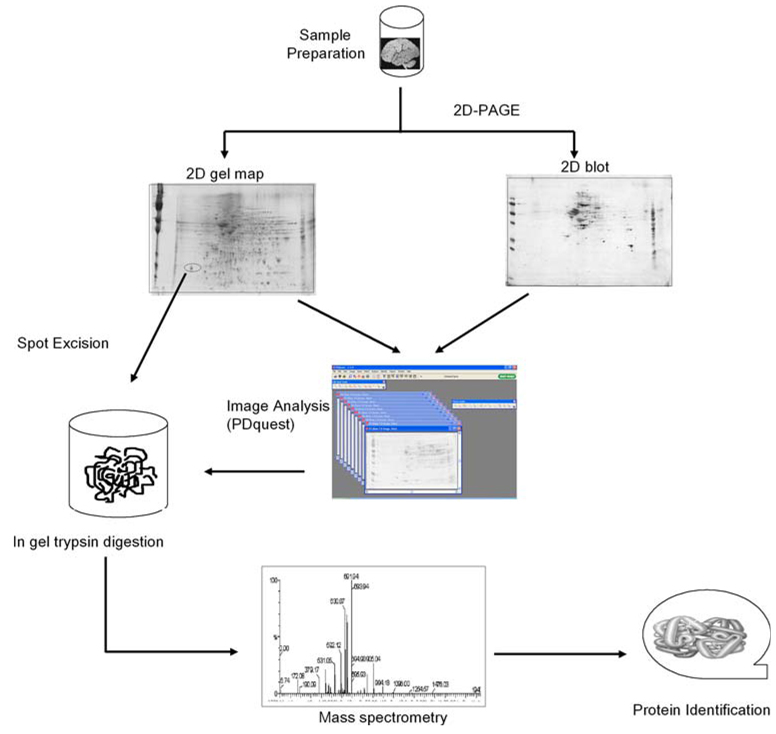
Redox proteomics techniques usually involve the coupling of two-dimensional (2D) gel electrophoresis techniques with mass spectrometry (MS) and 2D-Western blotting. This technique allows the identification and quantification of oxidatively modified proteins and is much advanced over traditional methods in that it allows for overall sample analysis for the detection of post-translational protein modifications such as protein carbonyls, protein-bound HNE, protein-resident 3-NT and glutathionylation, etc. [7, 48, 80, 81]. A detailed description of the applications and limitations of proteomics to the identification of brain proteins is provided elsewhere [19, 27, 159]. Briefly, some of the limitations of redox proteomics technique include solubilization of membrane proteins [142], highly basic proteins, and inability to detect low-abundance proteins [19]. Use of charged detergents like sodium dodecyl sulfate (SDS) in isoelectric focusing (IEF) may lead to artifacts, which should be avoided. If necessary, neutral detergents like NP-40 should be used. Figure 1 shows an experimental scheme of the overall approach followed in our laboratory for the identification of oxidatively modified proteins. In summary, brain samples are split into two equal aliquots and each separated by IEF followed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The gel is stained with Sypro Ruby and scanned at the appropriate wavelengths for total protein detection; the proteins from a second gel are transferred to a nitrocellulose membrane for 2D Western blot analysis, followed by probing with antibodies of the post-translational modification of interest and visualization with a colorimetric alkaline phosphatase detection system. Protein spots in gels and blots are aligned and matched utilizing PDQuest, a powerful image analysis software. Post-translational protein modifications of interest are calculated by normalization to total protein levels in the gel (i.e., the ratio of the spot intensity on the 2D blot to the spot intensity on the gel). This takes into account changes in protein levels that may influence the apparent post-translational protein modification.
Fig. 1.
Outline of redox proteomics approaches used in our laboratory for the identification of oxidatively modified brain proteins
Oxidatively modified proteins in AD brain
Alzheimer’s disease brain shows a regional difference in its pathology, and to large extent, AD-related pathology has been related to the amyloid load. For example, inferior parietal lobule and hippocampus have both high loads of amyloid and significantly increased oxidative damage and loss of cells, whereas cerebellum showed low levels of β-amyloid, no oxidative stress relative to controls, and essentially no neuronal loss [71, 155]. Our redox proteomics studies led to the identification of oxidatively modified brain proteins, indexed by increased carbonyl levels, protein-bound HNE, 3-NT, and glutathionylation, and the identification of these oxidatively modified proteins correlated with AD pathogenesis and strongly support a role of oxidative stress in AD pathogenesis. Using redox proteomics, we identified oxidatively modified proteins in the hippocampus (HP) and inferior parietal lobe (IPL) of the AD brain that are involved in different cellular functions such as energy metabolism, protein degradation, cellular defense, etc. (Table 1).
Table 1.
Oxidatively modified proteins identified in AD and MCI brain by redox proteomics
| Protein function | AD | MCI |
|---|---|---|
| Energy dysfunction/mitochondrial alterations |
α-enolase, TPI, PGM1, CK, γ-enolase, LDH GAPDH, aconitase, aldolase, VDAC, ATP synthase (α-chain) |
α-enolase, glucose regulated protein precursor, aldolase, malate dehydrogenase, pyruvate kinase, ATP synthase, LDH, phosphoglycerate kinase |
| Proteosomal dysfunction and synaptic dysfunction | UCHL-1, GAPDH | |
| Neuritic abnormalities | DRP-2, β-actin, α-tubulin | DRP-2, β-actin,Fascin 1, syntaxin binding protein 1 |
| Excitotoxicity | Glutamine synthetase, EAAT2 | Glutamine synthetase |
| Lipid abnormalities and cholinergic dysfunction | Neuropolypeptide h3 | Neuropolypeptide h3 |
| pH buffering and CO2 transport | CAII | CAII |
| Cell cycle; tau phosphorylation; Abeta production | Pin-1 | Pin-1 |
| Synaptic abnormalities and LTP | γ-SNAP | |
| Antioxidant defense/detoxification system | MnSOD, peroxiredoxin 6, GST, MRP-1, HSC 71 | peroxiredoxin 6, MRP3 protein, GSTM3, HSP70, carbonyl reductase |
| Cell signaling dysfunction | 14-3-3 gamma | |
| Protein synthesis alterations | Initiation factor alpha, elongation factor Tu |
Proteins that were oxidized in both AD and MCI brain are written in bold and are underlined
TPI triose phosphate isomerase, PGM1 phosphoglycerate mutase 1, CK creatine kinase, LDH lactate dehydrogenase, GAPDH glyceraldehyde 3-phosphate dehydrogenase, VDAC voltage dependent anion channel, UCHL-1 ubiqutin C-terminal hydrolase 1, DRP-2 dihydroxypyrimidine related protein-2, EAAT2 excitatory amino acid transporter 2, CAII carbonic anhydrase II, Pin-1 peptidyl prolys cis/trans isomerase, γ-SNAP gamma-soluble N-ethylmaleimide-sensitive factor (NSF) attachment proteins, MnSOD manganese superoxide dismutase, GST glutathione S-transferase, MRP-1 multidrug resistant protein 1, HSC 71 heat shock cognate, MRP3 protein multidrug resistant protein 3, GSTM3 glutathione S-transferase Mu 3, HSP70 heat shock protein 70
Functional classification of oxidatively modified brain proteins in AD
Energy dysfunction or mitochondrial alterations
The energy-related proteins, α-enolase, triose phosphate isomerase (TPI), phosphoglycerate mutase 1 (PGM1), creatine kinase (CK), γ-enolase, lactate dehydrogenase (LDH), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), aconitase, aldolase, voltage dependent anion channel (VDAC), and ATP synthase, were identified to be oxidatively modified and dysfunctional in AD brain, and these proteins normally are involved directly or indirectly in cellular energy production and related cellular functions [32, 33, 35, 128, 155, 159, 161]. Most of the proteins, such as α-enolase, TPI, PGM1, γ-enolase, LDH, GAPDH, and aldolase are related to the glycolytic pathway. This pathway is vitally important in the brain for energy production, since glucose is principle source of energy production in brain. The identification of these proteins as oxidatively modified and dysfunctional proteins in AD brain suggests an impaired energy metabolism pathway leading to less production of ATP. Decreased production of ATP may lead to loss of synapses and synaptic function ultimately leading to memory loss, since ATP levels are extremely important at nerve terminals for normal neuronal connections. Further, decreased levels of ATP may also lead to alterations in ion motive ATPases and glucose and glutamate transporters cholinergic defects, disturbances in cholesterol homeostasis, altered protein synthesis, and signal transduction, etc., eventually leading to cell death and consequently to cognitive decline in Alzheimer’s disease patients. Changes in cell potential, sequestration of Ca2+, and opening of voltage gated Ca2+ channels all would be expected to be altered by decreased levels of ATP secondary to altered energy metabolism in the brain. In addition, altered ATP levels could lead to exposure of phosphatidylserine to the outer membrane leaflet, a signal for apoptosis [34, 110]. Our proteomic studies are consistent with previous studies of others using positron emission tomography (PET), who showed reduced levels of cerebral metabolic rate of glucose utilization in the AD brain regions that are severely affected [174]. Further, VDAC and ATP synthase, related to mitochondria, were also found to be oxidatively modified [128, 161]. VDAC is a component of the mitochondrial permeability transition pore (MPTP), which plays an important role in importing and exporting various metabolites, including ATP, into the mitochondria. Being a part of MPTP, oxidation and functional impairment of this protein may lead to cell death via apoptosis pathways [100, 145]. Marin et al. showed that VDAC plays a role in Aβ-mediated neurotoxicity [100].
Among all the energy related proteins that were identified to be oxidatively modified, enolase appears to be more susceptible to oxidative modification, since it is the only protein that showed increased levels of protein carbonyls, enolase-bound HNE, and 3-NT in both hippocampus and IPL. However, it is not clear why enolase is more susceptible to all the three types of modifications [32, 33, 35, 128, 159, 161]. There are many diseases that are linked to enolase-dependent pathways, especially autoimmune and neurodegenerative disorders [122, 123], and initial studies suggest enolase is oxidatively modified in brain from subjects with familial AD [22].
Proteosomal dysfunction and synaptic dysfunction
UCHL-1 is a member of ubiquitin-proteasome pathway and plays an important role in protein degradation, thereby preventing the aggregation of damaged proteins within the cell. The identification of UCHL-1 as an oxidized and dysfunctional protein in AD brain [32, 39, 40, 159] suggests a dysfunctional ubiquitin-proteasome pathway and an increased accumulation of damaged and aggregated proteins and excess protein ubiquitinylation that may ultimately lead to synaptic degeneration in the AD brain [32, 78, 155]. Increased levels of protein aggregates have been reported in AD brain [78]. Further Gong et al. (2006) showed that the presence of UCHL-1 can recover synaptic function and contextual memory formation from Aβ-induced oxidation [63], indicating that UCH L-1 like enolase, may have more than one function. Taken together, oxidation of UCHL-1 may lead to altered protein degradation, synaptic function and memory in AD, and is consistent with known elevated ubiquitinylated proteins, proteosomal dysfunction, and protein accumulation in AD brain.
Neuritic abnormalities
DRP-2, β-actin, α-tubulin were identified to be oxidatively modified proteins in AD brain [32, 35, 128, 155, 159]. These proteins are crucial for maintaining the structure of brain cells, neuronal repair and also for proper neuronal connections that play a key role in the learning and memory process. Identification of these proteins as oxidatively modified suggests a role of oxidative modification in the loss of interneuronal connections, short dendritic length, impaired axonal transportation, and loss of structural integrity that might ultimately lead to neuronal death and AD-like pathology [6, 42, 149]. Further, DRP-2 has been reported to be down regulated in Down’s syndrome and AD patients [99].
Excitotoxicity
Glutamate is an excitatory neurotransmitter that, after its release in the synaptic cleft and performance of its function, is taken up by glia cells via excitatory amino acid neurotransmitter transporter receptor (EAAT2), where Glu is converted to glutamine by glutamine synthethase (GS). Any impairment in the function of EAAT2 and GS may lead to increased levels of glutamate on the external surface of neurons, which may lead to over-stimulation of neurons, e.g., excitotoxicity and Ca2+ entry leading to neuronal cell death [82]. Previous studies showed decreased activity of GS in AD brain [23, 71]. An in vitro study showed that Aβ(1–42) can led to HNE modification of EAAT2 [85]. The identification of both EAAT2 (by immunochemical method [85]) and GS (by proteomics) as oxidatively modified and dysfunctional proteins in AD brain is consistent with synaptic loss and neurodegeneration in AD [23, 32, 71, 128].
Lipid abnormalities and cholinergic dysfunction
Neuropolypeptide h3, also known as RAF kinase inhibitor (RKIP), phosphatidylethanolamine binding protein (PEBP), and hippocampal cholinergic neurostimulating protein (HCNP), is important for the production of choline acetyltransferase, an enzyme that play an important role in acetylcholine production, and also important in maintaining phospholipid asymmetry. Hence, decreased activity of this enzyme may lead to reduced levels of the neurotransmitter, acetylcholine, causing poor neurotransmission [120]. The oxidation and dysfunction of the neuropolypeptide h3 correlate well with the loss of cholinergic neurons in AD brain [44, 50, 129, 175]. Since neuropolypeptide h3 is also PEBP, it is possible that lipid asymmetry is also affected by oxidized PEBP, and studies from our laboratory showed that lipid asymmetry is lost in synaptic membranes treated with Aβ(1–42) or HNE [34, 110]. Both loss of lipid asymmetry and loss of cholinergic neurons have been reported in AD brain [5, 47, 61].
pH buffering and CO2 transport
Carbonic anhydrase II (CAII) is an enzyme that catalyzes the hydration of CO2 to HCO3− and regulates the transport of CO2 and HCO3−. The main function of CAII is maintenance of cellular pH, electrolytic and water balance [146]. Oxidative modification and impairment of CAII function may lead to altered cellular pH that may lead to altered enzyme activities, and/or impairment of the mitochondrial proton gradient for ATP synthesis. In addition, altered pH may also lead to increased aggregation of proteins as seen in AD [106, 155].
Cell cycle; tau phosphorylation; Abeta production
Peptidyl-prolyl cis/trans isomerase 1 (Pin-1) regulates biological functions of proteins involved in protein assembly, folding, intracellular transport, intracellular signaling, transcription, cell cycle progression and apoptosis [26, 64, 98]. Pin1 regulates the function of both APP and tau proteins in addition to maintenance of cell cycle [67, 93, 98, 124]. Pin1 regulates the phosphorylation of tau protein via its action on both kinases and phosphatases [26, 181]. Studies showed that Pin1 could bind to APP and regulate the production of Aβ [26, 124]. Hence, oxidation and reduced activity of Pin1 in AD brain will favor hyperphosphorylation of tau protein by dysregulation of kinases, such as GSK-3β, CDK5, etc., and by inhibiting the action of phosphatases, such as PP2A. Hyperphosphorylation of tau leads to neurofibrillary tangle formation, which kills neurons [26, 72, 154, 159]. This notion is supported by the study that showed that Pin1 protects neurons against age-related neurodegeneration [93]. Further, Pin1 was reported to co-localize with phosphorylated tau in AD brain, and showed an inverse relationship to the levels of tau [72, 137]. Taken together, oxidation of Pin1 could be related to major pathological hallmarks of AD: senile plaques, neurofibrillary tangles and synapse loss.
Synaptic abnormalities and LTP
Intracellular membrane fusion and vesicular trafficking are important for the proper function of the synapse. These functions are regulated by soluble N-ethylmaleimide-sensitive factor (NSF) attachment proteins. NSF proteins regulate neurotransmitter release, hormone secretion, mitochondrial organization, and vesicular transport, and thereby play an important role in learning and memory. γ-Soluble NSF attachment protein (γ-SNAP) is particularly involved in long-term potentiation, a process crucial for learning and memory [94]. Identification of γ-SNAP as one of the oxidized proteins in AD brain suggests impaired neurotransmission, and LTP learning and memory, and these alterations have a clear implications in AD symptoms [151, 155].
Antioxidant defense/detoxification system
Manganese superoxide dismutase (MnSOD), glutathione S-transferase (GSTM3), multidrug resistant proteins 1 (MRP1), multidrug resistant proteins 3 (MRP3), and peroxiredoxin 6 (PR VI) are found as oxidatively modified in AD brain [128, 156]. Studies from our laboratory clearly showed that oxidation of most proteins lead to functional impairment, and this has been clearly documented in AD brain [27, 71, 85, 128, 133, 159, 161]. Since these proteins above are involved in cellular defense, loss of functional activity of antioxidant proteins may lead to increased levels of oxidants and consequently to oxidative stress that may lead to AD-like pathology and neuronal death. GST is a detoxification enzyme that catalyses the conjugation of toxins (e.g., xenobiotics or HNE) with glutathione. The glutathione-conjugated toxic product is effluxed out from the cell by MRP [139, 156, 166]. Hence, loss of GST and MRP1 or 3 protein function will lead to accumulation of the toxicant levels in the cell resulting in increased production of ROS and toxic products with subsequent oxidative stress and ultimately to cell death. Previous studies reported increased levels of HNE in AD brain that support the loss of function of MRP and GST protein and their role in AD pathogenesis [156], consistent with proteomics identification of brain resident MRP3.
Another protein found to be oxidatively modified includes mitochondrial resident peroxiredoxin VI (PR VI). This enzyme normally catalyzes the transformation of H2O2 and the reduction of peroxynitrite. PR VI also is involved in detoxification, cell differentiation and apoptosis [135]. Alterations in peroxiredoxin, consequently, would lead to increased nitration of proteins and decreased detoxification effects that are detrimental to brain [134]. Loss of MnSOD activity leads to increase in production of superoxide leading to increased levels of ROS and consequently to a build up of oxidative stress in mitochondria. Mitochondrial function is known to be compromised in AD brain.
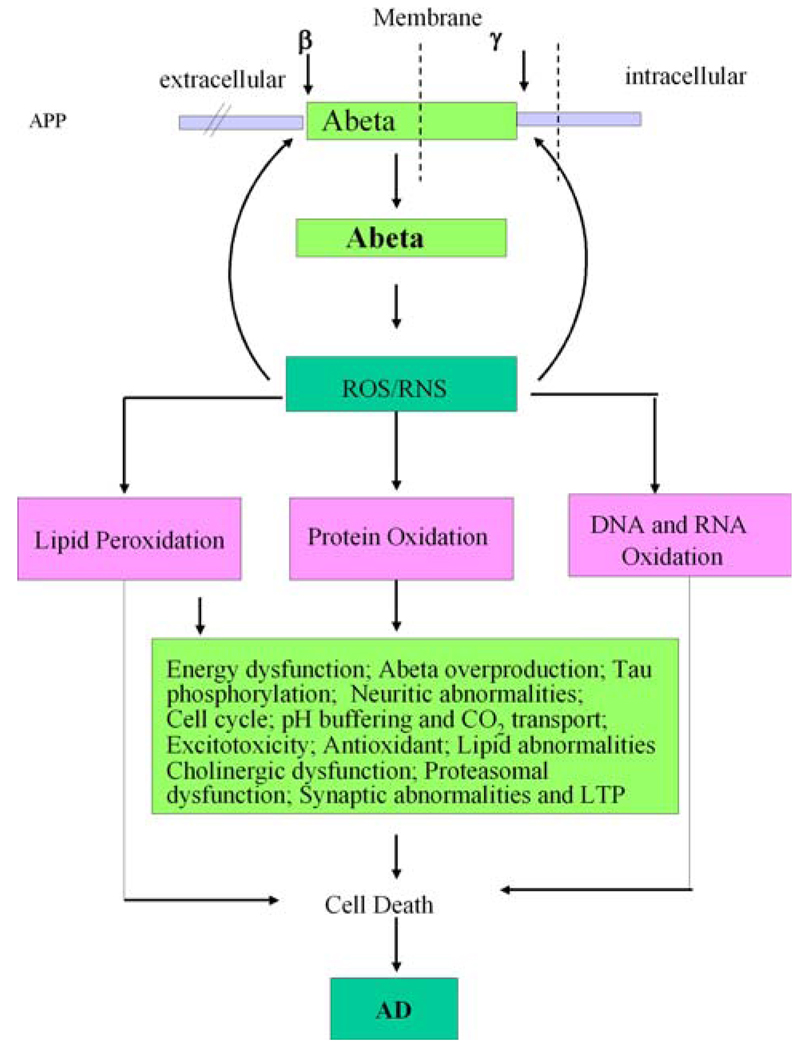
Taken together, our redox proteomics approach has allowed us to identify a number of the proteins that are excessively oxidized in AD brain and shows a direct or indirect association of the identified proteins with the pathological and/or biochemical alterations in AD. Further, our findings strongly support the notion that oxidative stress does play a role in AD pathogenesis. However, it is not clear if increased Aβ occurs before the onset of oxidative stress or the oxidative stress is the driving force for Aβ production. Both processes are interrelated. Our laboratory has hypothesized that oxidative stress induced by Aβ production is the driving force for neurodegeneration in AD and MCI (Fig. 2). In order to better understand the relation between Aβ and oxidative stress, we applied redox proteomics to identify oxidatively modified proteins in brain from subjects with MCI, arguably the earliest form of AD.
Fig. 2.
The diagram shows the formation and consequences of amyloid beta-peptide in AD pathogenesis. APP represents amyloid precursor protein. See text for details
Oxidatively modified brain proteins in MCI and comparison to AD
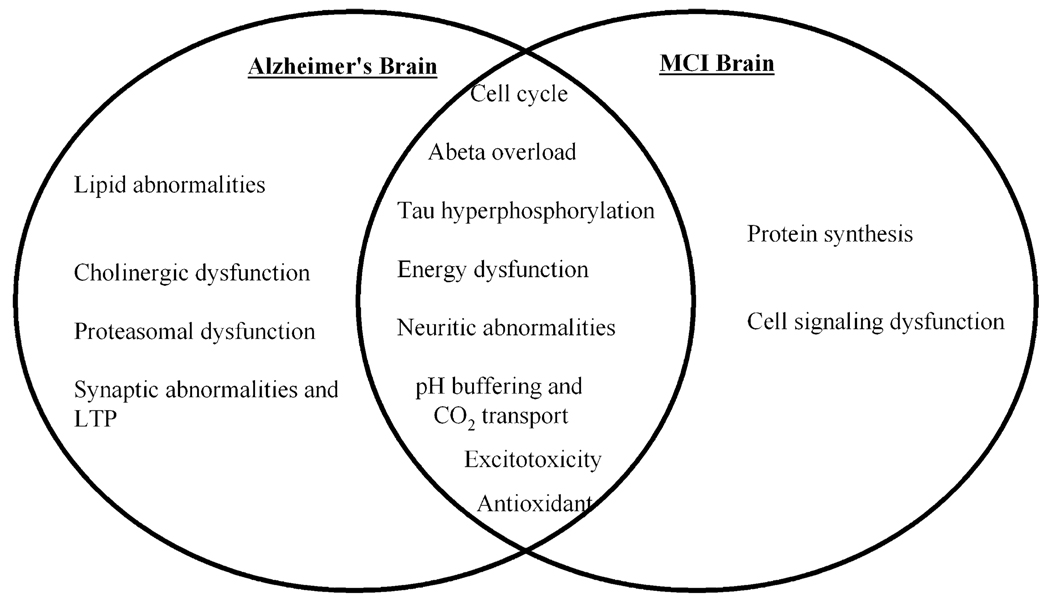
A large number of proteins were found to be oxidatively modified in MCI brain compared to controls (Table 1). These proteins were grouped based on their functional roles into various categories, such as energy dysfunction, neuritic abnormalities, excitotoxicity, lipid abnormalities and cholinergic dysfunction, pH buffering and CO2 transport, cell cycle and APP and tau protein regulation, antioxidant, cell signaling and protein synthesis [27, 138, 163]. Comparing the functional categories between AD and MCI, we observed an overlap in dysfunction of energy-related proteins, neuritic abnormalities, excitotoxicity, lipid abnormalities and cholinergic dysfunction, pH buffering and CO2 transport, cell cycle and APP and tau protein regulation, and antioxidant categories [27, 32, 33, 35, 128, 138, 159, 161, 163]. The appearance of oxidized brain proteins in common between AD and MCI suggests a possible involvement of critical pathways in the progression of MCI to AD (Fig. 3). However, all the proteins that were identified to be oxidatively modified in AD brain were not found in brain from subjects with MCI, and conversely, there were some brain proteins that were detected as oxidatively modified in MCI but were not identified as excessively oxidized in AD brain. This result raises question as what makes a protein oxidized early in MCI but not detected later in AD? However, this issue is currently unanswered and requires further studies. In addition to the protein categories of MCI brain that are similar to those in AD brain, and are therefore discussed above, there are two main functional categories of proteins found to be oxidatively modified in MCI that were not found in AD, which include proteins involved in cell signaling and protein synthesis (Table 1). These two categories are discussed here [27, 138, 163].
Fig. 3.
Venn diagram presents the functional categories of oxidatively modified brain proteins identified in AD, in MCI, and in common between the two disorders. See text for details
Two proteins, i.e., EF-Tu and eIF-α, are involved in protein synthesis and were found to be oxidatively modified in MCI brain [138]. Oxidation and functional impairment of EF-Tu protein may lead to impaired protein synthesis in cells, especially in mitochondria, leading to loss of differentiation in different cell types, energy impairment, and ultimately to cell death [90, 138] since EF-Tu aids protein translation in mitochondria, where it promotes protein chain elongation. eIF-α binds on aminoacyl-tRNA acceptor sites of ribosomes in a GTP-dependent manner during protein synthesis [130]. In addition, to its role in protein synthesis, eIF-α is reported to be involved in cytoskeletal organization, cell proliferation and senescence [168]. A number of studies have provided an indirect evidence of the impartment of brain protein synthesis in both AD and MCI [37, 53, 89]. Oxidation and impairment of eIF-α and EF-Tu may contribute to this loss of brain protein synthesis in MCI and AD and may lead to loss of cell homeostasis leading to cell death [138].
The second category of proteins in MCI brain discussed here includes cell signaling. One of the proteins that was identified to be oxidatively modified in MCI brain is 14-3-3 gamma, a member of the 14-3-3-protein family [138]. 14-3-3 proteins are involved in a number of cellular functions, including signal transduction, protein trafficking and metabolism. 14-3-3 gamma interacts with glycogen synthase kinase 3(GSK3β), and tau, hence, likely plays a role in the regulation of tau phosphorylation. Consequently, it is tempting to speculate that oxidative modification of this protein may lead to increased tangle formation in MCI and may contribute to progression of MCI to AD.
In vitro, ex vivo and in vivo model of Abeta
Animal models of Alzheimer’s disease
Animal models have been and continue to be important to understand the mechanisms of disease pathogenesis and progression and in developing therapeutic strategies for neurodegenerative diseases such as AD. Nevertheless, many animal models of AD do not closely resemble the pathology of human AD because they do not encompass all pathological features of AD, i.e., senile plaques and neuro-fibrillary tangles, and some do not demonstrate neuronal loss. Thus, it is important to study different animal models, each of which can provide important information to achieve a more comprehensive overview of the neurodegenerative mechanisms.
In this effort, the studies performed in our laboratory employed both invertebrate (Caenorhabditis elegans) models that allow the use of powerful genetic and high-through-put screening methods, and mammalian models that more closely mimic human AD. These combined strategies are expected to enable researchers to gain a greater understanding of disease pathways and develop new approaches to drug discovery.
Neuronal cell cultures
Previous studies from our laboratory reported that primary neuronal cell cultures treated with Aβ(1–42) showed increased production of reactive oxygen and nitrogen species (ROS/RNS), protein oxidation—as indexed by increased levels of carbonyls, HNE-bound proteins and formation of 3-NT—DNA and RNA oxidation and lipid peroxidation [12, 14–18, 20, 21, 25, 28]. Since protein oxidation has been shown to lead to conformational change in proteins, resulting in loss of protein function [152], we aimed to identify the specific target of Aβ-induced oxidative damage in neuronal cultures and to evaluate if this phenomenon could be prevented by pre-treatment with antioxidant compounds. For this purpose, we used proteomic techniques to identify neuronal proteins that were oxidized significantly by Aβ(1–42) treatment and also to test the protective effect against protein oxidation of γ-glutamylcysteine ethyl ester (GCEE), which has been shown to upregulate intracellular levels of GSH, and the GSH mimetic, D609. We found that 14-3-3ζ and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were significantly carbonylated. This finding is in agreement with results obtained on AD brain that reported dysfunction of both these proteins (Table 2) [35, 143, 161]. Interestingly, GCEE-induced upregulation of GSH or D609 protect both the proteins against oxidation after exposure to Aβ(1–42) [9, 12, 75, 157, 158].
Table 2.
Redox proteomics identification of oxidatively modified proteins in animal models of AD
| Oxidatively modified proteins | Function | |
|---|---|---|
| Cell culture plus Aβ(1–42) | 14-3-3ζ | Signal transduction |
| GAPDH, Pyruvate kinase, Malate dehydrogenase,glutamate dehydrogenase | Energy related | |
| Synaptosomes plus Aβ (1–42) | β and γ-actin, GFAP, DRP-2: H+-Transporting two-sector ATPase, syntaxin binding protein, elongation factor tu, Glutamate dehydrogenase |
Structural proteins, Energy-related and protein synthesis Excitotoxicity- and Neurotransmission- related |
| In vivo Aβ(1–42) models | Pyruvate dehydrogenase, GAPDH, Phosphoglycerate mutase-1 | Energy related |
| GS | Excitotoxicity-and Neurotransmission-related | |
| Tubulin chain 15/α, β-actin, β-Synuclein | Structural proteins | |
| 14-3-3ζ | Signal transduction | |
| Chaperonin 60 | Mitochondrial and proteosome | |
| C. elegans treated with Aβ(1–42) | Medium-chain and short-chain acyl-CoA dehydrogenase, Translation elongation factor EF-1, Malate dehydrogenase, Arginine kinase |
Protein synthesis- and Energy-related |
| Proteasome alpha and beta subunits | Proteoasome function | |
| Glutathione-S-tranferase (GST) | Antioxidant enzymes | |
| RACK1 ortholog, Adenosine kinase | Signaling proteins | |
| Lipid binding protein, Transketolase | Lipid metabolism | |
| Beta actin, Myosin regulatory light chain | Structural proteins | |
| Canine model | GST | Antioxidant enzymes |
| Glutamate dehydrogenase, GAPDH, α-enolase, Fascin actin bundling protein, neurofilament L protein |
Energy-, excitotoxic-, neurotransmission-, and structure- related |
GFAP glial fibrillary acidic protein, DRP-2 dihydroxypyrimidine related protein-2, GS Glutamine synthetase, GAPDH glyceraldehyde 3-phosphate dehydrogenase
14-3-3ζ protein is involved in kinase activation and chaperone activity, among other functions related to signaling pathways, in addition to key roles in cell cycle regulation and development [58]. As noted above, previous immunohistochemical studies have shown that 14-3-3 co-localizes with phospho-tau in NFTs in AD [86] and stimulates tau phosphorylation [141]. Interestingly, 14-3-3ζ was found to be a target of oxidative damage in AD human cortex as a result of Aβ-induced oxidative stress [143]. As previously shown in our laboratory, GAPDH was found to be oxidatively modified with decreased enzyme activity in AD brain [163], a finding replicated in AD fibroblasts [105].
Thus, our findings of increased oxidation of GAPDH and 14-3-3ζ in neuronal cell cultures treated with Aβ(1–42) demonstrated the central role played by this peptide which, also in vitro models of AD, lead to selective protein oxidation that closely resemble human pathology. In addition, since Aβ(1–42) is a mediator of oxidative stress, antioxidant therapies, such as upregulation of GSH or use of a GSH mimetic, may potentially be effective in slowing or ameliorating the neurodegenerative process as shown by protection of GAPDH and 14-3-3ζ from increased levels of oxidation induced by Aβ(1–42).
Synaptosomal system
Loss of synaptic terminals in AD brain demonstrates a higher correlation with decreased cognitive function than do cell death or plaque development that has led to the hypothesis that disappearance of synapses is a key event in early cognitive decline [104, 167]. The cellular location of initial amyloid-related damage is controversial, but a growing body of evidence suggests that intracellular accumulation of Aβ precedes plaque formation. However, the mechanisms of synapse loss in AD remain uncertain. Nevertheless, the damage in these regions is directly correlated with severity of dementia, oxidative stress, and deposition of Aβ(1–42). Therefore, the investigation of oxidative damage occurring in synaptosomes isolated from rodent brain has been shown to be a suitable experimental model to study the extent of Aβ-induced toxicity [85, 104]. Incubation of isolated synaptosomes with Aβ(1–42) leads to protein oxidation [14, 74, 126]; hence, we used a redox proteomic approach to identify proteins that are specific targets of oxidative damage induced by Aβ(1–42).
Our results contributed insights into the mechanisms leading to the synaptic alterations found in AD brain. As described above, we utilized two-dimensional gel electrophoresis, immunochemical detection of protein carbonyls, and mass spectrometry to identify proteins from synaptosomes isolated from Mongolian gerbils. Aβ(1–42) treatment leads to oxidative modification of β-actin, glial fibrillary acidic protein, dihydropyrimidinase-related protein-2, H+-transporting two-sector ATPase, syntaxin binding protein 1, glutamate dehydrogenase, γ-actin, and elongation factor Tu as indexed by increased carbonyl levels (Table 2). The listed proteins are involved in a wide variety of cellular functions including neuronal network formation and transmission, energy metabolism, excitotoxicity, protein synthesis, and cytoskeletal integrity. According to our previous findings in human samples [28] or animal models of AD [16], we suggest that oxidation and subsequent loss of function of the proteins identified would lead to excitotoxicity, the disruption of the synapse and neuronal communication, and inhibition of protein synthesis as shown in Table 1 and Table 2. Further, similar proteins were identified by proteomics and in AD and/or MCI brain to be oxidatively damaged. This experimental model provides a useful tool to establish a direct link between Abeta toxicity and synapse loss, consistent with the clinical impairment observed in AD subjects.
Intracerebral injection of Aβ(1–42) model (in vivo model)
Cholinergic hypofunction contributes to dementia-related cognitive decline and remains a target of therapeutic intervention for Alzheimer’s disease. The cholinergic hypothesis suggests that a dysfunction of acetylcholine-containing neurons in the basal forebrain contributes substantially to the cognitive decline observed in senescent and AD subjects [62, 176]. Many therapeutic strategies and drug development approaches for AD to date have been designed to prevent or attenuate the cholinergic dysfunction. However, disappointing clinical results have been reported with some muscarinic agonists in patients with Alzheimer’s disease [59]. A cholinergic hypofunction in Alzheimer’s disease can be linked to the formation of neurotoxic Aβ, which can further decrease the release of acetylcholine (a presynaptic effect) and impair the coupling of muscarinic receptors with G-proteins (a postsynaptic effect) [60]. This uncoupling leads to decreased signal transduction, impairments in cognition, a reduction in the levels of trophic-secreted amyloid precursor proteins, the generation of more neurotoxic β-amyloids and a further decrease in acetylcholine release. This ‘vicious cycle’ may be prevented, in principle, by M-selective agonists [60].
In order to investigate the impact of cholinergic dysfunction on AD neuropathology and clinical symptoms, a cholinergic animal model of AD was prepared by injecting into the nucleus basalis of the rat pre-aggregated Aβ(1–42). We and others have measured in this animal model, the levels of oxidative stress in different brain regions—hippocampus, cortex and nucleus basalis—and we found that hippocampus showed the highest level of oxidative damage—measured as the extent of protein oxidation—compared to that of other affected regions [11, 62]. In order to better understand the specific targets of oxidative damage, we used proteomic techniques to identify proteins that are specifically oxidized in different regions of rat brain injected with Aβ(1–42) into the nucleus basalis magnocellularis (NBM) compared with saline-injected control, 7 days post-injection. In the NBM, we found 14-3-3 ζ and chaperonin 60 to be significantly oxidized, while in the cortex, we identified GS and a mixture of tubulin β chain 15 and α-tubulin to be significantly oxidatively modified (Table 2). Finally, in the hippocampus, we identified β-synuclein, 14-3-3 ζ, GAPDH, pyruvate dehydrogenase, and PGM1 as specific targets of Aβ(1–42)-induced protein oxidation (Table 2). We further confirmed that oxidized proteins are involved in several cellular functions, including synaptic function and structure (β-synuclein), signal transduction (14-3-3ζ), energy metabolism (PGM1, pyruvate dehydrogenase, and GAPDH), and stress responses (HSP60) [11]. Interestingly, a number of proteins that were identified in this animal model were already reported to be oxidatively modified in AD brain, including PGM1, GAPDH, GS, 14-3-3 and tubulin [32, 35, 161]. These results showed not only selective protein damage in the NBM around the injection site, but also in other brain regions including the cortex and hippocampus. Moreover, dysfunction of these oxidatively modified proteins leads to excitotoxicty, oxidative stress, protein aggregation and cholinergic hypofunction all of which are characteristic pathological hallmarks of AD. In addition, these proteins have been identified to be oxidatively modified in AD brain [28, 32, 35, 163] and demonstrate the importance of ex vivo models of Aβ(1–42)-induced neurotoxicity.
Human Aβ(1–42)-expressing C. elegans
Transgenic C. elegans nematodes have been engineered to express potentially amyloidic human proteins. In this worm model, human Aβ(1–42) is expressed through a body-wall muscle myosin promoter and an Aβ minigene [92] in an attempt to generate significant extracellular levels of amyloid beta-peptide. This system mimics to some extent the situation postulated to exist in the human brain and might provide insights into Aβ-mediated neurotoxicity.
In our laboratory, we used this invertebrate model to gain insight into the toxic mechanisms associated to the oligomerization and deposition of Abeta which is considered to play a central role in AD pathogenesis. Consistent with this notion, we measured increased levels of protein oxidation in C. elegans expressing human Aβ(1–42). Substitution of the codon of the single Met residue of human Aβ(1–42) also suggested the involvement of methionine 35 of Aβ(1–42) in the mechanisms leading to increased levels of oxidative damage [179]. By using redox proteomics, we were able to identify which specific proteins underwent significant oxidative modifications thus leading to their dysfunction. C. elegans-containing Abeta, expressed for a specific period of time, showed 16 proteins that were oxidatively modified when compared to controls. These proteins included: ATP synthase α chain, glutamate dehydrogenase, proteasome α- and β-subunit, glutathione S-transferase (ε-class), medium and short-chain acyl-CoA dehydrogenase, arginine kinase, myosin regulatory light chain, actin, adenosine kinase, malate dehydrogenase, transketolase, translation elongation factor EF-1 γ, lipid binding protein, and RACK1 ortholog (Table 2). The impact of these results are multiple, since these identified proteins are involved in a variety of cellular functions including energy metabolism, protein degradation, cytoskeletal integrity, antioxidant system, signal transduction, and lipid metabolism. It is important to underline that many of these oxidized proteins, and their functional class also were found to be impaired in AD brain as described above.
Invertebrate models of neurodegenerative diseases, such as the C. elegans AD model, proved to be a viable approach for investigating the molecular processes underlying these diseases [91]. Parallel studies in different animal models and human samples support the relevance of this model and further confirm the pivotal role played by Aβ accumulation as one of the leading causes of AD neuropathology. However, the usefulness of the various invertebrate models of neurodegenerative diseases currently being studied will be further demonstrated with the identification of gene expression changes that can precede or overlap the Aβ toxic cascade.
Canine model
One of the characteristic features that correlates canine aging with human aging is the neuropathology associated with β-amyloid that naturally accumulates with age and is identical to human Aβ [46, 70]. In particular, aged beagles have the identical amino acid sequence of Aβ(1–42) as humans, and Aβ plaques form in aged beagle brain. Consequently, aged dogs can be used to study the events involved with the production and accumulation of Aβ without the introduction of genetic mutations that overexpress abnormal proteins. Plaques accumulate with a similar distribution and contain similar species of Aβ in dogs as in humans and thus, the proteins involved with Aβ production and effects are hypothesized as a common feature in dog and humans. In addition, dogs accumulate the longer, more toxic form of Aβ(1–42) prior to the deposition of the shorter, more soluble Aβ(1–40) in an age-dependent manner, which is consistent with human brain aging. Consistent with this neuropathologic feature, canines exhibit an age-dependent increase in protein carbonyl formation in brain [121] and similar results have been reported for aged rodents, humans, and also for subjects with AD [31, 71]. In addition, canine models absorb dietary nutrients in similar ways as humans, and therefore they can be used to test the protective role of antioxidant compounds against increased oxidative damage occurring in brain aging. Accordingly, previous studies performed in aged canines showed that long-term antioxidant administration reduces cognitive decline [69, 108] and leads to rapid improvements in complex learning ability in aging dogs [43, 109].
In order to evaluate the protective effects of an antioxidant-supplemented diet combined with behavioral enrichment (to enhance synapse formation), we used redox proteomics to identify brain proteins that showed decreased oxidation in aged beagles compared to control aged animals [121]. By following this approach, we were able to identify specific proteins that were protected from oxidative modification in aged beagles. These proteins were glutamate dehydrogenase (GDH), GAPDH, α-enolase, neurofilament triplet L protein, GST and fascin actin bundling protein (Table 2) [121]. These proteins are involved in energy metabolic pathways, maintenance and stabilization of cell structure and participate in cellular defense mechanisms.
At the same time, we were interested to see if the decreased oxidative damage also could be associated with the induction of protective enzymes. Interestingly, we observed an increased activity of key antioxidant enzymes such as glutathione-S-transferase (GST) and superoxide dismutase (SOD) in co-treated animals compared to controls. We suggest that the induction of these protective proteins contribute to the prevention of protein oxidative modification and their resultant dysfunction. Accordingly, protection of these specific proteins from oxidative modifications in aged beagles that received antioxidant supplementation resulted in an improvement of learning and memory [121].
The suitability of this animal model is further confirmed by the findings that enolase, GAPDH and GST were oxidized both in AD subjects and in aged beagle dogs. These results are consistent with a central role of Abeta-induced oxidative damage and offer a powerful model to test novel therapeutic approaches designed to delay the onset of AD pathology. We propose that pharmacological strategies should combine different approaches, such as behavioral enrichment with an antioxidant supplementation, since synergistic effects could result from these kind of approaches.
Future research
Redox proteomics is an emerging technique that allows the identification of oxidatively modified proteins in neurodegenerative diseases such as AD. Protein oxidation results from increased oxidative stress, which is considered to be crucially involved in the pathogenesis of several neurodegenerative disorders. Many studies have demonstrated the central role played by Aβ as a potent inducer or ROS production, as also shown by in vitro and in vivo animal models of AD. A dual relation exists between Aβ and the production of free radicals. Not only can the oxidative processes transform non-aggregated Aβ into aggregated Aβ in vitro [55], but also Aβ itself is a source of free radicals. Indeed, among the large array of pathological abnormalities seen in the AD brain, it seems increasingly likely that brain amyloidosis is the driving force underlying the involvement of oxidative stress in neurodegeneration and clinical impairments characteristic of AD. Further, the identification of a large number of oxidatively modified proteins in common among MCI, AD and AD animal models suggests that Aβ plays an important in AD pathogenesis (Table 3).
Table 3.
Oxidatively modified brain proteins found both in animal models of AD as well as in human samples (MCI or AD)
| Oxidatively modified proteins |
Human (MCI/AD) or animal model of AD |
|---|---|
| GAPDH | Human, cell cultures, in vivo Aβ model, canine |
| GS | Human, in vivo Aβ model |
| α-Enolase | Human, canine |
| DRP-2 | Human, synaptosomes |
| Glutamate dehydrogenase |
Human, cell cultures, synaptosomes, canine |
| GST | Human, C. elegans, canine |
| Actin | Human, synaptosomes, C. elegans, in vivo Aβ model |
| ATPsynthase | Human, C. elegans |
GST glutathione S-transferase, DRP-2 dihydroxypyrimidine related protein-2, GS Glutamine synthetase, GAPDH glyceraldehyde 3-phosphate dehydrogenase
Extensive oxidative damage observed in MCI brain regions [27–29, 79] suggest that oxidative stress may be an early event in the progression from normal brain to AD pathology. Based on these notions, it seems likely that increased production of ROS may act as important mediator of synaptic loss and eventually promote neurofibrillary tangles and senile plaque deposition [27, 35, 155, 156, 161]. Studies performed on MCI samples will enable researchers to better understand the mechanisms leading to AD by identifying early markers of the disease. Such information may be useful for developing therapeutic strategies with the hope that early treatment will slow or prevent the progression of AD. For this purpose, our laboratory is currently testing novel therapeutic approaches aimed to inhibit oxidative damage in MCI, AD, and models thereof [1, 4, 11, 28, 75, 127, 162]. We suggest that the use of novel, brain-accessible antioxidants or upregulation of endogenous antioxidants in AD therapy and prevention as either in a single compound or in a supplementary combination may be a safe and effective way of helping AD patients to improve the quality of their lives. However, targeting the Aβ-mediated oxidative stress reaction cascade, although an important goal of therapeutic approaches, still needs to be supported by combined strategies aimed to ameliorate other clinical symptoms that exacerbate AD progression.
Acknowledgments
This work was supported in part by grants from NIH to D.A.B. [AG-05119; AG-10836; AG-029839].
Contributor Information
Rukhsana Sultana, Department of Chemistry, Center of Membrane Sciences, University of Kentucky, Lexington, KY 40506-0055, USA; Sanders-Brown Center on Aging, University of Kentucky, Lexington, KY 40536, USA.
Marzia Perluigi, Department of Biochemical Sciences, University of Rome “La Sapienza”, Rome 00185, Italy.
D. Allan Butterfield, Department of Chemistry, Center of Membrane Sciences, University of Kentucky, Lexington, KY 40506-0055, USA dabcns@uky.edu; Sanders-Brown Center on Aging, University of Kentucky, Lexington, KY 40536, USA.
References
- 1.Abdul HM, Calabrese V, Calvani M, Butterfield DA. Acetyl-l-carnitine-induced up-regulation of heat shock proteins protects cortical neurons against amyloid-beta peptide 1–42-mediated oxidative stress and neurotoxicity: implications for Alzheimer’s disease. J Neurosci Res. 2006;84:398–408. doi: 10.1002/jnr.20877. doi: 10.1002/jnr.20877. [DOI] [PubMed] [Google Scholar]
- 2.Aksenova M, Butterfield DA, Zhang SX, Underwood M, Geddes JW. Increased protein oxidation and decreased creatine kinase BB expression and activity after spinal cord contusion injury. J Neurotrauma. 2002;19:491–502. doi: 10.1089/08977150252932433. doi: 10.1089/08977150252932433. [DOI] [PubMed] [Google Scholar]
- 3.Anantharaman M, Tangpong J, Keller JN, Murphy MP, Markesbery WR, Kiningham KK, St Clair DK. Beta-amyloid mediated nitration of manganese superoxide dismutase: implication for oxidative stress in a APPNLH/NLH X PS-1P264L/P264L double knock-in mouse model of Alzheimer’s disease. Am J Pathol. 2006;168:1608–1618. doi: 10.2353/ajpath.2006.051223. doi: 10.2353/ajpath.2006.051223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ansari MA, Joshi G, Huang Q, Opii WO, Abdul HM, Sultana R, Butterfield DA. In vivo administration of D609 leads to protection of subsequently isolated gerbil brain mitochondria subjected to in vitro oxidative stress induced by amyloid beta-peptide and other oxidative stressors: relevance to Alzheimer’s disease and other oxidative stress-related neurodegenerative disorders. Free Radic Biol Med. 2006;41:1694–1703. doi: 10.1016/j.freeradbiomed.2006.09.002. doi: 10.1016/j.freeradbiomed.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bader Lange ML, Cenini G, Piroddi M, Abdul HM, Sultana R, Galli F, Memo M, Butterfield DA. Loss of phospholipid asymmetry and elevated brain apoptotic protein levels in subjects with amnestic mild cognitive impairment and Alzheimer disease. Neurobiol Dis. 2008;29:456–464. doi: 10.1016/j.nbd.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beckman JS. Oxidative damage and tyrosine nitration from peroxynitrite. Chem Res Toxicol. 1996;9:836–844. doi: 10.1021/tx9501445. doi: 10.1021/tx9501445. [DOI] [PubMed] [Google Scholar]
- 7.Biroccio A, Del Boccio P, Panella M, Bernardini S, Di Ilio C, Gambi D, Stanzione P, Sacchetta P, Bernardi G, Martorana A, Federici G, Stefani A, Urbani A. Differential post-translational modifications of transthyretin in Alzheimer’s disease: a study of the cerebral spinal fluid. Proteomics. 2006;6:2305–2313. doi: 10.1002/pmic.200500285. doi: 10.1002/pmic.200500285. [DOI] [PubMed] [Google Scholar]
- 8.Boyd-Kimball D, Castegna A, Sultana R, Poon HF, Petroze R, Lynn BC, Klein JB, Butterfield DA. Proteomic identification of proteins oxidized by Abeta(1–42) in synaptosomes: implications for Alzheimer’s disease. Brain Res. 2005;1044:206–215. doi: 10.1016/j.brainres.2005.02.086. doi: 10.1016/j.brainres.2005.02.086. [DOI] [PubMed] [Google Scholar]
- 9.Boyd-Kimball D, Poon HF, Lynn BC, Cai J, Pierce WM, Jr, Klein JB, Ferguson J, Link CD, Butterfield DA. Proteomic identification of proteins specifically oxidized in Caenorhabditis elegans expressing human Abeta(1–42): implications for Alzheimer’s disease. Neurobiol Aging. 2006;27:1239–1249. doi: 10.1016/j.neurobiolaging.2005.07.001. doi: 10.1016/j.neurobiolaging.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Boyd-Kimball D, Sultana R, Mohmmad-Abdul H, Butterfield DA. Neurotoxicity and oxidative stress in D1M-substituted Alzheimer’s A beta(1–42): relevance to N-terminal methionine chemistry in small model peptides. Peptides. 2005;26:665–673. doi: 10.1016/j.peptides.2004.11.001. doi: 10.1016/j.peptides.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Boyd-Kimball D, Sultana R, Poon HF, Lynn BC, Casamenti F, Pepeu G, Klein JB, Butterfield DA. Proteomic identification of proteins specifically oxidized by intracerebral injection of amyloid beta-peptide (1–42) into rat brain: implications for Alzheimer’s disease. Neuroscience. 2005;132:313–324. doi: 10.1016/j.neuroscience.2004.12.022. doi: 10.1016/j.neuroscience.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 12.Boyd-Kimball D, Sultana R, Poon HF, Mohmmad-Abdul H, Lynn BC, Klein JB, Butterfield DA. Gamma-glutamylcysteine ethyl ester protection of proteins from Abeta(1–42)-mediated oxidative stress in neuronal cell culture: a proteomics approach. J Neurosci Res. 2005;79:707–713. doi: 10.1002/jnr.20393. doi: 10.1002/jnr.20393. [DOI] [PubMed] [Google Scholar]
- 13.Brunelle P, Rauk A. The radical model of Alzheimer’s disease: specific recognition of Gly29 and Gly33 by Met35 in a beta-sheet model of Abeta: an ONIOM study. J Alzheimers Dis. 2002;4:283–289. doi: 10.3233/jad-2002-4403. [DOI] [PubMed] [Google Scholar]
- 14.Butterfield DA. Amyloid beta-peptide (1–42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer’s disease brain. A review. Free Radic Res. 2002;36:1307–1313. doi: 10.1080/1071576021000049890. doi: 10.1080/1071576021000049890. [DOI] [PubMed] [Google Scholar]
- 15.Butterfield DA. beta-Amyloid-associated free radical oxidative stress and neurotoxicity: implications for Alzheimer’s disease. Chem Res Toxicol. 1997;10:495–506. doi: 10.1021/tx960130e. doi: 10.1021/tx960130e. [DOI] [PubMed] [Google Scholar]
- 16.Butterfield DA, Abdul HM, Newman S, Reed T. Redox proteomics in some age-related neurodegenerative disorders or models thereof. NeuroRx. 2006;3:344–357. doi: 10.1016/j.nurx.2006.05.003. doi: 10.1016/j.nurx.2006.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butterfield DA, Boyd-Kimball D. Amyloid beta-peptide(1–42) contributes to the oxidative stress and neurodegeneration found in Alzheimer disease brain. Brain Pathol. 2004;14:426–432. doi: 10.1111/j.1750-3639.2004.tb00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butterfield DA, Boyd-Kimball D. The critical role of methionine 35 in Alzheimer’s amyloid beta-peptide (1–42)-induced oxidative stress and neurotoxicity. Biochim Biophys Acta. 2005;1703:149–156. doi: 10.1016/j.bbapap.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 19.Butterfield DA, Boyd-Kimball D, Castegna A. Proteomics in Alzheimer’s disease: insights into potential mechanisms of neurodegeneration. J Neurochem. 2003;86:1313–1327. doi: 10.1046/j.1471-4159.2003.01948.x. doi: 10.1046/j.1471-4159.2003.01948.x. [DOI] [PubMed] [Google Scholar]
- 20.Butterfield DA, Castegna A, Lauderback CM, Drake J. Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer’s disease brain contribute to neuronal death. Neurobiol Aging. 2002;23:655–664. doi: 10.1016/s0197-4580(01)00340-2. doi: 10.1016/S0197-4580(01)00340-2. [DOI] [PubMed] [Google Scholar]
- 21.Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer’s disease brain: central role for amyloid beta-peptide. Trends Mol Med. 2001;7:548–554. doi: 10.1016/s1471-4914(01)02173-6. doi: 10.1016/S1471-4914(01)02173-6. [DOI] [PubMed] [Google Scholar]
- 22.Butterfield DA, Gnjec A, Poon HF, Castegna A, Pierce WM, Klein JB, Martins RN. Redox proteomics identification of oxidatively modified brain proteins in inherited Alzheimer’s disease: an initial assessment. J Alzheimers Dis. 2006;10:391–397. doi: 10.3233/jad-2006-10407. [DOI] [PubMed] [Google Scholar]
- 23.Butterfield DA, Hensley K, Cole P, Subramaniam R, Aksenov M, Aksenova M, Bummer PM, Haley BE, Carney JM. Oxidatively induced structural alteration of glutamine synthetase assessed by analysis of spin label incorporation kinetics: relevance to Alzheimer’s disease. J Neurochem. 1997;68:2451–2457. doi: 10.1046/j.1471-4159.1997.68062451.x. [DOI] [PubMed] [Google Scholar]
- 24.Butterfield DA, Kanski J. Brain protein oxidation in age-related neurodegenerative disorders that are associated with aggregated proteins. Mech Ageing Dev. 2001;122:945–962. doi: 10.1016/s0047-6374(01)00249-4. doi: 10.1016/S0047-6374(01)00249-4. [DOI] [PubMed] [Google Scholar]
- 25.Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic Biol Med. 2002;32:1050–1060. doi: 10.1016/s0891-5849(02)00794-3. doi: 10.1016/S0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- 26.Butterfield DA, Mohmmad-Abdul H, Opii W, Newman SF, Joshi G, Ansari MA, Sultana R. Role of Pin1 in Alzheimer’s disease. J Neurochem. 2006;98:1699–1706. doi: 10.1111/j.1471-4159.2006.03995.x. doi: 10.1111/j.1471-4159.2006.03995.x. [DOI] [PubMed] [Google Scholar]
- 27.Butterfield DA, Poon HF, St Clair D, Keller JN, Pierce WM, Klein JB, Markesbery WR. Redox proteomics identification of oxidatively modified hippocampal proteins in mild cognitive impairment: insights into the development of Alzheimer’s disease. Neurobiol Dis. 2006;22:223–232. doi: 10.1016/j.nbd.2005.11.002. doi: 10.1016/j.nbd.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Butterfield DA, Reed T, Newman SF, Sultana R. Roles of amyloid beta-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer’s disease and mild cognitive impairment. Free Radic Biol Med. 2007;43:658–677. doi: 10.1016/j.freeradbiomed.2007.05.037. doi: 10.1016/j.freeradbiomed.2007.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butterfield DA, Reed T, Perluigi M, De Marco C, Coccia R, Cini C, Sultana R. Elevated protein-bound levels of the lipid peroxidation product, 4-hydroxy-2-nonenal, in brain from persons with mild cognitive impairment. Neurosci Lett. 2006;397:170–173. doi: 10.1016/j.neulet.2005.12.017. doi: 10.1016/j.neulet.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 30.Butterfield DA, Stadtman ER. Protein Oxidation processes in aging brain. Adv Cell Aging Gerontol. 1997;2:161–191. doi: 10.1016/S1566-3124(08)60057-7. [Google Scholar]
- 31.Carney JM, Starke-Reed PE, Oliver CN, Landum RW, Cheng MS, Wu JF, Floyd RA. Reversal of age-related increase in brain protein oxidation, decrease in enzyme activity, and loss in temporal and spatial memory by chronic administration of the spin-trapping compound N-tert-butyl-alpha-phenylnitrone. Proc Natl Acad Sci USA. 1991;88:3633–3636. doi: 10.1073/pnas.88.9.3633. doi: 10.1073/pnas.88.9.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castegna A, Aksenov M, Aksenova M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, Markesbery WR, Butterfield DA. Proteomic identification of oxidatively modified proteins in Alzheimer’s disease brain. Part I: creatine kinase BB, glutamine synthase, and ubiquitin carboxy-terminal hydrolase L-1. Free Radic Biol Med. 2002;33:562–571. doi: 10.1016/s0891-5849(02)00914-0. doi: 10.1016/S0891-5849(02)00914-0. [DOI] [PubMed] [Google Scholar]
- 33.Castegna A, Aksenov M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, Markesbery WR, Butterfield DA. Proteomic identification of oxidatively modified proteins in Alzheimer’s disease brain. Part II: dihydropyrimidinase-related protein 2, alpha-enolase and heat shock cognate 71. J Neurochem. 2002;82:1524–1532. doi: 10.1046/j.1471-4159.2002.01103.x. doi: 10.1046/j.1471-4159.2002.01103.x. [DOI] [PubMed] [Google Scholar]
- 34.Castegna A, Lauderback CM, Mohmmad-Abdul H, Butterfield DA. Modulation of phospholipid asymmetry in synaptosomal membranes by the lipid peroxidation products, 4-hydroxynonenal and acrolein: implications for Alzheimer’s disease. Brain Res. 2004;1004:193–197. doi: 10.1016/j.brainres.2004.01.036. doi: 10.1016/j.brainres.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 35.Castegna A, Thongboonkerd V, Klein JB, Lynn B, Markesbery WR, Butterfield DA. Proteomic identification of nitrated proteins in Alzheimer’s disease brain. J Neurochem. 2003;85:1394–1401. doi: 10.1046/j.1471-4159.2003.01786.x. doi: 10.1046/j.1471-4159.2003.01786.x. [DOI] [PubMed] [Google Scholar]
- 36.Cenini G, Sultana R, Memo M, Butterfield DA. Elevated levels of pro-apoptotic p53 and its oxidative modification by the lipid peroxidation product, HNE, in brain from subjects with amnestic mild cognitive impairment and Alzheimer’s disease. J Cell Mol Med. 2008;12:987–994. doi: 10.1111/j.1582-4934.2008.00163.x. doi: 10.1111/j.1582-4934.2008.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang RC, Wong AK, Ng HK, Hugon J. Phosphorylation of eukaryotic initiation factor-2alpha (eIF2alpha) is associated with neuronal degeneration in Alzheimer’s disease. NeuroReport. 2002;13:2429–2432. doi: 10.1097/00001756-200212200-00011. doi: 10.1097/00001756-200212200-00011. [DOI] [PubMed] [Google Scholar]
- 38.Chertkow H, Bergman H, Schipper HM, Gauthier S, Bouchard R, Fontaine S, Clarfield AM. Assessment of suspected dementia. Can J Neurol Sci. 2001;28 Suppl 1:S28–S41. doi: 10.1017/s0317167100001189. [DOI] [PubMed] [Google Scholar]
- 39.Choi J, Levey AI, Weintraub ST, Rees HD, Gearing M, Chin LS, Li L. Oxidative modifications and down-regulation of ubiquitin carboxyl-terminal hydrolase L1 associated with idiopathic Parkinson’s and Alzheimer’s diseases. J Biol Chem. 2004;279:13256–13264. doi: 10.1074/jbc.M314124200. doi: 10.1074/jbc.M314124200. [DOI] [PubMed] [Google Scholar]
- 40.Choi J, Rees HD, Weintraub ST, Levey AI, Chin LS, Li L. Oxidative modifications and aggregation of Cu, Zn-superoxide dismutase associated with Alzheimer and Parkinson diseases. J Biol Chem. 2005;280:11648–11655. doi: 10.1074/jbc.M414327200. doi: 10.1074/jbc.M414327200. [DOI] [PubMed] [Google Scholar]
- 41.Clementi ME, Pezzotti M, Orsini F, Sampaolese B, Mezzogori D, Grassi C, Giardina B, Misiti F. Alzheimer’s amyloid beta-peptide (1–42) induces cell death in human neuroblastoma via bax/bcl-2 ratio increase: an intriguing role for methionine 35. Biochem Biophys Res Commun. 2006;342:206–213. doi: 10.1016/j.bbrc.2006.01.137. doi: 10.1016/j.bbrc.2006.01.137. [DOI] [PubMed] [Google Scholar]
- 42.Coleman PD, Flood DG. Neuron numbers and dendritic extent in normal aging and Alzheimer’s disease. Neurobiol Aging. 1987;8:521–545. doi: 10.1016/0197-4580(87)90127-8. doi: 10.1016/0197-4580(87)90127-8. [DOI] [PubMed] [Google Scholar]
- 43.Cotman CW, Head E, Muggenburg BA, Zicker S, Milgram NW. Brain aging in the canine: a diet enriched in antioxidants reduces cognitive dysfunction. Neurobiol Aging. 2002;23:809–818. doi: 10.1016/s0197-4580(02)00073-8. doi: 10.1016/S0197-4580(02)00073-8. [DOI] [PubMed] [Google Scholar]
- 44.Coyle JT, Price DL, DeLong MR. Alzheimer’s disease: a disorder of cortical cholinergic innervation. Science. 1983;219:1184–1190. doi: 10.1126/science.6338589. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- 45.Crouch PJ, Barnham KJ, Duce JA, Blake RE, Masters CL, Trounce IA. Copper-dependent inhibition of cytochrome c oxidase by Abeta(1–42) requires reduced methionine at residue 35 of the Abeta peptide. J Neurochem. 2006;99:226–236. doi: 10.1111/j.1471-4159.2006.04050.x. doi: 10.1111/j.1471-4159.2006.04050.x. [DOI] [PubMed] [Google Scholar]
- 46.Cummings BJ, Head E, Ruehl W, Milgram NW, Cotman CW. The canine as an animal model of human aging and dementia. Neurobiol Aging. 1996;17:259–268. doi: 10.1016/0197-4580(95)02060-8. doi: 10.1016/0197-4580(95)02060-8. [DOI] [PubMed] [Google Scholar]
- 47.Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc Natl Acad Sci USA. 2004;101:2070–2075. doi: 10.1073/pnas.0305799101. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dalle-Donne I, Scaloni A, Butterfield DA. Redox proteomics: from protein modifications to cellular dysfunction and diseases. Hoboken: Wiley; 2006. [DOI] [PubMed] [Google Scholar]
- 49.Dalle-Donne I, Scaloni A, Giustarini D, Cavarra E, Tell G, Lungarella G, Colombo R, Rossi R, Milzani A. Proteins as biomarkers of oxidative/nitrosative stress in diseases: the contribution of redox proteomics. Mass Spectrom Rev. 2005;24:55–99. doi: 10.1002/mas.20006. doi: 10.1002/mas.20006. [DOI] [PubMed] [Google Scholar]
- 50.Davies MJ, Fu S, Wang H, Dean RT. Stable markers of oxidant damage to proteins and their application in the study of human disease. Free Radic Biol Med. 1999;27:1151–1163. doi: 10.1016/s0891-5849(99)00206-3. doi: 10.1016/S0891-5849(99)00206-3. [DOI] [PubMed] [Google Scholar]
- 51.Demuro A, Mina E, Kayed R, Milton SC, Parker I, Glabe CG. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem. 2005;280:17294–17300. doi: 10.1074/jbc.M500997200. doi: 10.1074/jbc.M500997200. [DOI] [PubMed] [Google Scholar]
- 52.Ding Q, Markesbery WR, Cecarini V, Keller JN. Decreased RNA, and increased RNA oxidation, in ribosomes from early Alzheimer’s disease. Neurochem Res. 2006;31:705–710. doi: 10.1007/s11064-006-9071-5. doi: 10.1007/s11064-006-9071-5. [DOI] [PubMed] [Google Scholar]
- 53.Ding Q, Markesbery WR, Chen Q, Li F, Keller JN. Ribosome dysfunction is an early event in Alzheimer’s disease. J Neurosci. 2005;25:9171–9175. doi: 10.1523/JNEUROSCI.3040-05.2005. doi: 10.1523/JNEUROSCI.3040-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Drake J, Link CD, Butterfield DA. Oxidative stress precedes fibrillar deposition of Alzheimer’s disease amyloid beta-peptide (1–42) in a transgenic Caenorhabditis elegans model. Neurobiol Aging. 2003;24:415–420. doi: 10.1016/s0197-4580(02)00225-7. doi: 10.1016/S0197-4580(02)00225-7. [DOI] [PubMed] [Google Scholar]
- 55.Dyrks T, Dyrks E, Hartmann T, Masters C, Beyreuther K. Amyloidogenicity of beta A4 and beta A4-bearing amyloid protein precursor fragments by metal-catalyzed oxidation. J Biol Chem. 1992;267:18210–18217. [PubMed] [Google Scholar]
- 56.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 57.Farooqui AA, Horrocks LA. Phospholipase A2-generated lipid mediators in the brain: the good, the bad, and the ugly. Neuroscientist. 2006;12:245–260. doi: 10.1177/1073858405285923. doi: 10.1177/1073858405285923. [DOI] [PubMed] [Google Scholar]
- 58.Ferl RJ. 14-3-3 proteins and signal transduction. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:49–73. doi: 10.1146/annurev.arplant.47.1.49. doi: 10.1146/annurev.arplant.47.1.49. [DOI] [PubMed] [Google Scholar]
- 59.Fisher A. Cholinergic treatments with emphasis on m1 muscarinic agonists as potential disease-modifying agents for Alzheimer’s disease. Neurotherapeutics. 2008;5:433–442. doi: 10.1016/j.nurt.2008.05.002. doi: 10.1016/j.nurt.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fisher A. Muscarinic receptor agonists in Alzheimer’s disease: more than just symptomatic treatment? CNS Drugs. 1999;123:197–214. doi: 10.2165/00023210-199912030-00004. [Google Scholar]
- 61.Geula C, Nagykery N, Nicholas A, Wu CK. Cholinergic neuronal and axonal abnormalities are present early in aging and in Alzheimer disease. J Neuropathol Exp Neurol. 2008;67:309–318. doi: 10.1097/NEN.0b013e31816a1df3. doi: 10.1097/NEN.0b013e31816a1df3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Giovannini MG, Scali C, Prosperi C, Bellucci A, Vannucchi MG, Rosi S, Pepeu G, Casamenti F. Beta-amyloid-induced inflammation and cholinergic hypofunction in the rat brain in vivo: involvement of the p38MAPK pathway. Neurobiol Dis. 2002;11:257–274. doi: 10.1006/nbdi.2002.0538. doi: 10.1006/nbdi.2002.0538. [DOI] [PubMed] [Google Scholar]
- 63.Gong B, Cao Z, Zheng P, Vitolo OV, Liu S, Staniszewski A, Moolman D, Zhang H, Shelanski M, Arancio O. Ubiquitin hydrolase Uch-L1 rescues beta-amyloid-induced decreases in synaptic function and contextual memory. Cell. 2006;126:775–788. doi: 10.1016/j.cell.2006.06.046. doi: 10.1016/j.cell.2006.06.046. [DOI] [PubMed] [Google Scholar]
- 64.Gothel SF, Marahiel MA. Peptidyl-prolyl cis-trans isomerases, a superfamily of ubiquitous folding catalysts. Cell Mol Life Sci. 1999;55:423–436. doi: 10.1007/s000180050299. doi: 10.1007/s000180050299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guidi I, Galimberti D, Lonati S, Novembrino C, Bamonti F, Tiriticco M, Fenoglio C, Venturelli E, Baron P, Bresolin N, Scarpini E. Oxidative imbalance in patients with mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2006;27:262–269. doi: 10.1016/j.neurobiolaging.2005.01.001. doi: 10.1016/j.neurobiolaging.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 66.Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 67.Hamdane M, Dourlen P, Bretteville A, Sambo AV, Ferreira S, Ando K, Kerdraon O, Begard S, Geay L, Lippens G, Sergeant N, Delacourte A, Maurage CA, Galas MC, Buee L. Pin1 allows for differential Tau dephosphorylation in neuronal cells. Mol Cell Neurosci. 2006;32:155–160. doi: 10.1016/j.mcn.2006.03.006. doi: 10.1016/j.mcn.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 68.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 69.Head E, Callahan H, Muggenburg BA, Cotman CW, Milgram NW. Visual-discrimination learning ability and beta-amyloid accumulation in the dog. Neurobiol Aging. 1998;19:415–425. doi: 10.1016/s0197-4580(98)00084-0. doi: 10.1016/S0197-4580(98)00084-0. [DOI] [PubMed] [Google Scholar]
- 70.Head E, Torp R. Insights into Abeta and presenilin from a canine model of human brain aging. Neurobiol Dis. 2002;9:1–10. doi: 10.1006/nbdi.2002.0476. doi: 10.1006/nbdi.2002.0476. [DOI] [PubMed] [Google Scholar]
- 71.Hensley K, Hall N, Subramaniam R, Cole P, Harris M, Aksenov M, Aksenova M, Gabbita SP, Wu JF, Carney JM, et al. Brain regional correspondence between Alzheimer’s disease histopathology and biomarkers of protein oxidation. J Neurochem. 1995;65:2146–2156. doi: 10.1046/j.1471-4159.1995.65052146.x. [DOI] [PubMed] [Google Scholar]
- 72.Holzer M, Gartner U, Stobe A, Hartig W, Gruschka H, Bruckner MK, Arendt T. Inverse association of Pin1 and tau accumulation in Alzheimer’s disease hippocampus. Acta Neuropathol. 2002;104:471–481. doi: 10.1007/s00401-002-0581-1. [DOI] [PubMed] [Google Scholar]
- 73.Ischiropoulos H, al-Mehdi AB. Peroxynitrite-mediated oxidative protein modifications. FEBS Lett. 1995;364:279–282. doi: 10.1016/0014-5793(95)00307-u. doi: 10.1016/0014-5793(95)00307-U. [DOI] [PubMed] [Google Scholar]
- 74.Joshi G, Perluigi M, Sultana R, Agrippino R, Calabrese V, Butterfield DA. In vivo protection of synaptosomes by ferulic acid ethyl ester (FAEE) from oxidative stress mediated by 2,2-azobis(2-amidino-propane)dihydrochloride (AAPH) or Fe(2+)/H(2)O(2): insight into mechanisms of neuroprotection and relevance to oxidative stress-related neurodegenerative disorders. Neurochem Int. 2006;48:318–327. doi: 10.1016/j.neuint.2005.11.006. doi: 10.1016/j.neuint.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 75.Joshi G, Sultana R, Perluigi M, Allan Butterfield D. In vivo protection of synaptosomes from oxidative stress mediated by Fe2+/H2O2 or 2, 2-azobis-(2-amidinopropane) dihydrochloride by the glutathione mimetic tricyclodecan-9-yl-xanthogenate. Free Radic Biol Med. 2005;38:1023–1031. doi: 10.1016/j.freeradbiomed.2004.12.027. doi: 10.1016/j.freeradbiomed. 2004.12.027. [DOI] [PubMed] [Google Scholar]
- 76.Kanski J, Aksenova M, Schoneich C, Butterfield DA. Sub-stitution of isoleucine-31 by helical-breaking proline abolishes oxidative stress and neurotoxic properties of Alzheimer’s amyloid beta-peptide. Free Radic Biol Med. 2002;32:1205–1211. doi: 10.1016/s0891-5849(02)00821-3. doi: 10.1016/S0891-5849(02)00821-3. [DOI] [PubMed] [Google Scholar]
- 77.Kanski J, Varadarajan S, Aksenova M, Butterfield DA. Role of glycine-33 and methionine-35 in Alzheimer’s amyloid beta-peptide 1–42-associated oxidative stress and neurotoxicity. Biochim Biophys Acta. 2002;1586:190–198. doi: 10.1016/s0925-4439(01)00097-7. [DOI] [PubMed] [Google Scholar]
- 78.Keller JN, Hanni KB, Markesbery WR. Impaired proteasome function in Alzheimer’s disease. J Neurochem. 2000;75:436–439. doi: 10.1046/j.1471-4159.2000.0750436.x. doi: 10.1046/j.1471-4159.2000.0750436.x. [DOI] [PubMed] [Google Scholar]
- 79.Keller JN, Schmitt FA, Scheff SW, Ding Q, Chen Q, Butterfield DA, Markesbery WR. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64:1152–1156. doi: 10.1212/01.WNL.0000156156.13641.BA. [DOI] [PubMed] [Google Scholar]
- 80.Korolainen MA, Goldsteins G, Alafuzoff I, Koistinaho J, Pirttila T. Proteomic analysis of protein oxidation in Alzheimer’s disease brain. Electrophoresis. 2002;23:3428–3433. doi: 10.1002/1522-2683(200210)23:19<3428::AID-ELPS3428>3.0.CO;2-5. doi: 10.1002/1522-2683(200210)23:19<3428::AID-ELPS3428>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 81.Korolainen MA, Goldsteins G, Nyman TA, Alafuzoff I, Koistinaho J, Pirttila T. Oxidative modification of proteins in the frontal cortex of Alzheimer’s disease brain. Neurobiol Aging. 2006;27:42–53. doi: 10.1016/j.neurobiolaging.2004.11.010. doi: 10.1016/j.neurobiolaging.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 82.Lafon-Cazal M, Fagni L, Guiraud MJ, Mary S, Lerner-Natoli M, Pin JP, Shigemoto R, Bockaert J. mGluR7-like metabotropic glutamate receptors inhibit NMDA-mediated excitotoxicity in cultured mouse cerebellar granule neurons. Eur J NeuroSci. 1999;11:663–672. doi: 10.1046/j.1460-9568.1999.00475.x. doi: 10.1046/j.1460-9568.1999.00475.x. [DOI] [PubMed] [Google Scholar]
- 83.Lahiri DK, Ge YW, Maloney B, Wavrant-De Vrieze F, Hardy J. Characterization of two APP gene promoter polymerphisms that appear to influence risk of late-onset Alzheimer’s disease. Neurobiol Aging. 2005;26:1329–1341. doi: 10.1016/j.neurobiolaging.2004.11.005. doi: 10.1016/j.neurobiolaging.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 84.Lambert JC, Mann DM, Harris JM, Chartier-Harlin MC, Cumming A, Coates J, Lemmon H, StClair D, Iwatsubo T, Lendon C. The −48 C/T polymorphism in the presenilin 1 promoter is associated with an increased risk of developing Alzheimer’s disease and an increased Abeta load in brain. J Med Genet. 2001;38:353–355. doi: 10.1136/jmg.38.6.353. doi: 10.1136/jmg.38.6.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lauderback CM, Hackett JM, Huang FF, Keller JN, Szweda LI, Markesbery WR, Butterfield DA. The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-non-enal in the Alzheimer’s disease brain: the role of Abeta 1–42. J Neurochem. 2001;78:413–416. doi: 10.1046/j.1471-4159.2001.00451.x. doi: 10.1046/j.1471-4159.2001.00451.x. [DOI] [PubMed] [Google Scholar]
- 86.Layfield R, Fergusson J, Aitken A, Lowe J, Landon M, Mayer RJ. Neurofibrillary tangles of Alzheimer’s disease brains contain 14-3-3 proteins. Neurosci Lett. 1996;209:57–60. doi: 10.1016/0304-3940(96)12598-2. doi: 10.1016/0304-3940(96)12598-2. [DOI] [PubMed] [Google Scholar]
- 87.Levine RL, Williams JA, Stadtman ER, Shacter E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994;233:346–357. doi: 10.1016/s0076-6879(94)33040-9. doi: 10.1016/S0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- 88.Levy-Lahad E, Lahad A, Wijsman EM, Bird TD, Schellenberg GD. Apolipoprotein E genotypes and age of onset in early-onset familial Alzheimer’s disease. Ann Neurol. 1995;38:678–680. doi: 10.1002/ana.410380420. doi: 10.1002/ana.410380420. [DOI] [PubMed] [Google Scholar]
- 89.Li X, An WL, Alafuzoff I, Soininen H, Winblad B, Pei JJ. Phosphorylated eukaryotic translation factor 4E is elevated in Alzheimer brain. NeuroReport. 2004;15:2237–2240. doi: 10.1097/00001756-200410050-00019. doi: 10.1097/00001756-200410050-00019. [DOI] [PubMed] [Google Scholar]
- 90.Ling M, Merante F, Chen HS, Duff C, Duncan AM, Robinson BH. The human mitochondrial elongation factor tu (EF-Tu) gene: cDNA sequence, genomic localization, genomic structure, and identification of a pseudogene. Gene. 1997;197:325–336. doi: 10.1016/s0378-1119(97)00279-5. doi: 10.1016/S0378-1119(97)00279-5. [DOI] [PubMed] [Google Scholar]
- 91.Link CD. C. elegans models of age-associated neurodegenerative diseases: lessons from transgenic worm models of Alzheimer’s disease. Exp Gerontol. 2006;41:1007–1013. doi: 10.1016/j.exger.2006.06.059. doi: 10.1016/j.exger.2006.06.059. [DOI] [PubMed] [Google Scholar]
- 92.Link CD. Expression of human beta-amyloid peptide in transgenic Caenorhabditis elegans. Proc Natl Acad Sci USA. 1995;92:9368–9372. doi: 10.1073/pnas.92.20.9368. doi: 10.1073/pnas.92.20.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liou YC, Sun A, Ryo A, Zhou XZ, Yu ZX, Huang HK, Uchida T, Bronson R, Bing G, Li X, Hunter T, Lu KP. Role of the prolyl isomerase Pin1 in protecting against age-dependent neuro-degeneration. Nature. 2003;424:556–561. doi: 10.1038/nature01832. doi: 10.1038/nature01832. [DOI] [PubMed] [Google Scholar]
- 94.Lledo PM, Zhang X, Sudhof TC, Malenka RC, Nicoll RA. Postsynaptic membrane fusion and long-term potentiation. Science. 1998;279:399–403. doi: 10.1126/science.279.5349.399. doi: 10.1126/science.279.5349.399. [DOI] [PubMed] [Google Scholar]
- 95.Lovell MA, Markesbery WR. Oxidative damage in mild cognitive impairment and early Alzheimer’s disease. J Neurosci Res. 2007;85:3036–3040. doi: 10.1002/jnr.21346. doi: 10.1002/jnr.21346. [DOI] [PubMed] [Google Scholar]
- 96.Lovell MA, Markesbery WR. Ratio of 8-hydroxyguanine in intact DNA to free 8-hydroxyguanine is increased in Alzheimer disease ventricular cerebrospinal fluid. Arch Neurol. 2001;58:392–396. doi: 10.1001/archneur.58.3.392. doi: 10.1001/archneur.58.3.392. [DOI] [PubMed] [Google Scholar]
- 97.Lovell MA, Xie C, Markesbery WR. Acrolein is increased in Alzheimer’s disease brain and is toxic to primary hippocampal cultures. Neurobiol Aging. 2001;22:187–194. doi: 10.1016/s0197-4580(00)00235-9. doi: 10.1016/S0197-4580(00)00235-9. [DOI] [PubMed] [Google Scholar]
- 98.Lu KP, Hanes SD, Hunter T. A human peptidyl-prolyl isomerase essential for regulation of mitosis. Nature. 1996;380:544–547. doi: 10.1038/380544a0. doi: 10.1038/380544a0. [DOI] [PubMed] [Google Scholar]
- 99.Lubec G, Nonaka M, Krapfenbauer K, Gratzer M, Cairns N, Fountoulakis M. Expression of the dihydropyrimidinase related protein 2 (DRP-2) in Down syndrome and Alzheimer’s disease brain is downregulated at the mRNA and dysregulated at the protein level. J Neural Transm Suppl. 1999;57:161–177. doi: 10.1007/978-3-7091-6380-1_10. [DOI] [PubMed] [Google Scholar]
- 100.Marin R, Ramirez CM, Gonzalez M, Gonzalez-Munoz E, Zorzano A, Camps M, Alonso R, Diaz M. Voltage-dependent anion channel (VDAC) participates in amyloid beta-induced toxicity and interacts with plasma membrane estrogen receptor alpha in septal and hippocampal neurons. Mol Membr Biol. 2007;24:148–160. doi: 10.1080/09687860601055559. doi: 10.1080/09687860601055559. [DOI] [PubMed] [Google Scholar]
- 101.Mark RJ, Lovell MA, Markesbery WR, Uchida K, Mattson MP. A role for 4-hydroxynonenal, an aldehydic product of lipid peroxidation, in disruption of ion homeostasis and neuronal death induced by amyloid beta-peptide. J Neurochem. 1997;68:255–264. doi: 10.1046/j.1471-4159.1997.68010255.x. [DOI] [PubMed] [Google Scholar]
- 102.Markesbery WR. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic Biol Med. 1997;23:134–147. doi: 10.1016/s0891-5849(96)00629-6. doi: 10.1016/S0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- 103.Markesbery WR, Lovell MA. Four-hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer’s disease. Neurobiol Aging. 1998;19:33–36. doi: 10.1016/s0197-4580(98)00009-8. doi: 10.1016/S0197-4580(98)00009-8. [DOI] [PubMed] [Google Scholar]
- 104.Mattson MP, Partin J, Begley JG. Amyloid beta-peptide induces apoptosis-related events in synapses and dendrites. Brain Res. 1998;807:167–176. doi: 10.1016/s0006-8993(98)00763-x. doi: 10.1016/S0006-8993(98)00763-X. [DOI] [PubMed] [Google Scholar]
- 105.Mazzola JL, Sirover MA. Reduction of glyceraldehyde-3-phosphate dehydrogenase activity in Alzheimer’s disease and in Huntington’s disease fibroblasts. J Neurochem. 2001;76:442–449. doi: 10.1046/j.1471-4159.2001.00033.x. doi: 10.1046/j.1471-4159.2001.00033.x. [DOI] [PubMed] [Google Scholar]
- 106.Meier-Rugez W, Iwangoff P, Reichlmeier K. Neurochemical enzyme changes in Alzheimer’s and Pick’s disease. Arch Gerontol Geriatr. 1984;3:161–165. doi: 10.1016/0167-4943(84)90007-4. doi: 10.1016/0167-4943(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 107.Mello CF, Sultana R, Piroddi M, Cai J, Pierce WM, Klein JB, Butterfield DA. Acrolein induces selective protein carbonylation in synaptosomes. Neuroscience. 2007;147:674–679. doi: 10.1016/j.neuroscience.2007.04.003. doi: 10.1016/j.neuroscience.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Milgram NW, Head E, Zicker SC, Ikeda-Douglas CJ, Murphey H, Muggenburg B, Siwak C, Tapp D, Cotman CW. Learning ability in aged beagle dogs is preserved by behavioral enrichment and dietary fortification: a two-year longitudinal study. Neurobiol Aging. 2005;26:77–90. doi: 10.1016/j.neurobiolaging.2004.02.014. doi: 10.1016/j.neurobiolaging.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 109.Milgram NW, Zicker SC, Head E, Muggenburg BA, Murphey H, Ikeda-Douglas CJ, Cotman CW. Dietary enrichment counteracts age-associated cognitive dysfunction in canines. Neurobiol Aging. 2002;23:737–745. doi: 10.1016/s0197-4580(02)00020-9. doi: 10.1016/S0197-4580(02)00020-9. [DOI] [PubMed] [Google Scholar]
- 110.Mohmmad Abdul H, Butterfield DA. Protection against amyloid beta-peptide (1–42)-induced loss of phospholipid asymmetry in synaptosomal membranes by tricyclodecan-9-xanthogenate (D609) and ferulic acid ethyl ester: implications for Alzheimer’s disease. Biochim Biophys Acta. 2005;1741:140–148. doi: 10.1016/j.bbadis.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 111.Mohmmad Abdul H, Sultana R, Keller JN, St Clair DK, Markesbery WR, Butterfield DA. Mutations in amyloid precursor protein and presenilin-1 genes increase the basal oxidative stress in murine neuronal cells and lead to increased sensitivity to oxidative stress mediated by amyloid beta-peptide (1–42), HO and kainic acid: implications for Alzheimer’s disease. J Neurochem. 2006;96:1322–1335. doi: 10.1111/j.1471-4159.2005.03647.x. doi: 10.1111/j.1471-4159.2005.03647.x. [DOI] [PubMed] [Google Scholar]
- 112.Mohmmad Abdul H, Wenk GL, Gramling M, Hauss-Wegrzyniak B, Butterfield DA. APP and PS-1 mutations induce brain oxidative stress independent of dietary cholesterol: implications for Alzheimer’s disease. Neurosci Lett. 2004;368:148–150. doi: 10.1016/j.neulet.2004.06.077. doi: 10.1016/j.neulet.2004.06.077. [DOI] [PubMed] [Google Scholar]
- 113.Montine TJ, Neely MD, Quinn JF, Beal MF, Markesbery WR, Roberts LJ, Morrow JD. Lipid peroxidation in aging brain and Alzheimer’s disease. Free Radic Biol Med. 2002;33:620–626. doi: 10.1016/s0891-5849(02)00807-9. doi: 10.1016/S0891-5849(02)00807-9. [DOI] [PubMed] [Google Scholar]
- 114.Morris JC. Mild cognitive impairment and preclinical Alzheimer’s disease. Geriatrics Suppl. 2005:9–14. [PubMed] [Google Scholar]
- 115.Munch G, Thome J, Foley P, Schinzel R, Riederer P. Advanced glycation endproducts in ageing and Alzheimer’s disease. Brain Res Brain Res Rev. 1997;23:134–143. doi: 10.1016/s0165-0173(96)00016-1. doi: 10.1016/S0165-0173(96)00016-1. [DOI] [PubMed] [Google Scholar]
- 116.Murphy MP, Beckett TL, Ding Q, Patel E, Markesbery WR, St Clair DK, LeVine H, 3rd, Keller JN. Abeta solubility and deposition during AD progression and in APPxPS-1 knock-in mice. Neurobiol Dis. 2007;27:301–311. doi: 10.1016/j.nbd.2007.06.002. doi: 10.1016/j.nbd.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 117.Murray IV, Sindoni ME, Axelsen PH. Promotion of oxidative lipid membrane damage by amyloid beta proteins. Biochemistry. 2005;44:12606–12613. doi: 10.1021/bi050926p. doi: 10.1021/bi050926p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Negash S, Petersen LE, Geda YE, Knopman DS, Boeve BF, Smith GE, Ivnik RJ, Howard DV, Howard JH, Jr, Petersen RC. Effects of ApoE genotype and mild cognitive impairment on implicit learning. Neurobiol Aging. 2007;28:885–893. doi: 10.1016/j.neurobiolaging.2006.04.004. doi: 10.1016/j.neurobiolaging.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 119.Oda T, Wals P, Osterburg HH, Johnson SA, Pasinetti GM, Morgan TE, Rozovsky I, Stine WB, Snyder SW, Holzman TF, et al. Clusterin (apoJ) alters the aggregation of amyloid beta-peptide (A beta 1–42) and forms slowly sedimenting Abeta complexes that cause oxidative stress. Exp Neurol. 1995;136:22–31. doi: 10.1006/exnr.1995.1080. doi: 10.1006/exnr.1995.1080. [DOI] [PubMed] [Google Scholar]
- 120.Ojika K. Hippocampal cholinergic neurostimulating peptide Seikagaku. 1998;70:1175–1180. [PubMed] [Google Scholar]
- 121.Opii WO, Joshi G, Head E, Milgram NW, Muggenburg BA, Klein JB, Pierce WM, Cotman CW, Butterfield DA. Proteomic identification of brain proteins in the canine model of human aging following a long-term treatment with antioxidants and a program of behavioral enrichment: relevance to Alzheimer’s disease. Neurobiol Aging. 2008;29:51–70. doi: 10.1016/j.neurobiolaging.2006.09.012. doi: 10.1016/j.neurobiolaging.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pancholi V. Multifunctional alpha-enolase: its role in diseases. Cell Mol Life Sci. 2001;58:902–920. doi: 10.1007/PL00000910. doi: 10.1007/PL00000910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Parnetti L, Palumbo B, Cardinali L, Loreti F, Chionne F, Cecchetti R, Senin U. Cerebrospinal fluid neuron-specific enolase in Alzheimer’s disease and vascular dementia. Neurosci Lett. 1995;183:43–45. doi: 10.1016/0304-3940(94)11110-5. doi: 10.1016/0304-3940(94)11110-5. [DOI] [PubMed] [Google Scholar]
- 124.Pastorino L, Sun A, Lu PJ, Zhou XZ, Balastik M, Finn G, Wulf G, Lim J, Li SH, Li X, Xia W, Nicholson LK, Lu KP. The prolyl isomerase Pin1 regulates amyloid precursor protein processing and amyloid-beta production. Nature. 2006;440:528–534. doi: 10.1038/nature04543. doi: 10.1038/nature04543. [DOI] [PubMed] [Google Scholar]
- 125.Perluigi M, Fai Poon H, Hensley K, Pierce WM, Klein JB, Calabrese V, De Marco C, Butterfield DA. Proteomic analysis of 4-hydroxy-2-nonenal-modified proteins in G93A-SOD1 transgenic mice—a model of familial amyotrophic lateral sclerosis. Free Radic Biol Med. 2005;38:960–968. doi: 10.1016/j.freeradbiomed.2004.12.021. doi: 10.1016/j.freeradbiomed.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 126.Perluigi M, Joshi G, Sultana R, Calabrese V, De Marco C, Coccia R, Butterfield DA. In vivo protection by the xanthate tricyclodecan-9-yl-xanthogenate against amyloid beta-peptide (1–42)-induced oxidative stress. Neuroscience. 2006;138:1161–1170. doi: 10.1016/j.neuroscience.2005.12.004. doi: 10.1016/j.neuroscience.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 127.Perluigi M, Joshi G, Sultana R, Calabrese V, De Marco C, Coccia R, Cini C, Butterfield DA. In vivo protective effects of ferulic acid ethyl ester against amyloid-beta peptide 1–42-induced oxidative stress. J Neurosci Res. 2006;84:418–426. doi: 10.1002/jnr.20879. doi: 10.1002/jnr.20879. [DOI] [PubMed] [Google Scholar]
- 128.Perluigi M, Sultana R, Cenini G, Di Domenico F, Memo M, Pierce WM, Coccia R, Butterfield DA. Redox proteomics identification of HNE-modified brain proteins in Alzheimers disease: Role of lipid peroxidation in AD pathogenesis. Proteomics Clin Appli. 2009 doi: 10.1002/prca.200800161. (Accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Perry EK, Curtis M, Dick DJ, Candy JM, Atack JR, Bloxham CA, Blessed G, Fairbairn A, Tomlinson BE, Perry RH. Cholinergic correlates of cognitive impairment in Parkinson’s disease: comparisons with Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1985;48:413–421. doi: 10.1136/jnnp.48.5.413. doi: 10.1136/jnnp.48.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pestova TV, Hellen CU. The structure and function of initiation factors in eukaryotic protein synthesis. Cell Mol Life Sci. 2000;57:651–674. doi: 10.1007/PL00000726. doi: 10.1007/PL00000726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 132.Poon HF, Farr SA, Thongboonkerd V, Lynn BC, Banks WA, Morley JE, Klein JB, Butterfield DA. Proteomic analysis of specific brain proteins in aged SAMP8 mice treated with alpha-lipoic acid: implications for aging and age-related neurodegenerative disorders. Neurochem Int. 2005;46:159–168. doi: 10.1016/j.neuint.2004.07.008. doi: 10.1016/j.neuint.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 133.Poon HF, Shepherd HM, Reed TT, Calabrese V, Stella AM, Pennisi G, Cai J, Pierce WM, Klein JB, Butterfield DA. Proteomics analysis provides insight into caloric restriction mediated oxidation and expression of brain proteins associated with age-related impaired cellular processes: Mitochondrial dysfunction, glutamate dysregulation and impaired protein synthesis. Neurobiol Aging. 2006;27:1020–1034. doi: 10.1016/j.neurobiolaging.2005.05.014. doi: 10.1016/j.neurobiolaging.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 134.Power JH, Asad S, Chataway TK, Chegini F, Manavis J, Temlett JA, Jensen PH, Blumbergs PC, Gai WP. Peroxiredoxin 6 in human brain: molecular forms, cellular distribution and association with Alzheimer’s disease pathology. Acta Neuropathol. 2008;115:611–622. doi: 10.1007/s00401-008-0373-3. doi: 10.1007/s00401-008-0373-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ralat LA, Manevich Y, Fisher AB, Colman RF. Direct evidence for the formation of a complex between 1-cysteine peroxiredoxin and glutathione S-transferase pi with activity changes in both enzymes. Biochemistry. 2006;45:360–372. doi: 10.1021/bi0520737. doi: 10.1021/bi0520737. [DOI] [PubMed] [Google Scholar]
- 136.Ramakers IH, Visser PJ, Aalten P, Bekers O, Sleegers K, van Broeckhoven CL, Jolles J, Verhey FR. The association between APOE genotype and memory dysfunction in subjects with mild cognitive impairment is related to age and Alzheimer pathology. Dement Geriatr Cogn Disord. 2008;26:101–108. doi: 10.1159/000144072. doi: 10.1159/000144072. [DOI] [PubMed] [Google Scholar]
- 137.Ramakrishnan P, Dickson DW, Davies P. Pin1 colocalization with phosphorylated tau in Alzheimer’s disease and other tauopathies. Neurobiol Dis. 2003;14:251–264. doi: 10.1016/s0969-9961(03)00109-8. doi: 10.1016/S0969-9961(03)00109-8. [DOI] [PubMed] [Google Scholar]
- 138.Reed T, Perluigi M, Sultana R, Pierce WM, Klein JB, Turner DM, Coccia R, Markesbery WR, Butterfield DA. Redox proteomic identification of 4-hydroxy-2-nonenal-modified brain proteins in amnestic mild cognitive impairment: insight into the role of lipid peroxidation in the progression and pathogenesis of Alzheimer’s disease. Neurobiol Dis. 2008;30:107–120. doi: 10.1016/j.nbd.2007.12.007. doi: 10.1016/j.nbd.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 139.Renes J, de Vries EE, Hooiveld GJ, Krikken I, Jansen PL, Muller M. Multidrug resistance protein MRP1 protects against the toxicity of the major lipid peroxidation product 4-hydroxynonenal. Biochem J. 2000;350(Pt 2):555–561. doi: 10.1042/0264-6021:3500555. [PMC free article] [PubMed] [Google Scholar]
- 140.Rinaldi P, Polidori MC, Metastasio A, Mariani E, Mattioli P, Cherubini A, Catani M, Cecchetti R, Senin U, Mecocci P. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer’s disease. Neurobiol Aging. 2003;24:915–919. doi: 10.1016/s0197-4580(03)00031-9. doi: 10.1016/S0197-4580(03)00031-9. [DOI] [PubMed] [Google Scholar]
- 141.Sadik G, Tanaka T, Kato K, Yamamori H, Nessa BN, Morihara T, Takeda M. Phosphorylation of tau at Ser214 mediates its interaction with 14-3-3 protein: implications for the mechanism of tau aggregation. J Neurochem. 2008;108:33–43. doi: 10.1111/j.1471-4159.2008.05716.x. [DOI] [PubMed] [Google Scholar]
- 142.Santoni V, Molloy M, Rabilloud T. Membrane proteins and proteomics: un amour impossible? Electrophoresis. 2000;21:1054–1070. doi: 10.1002/(SICI)1522-2683(20000401)21:6<1054::AID-ELPS1054>3.0.CO;2-8. doi: 10.1002/(SICI)1522-2683(20000401)21:6<1054::AID-ELPS1054>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 143.Santpere G, Puig B, Ferrer I. Oxidative damage of 14-3-3 zeta and gamma isoforms in Alzheimer’s disease and cerebral amyloid angiopathy. Neuroscience. 2007;146:1640–1651. doi: 10.1016/j.neuroscience.2007.03.013. doi: 10.1016/j.neuroscience.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 144.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 145.Shimizu H, Banno Y, Sumi N, Naganawa T, Kitajima Y, Nozawa Y. Activation of p38 mitogen-activated protein kinase and caspases in UVB-induced apoptosis of human keratinocyte HaCaT cells. J Invest Dermatol. 1999;112:769–774. doi: 10.1046/j.1523-1747.1999.00582.x. doi: 10.1046/j.1523-1747.1999.00582.x. [DOI] [PubMed] [Google Scholar]
- 146.Sly WS, Hu PY. Human carbonic anhydrases and carbonic anhydrase deficiencies. Annu Rev Biochem. 1995;64:375–401. doi: 10.1146/annurev.bi.64.070195.002111. doi: 10.1146/annurev.bi.64.070195.002111. [DOI] [PubMed] [Google Scholar]
- 147.Smith MA, Richey Harris PL, Sayre LM, Beckman JS, Perry G. Widespread peroxynitrite-mediated damage in Alzheimer’s disease. J Neurosci. 1997;17:2653–2657. doi: 10.1523/JNEUROSCI.17-08-02653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Smith MA, Richey PL, Taneda S, Kutty RK, Sayre LM, Monnier VM, Perry G. Advanced Maillard reaction end products, free radicals, and protein oxidation in Alzheimer’s disease. Ann N Y Acad Sci. 1994;738:447–454. doi: 10.1111/j.1749-6632.1994.tb21836.x. [DOI] [PubMed] [Google Scholar]
- 149.Spires TL, Meyer-Luehmann M, Stern EA, McLean PJ, Skoch J, Nguyen PT, Bacskai BJ, Hyman BT. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J Neurosci. 2005;25:7278–7287. doi: 10.1523/JNEUROSCI.1879-05.2005. doi: 10.1523/JNEUROSCI.1879-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Stadtman ER, Berlett BS. Reactive oxygen-mediated protein oxidation in aging and disease. Chem Res Toxicol. 1997;10:485–494. doi: 10.1021/tx960133r. doi: 10.1021/tx960133r. [DOI] [PubMed] [Google Scholar]
- 151.Stenbeck G. Soluble NSF-attachment proteins. Int J Biochem Cell Biol. 1998;30:573–577. doi: 10.1016/s1357-2725(97)00064-2. doi: 10.1016/S1357-2725(97)00064-2. [DOI] [PubMed] [Google Scholar]
- 152.Subramaniam R, Roediger F, Jordan B, Mattson MP, Keller JN, Waeg G, Butterfield DA. The lipid peroxidation product, 4-hydroxy-2-trans-nonenal, alters the conformation of cortical synaptosomal membrane proteins. J Neurochem. 1997;69:1161–1169. doi: 10.1046/j.1471-4159.1997.69031161.x. [DOI] [PubMed] [Google Scholar]
- 153.Suh YH, Checler F. Amyloid precursor protein, presenilins, and alpha-synuclein: molecular pathogenesis and pharmacological applications in Alzheimer’s disease. Pharmacol Rev. 2002;54:469–525. doi: 10.1124/pr.54.3.469. doi: 10.1124/pr.54.3.469. [DOI] [PubMed] [Google Scholar]
- 154.Sultana R, Boyd-Kimball D, Poon HF, Cai J, Pierce WM, Klein JB, Markesbery WR, Zhou XZ, Lu KP, Butterfield DA. Oxidative modification and down-regulation of Pin1 in Alzheimer’s disease hippocampus: a redox proteomics analysis. Neurobiol Aging. 2006;27:918–925. doi: 10.1016/j.neurobiolaging.2005.05.005. doi: 10.1016/j.neurobiolaging.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 155.Sultana R, Boyd-Kimball D, Poon HF, Cai J, Pierce WM, Klein JB, Merchant M, Markesbery WR, Butterfield DA. Redox proteomics identification of oxidized proteins in Alzheimer’s disease hippocampus and cerebellum: an approach to understand pathological and biochemical alterations in AD. Neurobiol Aging. 2006;27:1564–1576. doi: 10.1016/j.neurobiolaging.2005.09.021. doi: 10.1016/j.neurobiolaging.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 156.Sultana R, Butterfield DA. Oxidatively modified GST and MRP1 in Alzheimer’s disease brain: implications for accumulation of reactive lipid peroxidation products. Neurochem Res. 2004;29:2215–2220. doi: 10.1007/s11064-004-7028-0. doi: 10.1007/s11064-004-7028-0. [DOI] [PubMed] [Google Scholar]
- 157.Sultana R, Newman S, Mohmmad-Abdul H, Keller JN, Butterfield DA. Protective effect of the xanthate, D609, on Alzheimer’s amyloid beta-peptide (1–42)-induced oxidative stress in primary neuronal cells. Free Radic Res. 2004;38:449–458. doi: 10.1080/1071576042000206478. doi: 10.1080/1071576042000206478. [DOI] [PubMed] [Google Scholar]
- 158.Sultana R, Newman SF, Abdul HM, Cai J, Pierce WM, Klein JB, Merchant M, Butterfield DA. Protective effect of D609 against amyloid-beta1–42-induced oxidative modification of neuronal proteins: redox proteomics study. J Neurosci Res. 2006;84:409–417. doi: 10.1002/jnr.20876. doi: 10.1002/jnr.20876. [DOI] [PubMed] [Google Scholar]
- 159.Sultana R, Perluigi M, Butterfield DA. Redox proteomics identification of oxidatively modified proteins in Alzheimer’s disease brain and in vivo and in vitro models of AD centered around Abeta(1–42) J Chromatogr B Analyt Technol Biomed Life Sci. 2006;833:3–11. doi: 10.1016/j.jchromb.2005.09.024. doi: 10.1016/j.jchromb.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 160.Sultana R, Piroddi M, Galli F, Butterfield DA. Protein levels and activity of some antioxidant enzymes in hippocampus of subjects with amnestic mild cognitive impairment. Neurochem Res. 2008;33:2540–2546. doi: 10.1007/s11064-008-9593-0. doi: 10.1007/s11064-008-9593-0. [DOI] [PubMed] [Google Scholar]
- 161.Sultana R, Poon HF, Cai J, Pierce WM, Merchant M, Klein JB, Markesbery WR, Butterfield DA. Identification of nitrated proteins in Alzheimer’s disease brain using a redox proteomics approach. Neurobiol Dis. 2006;22:76–87. doi: 10.1016/j.nbd.2005.10.004. doi: 10.1016/j.nbd.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 162.Sultana R, Ravagna A, Mohmmad-Abdul H, Calabrese V, Butterfield DA. Ferulic acid ethyl ester protects neurons against amyloid beta- peptide(1–42)-induced oxidative stress and neurotoxicity: relationship to antioxidant activity. J Neurochem. 2005;92:749–758. doi: 10.1111/j.1471-4159.2004.02899.x. doi: 10.1111/j.1471-4159.2004.02899.x. [DOI] [PubMed] [Google Scholar]
- 163.Sultana R, Reed T, Perluigi M, Coccia R, Pierce WM, Butterfield DA. Proteomic Identification of nitrated brain proteins in amnestic mild cognitive impairment: a regional study. J Cell Mol Med. 2007;11:839–851. doi: 10.1111/j.1582-4934.2007.00065.x. doi: 10.1111/j.1582-4934.2007.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tamagno E, Parola M, Guglielmotto M, Santoro G, Bardini P, Marra L, Tabaton M, Danni O. Multiple signaling events in amyloid beta-induced, oxidative stress-dependent neuronal apoptosis. Free Radic Biol Med. 2003;35:45–58. doi: 10.1016/s0891-5849(03)00244-2. doi: 10.1016/S0891-5849(03)00244-2. [DOI] [PubMed] [Google Scholar]
- 165.Tamagno E, Robino G, Obbili A, Bardini P, Aragno M, Parola M, Danni O. H2O2 and 4-hydroxynonenal mediate amyloid beta-induced neuronal apoptosis by activating JNKs and p38MAPK. Exp Neurol. 2003;180:144–155. doi: 10.1016/s0014-4886(02)00059-6. doi: 10.1016/S0014-4886(02)00059-6. [DOI] [PubMed] [Google Scholar]
- 166.Tchaikovskaya T, Fraifeld V, Urphanishvili T, Andorfer JH, Davies P, Listowsky I. Glutathione S-transferase hGSTM3 and ageing-associated neurodegeneration: relationship to Alzheimer’s disease. Mech Ageing Dev. 2005;126:309–315. doi: 10.1016/j.mad.2004.08.029. doi: 10.1016/j.mad.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 167.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 168.Thompson J, Dahlberg AE. Testing the conservation of the translational machinery over evolution in diverse environments: assaying Thermus thermophilus ribosomes and initiation factors in a coupled transcription-translation system from Escherichia coli. Nucleic Acids Res. 2004;32:5954–5961. doi: 10.1093/nar/gkh925. doi: 10.1093/nar/gkh925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tremblay C, Pilote M, Phivilay A, Emond V, Bennett DA, Calon F. Biochemical characterization of Abeta and tau pathologies in mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2007;12:377–390. doi: 10.3233/jad-2007-12411. [DOI] [PubMed] [Google Scholar]
- 170.Uchida K, Stadtman ER. Modification of histidine residues in proteins by reaction with 4-hydroxynonenal. Proc Natl Acad Sci USA. 1992;89:4544–4548. doi: 10.1073/pnas.89.10.4544. doi: 10.1073/pnas.89.10.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Viola KL, Velasco PT, Klein WL. Why Alzheimer’s is a disease of memory: the attack on synapses by A beta oligomers (ADDLs) J Nutr Health Aging. 2008;12:51S–57S. doi: 10.1007/BF02982587. doi: 10.1007/BF02982587. [DOI] [PubMed] [Google Scholar]
- 172.Walsh DM, Hartley DM, Kusumoto Y, Fezoui Y, Condron MM, Lomakin A, Benedek GB, Selkoe DJ, Teplow DB. Amyloid beta-protein fibrillogenesis. Structure and biological activity of protofibrillar intermediates. J Biol Chem. 1999;274:25945–25952. doi: 10.1074/jbc.274.36.25945. doi: 10.1074/jbc.274.36.25945. [DOI] [PubMed] [Google Scholar]
- 173.Walsh DM, Klyubin I, Fadeeva JV, Rowan MJ, Selkoe DJ. Amyloid-beta oligomers: their production, toxicity and therapeutic inhibition. Biochem Soc Trans. 2002;30:552–557. doi: 10.1042/bst0300552. doi: 10.1042/BST0300552. [DOI] [PubMed] [Google Scholar]
- 174.Watson GS, Craft S. Modulation of memory by insulin and glucose: neuropsychological observations in Alzheimer’s disease. Eur J Pharmacol. 2004;490:97–113. doi: 10.1016/j.ejphar.2004.02.048. doi: 10.1016/j.ejphar.2004.02.048. [DOI] [PubMed] [Google Scholar]
- 175.Wevers A, Witter B, Moser N, Burghaus L, Banerjee C, Steinlein OK, Schutz U, de Vos RA, Steur EN, Lindstrom J, Schroder H. Classical Alzheimer features and cholinergic dysfunction: towards a unifying hypothesis? Acta Neurol Scand Suppl. 2000;176:42–48. doi: 10.1034/j.1600-0404.2000.00306.x. doi: 10.1034/j.1600-0404.2000.00306.x. [DOI] [PubMed] [Google Scholar]
- 176.Whitehouse PJ, Price DL, Clark AW, Coyle JT, DeLong MR. Alzheimer disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann Neurol. 1981;10:122–126. doi: 10.1002/ana.410100203. doi: 10.1002/ana.410100203. [DOI] [PubMed] [Google Scholar]
- 177.Williams TI, Lynn BC, Markesbery WR, Lovell MA. Increased levels of 4-hydroxynonenal and acrolein, neurotoxic markers of lipid peroxidation, in the brain in Mild Cognitive Impairment and early Alzheimer’s disease. Neurobiol Aging. 2006;27:1094–1099. doi: 10.1016/j.neurobiolaging.2005.06.004. doi: 10.1016/j.neurobiolaging.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 178.Yatin SM, Varadarajan S, Butterfield DA. Vitamin E prevents Alzheimer’s amyloid beta-peptide (1–42)-induced neuronal protein oxidation and reactive oxygen species production. J Alzheimers Dis. 2000;2:123–131. doi: 10.3233/jad-2000-2212. [DOI] [PubMed] [Google Scholar]
- 179.Yatin SM, Varadarajan S, Link CD, Butterfield DA. In vitro and in vivo oxidative stress associated with Alzheimer’s amyloid beta-peptide (1–42) Neurobiol Aging. 1999;20:325–330. doi: 10.1016/s0197-4580(99)00056-1. doi: 10.1016/S0197-4580(99)00056-1 discussion 39–42. [DOI] [PubMed] [Google Scholar]
- 180.Zarkovic K. 4-hydroxynonenal and neurodegenerative diseases. Mol Aspects Med. 2003;24:293–303. doi: 10.1016/s0098-2997(03)00024-4. doi: 10.1016/S0098-2997(03)00024-4. [DOI] [PubMed] [Google Scholar]
- 181.Zhou XZ, Kops O, Werner A, Lu PJ, Shen M, Stoller G, Kullertz G, Stark M, Fischer G, Lu KP. Pin1-dependent prolyl isomerization regulates dephosphorylation of Cdc25C and tau proteins. Mol Cell. 2000;6:873–883. doi: 10.1016/s1097-2765(05)00083-3. doi: 10.1016/S1097-2765(05)00083-3. [DOI] [PubMed] [Google Scholar]