Abstract
Ras plays an important role in B cell development. However, the stage at which Ras governs B cell development remains unclear. Moreover, the upstream receptors and downstream effectors of Ras that govern B cell differentiation remain undefined. Using mice that express a dominant negative form of Ras, we demonstrate that Ras-mediated signaling plays a critical role in the development of common lymphoid progenitors (CLP). This developmental block parallels that found in flt3−/− mice, suggesting that Flt3 is an important upstream activator of Ras in early B cell progenitors. Ras inhibition impaired proliferation of CLP and pre-pro-B cells but not pro-B cells. Rather, Ras promotes STAT5-dependent pro-B cell differentiation by enhancing IL7Rα levels and suppressing socs2 and socs3 expression. Our results suggest a model in which Flt3/Ras-dependent signals play a critical role in B cell development by priming early B cell progenitors for subsequent STAT5-dependent B cell differentiation.
Introduction
The ubiquitously expressed Ras proteins play important roles in mammalian development. Three isoforms of Ras, K-, N- and H-Ras, are expressed in a tissue specific manner and are involved in proliferation and differentiation of various cell lineages in mammals. To elucidate the role of Ras in early B cell development, we made use of transgenic mice expressing a dominant-negative form of human H-ras (dnRas) throughout the B and T cell lineage (1). These dnRas mice have been previously shown to have a profound block at the pre-pro-B to pro-B transition in B cell development (1). However, whether Ras signaling plays a role in the earlier steps of B lymphopoiesis remains to be elucidated. Moreover, the upstream activators and key downstream targets of the Ras/MAPK pathway in B cell development remain unknown.
Early lymphopoiesis in adult bone marrow and fetal liver relies on cytokine signaling involving predominantly two receptors: FMS-like tyrosine kinase 3 (FLT3, also known as FLK2) and the interleukin 7 receptor (IL7R) (2-4). Mice deficient in both IL7Rα and Flt3 ligand (IL7Rα−/− × flt3L−/−) lack all B lineage cells (5). IL7 and IL7Rα deficient mice exhibit profound defects at the pro-B cell stage of early B cell development (2, 3). We and others have previously reported that the IL7R governs early B cell development through a Jak/Stat5 dependent pathway (6, 7), but could not provide any evidence for a role of Ras signaling in that process (6). In contrast to IL7Rα−/− mice, flt3−/− mice exhibit only a mild reduction of pro-B and pre-B cells (4). Bone marrow chimeras generated by mixing WT and flt3−/− bone marrow cells demonstrated that Flt3 is required for mature B cell generation under competitive circumstances (4). However, the reagents and tools available when this initial analysis was done did not allow for precise identification of the stages of B cell development that were affected by Flt3 deficiency. Moreover, the signaling pathways activated by Flt3 in vivo that drive B cell development have not been characterized.
In this study, we report that early B cell development in dnRas mice is blocked at the CLP stage in both adult and fetal mice, a much earlier stage than previously reported. Likewise, using mixed bone marrow chimeras, we observed an identical block at the CLP stage in flt3−/− cells. This developmental block is due to two distinct effects. First, Flt3/Ras-dependent signals govern CLP and pre-pro-B cell proliferation. Second, Flt3/Ras signals upregulate expression of the IL7Rα chain and suppress expression of socs2 and socs3. Our findings suggest a model in which Flt3-dependent Ras activation primes B cell development by inducing a state of STAT5 responsiveness, a key event required for subsequent B cell lineage commitment and differentiation (7).
Materials and Methods
Mice
C57BL/6 (B6) and B6.CD45.1 (B6.Ly5SJL) mice were purchased from the Jackson Laboratories (Bar Harbor, ME). dnRas, Raf-CAAX, Rag1-GFP, Bcl-xL and STAT5b-CA transgenic mice have been previously described (1, 6, 8, 9). flt3−/− mice were kindly provided by Dr. Rachel Gerstein. Mice used for analysis were 4-12 weeks old, unless otherwise noted. All animal experiments were approved by the University of Minnesota Institutional Animal Care and Use Committee.
Flow cytometry
Bone marrow cells isolated from both femurs and tibias were treated with ACK lysis buffer (Lonza, MD) to remove red blood cells. To enrich for early progenitors, cells were stained with a mixture of lineage specific antibodies described below and lineage positive cells were removed via magnetic depletion using LS MACS column and magnetic beads (Miltenyi Biotec, Auburn, CA). Cell preparations were stained with a panel of antibodies (listed below) and analyzed on a LSRII Flow Cytometer (BD Biosciences, Mountain View, CA). Antibodies used to define lineage positive cells included biotin-B220, CD3, CD8, CD11b/Mac-1, DX5, Gr-1, Ter-119. Additional antibodies used included FITC-CD43, PE-CD45R/B220, PerCP-Cy5.5-CD19, PE-CD71, APC-CD93/AA4.1, APC-CD117/c-Kit, PE-Cy5.5-Sca-1, PE-IL7R, Alexa700-CD45.2, and Pacific Blue-CD45.1. All antibodies used were obtained from eBioscience (San Diego, CA) except for FITC-CD43 and PE-CD71, which were obtained from BD Biosciences Pharmingen (San Diego, CA) and Pacific Blue-CD45.1, which was obtained from BioLegend (San Diego, CA).
Generation of bone marrow chimeras
Recipient mice were given 1000 rad of irradiation 4-6 hours before injection. Bone marrow from WT (CD45.1), dnRas (CD45.2) and flt3−/− (CD45.2) mice was depleted of CD3, CD8, CD11b, B220, DX5, Gr-1 and Ter-119 positive cells. Following depletion, WT (CD45.1) bone marrow was mixed with bone marrow from dnRas (CD45.2) or flt3−/− (CD45.2) at a 1:2 ratio. One million cells were then injected intravenously via the tail vein. Host mice were analyzed 6-8 weeks after reconstitution.
BrdU labeling assays
BrdU assays were conducted according to the manufacturer's instructions (BD Biosciences, San Jose, CA). Briefly, mice were injected intraperitoneally with 2 mg of BrdU 24 hours and 12 hours prior to analysis. Bone marrow cells were isolated and stained with surface markers to identify B cell progenitor populations. Intracellular staining was then conducted for BrdU. Cells were fixed with BD Cytofix/Cytoperm buffer, washed, and permeabilized with BD Cytoperm Plus buffer. Cells were refixed with BD Cytofix/Cytoperm buffer, resuspended in DNase (20 μg/sample), and incubated for 1 hour at 37°C. Intracellular staining for BrdU was conducted for 20 minutes at room temperature. Flow cytometry was conducted on a LSR II (BD Biosciences, San Jose, CA) to identify BrdU+ cells.
In vitro B cell differentiation
Bone marrow from WT and dnRas mice were isolated and depleted of B220, Mac-1, Gr-1 and Ter-119 positive cells. CLPs (dump− c-kitmedIL7R+) were sorted on a FACSVantage (BD Biosciences, Mountain View, CA) and plated in X-VIVO medium (BioWhittaker) supplemented with 5% BSA, 10 ng/ml IL7, 40 ng/ml SCF and 80 ng/ml Flt3L. Cells were harvested at the time points indicated in the figures and analyzed by flow cytometry.
Intracellular phospho-STAT5 staining
Bone marrow from WT, dnRas, STAT5b-CA and dnRas × STAT5b-CA mice were isolated and depleted of CD3, CD8, CD11b, CD25, IgM, DX5, Gr-1 and Ter-119 positive cells. Depleted cells were surface stained with APC-AA4.1 and streptavidin-Cascade Blue, rested for 20 min at 37°C and stimulated with IL7 (Peprotech). Samples were then stained for phospho-STAT5 as previously described (10, 11).
Real-time RT-PCR
RNA was prepared from sorted populations using an RNeasy kit (Qiagen, Valencia, CA). A two-step procedure was then conducted for real-time PCR assays (Invitrogen, Carlsbad, CA) using a Cepheid Smartcycler. All primer/probe mixtures were purchased from ABI (Applied Biosystems Inc, Foster City, CA) except for EBF and HPRT. HPRT primer/probe sequences were described previously (6). EBF primers: forward: AGGTTACAGAAGGTCATTCCTCG; reverse: CATACAGGGCTTCAACCAGATC; probe: AGCGCTTGCCAAAGGAAGTGATCC. All Real-time PCR assays were conducted for 1 cycle at 50°C for 2 min, 1 cycle at 95°C for 10 min followed by 40 cycles at 95°C (15 sec) and 60°C (1 min).
Results
Expression of a dnRas transgene impairs early B cell development in both fetal and adult mice
To study the role that the Ras signaling pathway plays in the earliest steps of B cell development, we crossed dominant-negative Ras (dnRas) transgenic mice with Rag1-GFP reporter mice. The dnRas mouse model has been previously described (1). Briefly, a human H-Ras transgene with a N17 point mutation was fused to the lck proximal promoter and the immunoglobulin intronic heavy chain (Eμ) enhancer. This results in expression of dnRas throughout B and T cell development. Consistent with this expression pattern, we observed decreased levels of phospho-Erk (pERK) in lymphocyte progenitor cells (lin−c-kithiFlt3+) from dnRas mice (data not shown). Likewise, expression of CD71, a downstream PI3K target gene (12), was significantly reduced (p=0.015) in early B cell progenitors (data not shown). Thus, dnRas mice have reduced activation of at least two of the primary downstream effectors of the Ras signaling pathway in early B cell progenitors.
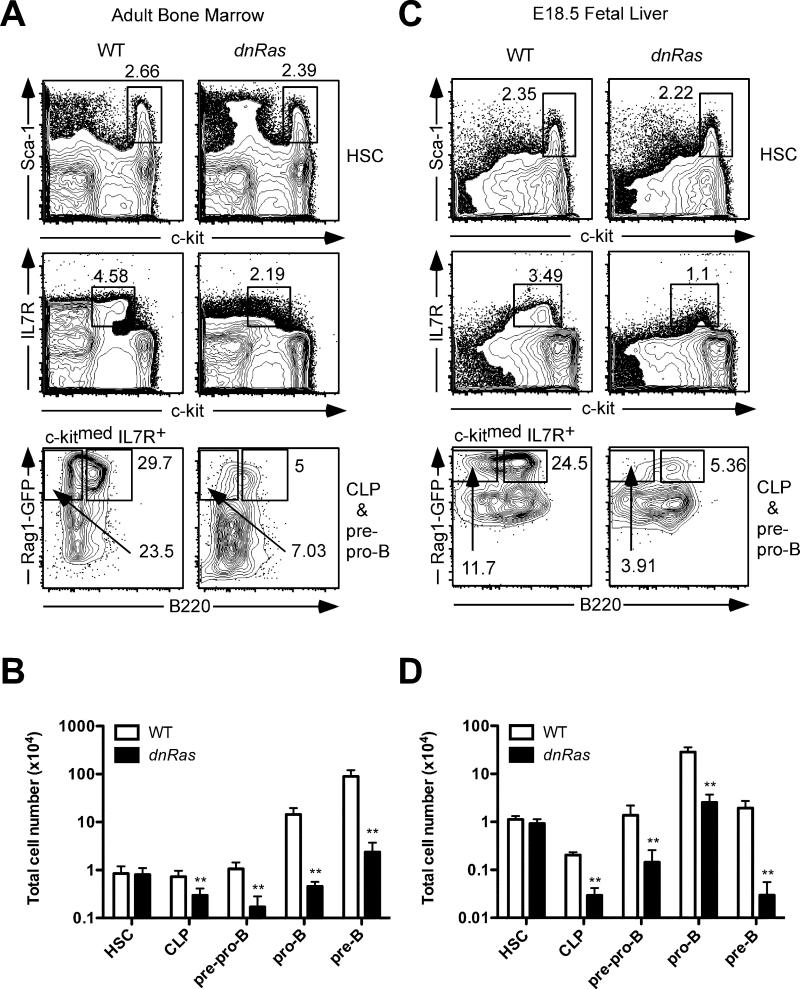
The Rag1-GFP knock-in model facilitates the precise identification of early lymphocyte progenitors by flow cytometry (8, 13). We used this system to analyze the precise developmental stages at which the dnRas transgene affects B cell development. Consistent with previous findings, we observed a significant reduction in the percentage and total cell number of pro-B and pre-B cells (32-fold and 38-fold, respectively; p<0.001) in dnRas × Rag1-GFP+/− mice compared to wild type littermate controls (Fig. 1A,B). Interestingly, we also observed a reduced percentage and total number of CLP (lin−Rag1-GFP+c-kitmedSca-1medIL7R+B220−, 2.5-fold↓, p<0.002) and pre-pro-B cells (lin−Rag1-GFP+c-kitmedSca-1medIL7R+B220+, 6.2-fold↓, p<0.001) in dnRas × Rag1-GFP+/− mice, while hematopoietic stem cell (HSC) frequency and total cell number remained unchanged (Fig. 1A,B). These results demonstrate that the actual effect of the dnRas transgene occurs at a much earlier stage than previously appreciated, namely the CLP stage.
Fig. 1. dnRas transgene expression blocks B cell development at the CLP stage.
A. The percentage of bone marrow HSC, CLP and pre-pro-B cells in dnRas and WT mice. Bone marrow cells were depleted for CD19 and non-B lineage markers. HSC were identified as Rag1-GFP−c-kithi Sca-1hi (upper panels). CLP and pre-pro-B cells were identified by first gating on c-kitmed IL7R+ cells (middle panels), and then further characterized as Rag1-GFP+B220− CLP and Rag1-GFP+B220+ pre-pro-B cells (bottom panels).
B. Total B cell progenitors recovered from the bone marrow of WT and dnRas × Rag1-GFP+/− mice. Cell numbers are plotted on a log scale. These results are derived from four independent experiments (11 WT mice, 11 dnRas mice). Error bars represent the mean ± SD; **p<0.01.
C. The percentage of fetal liver HSC, CLP and pre-pro-B cells in dnRas and WT mice. Fetal mice were analyzed at embryonic day 18.5. The same depletion and staining protocols were used as in panel A.
D. Total B cell progenitors recovered from the liver of WT and dnRas fetal mice. Cell numbers are plotted on a log scale. These results are representative of three independent experiments (11 WT mice, 15 dnRas mice). Error bars represent the mean ± SD; **p<0.01.
B cell development in adult versus fetal mice has been shown to differ in many ways including their dependence on cytokine signaling (14). To test whether Ras signaling also plays a role in fetal B cell development, we examined dnRas × Rag1-GFP+/− fetal liver at 18.5 days of gestation (E18.5). Consistent with our observations in adult bone marrow, we found a decrease in both the percentage and total cell number of fetal liver CLP, pre-pro-B, pro-B and pre-B cells (6.9-fold↓, 9.6-fold↓, 11.2-fold↓ and 66-fold↓, respectively; p<0.01; Fig. 1C and 1D). Taken together, our data demonstrate that Ras signaling plays important roles in the very earliest steps of B cell development in both fetal and adult mice.
flt3−/− and dnRas mice exhibit similar defects in B cell development
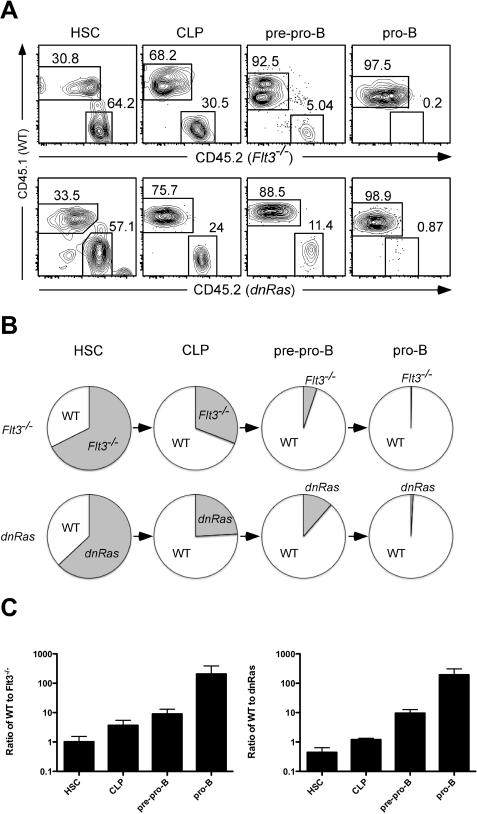
To identify the potential upstream activator of the Ras signaling pathway in developing B cells, we focused on the receptor tyrosine kinase Flt3. Flt3 ligand (Flt3L) has been previously reported to induce Erk phosphorylation in the Ba/F3 cell line transfected with Flt3 in vitro (15). Likewise, we observed that ex vivo stimulation of lineage negative bone marrow cells with Flt3L leads to elevated pERK levels in B cell progenitors (data not shown). Mice deficient for either flt3L or flt3 exhibit relatively subtle defects in early B cell development (4, 16). This most likely reflects compensatory signals mediated by other receptor tyrosine kinases, such as c-kit, that are also capable of activating the Ras/MAPK pathway (17). Therefore, to more robustly test the effect of Flt3 on B cell development, and to determine whether flt3−/− and dnRas mice had similar defects in B cell development, we generated mixed bone marrow chimeras. Bone marrow cells from either flt3−/− or dnRas mice were mixed with WT bone marrow at a 2:1 ratio and injected into lethally irradiated B6 host mice. Eight weeks after injection, the bone marrow of these hosts were analyzed for donor-derived cells. While the ratio of flt3−/− to WT cells in the HSC compartment remained unchanged (2:1), flt3−/− CLP, pre-pro-B and pro-B compartments exhibited a profound competitive disadvantage compared to WT cells (6.5-fold↓, 23-fold↓, and 686-fold↓, respectively; Fig. 2A, upper panels and Fig. 2B,C). We observed the same deficiencies in CLP, pre-pro-B and pro-B production by dnRas donors when compared to WT donors in similar mixed bone marrow chimera experiments (2.7-fold↓, 19-fold↓, and 313-fold↓, respectively; Fig 2A, lower panels and Fig. 2B,C). These data clearly demonstrate that flt3−/− and dnRas mice have a similar block at the CLP stage of B cell development, suggesting that Flt3-dependent Ras activation is essential for early B cell differentiation.
Fig. 2. flt3−/− mice exhibit similar defects in CLP as in dnRas mice.
A. Upper panels. Mixed bone marrow chimeras were generated by combining bone marrow from flt3−/−(CD45.2) and WT (CD45.1) mice at a 2:1 ratio and injecting these cells into lethally irradiated C57BL/6 host mice. Eight weeks after transfer, bone marrow cells from C57BL/6 host mice were isolated and analyzed for B cell progenitors. These results are representative of 15 hosts from three independent experiments. Lower panels. Mixed bone marrow chimeras were generated by combining bone marrow from dnRas (CD45.2) and WT (CD45.1) mice at a 2:1 ratio. The same analyses were performed as described for upper panels. These results are representative of 10 hosts from two independent experiments. B. The relative contribution of WT versus flt3−/− or dnRas cells to distinct stages of B cell development is depicted graphically via pie charts. C. The ratio of WT to flt3−/− or WT to dnRas cells in HSC, CLP, pre-pro-B and pro-B cells was plotted; error bars represent standard deviation. The ratio observed in HSC was similar to the original ratio at which cells were injected into host mice; the ratio's observed in CLP, pre-pro-B and pro-B cells was significantly greater than in HSC (p<0.05).
Ras regulates early B cell proliferation but not survival
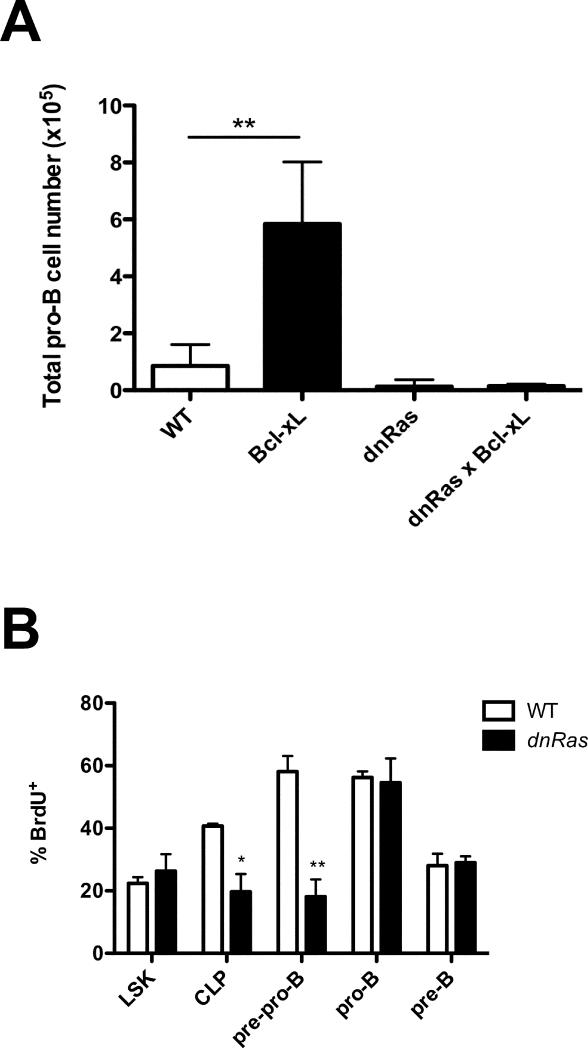
To test whether the decrease in B cell progenitors in dnRas mice was due to reduced cell survival, we first crossed dnRas mice with Bcl-xL transgenic mice. It has been previously reported that pro-B cell numbers are elevated 5 fold in Bcl-xL transgenic mice due to enhanced survival (9). We observed the same increase in pro-B cell numbers in Bcl-xL single transgenic mice relative to WT mice (Fig. 3A). In contrast, in dnRas × Bcl-xL double transgenic mice, the numbers of CLP, pre-pro-B and pro-B cells were identical to that observed in dnRas single transgenic mice (Fig. 3A and data not shown), indicating that the defects in dnRas mice could not be rescued by inhibiting apoptosis. Moreover, using caspase-3 staining to identify cells undergoing apoptosis, we observed the same percentages of apoptotic cells in WT and dnRas progenitor B cells (data not shown). Taken together, we conclude that the decrease in CLP in dnRas mice is not due to defects in cell survival.
Fig. 3. Reduced proliferation but not survival of dnRas B cell progenitors.
A. Bcl-xL transgene fails to rescue the defects in dnRas B progenitors. dnRas and Bcl-xL transgenic mice were bred to generate dnRas × Bcl-xL double transgenic mice. Total bone marrow cells from WT, Bcl-xL, dnRas or dnRas × Bcl-xL mice were isolated and depleted for non-B lineage cells as described in experimental procedures. Pro-B cells are defined as lin−CD43+B220+CD19+AA4.1+. The graph is derived from three independent experiments. Error bars represent the mean ± SD; *p<0.05.
B. Restricted proliferation of CLP and pre-pro-B in dnRas mice. BrdU incorporation in bone marrow cells was determined 24 hours after in vivo exposure to BrdU. Numbers indicate the percentage of BrdU+ cells. These results are representative of three independent experiments (8 WT mice, 8 dnRas mice). Error bars represent the mean ± SD; *p<0.05; **p<0.01.
We next examined whether cell proliferation is impaired in early B lineage cells in dnRas mice. For these studies, WT and dnRas mice were injected with BrdU and analyzed 24 hours later by flow cytometry for BrdU incorporation. As shown in Figure 3B, CLP and pre-pro-B cells from dnRas mice exhibit a 2-fold and 3-fold decrease, respectively, in the rate of BrdU incorporation when compared to WT littermate controls. In contrast, the percentages of BrdU+ pro-B and pre-B cells were not significantly different. These findings suggest that the reduced numbers of CLP and pre-pro-B cells in dnRas mice were due, at least in part, to defects in proliferation.
Decreased IL7Rα expression in dnRas and flt3−/− B cell progenitors
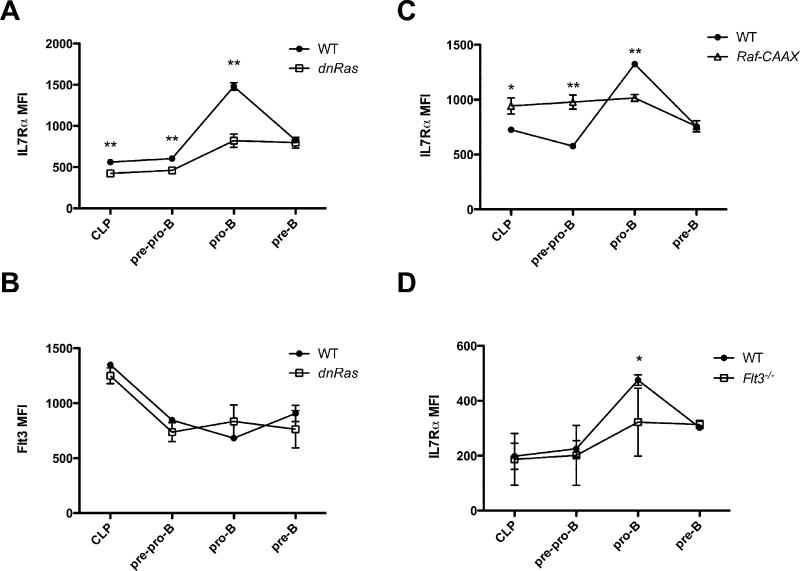
Although the proliferative defect we observed in the BrdU labeling studies provides an explanation for the diminished numbers of CLP and pre-pro-B cells, it is unlikely that this accounts entirely for the defect in pro-B cells, which is the most dramatically affected population in dnRas mice. Importantly, the pre-pro-B to pro-B transition requires signals sent by the IL7R (2). Thus, one potential explanation for the defect in pro-B cell generation in flt3−/− and dnRas mice would be if Flt3/Ras-dependent signals regulate expression of the IL7R. To test this hypothesis we measured the mean fluorescence intensity (MFI) for IL7Rα staining at various stages of B cell development. We observed modestly reduced levels of IL7Rα expression on CLP and pre-pro-B cells in dnRas mice. More importantly, IL7Rα expression is markedly upregulated during the pre-pro-B→ pro B cell transition in WT mice; this marked upregulation of the IL7Rα chain did not occur in dnRas pro-B cells (Fig. 4B). In contrast, we observed no difference in the MFI for Flt3 when comparing B cell progenitors from WT and dnRas mice (Fig. 4B). Similar results were seen when comparing IL7Rα expression levels on dnRas and WT B cell progenitors in the mixed bone marrow chimeras described earlier (data not shown). Supporting these findings, mice expressing a constitutively activated form of Raf (Raf-CAAX) showed the opposite result when examining IL7Rα expression on B cell progenitors. Specifically, IL7Rα expression was prematurely elevated in both CLP and pre-pro-B cells in Raf-CAAX mice relative to WT littermate controls (Fig. 4C). Finally, to test whether IL7Rα levels are also decreased in flt3−/− mice, we measured the MFI for IL7Rα expression in flt3−/− and WT mixed bone marrow chimeras (previously described; see Fig. 2). We found that IL7Rα levels on pro-B cells derived from flt3−/− donor cells were clearly reduced when compared to that seen on pro-B cells derived from WT donor cells (Fig. 4D). Taken together, our observation that dnRas and flt3−/− B cell progenitors fail to upregulate IL7Rα demonstrates that Flt3/Ras-dependent signals play an important role in regulating IL7Rα expression, which in turn regulates pro-B cell development.
Fig. 4. Flt3 and Ras regulate IL7Rα expression in early B cell development.
A, B. Bone marrow cells of WT and dnRas mice were isolated and stained for B cell progenitor populations. The mean fluorescence intensity (MFI) for IL7Rα and Flt3 expression on CLP, pre-pro-B, pro-B and pre-B cells was determined by FACS analysis. These results are representative of three independent experiments (8 WT mice, 8 dnRas mice). Error bars represent the mean ± SD; **p<0.01. C. Raf-CAAX mice exhibit higher IL7Rα expression in CLP and pre-pro-B cell subsets than WT mice. These results are representative of four independent experiments (9 WT mice, 9 Raf-CAAX mice). Error bars represent the mean ± SD; *p<0.05; **p<0.01. D. WT and flt3−/− mixed bone marrow chimeras were described in 2A. The mean fluorescent intensity (MFI) for IL7Rα expression on CLP, pre-pro-B, pro-B and pre-B cells was determined by FACS analysis. These results are representative of 15 hosts from three independent experiments. Error bars represent the mean ± SD; **p<0.01.
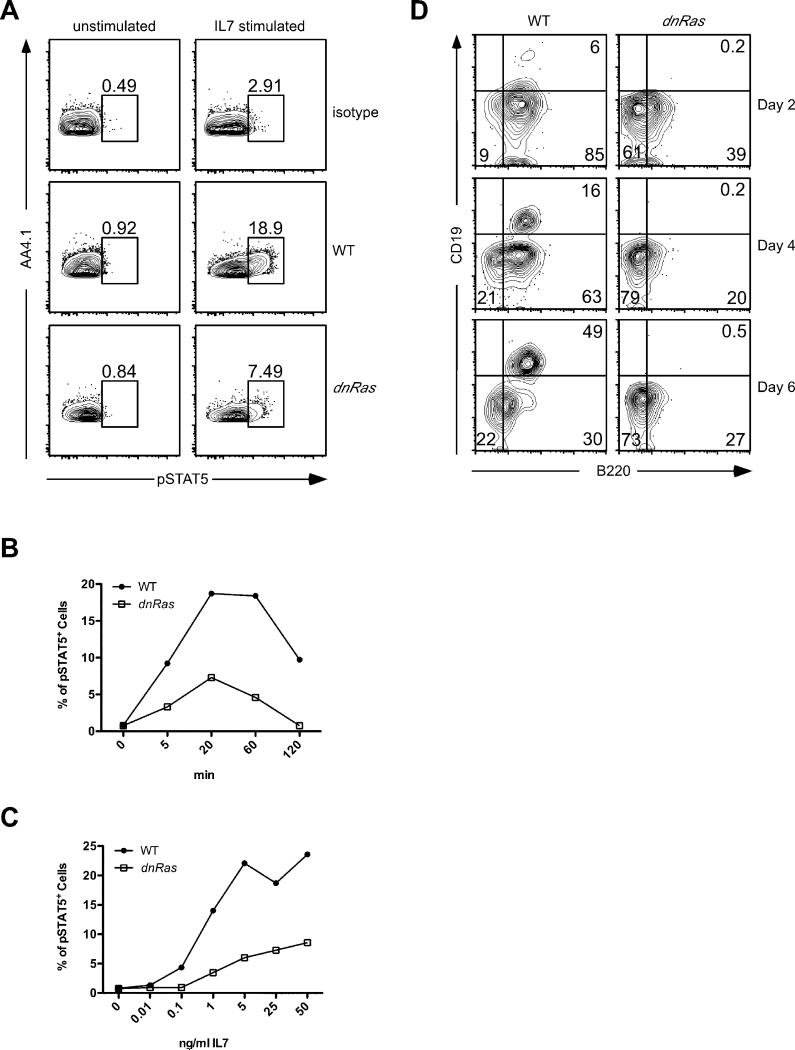
IL7 fails to induce the STAT5-dependent pre-pro-B to pro-B transition in dnRas mice
A key question is whether the reduced level of IL7Rα expression on B cell progenitors in dnRas mice is biologically relevant. To directly address this question, we first examined the activation of STAT5 in WT and dnRas mice. IL7 stimulation induced phosphorylation of STAT5 in early B cell progenitors in both WT and dnRas mice (Fig. 5A). Phosphorylation levels peaked 20 minutes after IL7 addition (Fig. 5B). However, the percentage of phospho-STAT5+ cells was significantly lower in dnRas mice compared to WT at all time points measured (Fig. 5B). Similar results were observed when we varied the dose of IL7 used (Fig. 5C). Importantly, at low IL7 concentrations (100 pg/mL), which are more likely to mimic physiological IL7 levels in vivo, WT but not dnRas progenitor B cells responded to IL7 by inducing STAT5 phosphorylation. Finally, we also examined expression of ebf1 in CLP and pro-B cells of dnRas and WT littermate control mice. Ebf1 is induced by IL7R-dependent signals and is required for the generation of pro-B cells. We found that ebf1 levels were reduced ~3-fold in CLP of dnRas mice. In contrast, ebf1 levels in the few pro-B cells that we could obtain from dnRas mice were comparable to that observed in pro-B cells from WT mice (Table I). Thus, in CLP, which do not require ebf1 for development, IL7R-dependent ebf1 expression was reduced. The failure to see reduced ebf1 in pro-B cells is likely due to a small number of cells that manage to upregulate ebf1 in the absence of IL7R signaling thereby allowing them to differentiate into pro-B cells. Taken together, these findings demonstrate that the reduced level of IL7Rα expression in dnRas B cell progenitors results in severely compromised IL7R-dependent signaling.
Fig. 5. Phospho-STAT5 expression in WT and dnRas B cell progenitors.
A. Phospho-STAT5 staining in WT and dnRas B cell progenitors. Depleted bone marrow cells from WT and dnRas mice were pooled, rested and stimulated with 25ng/ml IL-7 for 20 min. Phospho-STAT5 staining was conducted as described in experimental procedures. These results represent three independent experiments.
B, C. Time course and dose response of IL-7 stimulated phospho-STAT5. Depleted bone marrow cells were stimulated with 25ng/ml IL-7 for various time lengths (B) or various doses of IL-7 for 20 min (C) as indicated in the figures. The percentages of phospho-STAT5 positive cells within the dump−AA4.1+ population are summarized in these graphs. These results represent three independent experiments.
D. dnRas CLP fail to generate pro-B cells in vitro. Sorted bone marrow CLPs from WT and dnRas mice were cultured in vitro with IL7, SCF and Flt3L for 6 days. Cells were harvested, stained for B220 and CD19, and analyzed by flow cytometry at days 2, 4 and 6 in culture. 7AAD staining was used to discriminate live versus dead cells; panels shown are gated on 7AAD-negative cells (not shown). These results are representative of three independent experiments (pooled samples from 5 mice per group per experiment).
Table 1.
Real-time RT-PCR results
| Fold change | ||
|---|---|---|
| CLP and pre-pro-B | pro-B | |
| sfpi1 | 1.16 ± 0.11 | 1.00 ± 0.15 |
| gabpα | 1.10 ± 0.16 | 1.11 ± 0.17 |
| tyms | 0.88 ± 0.11 | 1.09 ± 0.11 |
| polα | 1.03 ± 0.09 | 1.17 ± 0.12 |
| skp2 | 0.76 ± 0.14** | 1.04 ± 0.13 |
| il7rα | 0.86 ± 0.29 | 0.95 ± 0.17 |
| ebf1 | 0.31 ± 0.39** | 1.27 ± 0.20 |
Fold change of sfpi1, gabpα, tyms, polα, skp2 il7rα, andebfı mRNA levels indnRas mice. Bone marrow cells from WT and dnRas mice were isolated and pooled. A mixture of CLP and pre-pro-B cells, or pro-B cells, were isolated by sorting on a FACSAria. Real time RT-PCR was performed as described in experimental procedures. Each reaction was conducted in triplicate. The fold change in mRNA levels in dnRas mice compared to WT was calculated by delta Ct. These results are derived from three or four independent experiments
p<0.01.
To further assess the effect of IL7Rα downregulation, we conducted in vitro B cell differentiation assays. Specifically, CLPs from WT and dnRas mice were sorted and cultured in vitro in the presence of IL7, SCF and Flt3L. After two days in culture a substantial percentage of CLP from WT mice had differentiated into B220+CD19− pre-pro-B cells. CLP derived from dnRas mice also gave rise to pre-pro-B cells although at a reduced level when compared to WT CLP (39% versus 85%, respectively)(Fig. 5D, top panel). After 4-6 days in culture, the WT pre-pro-B cells differentiated into B220+CD19+ pro-B cells (Fig. 5D, middle and bottom panels). In contrast, B cell development in the dnRas cultures was completely blocked at the pre-pro-B to pro-B transition (Fig. 5D, middle and bottom panels). Importantly, differentiation to the pro-B cell stage is exclusively dependent on IL7R signaling. Together, these studies demonstrate that the reduced level of IL7Rα expression on B cell progenitors in dnRas mice is not sufficient to entrain IL7-dependent pro-B cell differentiation.
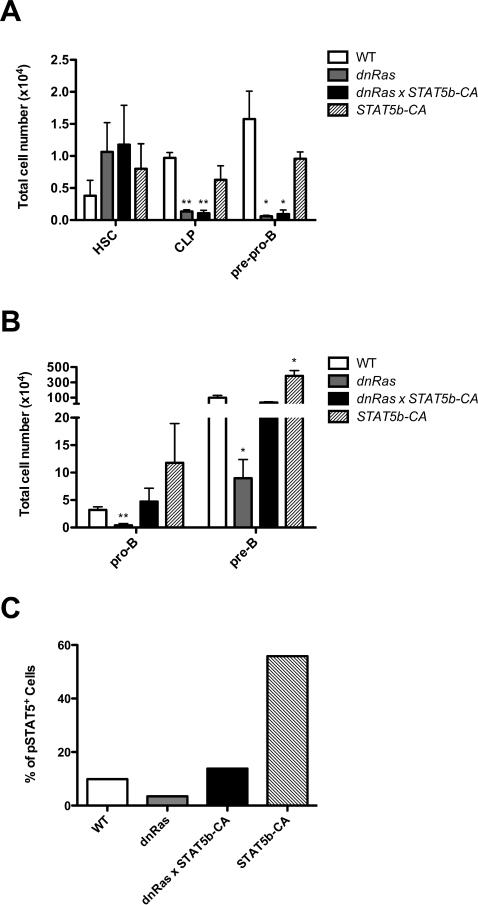
STAT5 restores pro-B cell differentiation in dnRas mice
Our results suggest that the defect in pro-B cell differentiation in dnRas mice is primarily due to a failure to induce IL7Rα expression, or activate IL7R-dependent signaling. This interpretation predicts that restoring expression of the IL7Rα chain, or inducing activation of its downstream effectors, should rescue B cell development in dnRas mice. To test this hypothesis, we crossed dnRas mice with mice expressing a constitutively active form of the key IL7 effector STAT5 (called STAT5b-CA). We and others have previously shown that STAT5 plays a critical role in early B cell differentiation (6, 7). Moreover, constitutively activated STAT5 can restore B cell development in IL7Rα deficient mice (6). Thus, if impaired IL7R/STAT5 signaling is responsible for the defect in pro-B cell development in dnRas mice, we would expect to see a rescue of pro-B cell differentiation in dnRas × STAT5b-CA double transgenic mice. As shown in Figure 6A, CLP and pre-pro-B cells numbers were still reduced in dnRas × STAT5b-CA mice, indicating that STAT5 failed to rescue the proliferative defects due to reduced Ras signaling in these cells. In contrast, STAT5 activation restored pro-B cell numbers in dnRas × STAT5b-CA mice to levels seen in WT littermate controls (Fig. 6B). This rescue was not due to an effect of STAT5b-CA on pro-B cell proliferation as BrdU incorporation studies demonstrated that dnRas and dnRas × STAT5b-CA pro-B cells incorporated BrdU at the same rate (data not shown). Finally, although IL7Rα levels remained low in dnRas × STAT5b-CA mice, STAT5 activation was restored to levels seen in WT controls (Fig. 6C and data not shown). Taken together, we conclude that Flt3/Ras regulates IL7 signaling either by governing IL7Rα expression alone or via additional effects on IL7R-dependent STAT5 activation.
Fig. 6. STAT5 activation rescues pro-B cell number in dnRas mice.
A, B. STAT5b-CA transgene rescues the defects in dnRas pro-B and pre-B subsets, but not in CLP and pre-pro-B cells. dnRas and STAT5b-CA transgenic mice were bred to generate dnRas × STAT5b-CA double transgenic mice. Total bone marrow cells from WT, STAT5b-CA, dnRas or dnRas × STAT5b-CA mice were isolated and depleted for non-B lineage cells as described in experimental procedures. Cell numbers are plotted on a log scale. The graph represents results of three independent experiments. Error bars represent the mean ± SD; *p<0.05; **p<0.01.
C. STAT5b-CA transgene rescues STAT5 phosphorylation in dnRas mice. Intracellular phospho-STAT5 levels of the dump-AA4.1+ population were measured after 20 min stimulation with 25ng/ml IL-7. These results are representative of three independent experiments.
GABPα and PU.1 expression levels are not altered in dnRas mice
A potential mechanism by which Flt3/Ras signaling could induce IL7Rα expression would be if they upregulated expression of the transcription factors GABPα and/or PU.1. Both of these factors have been shown to bind to the il7rα promoter and are required for IL7Rα expression in early pro-B cells (18-20). GABPα is additionally attractive as it has been shown to induce cell proliferation via induction of several genes such as tyms, skp2 and polα (21). To test this hypothesis, we measured mRNA levels of sfpi1 (i.e., PU.1) and gapbα by real-time RT-PCR using RNA isolated from sorted CLP, pre-pro-B and pro-B cells from WT and dnRas mice. No difference in mRNA levels for either sfpi1 or gabpα was observed (Table 1). Likewise, we observed no difference in the expression of the potential GABPα target genes tyms and polα in WT versus dnRas CLP/pre-pro-B cells or pro-B cells. A statistically significant reduction of skp2 mRNA levels was observed in CLP/pre-pro-B cells, although the effect was quite modest (~25% reduction) (Table 1). These results suggest that the effect of Flt3 and Ras on IL7Rα expression is not due to direct effects on PU.1 or GABPα expression levels. Consistent with these findings, we detected no difference in il7rα mRNA levels in WT and dnRas mice (Table 1). This latter result indicates that the Flt3/Ras pathway governs IL7Rα expression via a post-transcriptional mechanism.
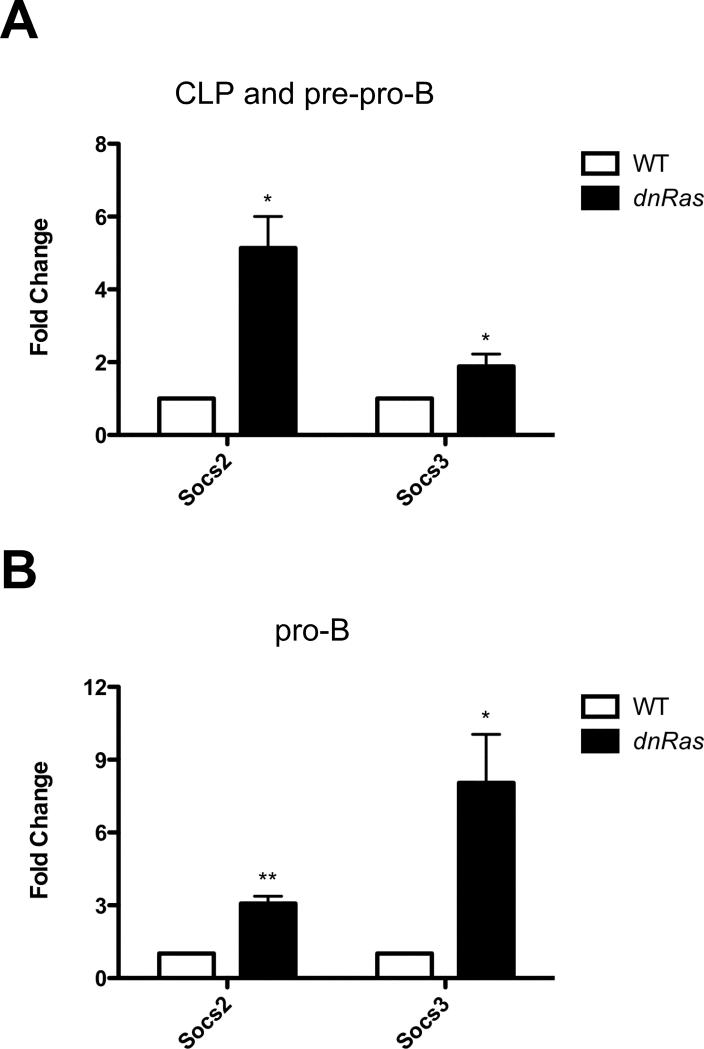
socs2 and socs3 expression are upregulated in dnRas mice
A recent study has demonstrated that SOCS2 and SOCS3 are two negative regulators of STAT5 signaling in mammary epithelial cells (22). To test the possibility that Ras signaling regulates expression of socs2 and socs3, we measured the mRNA level of these two genes in early B cell progenitors. Socs2 expression is upregulated 5-fold in CLP and pre-pro-B cells and 3-fold in pro-B cells in dnRas mice (Fig. 7A and B). Similarly, socs3 expression is increased 2-fold in CLP and pre-pro-B cells and 8-fold in pro-B cells in dnRas versus WT mice (Fig. 7A and B). Thus, increased Socs2 and Socs3 expression may contribute to the reduced ability of B cell progenitors from dnRas mice to induce STAT5 activation following stimulation with IL7.
Fig. 7.
Socs2 and socs3 mRNA levels are upregulated in CLP, pre-pro-B cells (A) and pro-B cells (B) in dnRas mice. Bone marrow cells from WT and dnRas mice were isolated and pooled. A mixture of CLP and pre-pro-B cells, or pro-B cells, were isolated by sorting on a FACSAria. Real time RT-PCR was performed as described in experimental procedures. Each reaction was conducted in triplicate. The fold change in mRNA levels in dnRas mice compared to WT was calculated by delta Ct. These results shown are derived from three independent experiments; *p<0.05; **p<0.01.
Discussion
Although B cell progenitors express a variety of receptor tyrosine kinases, only the IL7R has been shown to be absolutely essential for B cell development (2). Flt3 is known to act synergistically with the IL7R to promote B cell differentiation (5). However, Flt3 deficiency by itself results in only mild defects in B cell differentiation in vivo (4). One potential explanation for the relatively mild phenotype in flt3−/− mice is that other receptors, such as c-kit for example, may be able to substitute for Flt3 function in vivo. Herein we have clearly demonstrated, using mixed bone marrow chimeras, that Flt3 is essential for B cell development under competitive circumstances. In other words, when B cell progenitors are given the choice of using a Flt3-dependent versus a Flt3-independent pathway for generating pro-B cells, the Flt3-dependent pathway is essentially the only one used. Thus, under physiological circumstances (i.e., in WT Flt3+ mice) Flt3 signals play a critical role in normal B cell differentiation.
Although Flt3 has been demonstrated to activate numerous signaling pathways using in vitro cell lines, the downstream signaling pathways that drive Flt3-dependent B cell differentiation in vivo have not been identified. Using mice expressing a dominant-negative Ras transgene, we observed a developmental block at the CLP stage, which exactly parallels that observed in flt3−/− mice. In fact the developmental block observed in dnRas mice is much stronger than that seen in flt3−/− mice. This finding suggests that while there may be some redundancy with regard to the upstream receptors involved in driving early B cell differentiation, they all function via induction of Ras activity.
A key remaining question is how Ras signals entrain early B cell development. Ras has been shown to play an important role in regulating cell proliferation in a number of systems (23). Thus, one potential mechanism by which Ras could govern B cell differentiation is by regulating cell proliferation. As shown in Figure 3, this is in fact one of the key functions of Ras in both CLP and pre-pro-B cells. The exact mechanism by which Ras regulates cell cycle in CLP/pre-pro-B cells remains to be precisely determined. However, a potential mechanism may involve Skp2, an E3 ligase that mediates p27 degradation and thereby regulate S-phase entry (24). Consistent with this hypothesis, skp2 expression is reduced in CLP/pre-pro-B cells in dnRas mice. Thus, Flt3/Ras signals play an important role in expanding the pool of progenitor cells that are poised for B cell differentiation.
A second question involves which Ras effectors are required to drive B cell development. The Ras pathway activates a number of downstream signaling pathways including the Raf/Mek/Erk signaling cascade and the PI3K signaling pathway. The dnRas mice show reduced activation of both of those pathways. Our observation that mice expressing a constitutively active form of Raf (Raf-CAAX) have elevated IL7Rα expression in CLP and pre-pro-B cells suggested that Raf-dependent signals might be sufficient to drive Flt3-dependent B cell development. However, when we mixed bone marrow from WT and Raf-CAAX × flt3−/− mice and generated mixed bone marrow chimeras, we observed that Raf activation alone was completely unable to reverse the fitness of flt3−/− cells to compete with WT progenitors (data not shown). Thus, Flt3 and Ras likely act via multiple downstream effectors to promote early B cell development.
Pro-B cells are the most dramatically affected population in dnRas mice. However, unlike CLP and pre-pro-B cells, they do not exhibit a defect in proliferation in vivo. Pro-B cell differentiation requires signaling through an IL7R/STAT5-dependent pathway (2, 6, 7). Importantly, we demonstrate that B cell progenitors in dnRas mice show defects in IL7-dependent STAT5 activation and IL7-dependent pro-B cell differentiation. At least two potential mechanisms account for this Ras-dependent defect in B cell differentiation. First, dnRas mice fail to upregulate the IL7Rα chain during the pre-pro-B to pro-B transition. Ras appears to govern IL7Rα expression via a Raf-dependent pathway as Raf-CAAX mice show prematurely elevated levels of IL7Rα on CLP and pre-pro-B cells. A potential caveat to this explanation is that IL7Rα levels are lower in pro-B cells from Raf-CAAX versus WT mice. It is unclear why that should happen, although one potential explanation is that Ras/Raf-signals may enhance the ability of pro-B cells to respond to IL7 thereby allowing cells with lower IL7Rα expression to emerge. The effect of Ras does not involve alterations in the expression of PU.1 or GABPα, two previously characterized regulators of il7rα transcription (18-20, 25). Moreover, the fact that il7rα mRNA levels remain unchanged indicates that IL7Rα is regulated via a post-transcriptional mechanism in dnRas B cell progenitors. Second, dnRas mice also show dramatically increased expression of two negative regulators of STAT5 activation, socs2 and socs3. Consistent with these findings, we observed that expression of a constitutively active form of STAT5, which restores lymphocyte development in both IL7Rα−/− and socs1 overexpressing mice (6, 26), largely rescues pro-B cell differentiation in dnRas mice. Thus, a Flt3/Ras-dependent pathway governs the ability of early pro-B cell progenitors to respond to IL7. These findings suggest that IL7R signaling during pro-B cell differentiation is both limiting and tightly regulated. In addition, the finding that Ras regulates pre-pro-B→pro-B cell differentiation, and not proliferation, is consistent with the linear dose-response curve initially described between dnRas expression and pro-B cell numbers (1); if the defect at this stage was predominately due to an effect on cell proliferation we would have predicted to see a logarithmic relationship between these two parameters instead. Thus, our results suggest a model in which a Flt3/Ras-dependent pathway regulates B cell development via two distinct mechanisms. First, Flt3/Ras-dependent signals promote proliferation of CLP and pre-pro-B cells thereby expanding the pool of early B cell progenitors. Second, Flt3/Ras-dependent signals prime these B cell progenitors for subsequent IL7/STAT5-dependent pro-B cell differentiation by enhancing IL7Rα expression and suppressing inhibitors of STAT5 signaling, socs2 and socs3.
Our data clearly demonstrate that Flt3/Ras signals govern B cell development via effects on IL7R/STAT5 signaling. An important question is whether this is the only mechanism by which Flt3/Ras signals regulate B cell development. Our finding that Ras regulates socs3 expression in early B cell progenitors suggests that this is may not be the case. Specifically, SOCS3 was shown to negatively regulate CXCR4-dependent retention of B cell progenitors in the bone marrow (27). Socs3 is expressed at low levels in early B cell progenitors and is upregulated ~8-9 fold at the immature B cell stage. Furthermore, this study demonstrated that SOCS3 negatively regulates the ability of CXCR4 to induce Fak-dependent adhesion of B cell progenitors to VCAM. The conclusion of this study was that SOCS3 levels must be low in early B cell progenitors to allow for CXCR4-dependent retention of these cells in the bone marrow and that the subsequent increase in SOCS3 expression at the immature B cell stage allows for their emigration from the bone marrow. Interestingly, the increase in socs3 expression associated with immature B cell emigration, and defective CXCR4 activation of B cell adhesion, is virtually identical to the increase in socs3 expression that we observed in dnRas pro-B cells (~8-fold). Thus, Ras may regulate early B cell differentiation not just via effects on IL7R/STAT5-dependent B cell differentiation but possibly via CXCR4-dependent retention of B cell progenitors in the bone marrow environment required for appropriate B cell development.
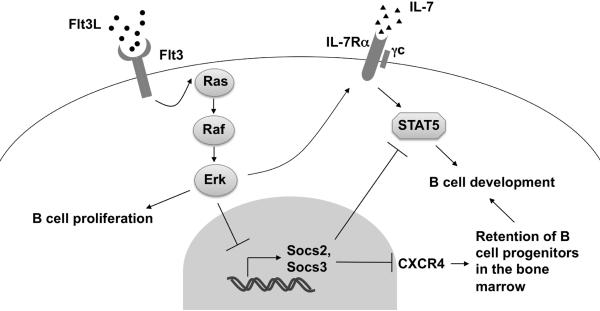
Fig. 8.
Model of Ras signaling in early B cell development. See text for details.
Acknowledgments
We thank Amanda Vegoe, Joshua Bednar and Christine Anderson for assistance with animal husbandry, Dr. Rachel Gerstein for providing flt3−/− mice, Dr. Paul Kincade for providing Rag1-GFP mice, Paul Champoux for assistance with flow cytometry, and Dr. Lynn Heltemes Harris for helpful discussions and critical review of the manuscript.
This work was supported by a grant from the NIH (AI050737), a Cancer Research Institute Investigator Award, a Pew Scholar Award, and a Leukemia and Lymphoma Society Scholar Award to M.A.F. The authors have no conflicting financial interests.
Abbreviations
- 7AAD
7-amino-actinomycin D
- CLP
common lymphoid progenitor
- dnRas
dominant negative Ras
- Flt3
FMS-like tyrosine kinase 3
- Flt3L
Flt3 ligand
- HSC
hematopoietic stem cell
- MFI
mean fluorescence intensity
- pErk
phospho-Erk
- SCF
stem cell factor
- SOCS
suppressor of cytokine signaling
References
- 1.Iritani BM, Forbush KA, Farrar MA, Perlmutter RM. Control of B cell development by Ras-mediated activation of Raf. EMBO J. 1997;16:7019–7031. doi: 10.1093/emboj/16.23.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peschon JJ, Morrissey PJ, Grabstein KI, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB, Meyer JD, Davison BL. Early lymphocyte expansion is severely impaired in interleukin-7 receptor deficient mice. The Journal of experimental medicine. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SEG, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. The Journal of experimental medicine. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mackarehtschian K, Hardin JD, Moore KA, Boast S, Goff SP, Lemischka IR. Targeted Disruption of the flk2/flt3 Gene Leads to Deficiencies in Primitive Hematopoietic Progenitors. Immunity. 1995;3:147–161. doi: 10.1016/1074-7613(95)90167-1. [DOI] [PubMed] [Google Scholar]
- 5.Sitnicka E, Brakebusch C, Martensson IL, Svensson M, Agace WW, Sigvardsson M, Buza-Vidas N, Bryder D, Cilio CM, Ahlenius H, Maraskovsky E, Peschon JJ, Jacobsen SE. Complementary signaling through flt3 and interleukin-7 receptor alpha is indispensable for fetal and adult B cell genesis. The Journal of experimental medicine. 2003;198:1495–1506. doi: 10.1084/jem.20031152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goetz CA, Harmon IR, O'Neil JJ, Burchill MA, Farrar MA. STAT5 activation underlies IL7 receptor-dependent B cell development. J Immunol. 2004;172:4770–4778. doi: 10.4049/jimmunol.172.8.4770. [DOI] [PubMed] [Google Scholar]
- 7.Yao Z, Cui Y, Watford WT, Bream JH, Yamaoka K, Hissong BD, Li D, Durum SK, Jiang Q, Bhandoola A, Hennighausen L, O'Shea JJ. Stat5a/b are essential for normal lymphoid development and differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1000–1005. doi: 10.1073/pnas.0507350103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuwata N, Igarashi H, Ohmura T, Aizawa S, Sakaguchi N. Cutting edge: absence of expression of RAG1 in peritoneal B-1 cells detected by knocking into RAG1 locus with green fluorescent protein gene. J Immunol. 1999;163:6355–6359. [PubMed] [Google Scholar]
- 9.Fang W, Mueller DL, Pennell CA, Rivard JJ, Li YS, Hardy RR, Schlissel MS, Behrens TW. Frequent aberrant immunoglobulin gene rearrangements in pro-B cells revealed by a bcl-xL transgene. Immunity. 1996;4:291–299. doi: 10.1016/s1074-7613(00)80437-9. [DOI] [PubMed] [Google Scholar]
- 10.Van De Wiele CJ, Marino JH, Murray BW, Vo SS, Whetsell ME, Teague TK. Thymocytes between the beta-selection and positive selection checkpoints are nonresponsive to IL-7 as assessed by STAT-5 phosphorylation. J Immunol. 2004;172:4235–4244. doi: 10.4049/jimmunol.172.7.4235. [DOI] [PubMed] [Google Scholar]
- 11.Will WM, Aaker JD, Burchill MA, Harmon IR, O'Neil J J, Goetz CA, Hippen KL, Farrar MA. Attenuation of IL-7 Receptor Signaling Is Not Required for Allelic Exclusion. J Immunol. 2006;176:3350–3355. doi: 10.4049/jimmunol.176.6.3350. [DOI] [PubMed] [Google Scholar]
- 12.Barata JT, Silva A, Brandao JG, Nadler LM, Cardoso AA, Boussiotis VA. Activation of PI3K is indispensable for interleukin 7-mediated viability, proliferation, glucose use, and growth of T cell acute lymphoblastic leukemia cells. The Journal of experimental medicine. 2004;200:659–669. doi: 10.1084/jem.20040789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Igarashi H, Gregory SC, Yokota T, Sakaguchi N, Kincade PW. Transcription from the RAG1 locus marks the earliest lymphocyte progenitors in bone marrow. Immunity. 2002;17:117–130. doi: 10.1016/s1074-7613(02)00366-7. [DOI] [PubMed] [Google Scholar]
- 14.Vosshenrich CA, Cumano A, Muller W, Di Santo JP, Vieira P. Thymic stromal-derived lymphopoietin distinguishes fetal from adult B cell development. Nature immunology. 2003;4:773–779. doi: 10.1038/ni956. [DOI] [PubMed] [Google Scholar]
- 15.Zhang S, Mantel C, Broxmeyer HE. Flt3 signaling involves tyrosylphosphorylation of SHP-2 and SHIP and their association with Grb2 and Shc in Baf3/Flt3 cells. J Leukoc Biol. 1999;65:372–380. doi: 10.1002/jlb.65.3.372. [DOI] [PubMed] [Google Scholar]
- 16.McKenna HJ, Stocking KL, Miller RE, Brasel K, De Smedt T, Maraskovsky E, Maliszewski CR, Lynch DH, Smith J, Pulendran B, Roux ER, Teepe M, Lyman SD, Peschon JJ. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95:3489–3497. [PubMed] [Google Scholar]
- 17.Linnekin D. Early signaling pathways activated by c-Kit in hematopoietic cells. The international journal of biochemistry & cell biology. 1999;31:1053–1074. doi: 10.1016/s1357-2725(99)00078-3. [DOI] [PubMed] [Google Scholar]
- 18.DeKoter RP, Schweitzer BL, Kamath MB, Jones D, Tagoh H, Bonifer C, Hildeman DA, Huang KJ. Regulation of the interleukin-7 receptor alpha promoter by the Ets transcription factors PU.1 and GA-binding protein in developing B cells. The Journal of biological chemistry. 2007;282:14194–14204. doi: 10.1074/jbc.M700377200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xue HH, Bollenbacher-Reilley J, Wu Z, Spolski R, Jing X, Zhang YC, McCoy JP, Leonard WJ. The transcription factor GABP is a critical regulator of B lymphocyte development. Immunity. 2007;26:421–431. doi: 10.1016/j.immuni.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Sharrocks AD. The ETS-domain transcription factor family. Nat Rev Mol Cell Biol. 2001;2:827–837. doi: 10.1038/35099076. [DOI] [PubMed] [Google Scholar]
- 21.Yang ZF, Mott S, Rosmarin AG. The Ets transcription factor GABP is required for cell-cycle progression. Nature cell biology. 2007;9:339–346. doi: 10.1038/ncb1548. [DOI] [PubMed] [Google Scholar]
- 22.Choi YS, Chakrabarti R, Escamilla-Hernandez R, Sinha S. Elf5 conditional knockout mice reveal its role as a master regulator in mammary alveolar development: failure of Stat5 activation and functional differentiation in the absence of Elf5. Developmental biology. 2009;329:227–241. doi: 10.1016/j.ydbio.2009.02.032. [DOI] [PubMed] [Google Scholar]
- 23.Buday L, Downward J. Many faces of Ras activation. Biochimica et biophysica acta. 2008;1786:178–187. doi: 10.1016/j.bbcan.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Carrano AC, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nature cell biology. 1999;1:193–199. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- 25.DeKoter RP, Lee HJ, Singh H. PU.1 regulates expression of the interleukin-7 receptor in lymphoid progenitors. Immunity. 2002;16:297–309. doi: 10.1016/s1074-7613(02)00269-8. [DOI] [PubMed] [Google Scholar]
- 26.Seki Y, Yang J, Okamoto M, Tanaka S, Goitsuka R, Farrar MA, Kubo M. IL-7/STAT5 cytokine signaling pathway is essential but insufficient for maintenance of naive CD4 T cell survival in peripheral lymphoid organs. J Immunol. 2007;178:262–270. doi: 10.4049/jimmunol.178.1.262. [DOI] [PubMed] [Google Scholar]
- 27.Le Y, Zhu BM, Harley B, Park SY, Kobayashi T, Manis JP, Luo HR, Yoshimura A, Hennighausen L, Silberstein LE. SOCS3 protein developmentally regulates the chemokine receptor CXCR4-FAK signaling pathway during B lymphopoiesis. Immunity. 2007;27:811–823. doi: 10.1016/j.immuni.2007.09.011. [DOI] [PubMed] [Google Scholar]